Abstract
Background and Purpose
The non-psychotropic cannabinoid cannabichromene is known to activate the transient receptor potential ankyrin-type1 (TRPA1) and to inhibit endocannabinoid inactivation, both of which are involved in inflammatory processes. We examined here the effects of this phytocannabinoid on peritoneal macrophages and its efficacy in an experimental model of colitis.
Experimental Approach
Murine peritoneal macrophages were activated in vitro by LPS. Nitrite levels were measured using a fluorescent assay; inducible nitric oxide (iNOS), cyclooxygenase-2 (COX-2) and cannabinoid (CB1 and CB2) receptors were analysed by RT-PCR (and/or Western blot analysis); colitis was induced by dinitrobenzene sulphonic acid (DNBS). Endocannabinoid (anandamide and 2-arachidonoylglycerol), palmitoylethanolamide and oleoylethanolamide levels were measured by liquid chromatography-mass spectrometry. Colonic inflammation was assessed by evaluating the myeloperoxidase activity as well as by histology and immunohistochemistry.
Key Results
LPS caused a significant production of nitrites, associated to up-regulation of anandamide, iNOS, COX-2, CB1 receptors and down-regulation of CB2 receptors mRNA expression. Cannabichromene significantly reduced LPS-stimulated nitrite levels, and its effect was mimicked by cannabinoid receptor and TRPA1 agonists (carvacrol and cinnamaldehyde) and enhanced by CB1 receptor antagonists. LPS-induced anandamide, iNOS, COX-2 and cannabinoid receptor changes were not significantly modified by cannabichromene, which, however, increased oleoylethanolamide levels. In vivo, cannabichromene ameliorated DNBS-induced colonic inflammation, as revealed by histology, immunohistochemistry and myeloperoxidase activity.
Conclusion and Implications
Cannabichromene exerts anti-inflammatory actions in activated macrophages – with tonic CB1 cannabinoid signalling being negatively coupled to this effect – and ameliorates experimental murine colitis.
Keywords: cannabichromene, cannabinoid receptors, endocannabinoids, inflammatory bowel disease, macrophages, nitric oxide, non-psychotropic cannabinoids, oleoylethanolamide, transient receptor potential (TRP) channels, TRPA1
Introduction
Preparations derived from Cannabis sativa have been used medicinally to treat inflammatory conditions since the earliest written records on pharmacobotany (Zurier, 2003). For example, Cannabis was reported to be helpful against rheumatisms about 4000 years ago in the ancient China (Zurier, 2003). The empirical traditional uses of Cannabis encountered scientific evidence nearly 40 years ago, when it was demonstrated that a crude Cannabis extract exerted anti-inflammatory actions in the carrageenan-induced paw oedema model of acute inflammation in rats (Sofia et al., 1974). The anti-inflammatory action of Cannabis was attributed to Δ9-tetrahydrocannabinol (Δ9-THC), the main psychotropic ingredient of the plant Cannabis, which activates both cannabinoid type 1 and 2 (CB1 and CB2) receptors, to cannabidiol (CBD), the main non-psychotropic component which does not efficiently activate cannabinoid receptors, and to cannabinol, a weak agonist of CB1 and CB2 receptors (Burstein and Zurier, 2009). More recently, Δ9-THC and CBD were shown to be beneficial in experimental models of chronic inflammation such as arthritis (Malfait et al., 2000; Cox and Welch, 2004) and have been administered as an oromucosal spray to patients with rheumatoid arthritis (Blake et al., 2006).
In addition to Δ9-THC, CBD and cannabinol, the plant C. sativa contains many other cannabinoids which could theoretically contribute to the anti-inflammatory effects of Cannabis preparations (Izzo et al., 2009; Russo, 2011). One of such compounds is cannabichromene (CBC), the isolation and structure elucidation of which was reported in 1966 (Izzo et al., 2009). CBC is one of four major cannabinoids in C. sativa and it is known to be abundant in high-grade drug-type marijuana, with little or no CBD (Holley et al., 1975). CBC represents 0.3% of the constituents from confiscated Cannabis preparations in the USA (Mehmedic et al., 2010). Despite the relative abundance of this phytocannabinoid, its pharmacological activity has been hardly investigated at all. Of relevance to the present study, CBC was shown to reduce carrageenan- and LPS-induced paw oedema in rodents (Wirth et al., 1980; Turner and Elsohly, 1981; DeLong et al., 2010). Pharmacodynamic studies have shown that CBC is an inhibitor of endocannabinoid cellular reuptake (Ligresti et al., 2006), a weak inhibitor of monoacylglycerol lipase (MAGL, i.e. the main enzyme involved in the inactivation of the endocannabinoid 2-arachydonoylglycerol) and a potent activator of transient receptor potential (TRP) ankyrin 1-type (TRPA1) channels (De Petrocellis et al., 2008; De Petrocellis et al., 2012). Both endocannabinoids and TRPA1 are known to be involved in inflammatory processes (McMahon and Wood, 2006; Burstein and Zurier, 2009).
Given the traditional use of Cannabis preparations in treating inflammatory conditions and in the light of the consideration that TRPA1 and endocannabinoids play an important role in inflammation, we have here evaluated the effect of CBC on activated macrophages. In addition, since macrophages play a pivotal role in inflammatory bowel disease (IBD) (Yoshino et al., 2010) and moreover, Cannabis preparations exert beneficial effects in IBD patients (Lahat et al., 2012), we also evaluated the effect of CBC in a murine colitis model.
Methods
Animals
Male ICR mice (Harlan Laboratories, S. Pietro al Natisone, Italy), weighing 28–32 g, were used after 1-week acclimation period (temperature 23 ± 2°C and humidity 60%). Mice were fed ad libitum with standard food, except for the 24-h period immediately preceding the administration of dinitrobenzene sulphonic acid (DNBS). All animal procedures were in conformity with the principles of laboratory animal care (NIH publication no. 86–23, revised 1985) and the Italian D.L. no. 116 of 27 January 1992 and associated guidelines in the European Communities Council Directive of 24 November 1986 (86/609/ECC). All studies involving animals are reported in accordance with the ARRIVE guidelines for reporting experiments involving animals (Kilkenny et al., 2010; McGrath et al., 2010).
Thioglycollate-elicited mouse peritoneal macrophages
Peritoneal macrophages were obtained from mice as previously described by Aviello et al. (2011). Briefly, to evoke the production of peritoneal exudates rich in macrophages, mice were injected intraperitoneally with 1 mL of 10% w/v sterile thioglycollate medium (Sigma-Aldrich, Milan, Italy). After 4 days, mice were killed and the peritoneal macrophages were collected and seeded in appropriate plates for performing in vitro experiments (Aviello et al., 2011).
Cell culture and inflammatory insult
Peritoneal macrophages were cultured in DMEM supplemented with 10% FBS. The inflammatory response in peritoneal macrophages was induced by LPS from Escherichia coli serotype O111 : B4 (1 μg·mL−1). The acute inflammatory response in macrophages required an LPS incubation time of 18 h (Aviello et al., 2011).
Cytotoxicity
Cell respiration was assessed by the mitochondrial dependent reduction of 3-(4,5-dimethylthiazol- 2-yl)-2,5-diphenyltetrazolium bromide (MTT) to formazan. After incubation with the tested compounds for 24 h, macrophages (1 × 105 cells per well seeded in a 96-well plate) were incubated with MTT (250 μg·mL−1) for 1 h. After solubilization in dimethyl sulfoxide (DMSO), the extent of reduction of MTT to formazan was quantitated by measuring the optical density at 490 nm (iMark™ Microplate Absorbance Reader, Bio-Rad, Milan, Italy). Treatments were compared with a reference cytotoxic drug (DMSO 20% v/v). Results are expressed as a percentage of the corresponding controls (without treatment).
Nitrite measurement and pharmacological treatment in vitro
Nitrites, stable metabolites of NO, were measured in macrophages medium as previously described (Aviello et al., 2011). Macrophages (5 × 105 cells per well seeded in a 24-well plate) were incubated with CBC (0.001–1 μM) for 30 min and subsequently with LPS (1 μg·mL−1) for 18 h. After reduction of nitrates to nitrites by cadmium, cell supernatants were incubated with 2,3-diaminonaphtalene (DAN) (50 μg·mL−1) for 7 min. After stopping the reaction with 2.8 N NaOH, nitrite levels were measured using a fluorescent microplate reader (LS55 Luminescence Spectrometer; PerkinElmer Life Sciences, Cambridge, UK; excitation–emission wavelengths of 365–450 nm).
In a subsequent set of experiments, cannabinoid receptor antagonists [0.1 μM 5-(4-Chlorophenyl)-1-(2,4-dichloro-phenyl)-4-methyl-N-(piperidin-1-yl)-1H-pyrazole-3-carboxamide (rimonabant), 1 μM N-(Piperidin-1-yl)-5-(4-iodophenyl)-1-(2,4-dichlorophenyl)-4-methyl-1H-pyrazole-3-carboxamide (AM251) and 0.1 μM N-[(1S)-endo-1,3,3-trimethylbicyclo [2.2.1]heptan2-yl]-5-(4-chloro-3-methylphenyl)-1-[(4-methylphenyl)methyl]-1H-pyrazole-3-carboxamide (SR144528) or a non-selective adenosine receptors antagonist [0.1 μM 9-Chloro-2-(2-furanyl)-[1,2,4]triazolo[1,5-c]quinazolin-5-amine (CGS 15943)] were incubated 30 min before CBC (1 μM). In some experiments, cells were also treated with arachidonyl-2′-chloroethylamide (ACEA) (0.001–0.1 μM, CB1 receptor agonists), (6aR,10aR)-3-(1,1-Dimethylbutyl)-6a,7,10,10a-tetrahydro-6,6,9-trimethyl-6H-dibenzo[b, d]pyran (JWH133) (0.001–0.1 μM, CB2 receptor agonist), 5-isopropyl-2-methylphenol 2-Methyl-5-(1-methylethyl)-phenol (carvacrol) (0.001–0.1 M, TRPA1 agonist), (2E)-3-phenylprop-2-enal (cinnamaldehyde) (0.001–0.1 μM, TRPA1 agonist), 4-(4-Chlorophenyl)-3-methyl-3-buten-2-one oxime (AP-18) (10 and 20 μM, TRPA1 antagonists) and 2-(1,3-Dimethyl-2,6-dioxo-1,2,3,6-tetrahydro-7H-purin-7-yl)-N-(4-isopropylphenyl)acetamide (HC-030031) (10 and 20 μM, TRPA1 antagonists), all incubated 30 min before LPS stimulation.
Western blot analysis
Cell lysates were separated on SDS polyacrylamide gel, and transferred to a nitrocellulose membrane (Protran®, Protran Nitrocellulose Transfer Membrane Schleicher & Schuell Bioscience, Germany) using a Bio-Rad Transblot as previously reported in detail (Aviello et al., 2010). Membranes were incubated with mouse anti-inducible nitric oxide (iNOS) and anti-cyclooxygenase-2 (COX-2) (BD Biosciences from Becton Dickinson, Buccinasco, Italy), and subsequently with mouse anti-peroxidase-conjugated goat IgG (Jackson ImmunoResearch from LiStarFish, Milan, Italy). The signals were visualized by enhanced chemiluminescence using ImageQuant 400 equipped with software ImageQuant Capture (GE Healthcare, Milan, Italy) and analysed using Quantity One Software version 4.6.3. The membranes were contextually probed with mouse monoclonal anti β-actin (Sigma-Aldrich), to normalize the results.
Interleukin-1β, interferon-γ and interleukin-10 levels
IL-1β, IFN-γ and IL-10 levels in cell medium after 18-h exposure to CBC 1 μM followed by LPS (1 μg·mL−1) were quantified using commercial ELISA kits (R&D Systems, from Space Import Export, Milan, Italy) according to the manufacturer's instructions.
Identification and quantification of endocannabinoids (anandamide and 2-AG) and related molecules
Endocannabinoid [(anandamide and 2-arachidonoylglycerol (2-AG)], palmitoylethanolamide (PEA) and oleoylethanolamide (OEA) levels were measured in peritoneal macrophages (treated or not with LPS, 1 μg·mL−1 for 18 h). CBC (1 μM) was added 30 min before LPS challenge. Cells were harvested in 70% methanol before cell processing, subsequently extracted, purified and analysed by isotope dilution liquid chromatography-atmospheric pressure-chemical ionization mass spectrometry as previously described (Aviello et al., 2012).
Quantitative (real-time) RT-PCR analysis
Peritoneal macrophages (treated or not with CBC, 1 μM, 30 min before LPS) were collected and homogenized in 1.0 mL of Trizol® (Invitrogen, Carlsbad, CA, USA). Total RNA was extracted according to the manufacturer's recommendations and further purified and DNA digested by the Micro RNA purification system (Invitrogen). Total RNA eluted from spin cartridge was UV-quantified by a Bio-Photometer® (Eppendorf, Santa Clara, CA, USA), and purity of RNA samples was evaluated by the RNA-6000-Nano® microchip assay using a 2100 Bioanalyzer® equipped with a 2100 Expert Software® (Agilent, Santa Clara, CA, USA) following the manufacturer's instructions. For all samples tested, the RNA integrity number was greater than 8 relative to a 0–10 scale. One microgram of total RNA, as evaluated by the 2100 Bioanalyzer. Retro transcription, primer design and qPCR were performed as previously reported in detail (Grimaldi et al., 2009). The amplification profile (10 ng of cDNA for assay) consisted of an initial denaturation of 2 min at 95°C and 40 cycles of 10 s at 96°C, annealing for 15 s at the optimal PCR annealing temperature and elongation for 25 s at 68°C. Assays were performed in quadruplicate, relative normalized expression was evaluated as previously described (Di Marzo et al., 2008).
Radioligand assays: cell lines
Chinese hamster ovarian (CHO) cells, stably transfected with complementary DNA encoding human cannabinoid CB1 receptors, were cultured in Eagle's medium nutrient mixture F-12 Ham supplemented with 1 mM L-glutamine, 10% v/v FBS and 0.6% penicillin-streptomycin together with geneticin (600 μg·mL−1).
Radioligand assays: membrane preparation
Binding assays with [35S]GTPγS were performed with CB1-CHO cell membranes (Brown et al., 2010). The cells were removed from flasks by scraping and then frozen as pellets at −20°C until required. Before use in a radioligand binding assay, cells were defrosted, diluted in Tris-buffer (50 mM Tris-HCl, 50 mM Tris-Base) and homogenized. Protein assays were performed using a Bio-Rad DC kit (Hercules, CA, USA).
Radioligand assays: [35S]GTPγS binding assays
Measurement of agonist-stimulated [35S]GTPγS binding to cannabinoid CB1 receptors was described previously (Brown et al., 2010). The assays were carried out with GTPγS binding buffer (50 mM Tris-HCl, 50 mM Tris-Base, 5 mM MgCl2, 1 mM EDTA, 100 mM NaCl, 1 mM DTT and 0.1% bovine serum albumin) in the presence of [35S]GTPγS and guanosine diphosphate (GDP), in a final volume of 500 μL. Binding was initiated by the addition of [35S]GTPγS to the wells. Non-specific binding was measured in the presence of 30 μM GTPγS. Cannabinoid receptor antagonists, rimonabant or AM251, were incubated 30 min before CBC, at 30°C. Total incubation time was 60 min. The reaction was terminated by a rapid vacuum filtration method using Tris-binding buffer, as described previously, and the radioactivity was quantified by liquid scintillation spectrometry. In all the [35S]GTPγS binding assays, we used 0.1 nM [35S]GTPγS, 30 μM GDP and 33 μg per well of proteins.
Induction of experimental colitis and pharmacological treatment
Colitis was induced by the intracolonic administration of DNBS (Borrelli et al., 2009). Briefly, mice were anaesthetized and DNBS (150 mg·kg−1) was inserted into the colon using a polyethylene catheter (1 mm in diameter) via the rectum (4.5 cm from the anus). Three days after DNBS administration, all animals were killed by asphyxiation with CO2, the mice abdomen was opened by a midline incision and the colon removed, isolated from surrounding tissues, opened along the antimesenteric border, rinsed, weighed and length measured (in order to determine the colon weight/colon length ratio). For biochemical analyses, tissues were kept at −80°C until use, while for histological examination and immunohistochemistry tissues were fixed in 10% v/v formaldehyde. The dose of DNBS was selected on the basis of preliminary experiments showing a remarkable colonic damage associated to high reproducibility and low mortality for the 150 mg·kg−1 dose. The time point of damage evaluation (i.e. 3 days after DNBS administration) was chosen because maximal DNBS-induced inflammation has been reported in mice after 3 days (Massa et al., 2004). Furthermore, previous studies have shown that 3 days after intracolonic DNBS administration in mice, the inflammatory response may be modulated by administration of cannabinoid drugs (Massa et al., 2004; Borrelli et al., 2009). CBC (0.1 and 1 mg·kg−1, i.p.) was injected for 2 consecutive days starting 24 h after DNBS administration. In some experiments, CBC (1 mg·kg−1, i.p.) was given as preventive treatment starting from 3 days before DNBS administration (once a day until the sacrifice).
Histology and immunohistochemistry
Histological and immunochemistry evaluations, performed 3 days after DNBS administration, was assessed on a segment of 1 cm of colon located 4 cm above the anal canal. After fixation for 24 h in saline 10% (v/v) formaldehyde, samples were dehydrated in graded ethanol and embedded in paraffin. Thereafter, 5-μm sections were deparaffinized with xylene, stained with hematoxylin–eosin, and observed in a DM 4000 B Leica microscope (Leica Microsystems, Milan, Italy). For microscopic scoring, we used a modified version of the scoring system reported by D'Argenio et al. (2006). Briefly, colon was scored considering (i) the submucosal infiltration (0, none; 1, mild; 2–3, moderate; 4–5 severe), (ii) the crypt abscesses (0, none, 1–2 rare; 3–5, diffuse) and (iii) the mucosal erosion (0, absent; 1, focus; 2–3, extended until the middle of the visible surface; 4–5, extended until the entire visible surface).
For immunohistochemical detection of Ki-67, paraffin-embedded slides were immersed in a Tris/EDTA buffer (pH 9.0), were heated in a decloaking chamber at 125°C for 3 min and were cooled at room temperature for 20 min. After adding 3% hydrogen peroxide, sections were incubated for 10 min. After washing the sections with Tris-buffered saline Tween-20 (pH 7.6), they were stained with rabbit monoclonal antibody to Ki-67 (Ventana Medical systems, Tucson, AZ, USA). Briefly, each tissue section was incubated with primary antibody to Ki-67 (1:100) for 30 min at room temperature. The slides were washed three times with Tris-buffered saline Tween-20 and were incubated with secondary antibody for 30 min. After the slides were reacted with streptavidin for 20 min, the reaction was visualized by 3,3′-diaminobenzidine tetrahydrochloride for 5 min, and the slides were counterstained with Mayer's hematoxylin. The intensity and localization of immunoreactivities against the primary antibody used were examined on all sections with a microscope (Leica Microsystems).
Myeloperoxidase (MPO) activity in the colon
MPO activity was determined as previously described (Goldblum et al., 1985). Full-thickness colons were homogenized in an appropriate lysis buffer [hexadecyltrimethylammonium bromide 0.5% in 3-(N-morpholino)propanesulfonic acid (MOPS) 10 mM] in ratio 50 mg tissue/1 mL MOPS. The samples were then centrifuged for 20 min at 15 000× g at 4°C. An aliquot of the supernatant was then incubated with NaPP (sodium phosphate buffer pH 5.5) e tetra-methyl-benzidine 16 mM. After 5 min, H2O2 (9.8 M) in NaPP was added and the reaction stopped adding acetic acid. The rate of exchange in absorbance was measured by a spectrophotometer at 650 nm. Different dilutions of human MPO enzyme of known concentration were used to obtain a standard curve (Sigma-Aldrich). MPO activity was expressed as Units·mL−1.
Intestinal permeability in the colon
Intestinal permeability was evaluated using a fluorescein isothiocyanate (FITC)-labelled-dextran method, as described previously (Osanai et al., 2007). Briefly, 2 days after DNBS administration, mice were gavaged with 0.6 mg·g−1 body weight of FITC-conjugated dextran. One day later, blood was collected and the serum was immediately analysed for FITC-derived fluorescence using a fluorescent microplate reader with an excitation–emission wavelengths of 485–520 nm (LS55 Luminescence Spectrometer, PerkinElmer Instruments). Preliminary experiments showed that FITC-dextran was stable after 24 h from its preparation. Serial-diluted FICT-dextran was used to generate a standard curve.
Statistical analysis
Results are expressed as mean ± SEM of n experiments. To determine statistical significance, Student's t-test was used for comparing a single treatment mean with a control mean, and a one-way anova followed by a Turkey–Kramer multiple comparisons test was used for analysis of multiple treatment means. P-values <0.05 were considered significant. Values obtained from the radioligand assays have been expressed as means and variability as SEM or as 95% confidence limits. Net agonist-stimulated [35S]GTPγS binding values were calculated by subtracting basal binding values (obtained in the absence of agonist) from agonist-stimulated values (obtained in the presence of agonist) as detailed elsewhere (Brizzi et al., 2005). Values for EC50, maximal effect (Emax) and SEM or 95% confidence limits of these values have been calculated by non-linear regression analysis using the equation for a sigmoid concentration-response curve (GraphPad Prism).
Materials
CBC, (purity by HPLC, 96.3%) was kindly supplied by GW Pharmaceuticals (Porton Down, Wiltshire, UK). Rimonabant and SR144528 were supplied by SANOFI Recherche, (Montpellier, France). ACEA, JWH133, AM251, AP-18, CGS15943 and HC-030031 were purchased from Tocris (Bristol, UK); LPS from E. coli serotype O111:B4, thioglycollate medium, cadmium, DAN, carvacrol, cinnamaldehyde, MTT, DNBS and FITC-conjugated dextran (molecular mass 3–5 kDa) were purchased from Sigma-Aldrich. All reagents for Western blot analysis and cell culture were obtained from Sigma-Aldrich, Bio-Rad Laboratories (Milan, Italy) and Microtech (Naples, Italy). For radioligand binding experiments, [35S]GTPγS (1250 Ci·mmol−1) was obtained from PerkinElmer Life Sciences, GTPγS from Roche Diagnostic (Indianapolis, IN), GDP from Sigma-Aldrich (UK). CBC was dissolved in ethanol (for in vitro experiments), in DMSO (for radioligand assays) or ethanol/Tween20/saline (1:1:8; for in vivo experiments). Rimonabant, SR144528, ACEA, JWH133, AM251, AP-18, CGS15943, HC-030031 were dissolved in DMSO. DNBS was dissolved in 50% ethanol (0.15 mL/mouse). The drug vehicles (0.01% ethanol in vitro; 0.1% DMSO for radioligand assays, 60 μL/mouse in vivo) had no significant effects on the responses under study.
Results
Cytotoxicity
Results are shown in Table 1. CBC, at concentration ranging from 0.001 to 1 μM, did not affect macrophage mitochondrial respiration after 24 h exposure. Similarly, TRP ligands such as carvacrol (0.001–0.1 μM), cinnamaldehyde (0.001–0.1 μM), AP-18 (10 and 20 μM) and HC-030031 (10 and 20 μM) as well as the CB1 receptor agonist ACEA (0.001–0.1 μM), the CB2 receptor agonist JWH133 (0.001–0.1 μM), the CB1 receptor antagonists rimonabant (0.1 μM) and AM251 (1 μM), the CB2 receptor antagonist SR144528 (0.1 μM) and the non-selective adenosine receptor antagonist CGS 15943 (0.1 μM) did not exert cytotoxic effects (Table 1).
Table 1.
Effect of cannabichromene, TRPA1 ligands (carvacrol, cinnamaldehyde, AP-18 and HC-030031), cannabinoid receptor (CB1 and CB2) ligands (ACEA, JWH133, rimonabant, AM251, SR144528), adenosine receptor antagonist CGS 15943 and DMSO (used as a positive control) on cell viability in murine peritoneal macrophages. Cytotoxicity was evaluated using the MTT assay
| Drugs | Concentration (μM) | Viability |
|---|---|---|
| Vehicle | 99.93 ± 4.7 | |
| Cannabichromene | 0.001 | 103.7 ± 8.0 |
| 0.01 | 101.3 ± 4.4 | |
| 0.1 | 96.29 ± 2.9 | |
| 1 | 103.8 ± 3.6 | |
| Vehicle | 100.1 ± 4.9 | |
| Carvacrol | 0.001 | 98.8 ± 5.1 |
| 0.01 | 96.2 ± 3.9 | |
| 0.1 | 90.6 ± 3.1 | |
| Vehicle | 100.2 ± 2.5 | |
| Cinnamaldehyde | 0.001 | 99.3 ± 2.1 |
| 0.01 | 104.9 ± 2.7 | |
| 0.1 | 94.1 ± 2.4 | |
| Vehicle | 99.9 ± 2.8 | |
| AP-18 | 10 | 99.4 ± 3.2 |
| 20 | 103.7 ± 3.2 | |
| Vehicle | 99.9 ± 2.8 | |
| HC-030031 | 10 | 100.5 ± 3.3 |
| 20 | 107.8 ± 2.9 | |
| Vehicle | 100.3 ± 2.2 | |
| ACEA | 0.001 | 99.5 ± 2.9 |
| 0.01 | 98.6 ± 1.5 | |
| 0.1 | 99.6 ± 3.1 | |
| Vehicle | 99.9 ± 2.8 | |
| JWH133 | 0.001 | 101.6 ± 3.9 |
| 0.01 | 97.9 ± 3.2 | |
| 0.1 | 100.7 ± 2.7 | |
| Vehicle | 99.93 ± 3.4 | |
| Rimonabant | 0.1 | 98.25 ± 1.8 |
| Vehicle | 99.93 ± 3.4 | |
| AM251 | 1 | 98.9 ± 2.7 |
| Vehicle | 100.0 ± 3.2 | |
| SR144528 | 0.1 | 94.9 ± 1.8 |
| Vehicle | 100.0 ± 4.1 | |
| CGS15943 | 0.1 | 95.8 ± 2.1 |
| DMSO | 20% v/v | 24.50 ± 1.783*** |
Results are mean ± SEM of three experiments (in triplicates).
P < 0.001 versus corresponding control (medium).
CBC reduces nitrite levels in LPS-stimulated macrophages
In cells not treated with LPS, CBC (0.001–1 μM) did not modify basal nitrite levels [nitrite levels (nM) ± SEM: control 614.4 ± 31.5, CBC 0.001 μM 620.5 ± 32.1, CBC 0.01 μM 618.4 ± 24.6, CBC 0.1 μM 612.7 ± 29.6, CBC 1 μM 626.9 ± 36.2; n = 12]. LPS (1 μg·mL−1 for 18 h) administration caused a significant increase in nitrite production (Figure 1). A pretreatment with CBC (0.001–1 μM), 30 min before LPS, significantly reduced LPS-increased nitrite levels (Figure 1). CBC was also effective when given 15 h after LPS challenge (i.e. 3 h before nitrite assay) (see insert to Figure 1). No significant differences were found in CBC effect when the compound was given 30 min before LPS or 15 h after LPS (i.e. 3 h before the nitrite assay, see overlapping curves in the insert to Figure 1). Like CBC, the CB1 receptor agonist ACEA (0.001–0.1 μM) and the CB2 receptor agonist JWH133 (0.001–0.1 μM) reduced the production of nitrites stimulated by LPS when given 30 min before LPS [nitrite levels (nM) ± SEM: control 642.2 ± 51.6, LPS 1 μg·mL−1 911.3 ± 42.4#, ACEA 0.001 μM 782.3 ± 12.0*, ACEA 0.01 μM 730.9 ± 20.4**, ACEA 0.1 μM 699.8 ± 18.1***; n = 6, #P < 0.01 vs. control, *P < 0.05, **P < 0.01 and ***P < 0.001 vs. LPS alone. Control 842.0 ± 18.4, LPS 1 μg·mL−1 1200 ± 55.3#, JWH133 0.001 μM 942.5 ± 70.7*, JWH133 0.01 μM 965.8 ± 58.7*, JWH133 0.1 μM 707.0 ± 83.6***; n = 6, #P < 0.001 vs. control, *P < 0.05 and ***P < 0.001 vs. LPS alone.].
Figure 1.
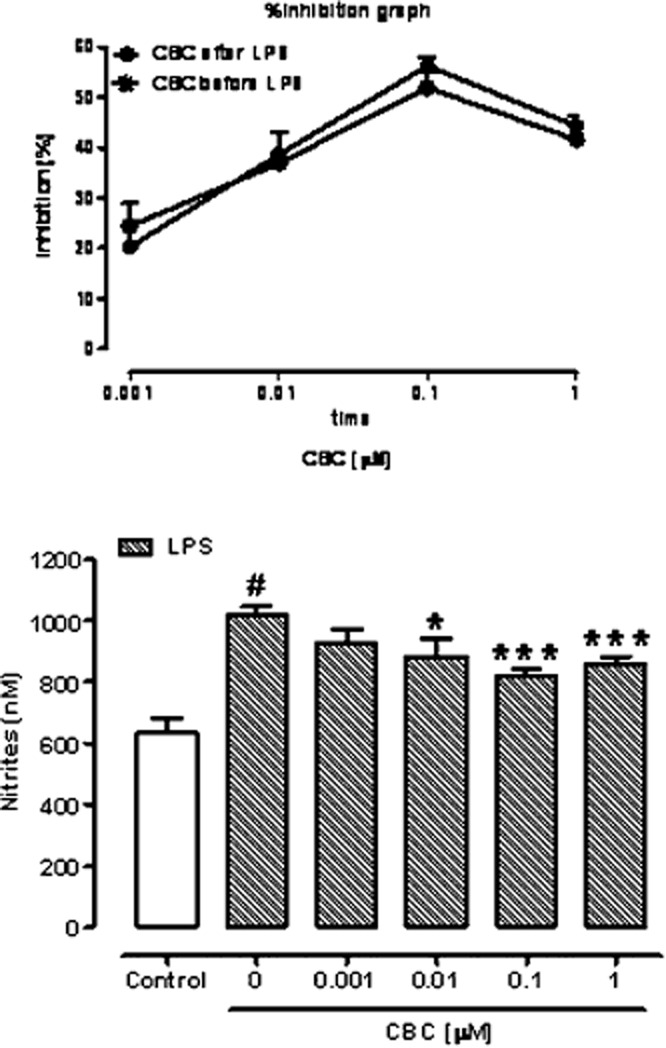
Inhibitory effect of cannabichromene on nitrite levels in the cell medium of murine peritoneal macrophages incubated with lipopolysaccharide (LPS, 1 μg·mL−1) for 18 h. Cannabichromene (CBC, 0.001–1 μM) was added to the cell media 30 min before LPS challenge (i.e. 18.5 h before nitrites assay). Results are mean ± SEM of six experiments (in triplicates). #P < 0.001 versus control; *P < 0.05 and ***P < 0.001 versus LPS alone. The insert (on top of the figure) shows the effect of CBC (expressed as percentage of inhibition of the corresponding control values, with the difference between LPS and control considered as 100%) when given 30 min before LPS (CBC before LPS) or 15 h after LPS (CBC after LPS). No statistically significant difference was observed between the two concentration–response curves reported in the insert.
CBC does not modify iNOS and COX-2 (mRNA and protein) levels in LPS-treated macrophages
In order to verify if the effect of CBC on the increased nitrite production was associated to changes in iNOS expression, we measured the mRNA and protein levels of this enzyme both by RT-PCR and by Western blot. LPS administration up-regulated iNOS mRNA and protein expression (Figure 2A,B). CBC (1 μM) incubated 30 min before LPS stimulation, did not modify LPS-induced changes in iNOS expression (Figure 2A,B). COX-2 is a key enzyme involved in the macrophages function. Similarly to iNOS, LPS administration caused up-regulation of COX-2 mRNA and protein expression (Figure 2C,D). CBC (1 μM) incubated 30 min before LPS stimulation, did not modify LPS-induced COX-2 up-regulation (Figure 2C,D).
Figure 2.
Inducible nitric oxide synthase (iNOS) (A, B) and cyclooxygenase-2 (COX-2) (C, D) mRNA and protein levels in cell lysates from macrophages incubated or not with lipopolysaccharide (LPS, 1 μg·mL−1) for 18 h. mRNA expression was evaluated by RT-PCR. The expression levels, normalized with respect to the reference genes, were scaled to the expression value of the control, considered as 1. The means of the quantitative-cycles (Cq) for the control were: 26.00 and 25.58 for iNOS and COX-2 respectively. The reaction background was N/A (see text) at 40 reaction cycles. Protein expression was evaluated by Western blot analysis. Cannabichromene (CBC, 1 μM) was added to the cell media 30 min before LPS challenge. #P < 0.001 versus control (n = 4–5 experiments).
CBC reduces IL-10 and INF-γ in LPS-treated macrophages
Interleukins and interferon-γ (INF-γ) are important cytokines involved in LPS-evoked responses in macrophages. The levels of IL-1β, INF-γ and IL-10 in macrophages medium were significantly increased after 18-h exposure to LPS (Figure 3A–C). A pretreatment with CBC (1 μM), incubated 30 min before LPS stimulation, significantly reduced INF-γ and IL-10 (but not IL-1β) levels in macrophages (Figure 3A–C).
Figure 3.
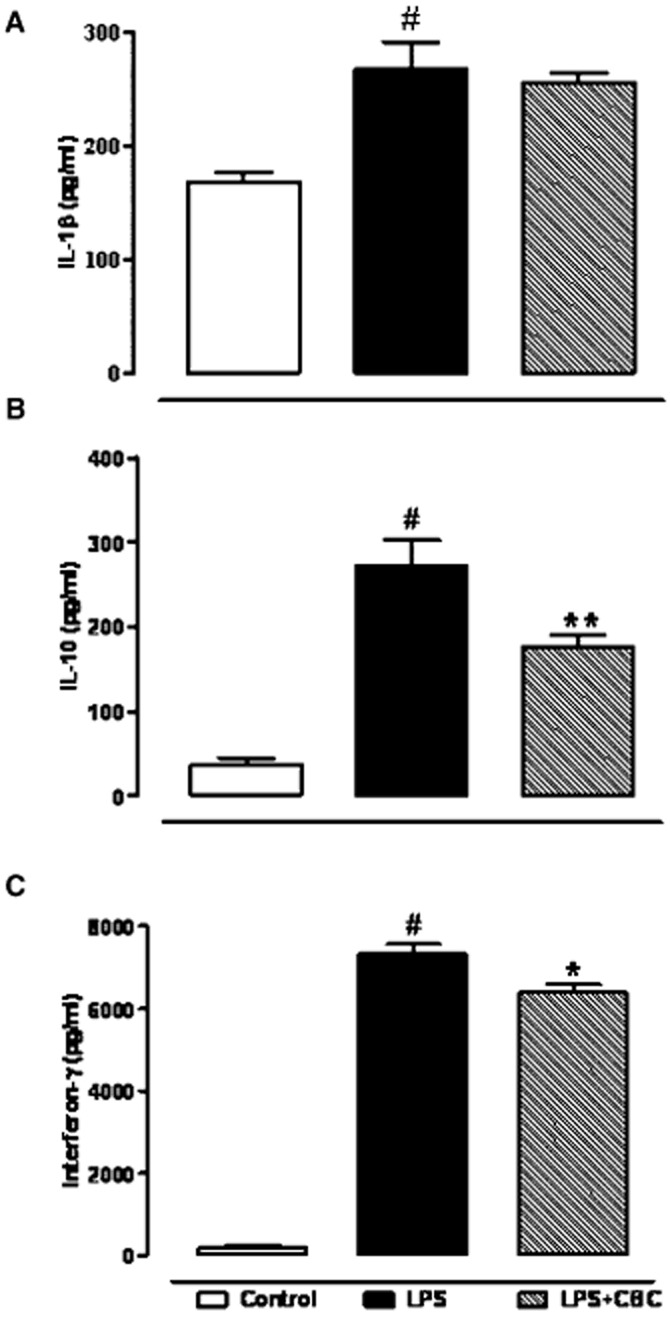
Effect of cannabichromene (CBC) on interleukin-1β (IL-1β) (A), interleukin-10 (IL-10) (B) and interferon-γ (C) levels detected in the cell media of macrophages incubated with lipopolysaccharide (LPS, 1 μg·mL−1) for 18 h. CBC (1 μM) was added to the media 30 min before LPS challenge. Results are means ± SEM of four experiments (in quadruplicates). #P < 0.001 versus control, *P < 0.05 and **P < 0.01 versus LPS.
The effect of CBC on nitrite production is modulated by selective CB1 receptor antagonists
Because CBC can inhibit endocannabinoid inactivation (De Petrocellis et al., 2011), in this set of experiments, we verified if CBC effect on nitrite production was reduced or counteracted by selective CB1 and CB2 receptor antagonists. We found that rimonabant (0.1 μM) and AM251 (1 μM) (two CB1 receptor antagonists) not only did not counteract but, instead, significantly enhanced the inhibitory effect of CBC (1 μM) on nitrite production (Figure 4A,B). By contrast, the CB2 receptor antagonist SR144528, at a concentration (0.1 μM) able to block the effect of the selective CB2 receptor agonist JWH133 (0.1 μM) on nitrite production (data not shown) did not modify CBC (1 μM)-induced changes in nitrite production (Figure 4C). Rimonabant, AM251 and SR144528, at the concentrations used, did not modify, per se, nitrite levels induced by LPS [nitrite levels (nM) ± SEM: control 611.9 ± 27.4, LPS 1 μg·mL−1 899.1 ± 25.2#, rimonabant 0.1 μM 863.1 ± 24.8, AM251 1 μM 881.8 ± 21.5, SR144528 0.1 μM 917.1 ± 27.2; n = 6, #P < 0.001 vs. control.].
Figure 4.

Effect of cannabichromene (CBC, 1 μM) alone and in presence of the cannabinoid CB1 receptor antagonists rimonabant (0.1 μM) (A) and AM251 (1 μM) (B) as well as in the presence of the cannabinoid CB2 receptor antagonist SR144528 (0.1 μM) (C) on nitrite levels in the cell medium of murine peritoneal macrophages incubated with lipopolysaccharide (LPS, 1 μg·mL−1) for 18 h. The antagonists were added to the cell media 30 min before CBC exposure. LPS (1 μg·mL−1for 18 h) was incubated 30 min after CBC. Results are means ± SEM of three experiments (in triplicates). #P < 0.001 versus control; *P < 0.05, **P < 0.01 and ***P < 0.001 versus LPS; °P < 0.05 versus LPS + CBC.
Next, using [35S]GTPγS binding assays, we found that when tested at concentrations from 1 nM up to 1 μM, CBC did not display any significant ability to stimulate or inhibit [35S]GTPγS binding to hCB1-CHO cell membranes (data not shown). In contrast, using the same experimental conditions, we found that, when incubated by themselves, 1 μM AM251 and 0.1 μM rimonabant each induced, as expected, a marked inhibition of [35S]GTPγS binding in this bioassay. When 1 μM CBC was added 30 min after 1 μM AM251 or 0.1 μM rimonabant, no significant change in Emax of either of these inverse agonists/antagonists was observed for their production of this inhibition (Figure 5A,B).
Figure 5.

Effect of cannabichromene (CBC, 1 μM) alone and in combination with AM251 (1 μM, CB1 receptor antagonist, A) or rimonabant (SR1, 0.1 μM, CB1 receptor antagonist, B) on [35S]GTPγS binding to hCB1- CHO cell membranes (n = 12–16). CBC was added 30 min after the CB1 antagonists. Symbols represent mean values ± SEM.
TRPA1 ligands reduce nitrite levels in LPS-treated macrophages
Because CBC can activate TRPA1 (De Petrocellis et al., 2008; 2011), we verified if other TRPA1 agonists mimicked the effect of CBC on nitrite production in macrophages treated with LPS. Like CBC, carvacrol and cinnamaldehyde (incubated 30 min before LPS stimulation), both in the 0.001–0.1 μM range, reduced, in a concentration-dependent fashion, nitrite production induced by LPS (Figure 6A,B). However, AP-18 (10 and 20 μM) and HC-030031 (10 and 20 μM), two selective TRPA1 antagonists, inhibited nitrites production induced by LPS administration in macrophages (Figure 6C), suggesting that the effects of the agonists (and possibly CBC) could also be due to activation and subsequent desensitization of TRPA1.
Figure 6.

Inhibitory effect of the selective transient receptor potential ankyrin type 1 (TRPA1) agonists carvacrol (0.001–0.1 μM) and cinnamaldehyde (0.001–0.1 μM) (A, B) and of the selective TRPA1 antagonists AP18 (10–20 μM) and HC-030031 (HC, 10–20 μM) (C) on nitrite levels detected in the cell media of macrophages incubated with lipopolysaccharide (LPS, 1 μg·mL−1) for 18 h. Both TRPA1 agonists and antagonists were added to the medium 30 min before LPS challenge. Results are mean ± SEM of two experiments (in triplicates). #P < 0.001 versus control; *P < 0.05, and ***P < 0.001 versus LPS.
CBC does not modify CB1, CB2 and TRPA1 mRNA alterations induced by LPS in macrophages
In contrast to CB1 and CB2 receptors mRNA, which were robustly expressed (see legend to Figure 7), TRPA1 mRNA was barely detectable in control macrophages (∼35 PCR quantitative-cycles (Cq) vs. background; mouse colon TRPA1 positive control was detectable at 27 Cq). LPS (1 μg·mL−1for 18 h) challenge caused up-regulation of CB1 receptors, down-regulation of CB2 receptors (Figure 7) and an even lower expression of TRPA1 mRNA, the expression of which resulted undetectable. CBC did not modify CB1 and CB2 (Figure 7) nor TRPA1 mRNA expression in LPS-treated macrophages.
Figure 7.
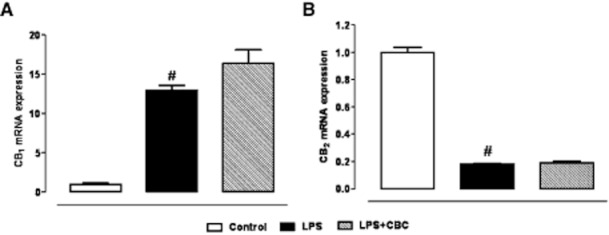
Relative mRNA expression of cannabinoid CB1 receptor (A) and cannabinoid CB2 receptor (B) in cell lysates from macrophages incubated or not with lipopolysaccharide (LPS, 1 μg·mL−1) for 18 h: effect of cannabichromene (CBC, 1 μM, added to the cell media or 30 min before LPS challenge). The expression levels of mRNA, evaluated by qRT-PCR and normalized with respect to the reference genes, was scaled for all conditions to the expression value of the control, considered as 1. The means of the quantitative-cycles (Cq) for the control values were: 31.2 (CB1 receptor) and 24.48 (CB2 receptor). The reaction background was 37.30 Cq and 36.60 Cq for CB1 receptor and CB2 receptor, respectively, at 40 reaction cycles. #P < 0.001 versus control (n = 4).
CBC increases OEA (but not endocannabinoid) levels in LPS-treated macrophages
Table 2 reports the levels of endocannabinoids, PEA and OEA in murine peritoneal macrophages treated with LPS. The exposure to LPS (1 μg·mL−1) for 18 h induced a significant increase in anandamide (but not 2-AG, PEA or OEA) levels. CBC (1 μM) did not change the levels of the endocannabinoids and PEA in control macrophages (i.e. not treated with LPS), nor in macrophages challenged with LPS (Table 2). By contrast, CBC significantly increased OEA levels in LPS-treated macrophages (Table 2).
Table 2.
Anandamide (AEA), 2-arachydonylglycerol (2-AG), palmitoylethanolamide (PEA) and oleoylethanolamide (OEA) levels in cell lysates from macrophages incubated or not with lipopolysaccharide (LPS, 1 μg·mL−1) for 18 h: effect of cannabichromene (CBC, 1 μM, added alone to the cell media or 30 min before LPS challenge)
| Drugs | AEA | 2-AG | PEA | OEA |
|---|---|---|---|---|
| Vehicle | 0.58 ± 0.13 | 102.1 ± 15.9 | 18.4 ± 2.9 | 9.52 ± 1.4 |
| CBC | 0.65 ± 0.22 | 121.8 ± 38.9 | 18.3 ± 5.5 | 7.7 ± 2.4 |
| LPS | 1.85 ± 0.55# | 122.7 ± 25.9 | 25.6 ± 6.0 | 9.73 ± 2.4 |
| LPS + CBC | 1.5 ± 0.72 | 173.9 ± 23.0 | 33.9 ± 4.2 | 20.4 ± 2.9* |
Results (pmol·mg−1 lipid) are mean ± SEM of three–six experiments. P < 0.01 versus control; *P < 0.05 versus LPS.
The effect of CBC on nitrite production is not counteracted by a adenosine receptors antagonist
Because some of the pharmacological action of CBC have been shown to be counteracted by the adenosine receptors antagonist CGS 15943 (Maione et al., 2011), we investigated the action of this antagonist on CBC-induced changes on nitrite production. We found that CGS 15943 (0.1 μM) did not modify CBC-induced reduction in nitrites levels in macrophages challenged with LPS [Nitrite levels (nM) ± SEM: control 809.3 ± 28.5, LPS 1 μg·mL−1 998.7 ± 25.7#, CBC 0.1 μM 841.1 ± 12.9***, CBC 0.1 μM + CGS15943 0.1 μM 810.6 ± 31.3; n = 12, #P < 0.001 vs. control; ***P < 0.001 vs. LPS alone]. CGS 15943 (0.1 μM), per se, did not modify nitrite productions in LPS-treated macrophages (data not shown).
CBC ameliorates DNBS-induced colitis (colon weight/colon length ratio, intestinal permeability MPO activity, histology and immunohistochemistry)
Because macrophages play a pivotal role in inflammatory diseases, including IBD, we investigated the effect of this phytocannabinoid in an experimental model of colitis. DNBS administration caused a significant increase in colon weight/colon length ratio (Figure 8). CBC, at the doses of 0.1 and 1 mg·kg−1 [given intraperitoneally after the inflammatory insult], significantly reduced the effects of DNBS on colon weight/colon length ratio. The effect was significant for the dose of 1 mg·kg−1. At the 1 mg·kg−1 dose, CBC significantly reduced DNBS-induced increase in intestinal permeability (Figure 9A) and MPO activity (Figure 9B). CBC (1 mg·kg−1) exerted protective effects also when given before the inflammatory insult [colon weight/colon length ratio ± SEM: control 26.7 ± 1.2, DNBS 41.6 ± 1.9#, CBC (1 mg·kg−1, i.p.) 30.6 ± 1.9**; n = 9 mice, #P < 0.001 vs. control; **P < 0.01 vs. DNBS].
Figure 8.

Dinitrobenzene sulfonic acid (DNBS)-induced colitis in mice. Colon weight/colon length ratio of colons from control and DNBS-treated mice in the presence or absence of cannabichromene (CBC). Tissues were analysed 3 days after vehicle or DNBS (150 mg·kg−1, intracolonically) administration. CBC (0.1 and 1 mg·kg−1) was administered (i.p.) once a day for 2 consecutive days starting 24-h after the inflammatory insult. Bars are mean ± SEM of 12–15 mice for each experimental group. #P < 0.001 versus control; **P < 0.01 versus DNBS alone.
Figure 9.
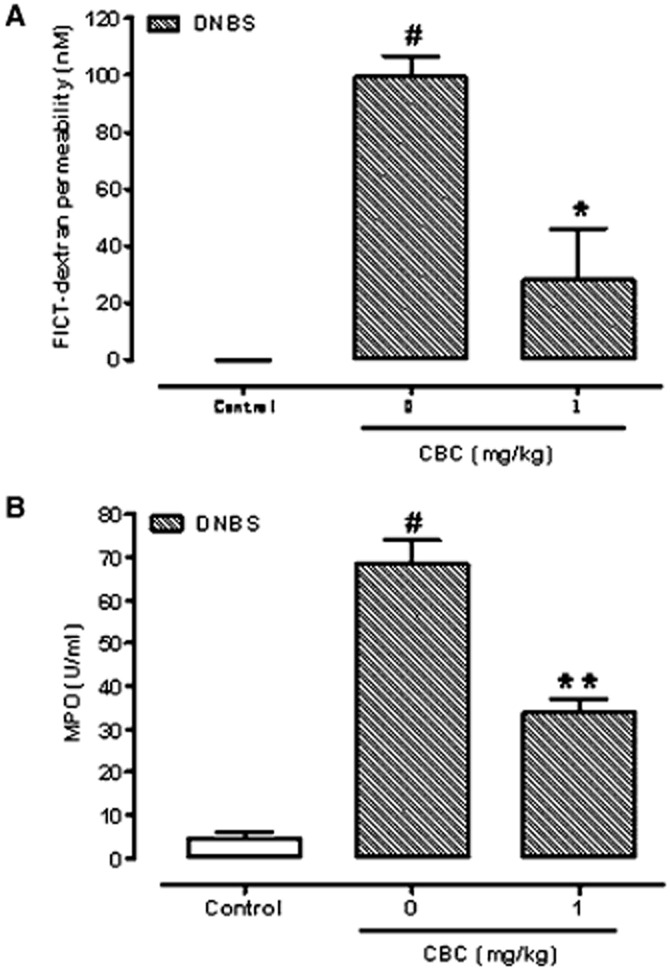
Inhibitory effect cannabichromene (CBC) on serum FICT-dextran concentration (a measure of intestinal barrier function) (A) and myeloperoxidase (MPO, a marker of intestinal inflammation) activity (B) in dinitrobenzene (DNBS)-induced colitis in mice. Permeability and MPO activity were measured on colonic tissues 3 days after vehicle or DNBS (150 mg·kg−1, intracolonically). CBC (1 mg·kg−1) was administered (i.p.) for 2 consecutive days starting 24 h after the inflammatory insult. Bars are mean ± SEM of five mice for each experimental group. #P < 0.001 versus control; *P < 0.05 and **P < 0.01 versus DNBS alone.
Histological analysis showed, in control mice, a normal appearance, with intact epithelium of the colonic mucosa (Figure 0A). In DNBS-treated mice, subtotal erosions of the mucosa, and diffuse lymphocyte infiltration involving the muscularis mucosae and the submucosa were observed (Figure 0B). CBC treatment (1 mg·kg−1, given i.p. after DNBS) resulted in a regenerative area surrounding the residual focal erosions (Figure 0C).
Figure 10.
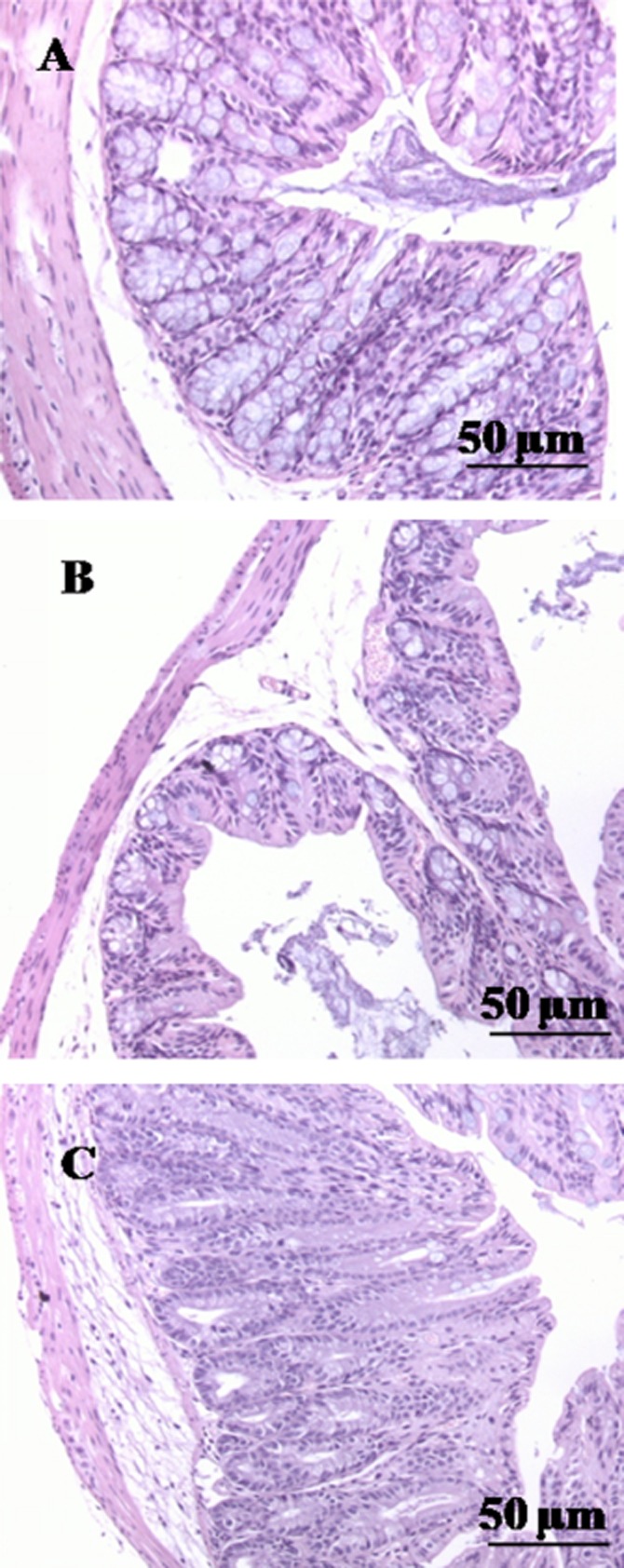
Histological evaluations of inflamed and non-inflamed colons: effect of cannabichromene (CBC). No histological modification was observed in the mucosa and submucosa of control mice (A); mucosal injury induced by dinitrobenzene sulfonic acid (DNBS) administration (B); treatment with CBC reduced colon injury stimulating a regeneration of the glands (C). CBC (1 mg·kg−1) was administered (i.p.) for 2 consecutive days starting 24 h after the inflammatory insult. Histological analysis was performed 3 days after DNBS (150 mg·kg−1, intracolonically). Original magnification ×200. The figure is representative of three experiments.
Immunohistochemical analyses confirmed the beneficial effect of CBC on inflamed colonic mucosa. In control tissues, Ki-67 immunoreactivity revealed proliferative activity on the fundus of the foveole glands (Figure 11A). In the colon from DNBS-treated mice, total necrosis with Ki-67 immunoreactivity on inflammatory cells was observed (Figure 11B). CBC (1 mg·kg−1, given i.p. after DNBS) reduced the effect of DNBS on cell proliferation, the mitotic activity being restricted to one half of the mucosa (Figure 11C).
Figure 11.

Different patterns of Ki-67 immunoreactivity in the colonic mucosa of control mice (A), dinitrobenzene sulfonic acid (DNBS)-treated mice (B) and mice treated with DNBS plus cannabichromene (C). (A) Ki-67 immunopositive cells localized to the lower of the crypts. (B) Ki-67 immunoreactivity was observed on inflammatory cells. (C) Ki-67 immunopositive cells observed only in the expanded basal zone. CBC (1 mg·kg−1) was administered (i.p.) for 2 consecutive days starting 24 h after the inflammatory insult. The figure is representative of three experiments.
Discussion
Preparations of Cannabis have been used since antiquity as medicinal agents to alleviate the symptoms of inflammation, including IBD (Zurier, 2003). The effect of Δ9-THC and CBD, two main Cannabis constituents, on the inflammatory response is well established and their effect on inflammation has been extensively reviewed (Burstein and Zurier, 2009; Booz, 2011). However, the issue of whether other Cannabis constituents contribute to the anti-inflammatory effect of the plant is still under investigation. CBC has been shown to reduce the paw oedema induced by carrageenan or LPS in rodents (Turner and Elsohly, 1981; DeLong et al., 2010) as well as to exert anti-inflammatory activity in the croton oil mouse ear dermatitis assay (Tubaro et al., 2010). In the present study, we have demonstrated that CBC inhibits nitric oxide production in LPS-stimulated murine macrophages and ameliorates experimental colitis in mice.
CBC inhibits nitric oxide production in macrophages
Macrophages play a central role in the inflammatory process. Stimulation of murine macrophages by LPS results in the expression of iNOS, which catalyses the production of large amounts of NO, a gaseous substance which is proinflammatory when produced in excess (Moncada et al., 1991). We have found here that CBC reduced the levels of nitrites, the stable metabolites of nitric oxide. It is unlikely that CBC affects the processes linked to the induction of iNOS since the phytocannabinoid (i) was pharmacologically active when given both 30 min before LPS as well as 15 h after the proinflammatory insult, that is once the enzyme had been already expressed and (ii) did not affect iNOS mRNA and protein expression, as revealed by RT-PCR and Western blot analyses. Likewise, CBC did not affect the expression of COX-2, another key enzyme involved in macrophage function. The latter result might be explained by the failure of CBC at reducing the LPS-induced IL-1β levels, since IL-1β represents one of the main proinflammatory cytokines able to induce COX-2 expression in macrophages (Samad et al., 2001; Liu et al., 2003). On the other hand, CBC reduced the levels of both IL-10 and INF-γ, two cytokines which limit or sustain, respectively, the inflammatory response in LPS-treated macrophages (Hawiger, 2001; Moore et al., 2001). The ability of macrophages to overproduce IL-10 (an anti-inflammatory cytokine) in response to LPS has been previously documented (Brightbill et al., 2000; Cao et al., 2005; Aviello et al., 2011) and can be considered as an adaptive reaction of the macrophages aiming at counteracting the inflammatory insult.
To investigate the mechanism of CBC-induced suppression of nitrite production in macrophages, we considered the possible involvement of cannabinoid (CB1 and CB2) and adenosine receptors as well as TRPA1 channels by evaluating the effect of selective receptor antagonists on CBC action as well as by exploring the expression of cannabinoid receptors and TRPA1 in LPS-treated macrophages.
Possible involvement of cannabinoid receptors in the CBC response in macrophages
CBC is a low (1 μM < Ki < 2 μM) and moderate (Ki∼0.1 μM) affinity ligand for human CB1 and CB2 receptors respectively (V. Di Marzo, unpubl. data). Furthermore, CBC inhibits endocannabinoid reuptake, and thus might indirectly activate – via increased extracellular endocannabinoid levels – the cannabinoid receptors (Ligresti et al., 2006; De Petrocellis et al., 2011). CBC was shown to stimulate descending pathways of antinociception and to cause analgaesia in rats in a manner partly attenuated by a CB1 receptor antagonist (Maione et al., 2011). We have shown here that the inhibitory effect of CBC was mimicked by selective CB1 and CB2 receptor agonists, suggesting that a direct pharmacological activation of such receptors results in inhibition of nitrite production. The ability of direct activation of both CB1 and CB2 receptor to reduce nitrite production in activated macrophages was previously documented (Ross et al., 2000; Aviello et al., 2011). Surprisingly, however, we observed that the inhibitory effect of CBC was further increased by rimonabant and AM251 (two CB1 receptor antagonists), at concentrations that, however, were inactive per se. These results, while confirming that exogenous activation of CB1 reduces NO formation in macrophages, negate the possibility that CBC acts via direct or indirect activation of CB1 receptors. This hypothesis is also supported by the results we obtained in the [35S]GTPγS binding assay performed with hCB1-CHO cell membranes. Thus, we found that CBC, at concentrations that included the one at which it significantly inhibits nitric oxide production (1 μM), did not induce any significant activation of cannabinoid CB1 receptors in this assay. Moreover, using the same assay, we also found that when CBC was administered 30 min after 1 μM AM251 or 0.1 μM rimonabant, it did not significantly affect the Emax of either of these compounds for their inhibition of [35S]GTPγS binding. It might be possible that an endogenous CB1 tone exists, which may couple negatively to the CBC signalling pathway and counteract CBC inhibition of nitrite production. Indeed, we found that LPS enhances anandamide levels in macrophages, and that CBC, instead, only elevates OEA levels. According to some authors, also OEA, but not PEA (the levels of which were not elevated by CBC) is taken up by cells through the same mechanism responsible for anandamide uptake (Hillard et al., 1997; Alhouayek and Muccioli, 2012). It is possible that CBC could not elevate anandamide levels because these were already maximally up-regulated by LPS. OEA, which is chemically related to anandamide, was previously shown to produce anti-inflammatory effects (Lo Verme et al., 2005) and hence, it is possible that a part of the beneficial effect of CBC observed here in macrophages could be due to its ability to increase OEA levels. It is also possible that CBC can merely synergize with rimonabant by unmasking the anti-inflammatory action of a per se inactive dose of this antagonist. In agreement with this hypothesis, rimonabant, but not the CB2 receptor antagonist SR144528, was previously shown to inhibit LPS-induced inflammation in wild-type mice, but not CB1 null mice (Croci et al., 2003). Accordingly, we also found here that SR144528 did not change the inhibitory effect of CBC on nitrite production in LPS-challenged macrophages. In another model of LPS-induced inflammation (LPS-induced paw oedema), DeLong et al. (2010) have recently shown that the anti-inflammatory action of CBC in vivo (LPS-induced paw oedema) was not blocked by either SR144528 or rimonabant.
In order to give further insights into the role of cannabinoid receptors in CBC action, we evaluated the effect of this plant constituent on cannabinoid receptors mRNA expression in macrophages. It was recently demonstrated that CBC alters the mRNA expression of cannabinoid receptors in the inflamed gut (Izzo et al., 2012). In the present study, we have shown that LPS causes up-regulation of CB1 receptors and down-regulation of CB2 receptors and that those changes were not modified by CBC. These results rule against the possibility that CBC could exert anti-inflammatory actions in macrophages by altering cannabinoid mRNA receptor expression.
Possible involvement of TRPA1 in the CBC response in macrophages
CBC was shown to potently activate TRPA1 channels, as revealed by the increase in [Ca2+] in human embryonic kidney (HEK)-293 cells overexpressing recombinant rat TRPA1 (De Petrocellis et al., 2008; De Petrocellis et al., 2011). CBC was also found to exert antinociceptive effects in the tail flick test via TRPA1 (Maione et al., 2011). Allyl isothiocyanate, a TRPA1 agonist, was previously shown to reduce nitrite production in LPS-activated macrophages (Ippoushi et al., 2002). In the present study, we have shown that the effect of CBC on nitrite production was mimicked by other TRPA1 agonists, namely carvacrol and cinnamaldehyde (Moran et al., 2011). However, also AP-18 (10 and 20 μM) and HC-030031 (10 and 20 μM), two well-established TRPA1 antagonists (Alexander et al., 2011; Moran et al., 2011), completely blocked nitrite production at a concentration (10 μM) below the IC50 value required to antagonize the agonists HEK-293 cells overexpressing TRPA1 channels (16.5 μM for AP-18 and 13.3 μM for HC-030031) (Capasso et al., 2012). The capability of the two TRPA1 antagonists to efficiently block nitrite production at very low concentrations precluded us the possibility to evaluate the effect of CBC in the presence of AP-18 or HC-030031. It should be also emphasized that the ability of TRPA1 agonists and antagonists to have a pharmacological effect in the same direction is not surprising since it is well known that TRP agonists (via a desensitizing mechanism) and antagonists (via receptor blockade) can interfere with TRP channel function (Moran et al., 2011). Accordingly, CBC was shown to desensitize TRPA1 receptors in HEK-293 cells overexpressing TRPA1 channels after an incubation as short as 5 min (De Petrocellis et al., 2012).
We also investigated TRPA1 mRNA expression in macrophages. Previous in vivo studies found that CBC may change TRPA1 as well as other TRP channel (i.e. transient receptor potential cation channel, subfamily V, member 3 and member 4) mRNA expression in the inflamed gut (De Petrocellis et al., 2012; Izzo et al., 2012), thus providing another potential mechanism – in addition to direct activation – through which this phytocannabinoid can exert pharmacological actions. In the present study, we have observed a very faint expression of TRPA1 mRNA expression in macrophages which was abolished by LPS and not restored by CBC treatment. It is possible that the further reduction of TRPA1 mRNA levels did not result in a complete removal also of TRPA1 protein (i.e. during in vitro culture of the cells the transcription of TRPA1 mRNA could be depressed while residual TRPA1 protein could be still present, a possibility that we could not investigate due to the lack of a commercially available specific and reliable antibody against the mouse TRPA1), thus explaining why the TRPA1 agonists and antagonists tested here did reduce LPS-induced inflammation.
In summary, we have shown that both TRPA1 antagonists and agonists, including CBC, inhibit nitrite productions in macrophages. Whether or not TRPA1 channels are involved in such action has not been conclusively demonstrated in the present study. While the similarity of the effect of TRPA1 agonists and antagonists could be explained by the ability of agonists, including CBC, to activate and subsequently desensitize TRPA1 channels, the undetectable TRPA1 mRNA signal in macrophages activated by LPS seems to rule against this possibility.
Possible involvement of adenosine receptors in the CBC response in macrophages
We also investigated the possible involvement of adenosine receptors. CBC was found to exerts analgesic actions in a CGS 15943 (adenosine receptors antagonist)-sensitive way (Maione et al., 2011). However, we found that the CGS 15943 did not modify the inhibitory effect of CBC on nitrite production, thus excluding a role of these receptors in CBC anti-inflammatory effects.
CBC ameliorates experimental murine colitis
We investigated the effect of CBC in an experimental model of IBD for a number of reasons. First, macrophage targeting treatment ameliorates colonic inflammation in experimental colitis models and the regulation of abnormal responses of macrophages appears to be a promising therapeutic approach for the treatment of IBD (Yoshino et al., 2010). Second, Cannabis is commonly used by IBD patients for symptom relief (Lal et al., 2011; Naftali et al., 2011) and Cannabis inhalation improves clinical disease activity and quality of life in patients with long-standing IBD (Lahat et al., 2012). Third, both endocannabinoids and TRPA1 (i.e. the main pharmacological targets of CBC) are involved in experimental colitis (D'Argenio et al., 2006; Di Marzo and Izzo, 2006; Izzo and Camilleri, 2009; Engel et al., 2011; Holzer, 2011). Fourth, we recently demonstrated that CBC inhibited gastrointestinal transit in an experimental model of intestinal inflammation (Izzo et al., 2012). Other Cannabis constituents, namely Δ9-THC and CBD, were previously shown to ameliorate experimental colitis in rodents after i.p. administration (Borrelli et al., 2009; Jamontt et al., 2010; Schicho and Storr, 2012), a route of administration able to bypass the hepatic metabolism, a limiting factor for oral cannabinoid use (Huestis, 2005). Interestingly, it has been recently reported that intrarectal or intraperitoneal (but not oral) administration of CBD, another plant-derived cannabinoid, improves murine colitis induced by trinitrobenzene sulfonic acid (Schicho and Storr, 2012). We have found here that intraperitoneal CBC exerts a therapeutic effect in the DNBS model of colitis as revealed by the reduction of colon weight/colon length ratio (a simple and reliable parameter of inflammation), intestinal permeability (a measure of intestinal epithelial integrity) (Osanai et al., 2007) and MPO, an index of neutrophil infiltration (Krawisz et al., 1984) as well as by histology and immunohistochemistry. Importantly, histological and immunohistochemical analyses showed the ability of CBC to induce tissue regeneration in the inflamed gut.
CBC significantly cured experimental colitis at the 1 mg·kg−1daily dose, which is a dose more than 100-fold lower than the subacute LD50 dose calculated in mice receiving, for 7 days, repeated intraperitoneal daily dosing of CBC (Krawisz et al., 1984). In other in vivo assays (i.e. paw oedema and intestinal motility in the inflamed gut), CBC was shown to exert statistically significant pharmacological actions starting from the 10 mg·kg−1dose (DeLong et al., 2010; Izzo et al., 2012).
Conclusions
In the present study, we have shown that the non-psychotropic Cannabis constituent CBC reduced nitric oxide, IL-10 and interferon-γ levels in peritoneal macrophages activated by LPS. The effect of CBC on nitric oxide production is mimicked by other TRPA1 ligands and does not appear to be mediated by direct or indirect cannabinoid receptor activation, although it is apparently modulated by CB1 receptors. Specifically, since the CBC response was exacerbated in the presence of CB1 antagonists, it is possible that an endogenous cannabinoid ‘tone’ at CB1 is coupled negatively to CBC pharmacological actions. In vivo, CBC exerted protective effects in experimental colitis at a dose more than 100-fold lower than the LD50 value previously reported. At least two significant therapeutic implications are evidenced by the present study: first, by decreasing NO production, CBC might limit tissue destruction caused by NO in autoimmune diseases; and second, in the light of its curative effect on murine colitis in vivo, CBC can be regarded as a promising candidate for clinical evaluation in IBD patients.
Acknowledgments
We are grateful to Mrs Lesley A. Stevenson for technical support.
Glossary
- [35S]GTPγS
[35S] guanosine 5″-(gamma-thio) triphosphate
- 2-AG
2-arachidonoylglycerol
- ACEA
arachidonyl-2′-chloroethylamide
- AM251
N-(Piperidin-1-yl)-5-(4-iodophenyl)-1-(2,4-dichlorophenyl)-4-methyl-1H-pyrazole-3-carboxamide
- AP-18
4-(4-Chlorophenyl)-3-methyl-3-buten-2-one oxime
- carvacrol
5-isopropyl-2-methylphenol 2-Methyl-5-(1-methylethyl)-phenol
- CB1
cannabinoid receptor type 1
- CB2
cannabinoid receptor type 2
- CBC
cannabichromene
- CBD
cannabidiol
- CGS 15943
9-Chloro-2-(2-furanyl)-[1,2,4]triazolo[1,5-c]quinazolin-5-amine
- Cq
PCR quantitative-cycles
- DAN
2,3-diaminonaphtalene
- DNBS
dinitrobenzene sulphonic acid
- FITC
fluorescein isothiocyanate
- GDP
guanosine diphosphate
- HC-030031
2-(1,3-Dimethyl-2,6-dioxo-1,2,3,6-tetrahydro-7H-purin-7-yl)-N-(4-isopropylphenyl)acetamide
- HEK-293
human embryonic kidney 293
- IBD
inflammatory bowel disease
- JWH133
(6aR,10aR)-3-(1,1-Dimethylbutyl)-6a,7,10,10a-tetrahydro-6,6,9-trimethyl-6H-dibenzo[b, d] pyran
- MAGL
monoacylglycerol lipase
- MOPS
3-(N-morpholino)propanesulfonic acid
- MPO
myeloperoxidase
- MTT
3-(4,5-dimethylthiazol- 2-yl)-2,5-diphenyltetrazolium bromide
- NaPP
sodium diphosphate
- OEA
oleoylethanolamide
- PEA
palmitoylethanolamide
- rimonabant
5-(4-Chlorophenyl)-1-(2,4-dichloro-phenyl)-4-methyl-N-(piperidin-1-yl)-1H-pyrazole-3-carboxamide
- SR144528
[2.2.1]heptan2-yl]-5-(4-chloro-3-methylphenyl)-1-[(4-methylphenyl)methyl]-1H-pyrazole-3-carboxamide
- TRPA1
transient receptor potential ankyrin-type1
- Δ9-THC
Δ9-tetrahydrocannabinol
Conflict of interest
This investigation was partly supported by grants from GW Pharmaceuticals (Porton Down, Wiltshire, UK).
References
- Alexander SP, Mathie A, Peters JA. Guide to receptors and channels (GRAC), 5th edition. Br J Pharmacol. 2011;164:S1–S324. doi: 10.1111/j.1476-5381.2011.01649_1.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alhouayek M, Muccioli GG. The endocannabinoid system in inflammatory bowel diseases: from pathophysiology to therapeutic opportunity. Trends Mol Med. 2012;18:615–625. doi: 10.1016/j.molmed.2012.07.009. [DOI] [PubMed] [Google Scholar]
- Aviello G, Rowland I, Gill CI, Acquaviva AM, Capasso F, McCann M, et al. Anti-proliferative effect of rhein, an anthraquinone isolated from Cassia species, on Caco-2 human adenocarcinoma cells. J Cell Mol Med. 2010;14:2006–2014. doi: 10.1111/j.1582-4934.2009.00815.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aviello G, Borrelli F, Guida F, Romano B, Lewellyn K, De Chiaro M, et al. Ultrapotent effects of salvinorin A, a hallucinogenic compound from Salvia divinorum, on LPS-stimulated murine macrophages and its anti-inflammatory action in vivo. J Mol Med (Berl) 2011;89:891–902. doi: 10.1007/s00109-011-0752-4. [DOI] [PubMed] [Google Scholar]
- Aviello G, Romano B, Borrelli F, Capasso R, Gallo L, Piscitelli F, et al. Chemopreventive effect of the non-psychotropic phytocannabinoid cannabidiol on experimental colon cancer. J Mol Med (Berl) 2012;90:925–934. doi: 10.1007/s00109-011-0856-x. [DOI] [PubMed] [Google Scholar]
- Blake DR, Robson P, Ho M, Jubb RW, McCabe CS. Preliminary assessment of the efficacy, tolerability and safety of a cannabis-based medicine (Sativex) in the treatment of pain caused by rheumatoid arthritis. Rheumatology (Oxford) 2006;45:50–52. doi: 10.1093/rheumatology/kei183. [DOI] [PubMed] [Google Scholar]
- Booz GW. Cannabidiol as an emergent therapeutic strategy for lessening the impact of inflammation on oxidative stress. Free Radic Biol Med. 2011;51:1054–1061. doi: 10.1016/j.freeradbiomed.2011.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borrelli F, Aviello G, Romano B, Orlando P, Capasso R, Maiello F, et al. Cannabidiol, a safe and non-psychotropic ingredient of the marijuana plant Cannabis sativa, is protective in a murine model of colitis. J Mol Med (Berl) 2009;87:1111–1121. doi: 10.1007/s00109-009-0512-x. [DOI] [PubMed] [Google Scholar]
- Brightbill HD, Plevy SE, Modlin RL, Smale ST. A prominent role for Sp1 during lipopolysaccharide-mediated induction of the IL-10 promoter in macrophages. J Immunol. 2000;164:1940–1951. doi: 10.4049/jimmunol.164.4.1940. [DOI] [PubMed] [Google Scholar]
- Brizzi A, Brizzi V, Cascio MG, Bisogno T, Siriani R, Di Marzo V. Design, synthesis, and binding studies of new potent ligands of cannabinoid receptors. J Med Chem. 2005;48:7343–7350. doi: 10.1021/jm0501533. [DOI] [PubMed] [Google Scholar]
- Brown I, Cascio MG, Wahle KW, Smoum R, Mechoulam R, Ross RA, et al. Cannabinoid receptor-dependent and -independent anti-proliferative effects of omega-3 ethanolamides in androgen receptor-positive and -negative prostate cancer cell lines. Carcinogenesis. 2010;31:1584–1591. doi: 10.1093/carcin/bgq151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burstein SH, Zurier RB. Cannabinoids, endocannabinoids, and related analogs in inflammation. AAPS J. 2009;11:109–119. doi: 10.1208/s12248-009-9084-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao S, Liu J, Song L, Ma X. The protooncogene c-Maf is an essential transcription factor for IL-10 gene expression in macrophages. J Immunol. 2005;74:3484–3492. doi: 10.4049/jimmunol.174.6.3484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capasso R, Aviello G, Romano B, Borrelli F, De Petrocellis L, Di Marzo V, et al. Modulation of mouse gastrointestinal motility by allyl isothiocyanate, a constituent of cruciferous vegetables (Brassicaceae): evidence for TRPA1-independent effects. Br J Pharmacol. 2012;165:1966–1977. doi: 10.1111/j.1476-5381.2011.01703.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox ML, Welch SP. The antinociceptive effect of Delta9-tetrahydrocannabinol in the arthritic rat. Eur J Pharmacol. 2004;16:65–74. doi: 10.1016/j.ejphar.2004.04.022. [DOI] [PubMed] [Google Scholar]
- Croci T, Landi M, Galzin AM, Marini P. Role of cannabinoid CB1 receptors and tumor necrosis factor-alpha in the gut and systemic anti-inflammatory activity of SR 141716 (rimonabant) in rodents. Br J Pharmacol. 2003;140:115–122. doi: 10.1038/sj.bjp.0705412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Argenio G, Valenti M, Scaglione G, Cosenza V, Sorrentini I, Di Marzo V. Up-regulation of anandamide levels as an endogenous mechanism and a pharmacological strategy to limit colon inflammation. FASEB J. 2006;20:568–570. doi: 10.1096/fj.05-4943fje. [DOI] [PubMed] [Google Scholar]
- De Petrocellis L, Vellani V, Schiano-Moriello A, Marini P, Magherini PC, Orlando P, et al. Plant-derived cannabinoids modulate the activity of transient receptor potential channels of ankyrin type-1 and melastatin type-8. J Pharmacol Exp Ther. 2008;325:1007–1015. doi: 10.1124/jpet.107.134809. [DOI] [PubMed] [Google Scholar]
- De Petrocellis L, Ligresti A, Moriello AS, Allarà M, Bisogno T, Petrosino S, et al. Effects of cannabinoids and cannabinoid-enriched Cannabis extracts on TRP channels and endocannabinoid metabolic enzymes. Br J Pharmacol. 2011;163:1479–1494. doi: 10.1111/j.1476-5381.2010.01166.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Petrocellis L, Orlando P, Moriello AS, Aviello G, Stott C, Izzo AA, et al. Cannabinoid actions at TRPV channels: effects on TRPV3 and TRPV4 and their potential relevance to gastrointestinal inflammation. Acta Physiol (Oxf) 2012;204:255–266. doi: 10.1111/j.1748-1716.2011.02338.x. [DOI] [PubMed] [Google Scholar]
- DeLong GT, Wolf CE, Poklis A, Lichtman AH. Pharmacological evaluation of the natural constituent of Cannabis sativa, cannabichromene and its modulation by Δ(9)-tetrahydrocannabinol. Drug Alcohol Depend. 2010;112:126–133. doi: 10.1016/j.drugalcdep.2010.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Marzo V, Izzo AA. Endocannabinoid overactivity and intestinal inflammation. Gut. 2006;55:1373–1376. doi: 10.1136/gut.2005.090472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Marzo V, Capasso R, Matias I, Aviello G, Petrosino S, Borrelli F, et al. The role of endocannabinoids in the regulation of gastric emptying: alterations in mice fed a high-fat diet. Br J Pharmacol. 2008;153:1272–1280. doi: 10.1038/sj.bjp.0707682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel MA, Leffler A, Niedermirtl F, Babes A, Zimmermann K, Filipović MR, et al. TRPA1 and substance P mediate colitis in mice. Gastroenterology. 2011;141:1346–1358. doi: 10.1053/j.gastro.2011.07.002. [DOI] [PubMed] [Google Scholar]
- Goldblum SE, Wu KM, Jay M. Lung myeloperoxidase as a measure of pulmonary leukostasis in rabbits. J Appl Physiol. 1985;59:1978–1985. doi: 10.1152/jappl.1985.59.6.1978. [DOI] [PubMed] [Google Scholar]
- Grimaldi P, Orlando P, Di Siena S, Lolicato F, Petrosino S, Bisogno T, et al. The endocannabinoid system and pivotal role of the CB2 receptor in mouse spermatogenesis. Proc Natl Acad Sci U S A. 2009;106:11131–11136. doi: 10.1073/pnas.0812789106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawiger J. Innate immunity and inflammation: a transcriptional paradigm. Immunol Res. 2001;23:99–109. doi: 10.1385/IR:23:2-3:099. [DOI] [PubMed] [Google Scholar]
- Hillard CJ, Edgemond WS, Jarrahian A, Campbell WB. Accumulation of N-arachidonoylethanolamine (anandamide) into cerebellar granule cells occurs via facilitated diffusion. J Neurochem. 1997;69:631–638. doi: 10.1046/j.1471-4159.1997.69020631.x. [DOI] [PubMed] [Google Scholar]
- Holley JH, Hadley KW, Turner CE. Constituents of Cannabis sativa L. XI: cannabidiol and cannabichromene in samples of known geographical origin. J Pharm Sci. 1975;64:892–894. doi: 10.1002/jps.2600640546. [DOI] [PubMed] [Google Scholar]
- Holzer P. Transient receptor potential (TRP) channels as drug targets for diseases of the digestive system. Pharmacol Ther. 2011;131:142–170. doi: 10.1016/j.pharmthera.2011.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huestis MA. Pharmacokinetics and metabolism of the plant cannabinoids, delta9-tetrahydrocannabinol, cannabidiol and cannabinol. Handb Exp Pharmacol. 2005;168:657–690. doi: 10.1007/3-540-26573-2_23. [DOI] [PubMed] [Google Scholar]
- Ippoushi K, Itou H, Azuma K, Higashio H. Effect of naturally occurring organosulfur compounds on nitric oxide production in lipopolysaccharide-activated macrophages. Life Sci. 2002;71:411–419. doi: 10.1016/s0024-3205(02)01685-5. [DOI] [PubMed] [Google Scholar]
- Izzo AA, Camilleri M. Cannabinoids in intestinal inflammation and cancer. Pharmacol Res. 2009;60:117–125. doi: 10.1016/j.phrs.2009.03.008. [DOI] [PubMed] [Google Scholar]
- Izzo AA, Borrelli F, Capasso R, Di Marzo V, Mechoulam R. Non-psychotropic plant cannabinoids: new therapeutic opportunities from an ancient herb. Trends Pharmacol Sci. 2009;30:515–527. doi: 10.1016/j.tips.2009.07.006. [DOI] [PubMed] [Google Scholar]
- Izzo AA, Capasso R, Aviello G, Borrelli F, Romano B, Piscitelli F, et al. Inhibitory effect of cannabichromene, a major non-psychotropic cannabinoid extracted from Cannabis sativa, on inflammation-induced hypermotility in mice. Br J Pharmacol. 2012;166:1444–1460. doi: 10.1111/j.1476-5381.2012.01879.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamontt JM, Molleman A, Pertwee RG, Parsons ME. The effects of Delta-tetrahydrocannabinol and cannabidiol alone and in combination on damage, inflammation and in vitro motility disturbances in rat colitis. Br J Pharmacol. 2010;160:712–723. doi: 10.1111/j.1476-5381.2010.00791.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG. NC3Rs Reporting Guidelines Working Group. Br J Pharmacol. 2010;160:1577–1579. doi: 10.1111/j.1476-5381.2010.00872.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krawisz JE, Sharon P, Stenson WF. Quantitative assay for acute intestinal inflammation based on myeloperoxidase activity. Assessment of inflammation in rat and hamster models. Gastroenterology. 1984;87:1344–1350. [PubMed] [Google Scholar]
- Lahat A, Lang A, Ben-Horin S. Impact of cannabis treatment on the quality of life, weight and clinical disease activity in inflammatory bowel disease patients: a pilot prospective study. Digestion. 2012;85:1–8. doi: 10.1159/000332079. [DOI] [PubMed] [Google Scholar]
- Lal S, Prasad N, Ryan M, Tangri S, Silverberg MS, Gordon A, et al. Cannabis use amongst patients with inflammatory bowel disease. Eur J Gastroenterol Hepatol. 2011;23:891–896. doi: 10.1097/MEG.0b013e328349bb4c. [DOI] [PubMed] [Google Scholar]
- Ligresti A, Cascio MG, Pryce G, Kulasegram S, Beletskaya I, De Petrocellis L, et al. New potent and selective inhibitors of anandamide reuptake with antispastic activity in a mouse model of multiple sclerosis. Br J Pharmacol. 2006;147:83–91. doi: 10.1038/sj.bjp.0706418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W, Reinmuth N, Stoeltzing O, Parikh AA, Tellez C, Williams S, et al. Cyclooxygenase-2 is up-regulated by interleukin-1 beta in human colorectal cancer cells via multiple signaling pathways. Cancer Res. 2003;63:3632–3636. [PubMed] [Google Scholar]
- Lo Verme J, Fu J, Astarita G, La Rana G, Russo R, Calignano A, et al. The nuclear receptor peroxisome proliferator-activated receptor-alpha mediates the anti-inflammatory actions of palmitoylethanolamide. Mol Pharmacol. 2005;67:15–19. doi: 10.1124/mol.104.006353. [DOI] [PubMed] [Google Scholar]
- McGrath J, Drummond G, McLachlan E, Kilkenny C, Wainwright C. Guidelines for reporting experiments involving animals: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1573–1576. doi: 10.1111/j.1476-5381.2010.00873.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon SB, Wood JN. Increasingly irritable and close to tears: TRPA1 in inflammatory pain. Cell. 2006;124:1123–1125. doi: 10.1016/j.cell.2006.03.006. [DOI] [PubMed] [Google Scholar]
- Maione S, Piscitelli F, Gatta L, Vita D, De Petrocellis L, Palazzo E, et al. Non-psychoactive cannabinoids modulate the descending pathway of antinociception in anaesthetized rats through several mechanisms of action. Br J Pharmacol. 2011;162:584–596. doi: 10.1111/j.1476-5381.2010.01063.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malfait AM, Gallily R, Sumariwalla PF, Malik AS, Andreakos E, Mechoulam R, et al. The nonpsychoactive cannabis constituent cannabidiol is an oral anti-arthritic therapeutic in murine collagen-induced arthritis. Proc Natl Acad Sci U S A. 2000;15:9561–9566. doi: 10.1073/pnas.160105897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massa F, Marsicano G, Hermann H, Cannich A, Monory K, Cravatt BF, et al. The endogenous cannabinoid system protects against colonic inflammation. J Clin Invest. 2004;113:1202–1209. doi: 10.1172/JCI19465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehmedic Z, Chandra S, Slade D, Denham H, Foster S, Patel AS, et al. Potency trends of Δ9-THC and other cannabinoids in confiscated cannabis preparations from 1993 to 2008. J Forensic Sci. 2010;55:1209–1217. doi: 10.1111/j.1556-4029.2010.01441.x. [DOI] [PubMed] [Google Scholar]
- Moncada S, Palmer RM, Higgs EA. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991;43:109–142. [PubMed] [Google Scholar]
- Moore KW, de Waal MR, Coffman RL, O'Garra A. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol. 2001;19:683–765. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- Moran MM, McAlexander MA, Bíró T, Szallasi A. Transient receptor potential channels as therapeutic targets. Nat Rev Drug Discov. 2011;10:601–620. doi: 10.1038/nrd3456. [DOI] [PubMed] [Google Scholar]
- Naftali T, Lev LB, Yablecovitch D, Half E, Konikoff FM. Treatment of Crohn's disease with cannabis: an observational study. Isr Med Assoc J. 2011;13:455–582. [PubMed] [Google Scholar]
- Osanai M, Nishikiori N, Murata M, Chiba H, Kojima T, Sawada N. Cellular retinoic acid bioavailability determines epithelial integrity: role of retinoic acid receptor alpha agonists in colitis. Mol Pharmacol. 2007;71:250–258. doi: 10.1124/mol.106.029579. [DOI] [PubMed] [Google Scholar]
- Ross RA, Brockie HC, Pertwee RG. Inhibition of nitric oxide production in RAW264.7 macrophages by cannabinoids and palmitoylethanolamide. Eur J Pharmacol. 2000;401:121–130. doi: 10.1016/s0014-2999(00)00437-4. [DOI] [PubMed] [Google Scholar]
- Russo EB. Taming THC: potential cannabis synergy and phytocannabinoid-terpenoid entourage effects. Br J Pharmacol. 2011;163:1344–1364. doi: 10.1111/j.1476-5381.2011.01238.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samad TA, Moore KA, Sapirstein A, Billet S, Allchorne A, Poole S, et al. Interleukin-1beta-mediated induction of Cox-2 in the CNS contributes to inflammatory pain hypersensitivity. Nature. 2001;410:471–475. doi: 10.1038/35068566. [DOI] [PubMed] [Google Scholar]
- Schicho R, Storr M. Topical and systemic cannabidiol improves trinitrobenzene sulfonic acid colitis in mice. Pharmacology. 2012;89:149–155. doi: 10.1159/000336871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofia RD, Nalepa SD, Vassar HB, Knobloch LC. Comparative anti-phlogistic activity of delta 9-tetrahydrocannabinol, hydrocortisone and aspirin in various rat paw edema models. Life Sci. 1974;15:251–260. doi: 10.1016/0024-3205(74)90214-8. [DOI] [PubMed] [Google Scholar]
- Tubaro A, Giangaspero A, Sosa S, Negri R, Grassi G, Casano S, et al. Comparative topical anti-inflammatory activity of cannabinoids and cannabivarins. Fitoterapia. 2010;81:816–819. doi: 10.1016/j.fitote.2010.04.009. [DOI] [PubMed] [Google Scholar]
- Turner CE, Elsohly MA. Biological activity of cannabichromene, its homologs and isomers. J Clin Pharmacol. 1981;21:283S–291S. doi: 10.1002/j.1552-4604.1981.tb02606.x. [DOI] [PubMed] [Google Scholar]
- Wirth PW, Watson ES, ElSohly M, Turner CE, Murphy JC. Anti-inflammatory properties of cannabichromene. Life Sci. 1980;26:1991–1995. doi: 10.1016/0024-3205(80)90631-1. [DOI] [PubMed] [Google Scholar]
- Yoshino T, Nakase H, Honzawa Y, Matsumura K, Yamamoto S, Takeda Y, et al. Immunosuppressive effects of tacrolimus on macrophages ameliorate experimental colitis. Inflamm Bowel Dis. 2010;16:2022–2033. doi: 10.1002/ibd.21318. [DOI] [PubMed] [Google Scholar]
- Zurier RB. Prospects for cannabinoids as anti-inflammatory agents. J Cell Biochem. 2003;88:462–466. doi: 10.1002/jcb.10291. [DOI] [PubMed] [Google Scholar]



