Abstract
Background and Purpose
α1-adrenoceptor (-AR) antagonists may facilitate ureter stone passage in humans. We aimed to study effects by the α1A-AR selective antagonist silodosin (compared to tamsulosin and prazosin) on ureter pressures in a rat model of ureter obstruction, and on contractions of human and rat isolated ureters.
Experimental Approach
After ethical approval, ureters of male rats were cannulated beneath the kidney pelvis for in vivo ureteral intraluminal recording of autonomous peristaltic pressure waves. A partial ureter obstruction was applied to the distal ureter. Mean arterial blood pressure (MAP) was recorded. Approximate clinical and triple clinical doses of the α1-AR antagonists were given intravenously. Effects by the α1-AR antagonists on isolated human and rat ureters were studied in organ baths.
Key Results
Intravenous silodosin (0.1–0.3 mg kg−1) or prazosin (0.03–0.1 mg kg−1) reduced obstruction-induced increases in intraluminal ureter pressures by 21–37% or 18–40% respectively. Corresponding effects by tamsulosin (0.01 or 0.03 mg kg−1) were 9–20%. Silodosin, prazosin and tamsulosin reduced MAP by 10–12%, 25–26% (P < 0.05), or 18–25% (P < 0.05) respectively. When effects by the α1A-AR antagonists on obstruction-induced ureter pressures were expressed as a function of MAP, silodosin had six- to eightfold and 2.5- to eightfold better efficacy than tamsulosin or prazosin respectively. Silodosin effectively reduced contractions of both human and rat isolated ureters.
Conclusions and Implications
Silodosin inhibits contractions of the rat and human isolated ureters and has excellent functional selectivity in vivo to relieve pressure-load of the rat obstructed ureter. Silodosin as pharmacological ureter stone expulsive therapy should be clinically further explored.
Keywords: ureter, urolithiasis, pressure, in vivo, α1A-adrenoceptor, antagonist, uroselectivity
Introduction
Urolithiasis is a multifactorial disease that often is encountered in daily urological practice and that is increasing in Western countries (Stamatelou et al., 2003; Pearle et al., 2005). Mechanical obstruction, ureteral spasms and luminal dilation are causes for significant discomfort in patients with urolithiasis. When calculi are small (≤10 mm), located in the distal part of the ureter, and with no clinical evidence of infection and pain, conservative pharmacological expulsive therapy may be indicated to accelerate spontaneous passage of ureter stones (Hollingsworth et al., 2006; Seitz et al., 2009).
Several transmitter systems or receptors may be considered to be putative pharmacological targets to modify ureteral peristalsis (Canda et al., 2007). Adrenergic nerve fibres that are similarly distributed in ureters from different species, including humans, exhibit most abundant expression in the distal ureter, (Rolle et al., 2008) localized in the muscular layers, within the perivascular networks, as well as, to a lower extent, in the suburothelial space (Rolle et al., 2008). Alpha1-adrenoceptors (α1-ARs) have been detected in ureters from both animals and humans (Morita et al., 1994; Sigala et al., 2005; Park et al., 2007; Kobayashi et al., 2009b). The density of α1-ARs in the ureteral smooth muscle appears to be greater than other adrenoceptors (Morita et al., 1994; Sigala et al., 2005). A rather homogenous distribution of different α1-AR subtypes along of the human ureter is reported (Park et al., 2007). Various α1-AR antagonists that are available in clinics, for example, alfuzosin, doxazosin, tamsulosin and silodosin, exhibit inhibitory effects on contractions on isolated ureter of a variety of species (Kobayashi et al., 2009a; 2009b), including humans (Rajpathy et al., 2008; Sasaki et al., 2011) and appear to reduce human ureteral activity in vivo (Davenport et al., 2007). Results obtained from randomized controlled trials suggest that α1-AR antagonists may be used in patients to facilitate passage of ureter stones (Hollingsworth et al., 2006; Seitz et al., 2009). On the other hand, recently presented contradictory results indicate that tamsulosin, an α1- AR antagonist with some selectivity for α1A- and α1D-AR, is not efficacious as medical ureter stone expulsion therapy (Vincendeau et al., 2010). It is currently not clear if α1-AR antagonists with different receptor-subtype preferences other than tamsulosin may have better effects on ureteral contractile functions or ureteral peristaltic activity. Even so, silodosin, a highly selective α1A-AR antagonist, was recently proposed as a putative drug for expulsive therapy for ureter stones (Itoh et al., 2011).
Whereas only few studies have compared the effect of different α-blockers on ureteral activity of isolated human tissues (Rajpathy et al., 2008; Sasaki et al., 2011), several studies have addressed the effect of α1-AR antagonist in dogs and mice using an α-AR agonist-induced ureteral contraction model (Kobayashi et al., 2009a; 2010). Mechanical and complete unilateral acute ureteral obstruction has been utilized in pigs to evaluate the potential of β-AR agonists as putative new ureter relaxing drugs for the management of stone disease (Wanajo et al., 2011). These models however, do not allow for registration of the endogenously generated ureter contractions that propel urine from the kidney to the bladder. This peristaltic ureter activity occurs spontaneously along a functional and not completely obliterated ureter and can be continuously recorded as regular pressure waves. To better understand how different α1-AR antagonists may affect ureteral contractions and spontaneous peristaltic propulsive pressure waves, one goal of our project was to further develop a rat model of acute unilateral ureteral obstruction. In this model, the main aim of our study was to compare the effects by intravenous administration of approximate relevant clinical doses of silodosin, tamsulosin and prazosin on intraluminal ureter pressure parameters and systemic blood pressures. In separate in vitro experiments, we also aimed to compare the effects of the selected α1-AR antagonists on contractions of isolated rat and human distal ureters.
Methods
Ethical approvals
All experiments involving animals were approved by the Institutional Animal Care and Use Committee, San Raffaele Scientific Institute, Milan, Italy and carried out in accordance with the ARRIVE Guidelines (Kilkenny et al., 2010). Human specimens were obtained after the signature of an informed consent, as specified by the Institutional Ethical Committee approved Protocol n. URI003-2010 (approval date 9 December 2010).
Animals
A total of 55 male Sprague Dawley rats (250–300 g, Charles River, Italy) were used. The animals were maintained under standard laboratory conditions with a 12:12 h light : dark cycle and free access to food pellets and tap water. For surgery, isofluorane (alveolar concentration 2%) was used as inhalation anaesthesia. Forty rats were treated with different α-blockers; (i) silodosin (α1A-AR selective) at 0.1 and 0.3 mg kg−1 (n = 6 for both doses), (ii) tamsulosin (‘α1A-D-AR selective’) at 0.01 and 0.03 mg kg−1 (n = 7 for both doses) and (iii) prazosin (non-α-AR selective) at 0.03 and 0.1 mg kg−1 (n = 7 for both doses). Fifteen animals were used as controls to record the effect of vehicles. Rats were killed by carbon dioxide asphyxia and ureters were harvested for immediate functional investigations in organ baths.
Human tissues
Macroscopically, normal human ureter was obtained from patients (n = 13, age: 59 ± 4 years) who had undergone surgery for urological malignancies, but not exposed to chemotherapy, radiotherapy or immunotherapy before the surgical procedure. The normal tissue was isolated by an uropathologist distally from the tumour lesion, immediately placed in a chilled Krebs solution and transported to the laboratory. All tissues were immediately used in organ bath experiments.
Partial ureteral obstruction and in vivo pressure recording in rats
Under surgical anaesthesia, a midline abdominal incision was performed and the left ureter was visualized from the renal pelvis to the bladder. The proximal ureter was carefully isolated by microsurgical dissection. A small incision was made just below the ureteropelvic junction to allow insertion of a polyethylene catheter (PE-10, Clay-Adams, Parsippany, NJ, USA) into the ureter. The catheter was then attached to a microsyringe pump (CMA 100; Carnegie Medicine AB, Solna, Sweden) and connected to a pressure transducer. While room temperature physiological saline was continuously infused at a speed of 0.4 mL h−1 to simulate normal urine production, (Schmidt et al., 2001) ureteral intraluminal pressure oscillations were constantly recorded. A partial ureteral obstruction was created by suturing the underlying psoas muscle around the distal 1/5 portion of the ureter just before the vesicoureteric junction (Ulm and Miller, 1962). As previously described (Becker et al., 1998), the urinary bladder was incised at the dome to avoid interference on ureter pressures from detrusor contractions.
The partial obstruction was considered optimal when the ureteral reproducibly and stably regained its spontaneous peristaltic activity over time and the minimal ureteral pressure achieved was greater than the maximal pressure before the obstruction.
In order to monitor systemic blood pressure, a heparinized (5 IU mL−1) saline-filled polyethylene catheter (PE-50, Clay-Adams) was positioned in the carotid artery for recording of mean arterial pressure (MAP). An additional saline-filled polyethylene catheter (PE-10, Clay-Adams) was positioned in the femoral vein to allow drugs administration. All pressure data were acquired continuously with Acq Knowledge 3.8.1 software and a MP100 data acquisition system (BIOpac Syst. Inc. Santa Barbara, CA, USA) connected to a Grass polygraph (Model 7E, Grass Technologies, Warwick, RI, USA). The following ureteral parameters were analysed: frequency (contractions per minute) and amplitude (cmH2O) of autonomous peristaltic pressure waves, minimum pressure (MinP; cmH2O), maximum pressure (MaxP; cmH2O) and the AUC per second (cmH2·s−1). An example of the in vivo ureteral pressure recording after partial obstruction is depicted in Figure 1, showing representative original traces for each tested drug.
Figure 1.
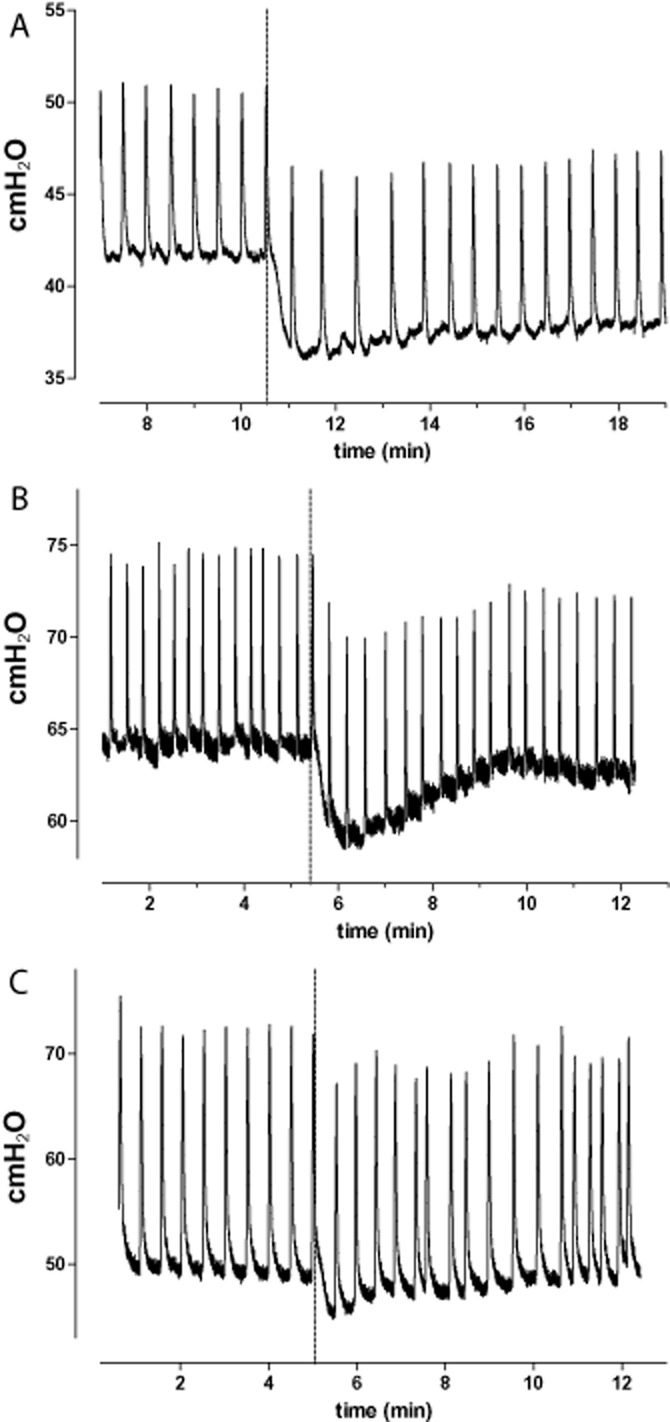
Representative original ureteral pressure traces recorded after partial obstruction and administration of silodosin (A), tamsulosin (B) and prazosin (C) given at the maximal tested dose. Vertical dashed line indicates the timing of intravenous drug injection.
Isolated ureteral tissue
Tissue strips (3 × 3 × 6 mm) were dissected from the proximal part of ureters obtained from surgical human specimens and naïve rats never exposed before to pharmacological treatments. Silk ligatures were applied at both ends of the preparations and then mounted in a 5 mL aerated (95% O2 and 5% CO2) organ bath chamber, containing Krebs solution (37°C, pH 7.4). The working Krebs solution was routinely replaced every 30 min. Isometric tension was registered with a Grass Polygraph model 7E (Grass Technologies). The human preparations were stretched to a tension approximately 5 mN and left to equilibrate for 30 min to attain a stable resting tone of 1.82 ± 0.2 mN (n = 13). The rat ureteral preparations were stretched to a tension approximately 2 mN and left to equilibrate for 30 min to obtain a stable tension of 0.358 ± 0.02 mN (n = 18). The viability of the preparations was verified by addition of high K+ solution (60 mM KCl) to the organ baths to induce force displacement of 0.42 ± 0.07 mN (n = 13) and 0.15 ± 0.01 mN (n = 18) for human and rat ureteral preparations respectively. Electrical field stimulation (EFS) was performed with two platinum electrodes, placed in parallel to the tissue strips. A Grass S48 stimulator delivered single 0.5 ms square-wave pulses at supra-maximum voltage. The train duration was 5 s and the train interval 120 s. Scouting frequency-response (0.5–40 Hz) experiments were preliminarily carried out on separate ureter specimens to evaluate the optimal stimulation frequency needed to obtain the 75% of maximal contractile effect (data not shown). Effects by cumulative addition of single concentrations of silodosin (range 10−7 to 10−4 M), tamsulosin (10−7 to 10−4 M) or prazosin (10−7 to 10−4 M) were studied on continuous EFS contractions (20 Hz). The –logIC30 values were calculated by graphical interpolation. In separate experiments, vehicles for silodosin, tamsulosin and prazosin were tested on EFS-induced contractions (each n = 5).
Drugs and solutions
The composition of the Krebs solution was (mM): NaCl 119, KCl 4.6, CaCl2 1.5, MgCl2 1.2, NaHCO3 15, NaH2PO4 1.2, glucose 5.5. A K+ solution (60 mM) was used, in which the NaCl in the normal Krebs solution was replaced by equimolar KCl. Silodosin (Recordati, Milan, Italy), tamsulosin (Sigma-Aldrich, Milan, Italy) and prazosin (Sigma Aldrich) were used. Silodosin and tamsulosin were dissolved in ethanol (Merck Chemicals, Darmstadt, Germany), prazosin in methanol (Merck Chemicals) and kept as stock solutions (10−2 M). Silodosin (0.1 or 0.3 mg kg−1), tamsulosin (0.01 or 0.03 mg kg−1) or prazosin (0.03 or 0.1 mg kg−1) were given i.v. as a single dose as soon as the ureter recovered a stable, post obstruction, spontaneous peristaltic activity for at least 5 min. The dosing range in rats was estimated according to the allometric scaling calculation of metabolic rate and body mass using the clinical dosage in humans (Sharma and McNeill, 2009). In separate experiments, control animals (n = 5 for each drug) were injected i.v. with the appropriate vehicles.
Calculations
Values are given as mean ± SEM. For multiple comparisons, Student Newman–Keuls analysis of variance was used. Pairwise and non-pairwise comparisons were made by Student's t-test. All statistical calculations were based on the number of individual animals. Differences were considered significant when P < 0.05.
Results
In vivo ureteral pressures at baseline and after obstruction
For each animal, baseline autonomous peristaltic ureteral activities were recorded before obstruction (n = 55). MinP and MaxP amounted to 18.7 ± 0.9 cmH2O and 36.2 ± 1.6 cmH2O respectively. The amplitude and frequency of the peristaltic pressure waves were 16.1 ± 1.2 cmH2O and 3.8 ± 0.2 contractions per minute, and the AUC was 22.5 ± 0.9 cmH2O·s−1. Upon obstruction of the ureter (n = 50), MinP and MaxP increased to 41.8 ± 1.9 cmH2O (P < 0.001) and 57.2 ± 2.1 cmH2O (P < 0.001), respectively and the AUC increased by approximately 50% to 45.3 ± 1.8 cmH2O·s−1 (P < 0.001). Obstruction did not affect the amplitude of peristaltic pressure waves (14.6 ± 1.3 cmH2O, P = 0.1), and no changes were noted for frequency (3.8 ± 0.2 contractions per minute). Baseline mean arterial blood pressure amounted to 120.6 ± 3.4 cmH2O and 120.4 ± 3.5 cmH2O after obstruction.
In vivo effects of α1-AR antagonists on the obstructed ureter
None of the α1-AR antagonists altered the amplitude or frequency of peristaltic pressure waves after obstruction (data not shown). The results of the different α-blockers on ureteral MinP, MaxP, AUC and MAP are shown in Figure 2.
Figure 2.
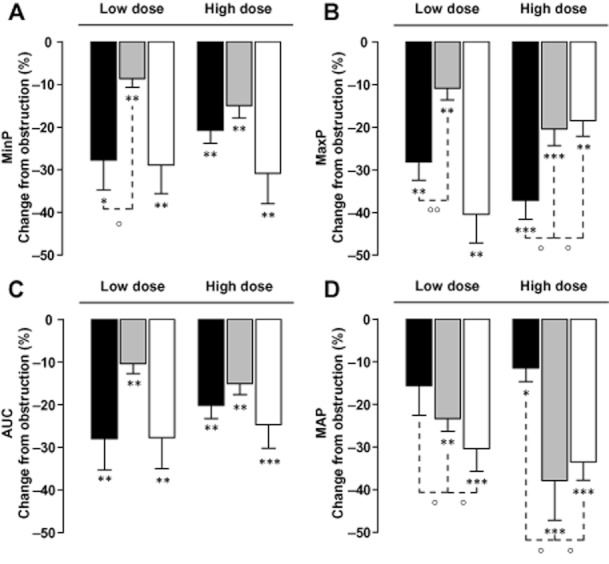
Ureter pressure parameters in vivo. Inhibitory effects of different doses of silodosin (black bars), tamsulosin (grey bars) or prazosin (white bars) on minimal (A) and maximal (B) ureter pressure, and AUC (C). Data are expressed as percent reduction of the increase in intraluminal ureter pressure due to obstruction. Panel D shows the effect of the investigated α1-AR antagonists on mean arterial pressure (MAP) expressed as percent reduction of MAP before drug administration. *P < 0.05, **P < 0.01, ***P < 0.001 versus obstruction alone. °P < 0.05 °°P < 001 versus silodosin, two-way Student's T-test.
Silodosin (i.v.) reduced the obstruction-induced increases in MinP (Figure 2A) by 27.7 ± 7.6% (0.1 mg kg−1; n = 6; P < 0.05 vs. obstruction) and 20.8 ± 3.3% (0.3 mg kg−1; n = 6; P < 0.01 vs. obstruction). Similarly, prazosin reduced MinP by 28.8 ± 6.3% (0.03 mg kg−1; n = 7; P < 0.01 vs. obstruction) and 30.8 ± 6.3% (0.1 mg kg−1; n = 7; P < 0.01 vs. obstruction). Corresponding effects by tamsulosin on MinP were 8.6 ± 2.4% (0.01 mg kg−1; n = 7; P < 0.01 vs. obstruction) and 14.9 ± 3.4% (0.03 mg kg−1; n = 7; P < 0.01 vs. obstruction). Effect by 0.1 mg kg−1 of silodosin on MinP was similar to prazosin (0.03 mg kg−1; P = 0.9) but larger that tamsulosin (0.01 mg kg−1; P < 0.05). When comparing the inhibitory effects on MinP by the highest investigated doses of the α1-AR antagonists, no differences were noted.
Silodosin treatment appeared to dose-dependently reduce the obstruction-induced increases in MaxP (Figure 2B) by 28.2 ± 4.3% (0.1 mg kg−1; n = 6; P < 0.01 vs. obstruction) and 37.2 ± 4.4% (0.3 mg kg−1; n = 6; P < 0.001 vs. obstruction). Prazosin reduced MaxP by 40.4 ± 6.8% (0.03 mg kg−1; n = 7; P < 0.01 vs. obstruction) and 18.4 ± 3.6% (0.1 mg kg−1; n = 7; P = 0.01 vs. obstruction). The effects by tamsulosin on MaxP were 10.9 ± 2.7% (0.01 mg kg−1; n = 7; P < 0.01 vs. obstruction) and 20.49 ± 3.9% reduction (0.03 mg kg−1; n = 7; P < 0.001 vs. obstruction). Overall, the effect of 0.1 mg kg−1 of silodosin on MaxP was similar to prazosin (0.03 mg kg−1; P = 0.2) but better than tamsulosin (0.01 mg kg−1; P < 0.01). Nonetheless, at the highest investigated doses, silodosin exerted a greater inhibitory effect on MaxP than prazosin and tamsulosin (all P < 0.05).
Silodosin (i.v.) also reduced the obstruction-induced increases in AUC (Figure 2C) by 28 ± 7.3% (0.1 mg kg−1; n = 6; P < 0.01 vs. obstruction) and 20.1 ± 3.1% (0.3 mg kg−1; n = 6; P < 0.01 vs. obstruction). This effect was comparable to prazosin that diminished AUC by 27.7 ± 7.3% (0.03 mg kg−1; n = 7; P < 0.01 vs. obstruction) and 24.7 ± 5.5% (0.1 mg kg−1; n = 7; P < 0.01 vs. obstruction). Corresponding effects by tamsulosin on AUC were a reduction of 10.4 ± 2.4% (0.01 mg kg−1; n = 7; P < 0.01 vs. obstruction) and 15.1 ± 2.5% (0.03 mg kg−1; n = 7; P < 0.001 vs. obstruction). Effect by 0.1 mg kg−1 of silodosin on AUC was similar to prazosin 0.03 mg kg−1 (P = 0.9) but greater than tamsulosin 0.01 mg kg−1 (P < 0.05). When comparing the inhibitory effects on AUC by the highest investigated doses of the different α1-AR antagonists, no differences were noted.
Silodosin 0.1 mg kg−1 reduced the MAP (Figure 2D) to 108.7 ± 5.5cmH2O (11.7 ± 4.4%, n = 6; P = 0.07 vs. obstruction) and to 97.3 ± 9.7cmH2O (10.2 ± 2.5%, 0.3 mg kg−1; n = 6; P < 0.05 vs. obstruction). Prazosin 0.03 mg kg−1 diminished MAP to 90.1 ± 7 cmH2O (25.4 ± 4%; n = 7; P = 0.001 vs. obstruction) and to 95.9 ± 11.5 cmH2O (26.4 ± 3.9% 0.1 mg kg−1; n = 7; P < 0.001 vs. obstruction). Similarly, tamsulosin 0.01 mg kg−1 lowered MAP to 105.7 ± 4.7 cmH2O (25.1 ± 3.1% n = 7; P < 0.01 vs. obstruction) and to 100.4 ± 3.3 cmH2O (17.5 ± 1.6%, 0.03 mg kg−1; n = 7; P < 0.001 vs. obstruction). At the investigated doses, effects by silodosin on MAP were less than those of prazosin and tamsulosin (all P < 0.05). When expressed as a function of MAP (percent effect per cmH2O reduction of MAP; Figure 3), silodosin demonstrated at the investigated doses significantly better inhibition of obstruction-induced increases in ureter pressure parameters than did tamsulosin or prazosin. The gain of effect for silodosin (Figure 3) ranged from six to eightfold higher than tamsulosin and from 2.5- to eightfold higher than prazosin for MaxP, MinP and AUC.
Figure 3.
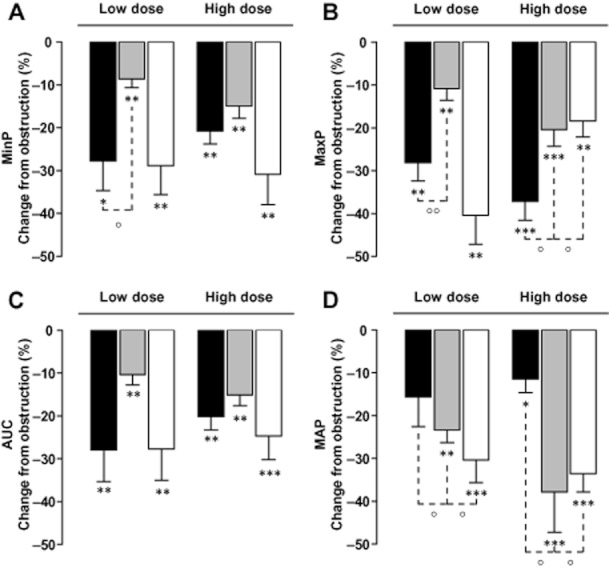
Ureter pressure parameters in vivo. Inhibitory effects of silodosin (black bars), tamsulosin (grey bars) or prazosin (white bars) on minimal ureter pressure (MinP) and maximal ureter pressure (MaxP) and AUC. Data are expressed as percent reduction of the increase in intraluminal ureter pressure due to obstruction per reduction of mean arterial pressure (MAP; cmH2O) at the lowest (silodosin 0.1, tamsulosin 0.01, prazosin 0.03, A) and highest (silodosin 0.3, tamsulosin 0.03, prazosin 0.1, B) investigated doses (mg·kg−1). °P < 0.05 °°P < 001 versus silodosin, two-way Student's T test.
In vitro activities of α1-AR antagonists on rat and human isolated ureteral tissues
All α1-AR antagonists exhibited concentration-dependent inhibitory effects on EFS-induced contractions of isolated rat (Figure 4A) and human (Figure 4B) ureters. Inhibitory effects by silodosin on EFS-induced contractions of the rat ureter amounted to 7 ± 5% (10−7 M), 27 ± 15% (10−6 M), 74 ± 8% (10−5 M, P < 0.01 vs. tamsulosin and prazosin), with a maximal effect of 88.9 ± 7% obtained at the highest investigated concentration of 10−4 M (P < 0.05 vs. tamsulosin). Tamsulosin showed no inhibitory effect on EFS-induced contractions of the rat ureter at 10−7 M, whereas the drug inhibited contractions by 12 ± 6%, 16 ± 6% and 42.6 ± 12% at 10−6 M, 10−5 M and 10−4 M respectively. Prazosin inhibited EFS-induced contractions of the rat ureter only at 10−5 M and 10−4 M by 31 ± 12% and 76 ± 9% (P < 0.05 vs. tamsulosin) respectively. The calculated –logIC30 values of the differentα1-AR antagonists amounted to 5.98 ± 0.30 (silodosin, P < 0.05 vs. tamsulosin), 4.61 ± 0.46 (tamsulosin) and 5.39 ± 0.41 (prazosin). The order of potency and efficacy at the currently investigated concentrations for the drugs on the rat ureter may be summarized as silodosin = prazosin > tamsulosin.
Figure 4.
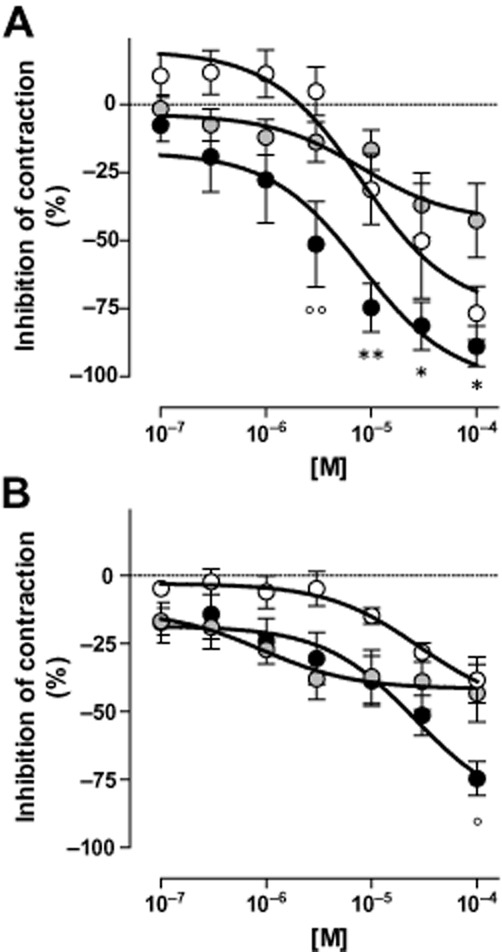
Contractions of isolated ureter preparations. Inhibition of contractions induced by electrical field stimulation (EFS; 0.5 ms, 50 V, 20 Hz) of rat (A) and human (B) isolated ureter preparations upon exposure to 10−7 to 10−4 M of silodosin ( ), tamsulosin (
), tamsulosin ( ) or prazosin (○). Effects by the investigated drugs on contractions induced by EFS are expressed as percent inhibition of contractions before drug treatment. (*P < 0.05 **P < 0.01 versus tamsulosin, °P < 0.05 °°P < 001 vs. prazosin; two-way anova, Bonferroni post test). Non-linear regression calculation and statistical comparisons were made by using Graphpad Prism 5.0 software.
) or prazosin (○). Effects by the investigated drugs on contractions induced by EFS are expressed as percent inhibition of contractions before drug treatment. (*P < 0.05 **P < 0.01 versus tamsulosin, °P < 0.05 °°P < 001 vs. prazosin; two-way anova, Bonferroni post test). Non-linear regression calculation and statistical comparisons were made by using Graphpad Prism 5.0 software.
In isolated human ureteral preparations (Figure 4B), silodosin inhibited contractions by 17 ± 7% (10−7 M), 24 ± 8% (10−6 M; P = 0.05 vs. prazosin), 38 ± 9% (10−5 M) and 74 ± 6% (10−4 M; P < 0.05 vs. prazosin and tamsulosin). Tamsulosin (Figure 4B) exhibited inhibitory effects of 17 ± 5% (10−7 M), 27 ± 5% (10−6 M), 37 ± 9% (10−5 M) and 43 ± 10% (10−4 M). Prazosin, inhibited contractions by 5 ± 2% (10−7 M), 6.1 ± 6% (10−6 M), 14.8 ± 3% (10−5 M) and 38.5 ± 9% (10−4 M). The –logIC30 values amounted to 6.22 ± 0.53, 5.64 ± 0.49 and 4.32 ± 0.23 for silodosin, tamsulosin and prazosin (P = 0.06 vs. silodosin) respectively. For the human ureter, the order of potency at the currently investigated drug concentrations was silodosin = tamsulosin > prazosin, and the order of efficacy was silodosin > tamsulosin = prazosin. All together, silodosin appeared to perform better than tamsulosin and prazosin in reducing the EFS-induced ureteral contractions, both in rat and human isolated preparations. Vehicles did not exhibit any effects on EFS-induced contractions of the isolated rat or human ureters (not shown).
Discussion and conclusions
Medical expulsive therapy represents a valuable alternative to interventional approaches. Several clinical trials have investigated clinically the use of the α1A/D-selective α-blockers such as tamsulosin, naftopidil and silodosin, demonstrating an overall benefit in enhancing stone expulsion. Given the evidence that generally α-blockers are comparably effective in treating ureteral obstructions, more studies are required to address the uroselectivity and the potential adverse effects that may help to select the most appropriate molecule. To this respect, appropriate animal modelling is critical towards achievement of meaningful results. Initial models of ureteral obstruction were specifically designed to mimic congenital hydronephrosis, not to evaluate in vivo ureter pharmacology (Ulm and Miller, 1962; Wen et al., 1998) Different approaches in various species developed experimental models to study the ureteral peristalsis either in absence of obstruction (Becker et al., 1998) or in a pharmacologically induced increase of intraluminal ureter pressure to the goal of evaluating the relaxant effects of selective α1A D-AR antagonists (Kobayashi et al., 2010). Even though previous studies demonstrated effective action of various α-blockers on ureteral relaxation and differential side effects at the circulatory level, we sought to develop an original rat model in which, while inducing a mechanical partial obstruction of the ureter, the spontaneous ureteral peristalsis activity were maintained and measurable. In the present study indeed, the ureter activity was modulated only by a non-complete obstruction obtained by psoas muscle ligation, without pharmacological induction of ureteral contractions. We believe that this model better recapitulates the physio-dynamics of an obstructed ureter towards the evaluation of effective treatments for expulsive therapy in stone disease, which is after all a mechanically induced dysfunction. We definitely consider that this approach better simulates the in vivo pathophysiology of urolithiasis. Also, in order to avoid any interference on the peristaltic cycles or intraluminal pressures by retrograde pressure inclines originating from the bladder during micturitions, the bladder dome of the rats was notched open to equilibrate the internal back pressure. This procedure allows the measure of the genuine intra-ureteral pressure (IUP) and the spontaneous ureteral dynamics as previously described (Becker et al., 1998).
While tamsulosin and alfuzosin have been reported to increase stones expulsion rate and reduce stone expulsion time in patients with urolithiasis (Agrawal et al., 2009), some controversial data on the real-life efficacy of α1-AR antagonists in ureteral stone disease have been more recently reported in a multicentre placebo-controlled clinical trial that demonstrated no significant effects by tamsulosin treatment on acceleration of stone expulsion in patients with ureteral colic (Vincendeau et al., 2010). Although these last findings show a not significant numerical superiority of tamsulosin compared to placebo in facilitating stone expulsion, successive clinical reports suggest that α1A-AR antagonists, such as silodosin, perform better than α1D-specific blockers such as naftopidil in the medical expulsion therapy for ureteral stone in a Japanese population (Itoh et al., 2011; Tsuzaka et al., 2011). There seem to be some discrepancies in the efficacy rates of α1-AR antagonists in the therapy of ureteral stone, which deserve further clinical investigations and possibly more accurate patient stratification (Vincendeau et al., 2010). Even so, our present results are supportive of a beneficial effect of α1-AR antagonists in improving peristaltic dynamics in obstructed ureters in vivo and that α1-AR antagonists modify contractile functions of the isolated rat and human ureter. Our findings show that, at doses approximately corresponding to normal and triple human therapeutic use, each tested drug significantly reduced in vivo the obstruction-induced increase of IUP, without affecting contraction frequency or wave amplitude. Hence, following systemic administration of α1-AR antagonists, a main effect seems to be reduction of intraluminal pressure burden without altering spontaneous peristaltic work by the ureter. It may be speculated if this reduces the metabolic demand on the obstructed ureter with improved propulsive capacity. If translatable to the clinical situation, this might lead to an easier and less painful passage of the ureteral stone.
In the current investigation, the α1A-AR selective antagonist silodosin seemed to display a better efficacy-to-safety ratio compared to tamsulosin and prazosin. Significant reductions of MAP were always observed immediately after i.v. administration of prazosin and tamsulosin, while silodosin exerted a smaller hypotensive effect. Indeed, when the effect of the investigated drugs on ureteral pressures is reported as a function of the effect on MAP, silodosin exerted significantly larger inhibitory effect on IUP than prazosin and tamsulosin. These findings confirm the results obtained previously in anesthetized dogs (Kobayashi et al., 2010) and strongly suggest that the α1A-AR antagonist silodosin has a better in vivo uroselectivity than other α1-AR antagonists. Several other preclinical reports also demonstrate that silodosin has a high uroselectivity, as shown by, for example, the tissue discrimination of its binding on liver and aortic α1A and α1B receptors versus prostate α1A-ARs (Russo et al., 2011). Previous data demonstrated a clear reduced affinity and selectivity of silodosin towards human vascular structures expressing α1B-ARs, accounting for an approximately 200-fold less affinity compared to prostatic tissue. In addition, functional studies carried out on noradrenaline-induced tissue contraction also demonstrated that silodosin inherently possesses a very high affinity and selectivity for the prostate, mainly mediated by α1L-AR subtype (Murata et al., 2000), a functional phenotype of α1A-AR. The reference drug tamsulosin also displayed good affinity for the prostate but a comparatively higher affinity for the mesenteric aorta was reported (Tatemichi et al., 2006), suggesting relatively higher cardiovascular systemic side effects in comparison with silodosin.
Functional selectivity by silodosin towards the urinary system has also been described in other animal models, and upon systemic administration, silodosin has been reported to display little cardiovascular effects when compared to other α1-AR antagonists. In addition, as previously reported in isolated ureteral tissues from animals (Kobayashi et al., 2009b) and humans (Sasaki et al., 2011), where silodosin and prazosin exerted similar effects in inhibiting phenylephrine-induced contraction on isolated ureter preparations, we further verify that silodosin has indeed a higher efficacy on EFS-induced contractions of isolated human and rat ureter than tamsulosin or prazosin.
As a limitation remark, however, we cannot completely exclude that, although human ureter specimens were evaluated by a pathologist as normal, any undiagnosed kidney or ureter dysfunctions might have influenced the ureter function.
Taken together, our data suggest that the α1A-AR subtype, without interfering with the autonomous peristaltic activity, plays an important role in the contractile function of the ureter. Since ureteral relaxation and maintenance of spontaneous contraction are main physiological requirements for enhancing stone passage, a pharmacological approach that selectively blocks α1A-AR activity may represent a valid treatment option for patients with distal ureteral stones. Moreover, by targeting the α1A-AR subtype, a minimal hypotensive systemic effect is possibly anticipated.
In our in vivo rat model of ureteral obstruction, silodosin reduces ureteral pressure with less systemic side effects than tamsulosin and prazosin. Silodosin exhibits better inhibitory efficacy on EFS-induced contraction of human and rat isolated ureters than tamsulosin and prazosin. Selective inhibition of the α1A-AR subtype with silodosin should be further considered and evaluated clinically as an option for pharmacological expulsive therapy in patients with distal ureteral stones.
Acknowledgments
Authors thank the Urological Research Institute and the Gester Foundation for partly supporting the research activities.
Glossary
- -AR
-adrenoceptor
- EFS
electric field stimulation
- IUP
intra-ureteral pressure
- MAP
mean arterial pressure
- MaxP
maximum ureter pressure
- MinP
minimum ureter pressure
Conflicts of interest
This study was supported by an unrestricted grant from Recordati SpA, which only supported the project financially and was not involved in the design, production or handling of the intellectual content of the manuscript.
References
- Agrawal M, Gupta M, Gupta A, Agrawal A, Sarkari A, Lavania P. Prospective randomized trial comparing efficacy of alfuzosin and tamsulosin in management of lower ureteral stones. Urology. 2009;73:706–709. doi: 10.1016/j.urology.2008.11.013. [DOI] [PubMed] [Google Scholar]
- Becker AJ, Stief CG, Meyer M, Truss MC, Forssmann WG, Jonas U. The effect of the specific phosphodiesterase-IV-inhibitor rolipram on the ureteral peristalsis of the rabbit in vitro and in vivo. J Urol. 1998;160(3 Pt 1):920–925. doi: 10.1016/S0022-5347(01)62833-7. [DOI] [PubMed] [Google Scholar]
- Canda AE, Turna B, Cinar GM, Nazli O. Physiology and pharmacology of the human ureter: basis for current and future treatments. Urol Int. 2007;78:289–298. doi: 10.1159/000100830. [DOI] [PubMed] [Google Scholar]
- Davenport K, Timoney AG, Keeley FX., Jr Effect of smooth muscle relaxant drugs on proximal human ureteric activity in vivo: a pilot study. Urol Res. 2007;35:207–213. doi: 10.1007/s00240-007-0100-x. [DOI] [PubMed] [Google Scholar]
- Hollingsworth JM, Rogers MA, Kaufman SR, Bradford TJ, Saint S, Wei JT, et al. Medical therapy to facilitate urinary stone passage: a meta-analysis. Lancet. 2006;368:1171–1179. doi: 10.1016/S0140-6736(06)69474-9. [DOI] [PubMed] [Google Scholar]
- Itoh Y, Okada A, Yasui T, Hamamoto S, Hirose M, Kojima Y, et al. Efficacy of selective α1A adrenoceptor antagonist silodosin in the medical expulsive therapy for ureteral stones. Int J Urol. 2011;18:672–674. doi: 10.1111/j.1442-2042.2011.02810.x. [DOI] [PubMed] [Google Scholar]
- Kilkenny C, Browne WJ, Cuthill IC, Emerson M, Altman DG. Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. PLoS Biol. 2010;8:e1000412. doi: 10.1371/journal.pbio.1000412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi S, Tomiyama Y, Hoyano Y, Yamazaki Y, Kusama H, Itoh Y, et al. Gene expressions and mechanical functions of α1-adrenoceptor subtypes in mouse ureter. World J Urol. 2009a;27:775–780. doi: 10.1007/s00345-009-0396-y. [DOI] [PubMed] [Google Scholar]
- Kobayashi S, Tomiyama Y, Hoyano Y, Yamazaki Y, Kusama H, Kubota Y, et al. Mechanical function and gene expression of alpha(1)-adrenoceptor subtypes in dog intravesical ureter. Urology. 2009b;74:458–462. doi: 10.1016/j.urology.2009.01.009. [DOI] [PubMed] [Google Scholar]
- Kobayashi S, Tomiyama Y, Hoyano Y, Yamazaki Y, Sasaki S, Kohri K. Effects of silodosin and naftopidil on the distal ureter and cardiovascular system in anesthetized dogs: comparison of potential medications for distal ureteral stone passage. J Urol. 2010;183:357–361. doi: 10.1016/j.juro.2009.08.106. [DOI] [PubMed] [Google Scholar]
- Morita T, Ando M, Kihara K, Oshima H. Function and distribution of autonomic receptors in canine ureteral smooth muscle. Neurourol Urodyn. 1994;13:315–321. doi: 10.1002/1520-6777(1994)13:3<315::aid-nau1930130313>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Murata S, Taniguchi T, Takahashi M, Okada K, Akiyama K, Muramatsu I. Tissue selectivity of KMD-3213, an alpha(1)-adrenoreceptor antagonist, in human prostate and vasculature. J Urol. 2000;164:578–583. [PubMed] [Google Scholar]
- Park HK, Choi EY, Jeong BC, Kim HH, Kim BK. Localizations and expressions of alpha-1A, alpha-1B and alpha-1D adrenoceptors in human ureter. Urol Res. 2007;35:325–329. doi: 10.1007/s00240-007-0118-0. [DOI] [PubMed] [Google Scholar]
- Pearle MS, Calhoun EA, Curhan GC. Urologic diseases in America project: urolithiasis. J Urol. 2005;173:848–857. doi: 10.1097/01.ju.0000152082.14384.d7. [DOI] [PubMed] [Google Scholar]
- Rajpathy J, Aswathaman K, Sinha M, Subramani S, Gopalakrishnan G, Kekre NS. An in vitro study on human ureteric smooth muscle with the alpha1-adrenoceptor subtype blocker, tamsulosin. BJU Int. 2008;102:1743–1745. doi: 10.1111/j.1464-410X.2008.08022.x. [DOI] [PubMed] [Google Scholar]
- Rolle U, Brylla E, Tillig B, Chertin B, Cascio S, Puri P. Demonstration of intrinsic innervation of the guinea pig upper urinary tract using whole-mount preparation. Neurourol Urodyn. 2008;27:341–347. doi: 10.1002/nau.20496. [DOI] [PubMed] [Google Scholar]
- Russo A, Hedlund P, Montorsi F. Silodosin from bench to bedside: selectivity, safety, and sustained efficacy. Eur Urol Suppl. 2011;10:445–450. [Google Scholar]
- Sasaki S, Tomiyama Y, Kobayashi S, Kojima Y, Kubota Y, Kohri K. Characterization of alpha1-adrenoceptor subtypes mediating contraction in human isolated ureters. Urology. 2011;77:762 e713–762 e767. doi: 10.1016/j.urology.2010.09.034. [DOI] [PubMed] [Google Scholar]
- Schmidt F, Yoshimura Y, Ni RX, Kneesel S, Constantinou CE. Influence of gender on the diurnal variation of urine production and micturition characteristics of the rat. Neurourol Urodyn. 2001;20:287–295. doi: 10.1002/nau.1006. [DOI] [PubMed] [Google Scholar]
- Seitz C, Liatsikos E, Porpiglia F, Tiselius HG, Zwergel U. Medical therapy to facilitate the passage of stones: what is the evidence? Eur Urol. 2009;56:455–471. doi: 10.1016/j.eururo.2009.06.012. [DOI] [PubMed] [Google Scholar]
- Sharma V, McNeill JH. To scale or not to scale: the principles of dose extrapolation. Br J Pharmacol. 2009;157:907–921. doi: 10.1111/j.1476-5381.2009.00267.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigala S, Dellabella M, Milanese G, Fornari S, Faccoli S, Palazzolo F, et al. Evidence for the presence of alpha1 adrenoceptor subtypes in the human ureter. Neurourol Urodyn. 2005;24:142–148. doi: 10.1002/nau.20097. [DOI] [PubMed] [Google Scholar]
- Stamatelou KK, Francis ME, Jones CA, Nyberg LM, Curhan GC. Time trends in reported prevalence of kidney stones in the United States: 1976–1994. Kidney Int. 2003;63:1817–1823. doi: 10.1046/j.1523-1755.2003.00917.x. [DOI] [PubMed] [Google Scholar]
- Tatemichi S, Tomiyama Y, Maruyama I, Kobayashi S, Kobayashi K, Maezawa A, et al. Uroselectivity in male dogs of silodosin (KMD-3213), a novel drug for the obstructive component of benign prostatic hyperplasia. Neurourol Urodyn. 2006;25:792–799. doi: 10.1002/nau.20312. discussion 800–791. [DOI] [PubMed] [Google Scholar]
- Tsuzaka Y, Matsushima H, Kaneko T, Yamaguchi T, Homma Y. Naftopidil vs silodosin in medical expulsive therapy for ureteral stones: a randomized controlled study in Japanese male patients. Int J Urol. 2011;18:792–795. doi: 10.1111/j.1442-2042.2011.02850.x. [DOI] [PubMed] [Google Scholar]
- Ulm AH, Miller F. An operation to produce experimental reversible hydronephrosis in dogs. J Urol. 1962;88:337–341. doi: 10.1016/S0022-5347(17)64796-7. [DOI] [PubMed] [Google Scholar]
- Vincendeau S, Bellissant E, Houlgatte A, Dore B, Bruyere F, Renault A, et al. Tamsulosin hydrochloride vs placebo for management of distal ureteral stones: a multicentric, randomized, double-blind trial. Arch Intern Med. 2010;170:2021–2027. doi: 10.1001/archinternmed.2010.447. [DOI] [PubMed] [Google Scholar]
- Wanajo I, Tomiyama Y, Yamazaki Y, Kojima M. Ureteral selectivity of intravenous beta-adrenoceptor agonists in pig model of acute ureteral obstruction: comparison of KUL-7211, a selective beta2/beta3 agonist, with isoproterenol, terbutaline, and CL-316243. Urology. 2011;77:1266 e1261–1266 e1266. doi: 10.1016/j.urology.2010.12.045. [DOI] [PubMed] [Google Scholar]
- Wen JG, Chen Y, Frokiaer J, Jorgensen TM, Djurhuus JC. Experimental partial unilateral ureter obstruction. I. Pressure flow relationship in a rat model with mild and severe acute ureter obstruction. J Urol. 1998;160:1567–1571. [PubMed] [Google Scholar]


