Abstract
Cell-based therapies are being developed for myocardial infarction (MI) and its consequences (e.g. heart failure) as well as refractory angina and critical limb ischemia. The promising results obtained in pre-clinical studies led to the translation of this strategy to clinical studies. To date, the initial results have been mixed: some studies showed benefit, while in others no benefit was observed. There is a growing consensus among the scientific community that a better understanding of the fate of transplanted cells (e.g., cell homing and viability over time) will be critical for the long term success of these strategies and that future studies should include an assessment of cell homing, engraftment and fate as an integral part of the trial design. In this review, different imaging methods and technologies will be discussed within the framework of the physiological answers that the imaging strategies can provide, with special focus on the inherent regulatory issues.
Cell-based therapies are being developed for cardiac dysfunction as well as refractory angina and critical limb ischemia. Promising results obtained in pre-clinical studies led to the translation of this strategy to clinical studies. To date, several clinical trials of cell therapy after MI have been completed, providing initial evidence of the safety of stem cell delivery of many cell types -including BMCs (1) and mesenchymal stem cells (MSCs) (2). In terms of recovery of cardiac function, the initial results have been mixed: some studies have shown an improvement in cardiac function (3), while others have been neutral (4) or associated with a transient improvement in LVEF (5). Meta-analysis of these trials (6, 7) showed that cell therapy after MI has potential benefit, by increasing LVEF, reducing LV end-systolic volume, infarct size, and a trend towards a reduction in major adverse cardiac events.
The Cardiovascular Cell Therapy Research Network (CCTRN) was established by the National Heart, Lung and Blood Institute (NHLBI) to develop, coordinate, and conduct multiple collaborative protocols testing the effects of stem cell therapy on cardiovascular disease. The initial step is to prove that these therapies are safe for use in patients and will not lead to adverse events, such as arrhythmias (as previously seen with skeletal myoblasts). The Network builds on contemporary findings of the cell therapy basic science community, translating newly acquired information to the cardiac clinical setting in the Phase I/II study paradigm (8).
The CCTRN is simultaneously conducting two trials in patients with acute myocardial infarction, TIME (9) and LateTIME (10), and one trial in patients with chronic heart failure and ongoing ischemia, FOCUS (11). In these initial studies, the CCTRN initial focus is on the clinical feasibility and safety of these strategies, together with measuring their effect on LV function. The variability in the response to cell transplantation underscores the importance of determining the fate of transplanted stem cells and whether it correlates with changes in cardiac function. There is a general consensus among the CCTRN and the scientific community that a better understanding of the fate of transplanted cells (e.g., cell homing and viability over time) (12, 13) will be critical for the long term success of these strategies and that future studies should include an assessment of cell homing, engraftment and fate as an integral part of the trial design.
In this review, the different imaging methods and technologies available will be discussed within the framework of the physiological answers that they can provide. Furthermore, focus will be placed on the advantages and disadvantages of each strategy and the inherent regulatory issues.
Unanswered questions in cell therapy following MI
Currently, the evaluation of cell delivery for MI has been based on evaluating the recovery of cardiac function (14), as well as myocardial perfusion and ischemia (15). However, the efficacy of delivery, homing and fate of these cells remain poorly understood. Hou et al delivered BMCs, labeled with 111In, to a swine model of myocardial ischemia and showed that cell retention varied with the delivery route with a high percentage of pulmonary cell trapping (16). Kraitchman confirmed these findings and showed that within days, cells ultimately homed in the myocardium and other organs (17, 18). Furthermore, the effect of other factors, such as vascular leakage (19), extravasation and lymphatic drainage can account for the variability observed in cell therapy studies.
The original premise was that BMC delivery after MI had a direct regenerative effect (20). More recently it has been postulated that the improvement can be achieved through a paracrine effect and by accelerating the healing process after MI (21). It is likely that the ratio of direct/paracrine beneficial effect depends, among other biological variables, on the cell type used as well as the conditions of the host tissue. Regardless of the mechanisms of the beneficial response, whether through a direct regenerative effect or a paracrine effect, the presence (even if brief) of transplanted cells in the damaged myocardium appears to be an important factor. Furthermore, numerous questions, such as the ideal timing, dose and delivery route (e.g., intracoronary, intravenous, coronary sinus, intramyocardial) remain to be answered. To better understand these factors and to optimize the beneficial effect of these therapies, it is important to be able to monitor the presence of transplanted cells as well as the kinetics and biology of transplanted cells over time and to integrate this with the evaluation of LV structure and function.
Strategies to address these questions can be broadly divided into short- and long-term assessments of cell therapy. Short-term assessment can include the study of the retention and homing of transplanted cells. The long-term assessment includes the monitoring of the viability of transplanted cells over time as well as the post-engraftment biology of the transplanted cells. Understanding issues like the functionality of transplanted cells (e.g., differentiation, interaction of cells with the host tissue) will be of critical importance for the optimal translation of these approaches. However, short- and long-term assessment should not be considered as separate concepts, as they are closely connected. For example, the functionality of injected cells (long-term assessment) may not be relevant if those cells do not initially home and engraft (short-term assessment).
Short term assessment of transplanted cells
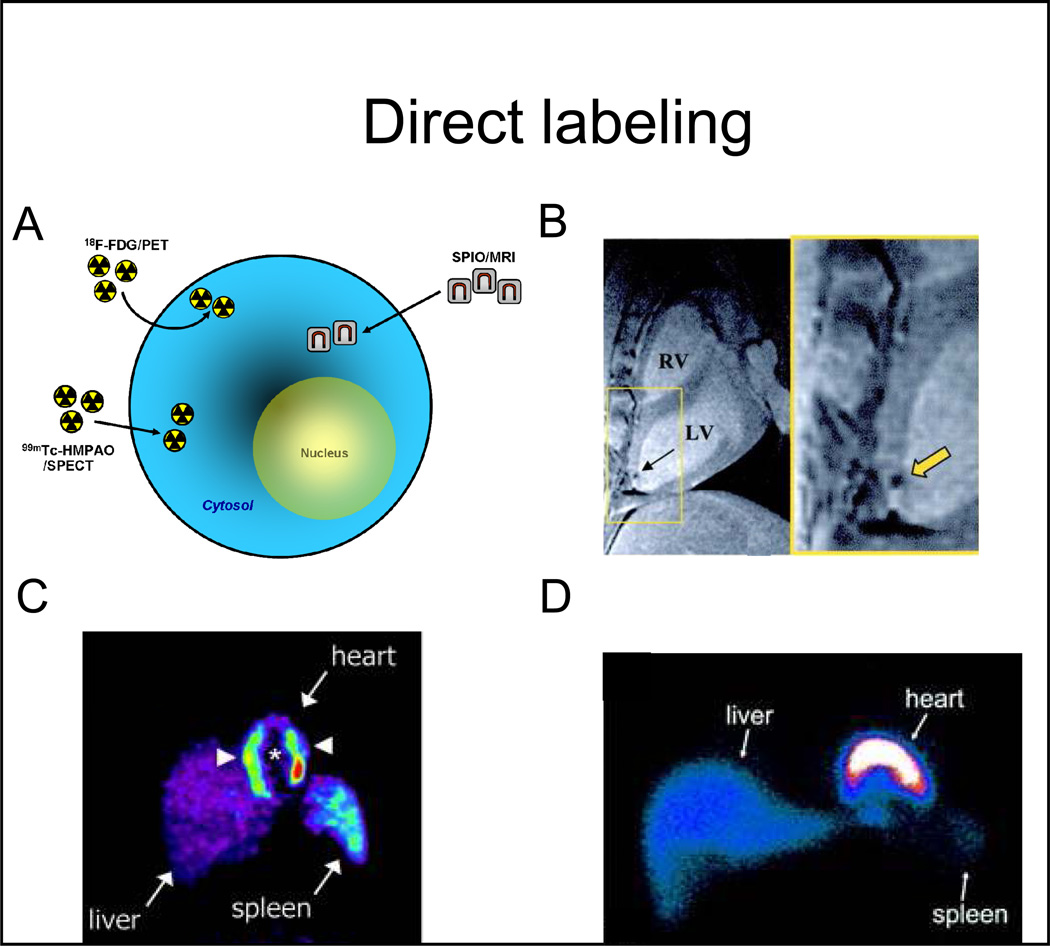
To assess homing and engraftment, the most commonly used monitoring strategy is that of direct labeling (22, 23), when different labeling agents are introduced into the cells exogenously (Figure 1A) and cells are then transplanted and imaged in the living subject (Figure 1). Imaging of the introduced molecules is performed, and the signal obtained is used as a surrogate for the number of stem cells. In direct labeling strategies, signal originates from the labeling compounds and is independent of progenitor cell viability. Direct strategies have the advantage of relative ease of labeling and that many probes are already used clinically (albeit for different purposes), facilitating their clinical translation. Notably, the signal from direct labeling strategies may decrease over time due to cell division and ”dilution”, that will reduce the sensitivity of the technique for serial imaging. Imaging of direct labels may include MRI and nuclear techniques (SPECT and PET).
Figure 1. Direct cell labeling strategies.
A, labeling agents (for either magnetic resonance or radionuclide imaging) are first introduced into the stem cells exogenously, and are then transplanted to the tissue and/or organ of interest. Non-invasive imaging is subsequently performed. B, 2.8×107 MSCs, labeled with super paramagnetic particles (Feridex, 25 µg Fe/mL), were imaged, after direct transmyocardial delivery, using a 1.5T MRI. The black signal (yellow arrow) represents the super paramagnetic signal, which has been used to monitor the delivery of stem cells. C, 1.25×108 BMCs, labeled with 18F-FDG (100MBq), were delivered to the myocardium via intracoronary injection, and then imaged using PET. The white arrowheads point to the transplanted cells in the heart. There is also liver and spleen uptake (route of tracer elimination). D, 8×108 BMCs were labeled with 99Tc-HMPAO (100MBq/1×108 cells) and infused via intracoronary injection to patients with chronic ischemic cardiomyopathy and imaged with SPECT at different times after delivery (shown is a representative image obtained one hour after cell delivery).
Abbreviations: SPIO: superparamagnetic iron oxide particles, 18F-FDG: 18F-fluorodeoxyglucose, 99Tc-HMPAO: Tc99m-hexamethylpropylenamineoxime, MSCs: mesenchymal stem cells, MRI: magnetic resonance imaging, PET: positron emission tomography, SPECT: single-photon emission computed tomography, RV: right ventricle, LV: left ventricle. Adapted from Kraitchman et al. Circulation 2003 13;107(18):2290–3, Gousettis et al. Stem Cells 2006; 24: 2279–2283, and Hofmann et al. Circulation 2005 111: 2198–2202 with permission.
Monitoring of stem cells using MRI is based on the imaging of SPIOs particles, which are highly magnetic particles that cause magnetic field perturbations that can be identified on T2* weighted images (24, 25) (Figure 1B). The detected signal is used as a surrogate for the number of cells. However, SPIOs may not stay inside the transplanted cells over time (26), but may be phagocytized by macrophages, resulting in an uncoupling between the MRI signal and the viability of stem cells (26, 27). Furthermore, considerations should be given to the potential toxicity of ferromagnetic compounds and transfection agents (28, 30) as well as the potential interaction between certain SPIOs with metalloproteins (28). As MRI has high spatial resolution, this strategy appears as a good modality to define cardiac delivery and short-term (e.g., 1–2 days) homing of transplanted cells (Figure 1B) (23, 25). MRI labeling agents and/or the transfection agents used to introduce iron particles can affect cell viability of stem cells (27), while others have not (29), likely depending on the dose and cell type used. Although used in animal and small patient studies (30, 31), direct labeling-MRI tracking has not yet been used in clinical studies.
Radionuclide labeling of cells has also been used for direct cell labeling and imaging (Figure 1C and 1D). The half-life of the radionuclides used (e.g., 6 hours for 99mTc, 109 minutes for 18F) determines the duration of time that cells can be monitored after labeling.
SPECT and PET imaging are more sensitive (nano- and femto-molar detection, respectively) compared to SPIO-MRI (micromolar), (12, 13, 32). However, the cellular detection sensitivity should be considered together with the spatial resolution (MRI> SPECT or PET). The recent development of integrated PET-Computed Tomography (CT) and SPECT-CT provides a better anatomical guide for the location of the PET or SPECT signal.
Hofmann and colleagues (22), using 18F-FDG as the label and PET as the imaging modality, monitored cells after intravenous or intracoronary delivery of unselected BMCs or CD34-enriched cells (Figure 1C), demonstrating that intracoronary delivery, specially of CD34-enriched populations, enhanced homing to the infarct border zone compared with unselected populations. Also noted was signal from non-cardiac sites such as liver and spleen, that could represent free 18F or actual labeled cells.
Another consideration is that the radionuclide’s biological half-life, or the amount of time that the radionuclide stays in the intracellular compartment, may vary depending on the radionuclide, and may differ between cell types and characteristics of the cell (e.g., senescence, phenotype). Furthermore, all radionuclides emit a certain level of ionizing radiation, with its potential toxicity to both the cell and host. Previous studies have used an average of 100MBq to label 1x108 BMCs and have not observed significant cell toxicity (22, 33). The potential harmful risk of ionizing radiation from medical procedures is a hypothetical one, and stems from studies of the radiation exposure experienced by survivors from the atomic bombs in Hiroshima and Nagasaki. However, there are no definitive studies on the effects of ionizing radiation from medical procedures (34). Further studies are needed to precisely and accurately determine the consequence that this level of low radiation may or may not have on the host. Therefore, the use of as low as reasonably appropriate amounts of radionuclides appears as a reasonable strategy.
In summary, direct labeling methods are good strategies to confirm successful cell delivery and short term retention of transplanted cells. Furthermore, their implementation is relatively straightforward, and have already been used in clinical studies (Figure 1C) (22). However, these imaging modalities are less suitable to provide answers on the long-term viability and biology of transplanted cells.
Long-term assessment of cell therapy
To address issues such as cell functionality and/or long-term viability, imaging modalities that are dependent on the viability of the cell may be used. Recent advances in non-invasive imaging and reporter gene technology have provided with novel tools to study trans-gene expression non-invasively (13, 32, 35). Reporter gene constructs produce proteins which interact with an exogenously given probe, producing a signal that can be monitored non-invasively (13, 32, 35, 36).
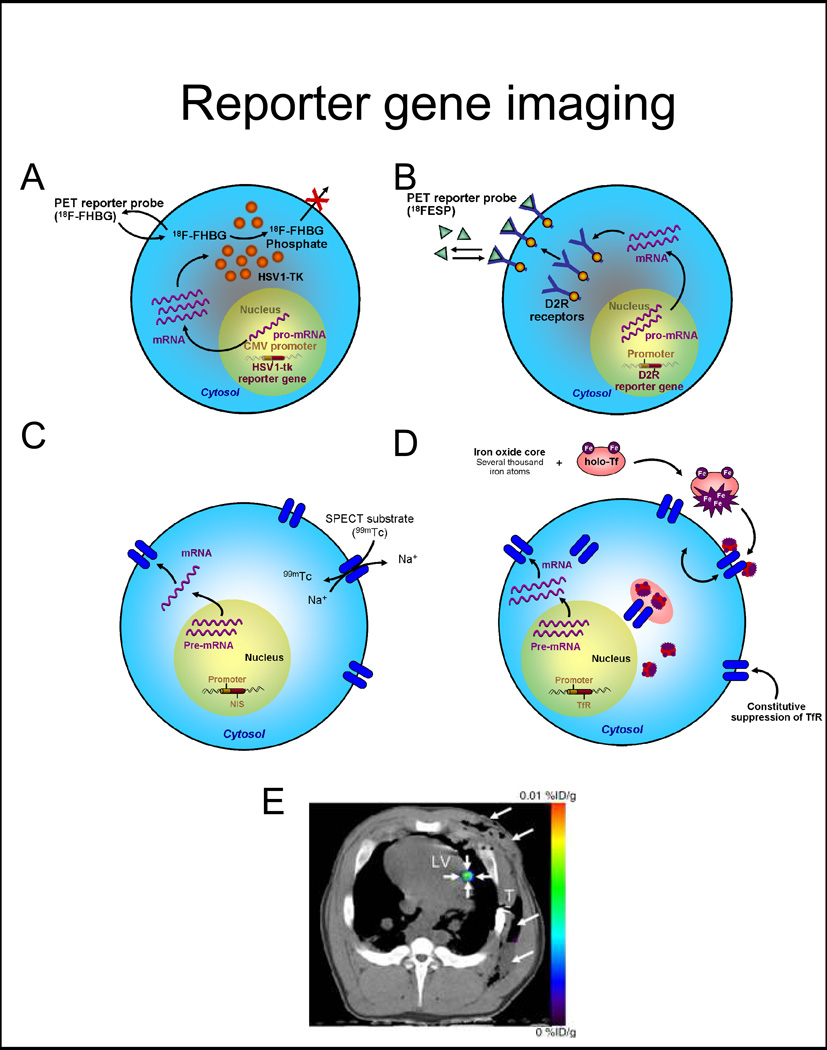
The most common use of reporter genes in vivo is for the longitudinal study of cell viability (11, 37–39), and this strategy can be used to investigate the activity of a specific biological pathway, when a reporter gene is driven by a cell-specific promoter (40). Commonly used reporter gene systems are either based on an intracellular enzyme (e.g., HSV1-tk, an enzyme that phosphorylates an exogenously administered substrate which in turn is retained inside the cell and imaged with PET, Figure 2A), a cell membrane receptor, such as mutant dopamine receptor D2R, imaged with PET (Figure 2B) (41) or the cell membrane sodium-iodine symporter, NIS (Figure 2C), whose activity can be imaged with PET or SPECT (40, 42). Recently, efforts have been devoted to developing MR reporter genes (43), based on the production of different proteins, mostly intracellular metalloproteins (transferrin, ferritin, tyrosinase, Figure 2D) (44), that accumulate iron intra-cellularly, creating signal that can be detected on T2* weighted images. Many of MR reporter genes are based on the intracellular accumulation of iron for signal production, thus necessitating a critical steady intracellular iron level and having also potentially experiencing a dilution effect of ferritin iron when cells divide (44). Novel MR-reporter genes are targeted to produce amino acids with specific diamagnetic characteristics (chemical exchange saturation transfer, CEST) (45). Currently, MRI-based reporter genes have not yet become widely available (44).
Figure 2. Reporter gene imaging strategies.
A, Enzyme-based PET imaging. 18F-FHBG is a substrate molecular probe that is phosphorylated by the HSV1-tk enzyme resulting in intracellular trapping of the probe in cells expressing the HSV1-tk gene. B, Receptor-based PET imaging. 18F-FESP is a ligand molecular probe interacting with the D2R to result in trapping of the probe in cells expressing the D2R gene. C, Symporter-based SPECT imaging. 99Tc is uptaken by the progenitor cell expressing the NIS reporter gene in exchange for Na+. D, Receptor-based MR imaging. Iron enters the cell through transferrin receptors. The signal detection by MRI is based on the T2* effect (as in direct labeling). E, Representative PET-CT image of 3×107 MSCs, transduced with Ad-CMV-HSV1-sr39tk, and transplanted to the myocardium of swine. 18F-FHBG was administered intravenously and transverse non-enhanced PET-CT imaging was performed after four hours. Small arrows depict the signal at the intramyocardial injection site, while large arrows point to the post-operative changes following delivery.
Abbreviations: 18F-FHBG: 9-[4-[18F]fluoro-3-(hydroxymethyl)butyl]guanine, HSV1-sr39tk: mutant herpes simplex virus type 1 thymidine kinase, 18F-FESP: 3-N-(2-[18F]Fluoroethyl)spiperone, D2R: dopamine-2 receptor, NIS: sodium iodide symporter, TfR: transferrin receptor, PET-CT: positron emission tomography-computed tomography. Adapted from Wu et al. J Nucl Cardiol 2004 Jul–Aug 11(4):491–505 and Willmann et al. Radiology 2009; 252:117–127 with permission.
Different from direct labeling, reporter gene systems have the advantage that the signal emitted is based on the viability and biology of the cell. Reporter gene technology is mostly efficacious when randomly stably integrated into the genome. Although there are risks of insertional mutagenesis, the risk may be low (46, 47). Novel developments in site-specific integration technology may even circumvent this issue (48).
Currently, there are a larger number of reporter genes for PET (compared to SPECT) that have been used for cell imaging, which gives PET-based reporter gene imaging more flexibility in the number of biological events that can be studied in a single subject, albeit not simultaneously. However, PET probe production is more complex, needing advanced radiochemistry, and in many cases it requires an on-site or nearby cyclotron. SPECT, on the other hand, can detect simultaneous signals of different energies by varying the detection windows, allowing the monitoring of cell therapies together with tissue perfusion with 201Tl or 99Tc, or even the concomitant monitoring of multiple cell types. SPECT tracer labeling is less complex but more limited and, for the most part, can be performed in a radionuclide pharmacy.
Reporter gene systems have been used in small animal studies under different pathophysiological conditions. In 2003, Wu et al demonstrated the feasibility of PET reporter genes to monitor the survival of murine cardiomyoblasts transfected with a mutant of the HSV1-tk after transplantation to the myocardium (38). Since then, a number of studies have used reporter genes to monitor the survival and biology of cells after transplantation to the myocardium (38–40, 49–51), also combined with studies of myocardial perfusion (38, 40). However, due to the complexity of the system and the multidisciplinary approach need, there is limited experience in large animals on the monitoring of trans-gene expression (52, 53), the assessment of cell viability (Figure 2E) (19, 54), and only one reported experience (in oncology) in the use of reporter genes to monitor cell survival of T cells expressing HSV1-tk in patients by PET (55). In summary, reporter genes offer a promising alternative for long-term assessment of cellular viability and functionality.
A multimodality imaging approach may prove useful to better characterize the success of cardiac cell delivery. The success of delivery might be assessed by direct labeling using SPIO-MRI or 18F-FDG-PET, while viability might be assessed using reporter gene techniques (e.g. HSV1-tk-PET). This information can be complemented with the evaluation of myocardial perfusion and the assessment of cardiac structure, and function.
Regulatory issues
It is important to assure that any imaging strategy does not alter the survival, viability and phenotype of the transplanted cells, the host organ or the patient. For direct imaging approaches, most of the labeling compounds that will be useful clinically have been previously used. For example, 111In (56) and 18F-FDG (22, 33) have been used for labeling of leukocytes and for studies of myocardial viability, respectively. Although previous experience may be reassuring relating to the safety of these compounds, we anticipate that each strategy will need to be tested in the specific cell of interest, as not all cells may behave similarly. Thus, if direct labeling agents (for SPECT, PET or MRI) are to be used, it seems reasonable to test each cell type for toxicity before clinical implementation. Focus should be placed on cell viability, survival and/or phenotype, including the assessment of the functions that are expected from the transplanted cells. Preclinical studies of these labeling compounds will be an important aspect of any Investigational New Drug (IND) application to the Food and Drug Administration (FDA).
Reporter gene strategies also present some regulatory issues that need to be addressed. In addition to the concepts related to the radionuclide probes described above, it is important to evaluate the potential effect of the introduction of reporter genes into the cell of interest. Pre-clinical studies have shown that the introduction of reporter genes did not significantly alter the phenotype of embryonic stem cells (46), but caution should be exercised when using different reporter genes and different vectors and different cell types. Successful use of these strategies in other patient population (e.g., oncologic) may pave the road for cardiac applications. A possible approach will be that, after defining the cell and the reporter gene vector to be used, studies be performed to test the safety of the strategy. Genetic manipulation of cells will also necessitate the review by the Recombinant DNA Advisory Committee (RAC) of the NIH, a step that can take place in parallel with review by the FDA but must be complete prior to initiation of the study.
Conclusion
Cell therapy has great potential for the treatment of cardiovascular diseases, but many questions remain about the efficacy of cell delivery and the fate of delivered cells. Direct labeling and reporter gene strategies may be used to begin to define and track cell fate and should be strongly considered in early phase clinical trials of cardiovascular cell delivery.
Abbreviations
- MI
myocardial infarction
- BMCs
bone marrow cells
- LVEF
left ventricular ejection fraction
- MRI
magnetic resonance imaging
- SPECT
single photon emission computed tomography
- PET
positron emission tomography
- SPIO
super paramagnetic iron oxide
- 18F-FDG
18-Fluorine-Fluorodeoxyglucose
- HSV1-tk
herpes symplex virus type 1 thymidine kinase
BIBLIOGRAPHY
- 1.Wollert KC, Meyer GP, Lotz J, et al. Intracoronary autologous bone-marrow cell transfer after myocardial infarction: the BOOST randomised controlled clinical trial. Lancet. 2004;364:141–148. doi: 10.1016/S0140-6736(04)16626-9. [DOI] [PubMed] [Google Scholar]
- 2.Hare JM, Traverse JH, Henry TD, et al. A randomized, double-blind, placebo-controlled, dose-escalation study of intravenous adult human mesenchymal stem cells (prochymal) after acute myocardial infarction. J Am Coll Cardiol. 2009;54:2277–2286. doi: 10.1016/j.jacc.2009.06.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Erbs S, Linke A, Schachinger V, et al. Restoration of microvascular function in the infarct-related artery by intracoronary transplantation of bone marrow progenitor cells in patients with acute myocardial infarction: the Doppler Substudy of the Reinfusion of Enriched Progenitor Cells and Infarct Remodeling in Acute Myocardial Infarction (REPAIR-AMI) trial. Circulation. 2007;116:366–374. doi: 10.1161/CIRCULATIONAHA.106.671545. [DOI] [PubMed] [Google Scholar]
- 4.Lunde K, Solheim S, Aakhus S, et al. Intracoronary injection of mononuclear bone marrow cells in acute myocardial infarction. N Engl J Med. 2006;355:1199–1209. doi: 10.1056/NEJMoa055706. [DOI] [PubMed] [Google Scholar]
- 5.Meyer GP, Wollert KC, Lotz J, et al. Intracoronary bone marrow cell transfer after myocardial infarction: eighteen months' follow-up data from the randomized, controlled BOOST (BOne marrOw transfer to enhance ST-elevation infarct regeneration) trial. Circulation. 2006;113:1287–1294. doi: 10.1161/CIRCULATIONAHA.105.575118. [DOI] [PubMed] [Google Scholar]
- 6.Lipinski MJ, Biondi-Zoccai GG, Abbate A, et al. Impact of intracoronary cell therapy on left ventricular function in the setting of acute myocardial infarction: a collaborative systematic review and meta-analysis of controlled clinical trials. J Am Coll Cardiol. 2007;50:1761–1767. doi: 10.1016/j.jacc.2007.07.041. [DOI] [PubMed] [Google Scholar]
- 7.Martin-Rendon E, Brunskill SJ, Hyde CJ, Stanworth SJ, Mathur A, Watt SM. Autologous bone marrow stem cells to treat acute myocardial infarction: a systematic review. Eur Heart J. 2008;29:1807–1818. doi: 10.1093/eurheartj/ehn220. [DOI] [PubMed] [Google Scholar]
- 8.Simari RD, Moye LA, Skarlatos SI, et al. Development of a network to test strategies in cardiovascular cell delivery: the NHLBI-sponsored Cardiovascular Cell Therapy Research Network (CCTRN) J Cardiovasc Transl Res. 2010;3:30–36. doi: 10.1007/s12265-009-9160-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Traverse JH, Henry TD, Vaughan DE, et al. Rationale and design for TIME: A phase II, randomized, double-blind, placebo-controlled pilot trial evaluating the safety and effect of timing of administration of bone marrow mononuclear cells after acute myocardial infarction. Am Heart J. 2009;158:356–363. doi: 10.1016/j.ahj.2009.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Traverse JH, Henry TD, Vaughan DE, et al. LateTIME: a phase-II, randomized, double-blinded, placebo-controlled, pilot trial evaluating the safety and effect of administration of bone marrow mononuclear cells 2 to 3 weeks after acute myocardial infarction. Tex Heart Inst J. 2010;37:412–420. [PMC free article] [PubMed] [Google Scholar]
- 11.Willerson JT, Perin EC, Ellis SG, et al. Intramyocardial injection of autologous bone marrow mononuclear cells for patients with chronic ischemic heart disease and left ventricular dysfunction (First Mononuclear Cells injected in the US [FOCUS]): Rationale and design. Am Heart J. 2010;160:215–223. doi: 10.1016/j.ahj.2010.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bengel FM, Schachinger V, Dimmeler S. Cell-based therapies and imaging in cardiology. Eur J Nucl Med Mol Imaging. 2005;32(Suppl 2):S404–S416. doi: 10.1007/s00259-005-1898-5. [DOI] [PubMed] [Google Scholar]
- 13.Rodriguez-Porcel M. In vivo imaging and monitoring of transplanted stem cells: clinical applications. Curr Cardiol Rep. 2010;12:51–58. doi: 10.1007/s11886-009-0073-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schachinger V, Erbs S, Elsasser A, et al. Intracoronary bone marrow-derived progenitor cells in acute myocardial infarction. N Engl J Med. 2006;355:1210–1221. doi: 10.1056/NEJMoa060186. [DOI] [PubMed] [Google Scholar]
- 15.van Ramshorst J, Bax JJ, Beeres SL, et al. Intramyocardial bone marrow cell injection for chronic myocardial ischemia: a randomized controlled trial. JAMA. 2009;301:1997–2004. doi: 10.1001/jama.2009.685. [DOI] [PubMed] [Google Scholar]
- 16.Hou D, Youssef EA, Brinton TJ, et al. Radiolabeled cell distribution after intramyocardial, intracoronary, and interstitial retrograde coronary venous delivery: implications for current clinical trials. Circulation. 2005;112:I150–I156. doi: 10.1161/CIRCULATIONAHA.104.526749. [DOI] [PubMed] [Google Scholar]
- 17.Kraitchman DL, Tatsumi M, Gilson WD, et al. Dynamic imaging of allogeneic mesenchymal stem cells trafficking to myocardial infarction. Circulation. 2005;112:1451–1461. doi: 10.1161/CIRCULATIONAHA.105.537480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chin BB, Nakamoto Y, Bulte JW, Pittenger MF, Wahl R, Kraitchman DL. 111In oxine labelled mesenchymal stem cell SPECT after intravenous administration in myocardial infarction. Nucl Med Commun. 2003;24:1149–1154. doi: 10.1097/00006231-200311000-00005. [DOI] [PubMed] [Google Scholar]
- 19.Willmann JK, Paulmurugan R, Rodriguez-Porcel M, et al. Imaging Gene Expression in Human Mesenchymal Stem Cells: From Small to Large Animals. Radiology. 2009;252:117–127. doi: 10.1148/radiol.2513081616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Behfar A, Terzic A. Derivation of a cardiopoietic population from human mesenchymal stem cells yields cardiac progeny. Nat Clin Pract Cardiovasc Med. 2006;3(Suppl 1):S78–S82. doi: 10.1038/ncpcardio0429. [DOI] [PubMed] [Google Scholar]
- 21.Gnecchi M, He H, Liang OD, et al. Paracrine action accounts for marked protection of ischemic heart by Akt-modified mesenchymal stem cells. Nat Med. 2005;11:367–368. doi: 10.1038/nm0405-367. [DOI] [PubMed] [Google Scholar]
- 22.Hofmann M, Wollert KC, Meyer GP, et al. Monitoring of bone marrow cell homing into the infarcted human myocardium. Circulation. 2005;111:2198–2202. doi: 10.1161/01.CIR.0000163546.27639.AA. [DOI] [PubMed] [Google Scholar]
- 23.Kraitchman DL, Heldman AW, Atalar E, et al. In vivo magnetic resonance imaging of mesenchymal stem cells in myocardial infarction. Circulation. 2003;107:2290–2293. doi: 10.1161/01.CIR.0000070931.62772.4E. [DOI] [PubMed] [Google Scholar]
- 24.Bos C, Delmas Y, Desmouliere A, et al. In vivo MR imaging of intravascularly injected magnetically labeled mesenchymal stem cells in rat kidney and liver. Radiology. 2004;233:781–789. doi: 10.1148/radiol.2333031714. [DOI] [PubMed] [Google Scholar]
- 25.Dick AJ, Guttman MA, Raman VK, et al. Magnetic resonance fluoroscopy allows targeted delivery of mesenchymal stem cells to infarct borders in Swine. Circulation. 2003;108:2899–2904. doi: 10.1161/01.CIR.0000095790.28368.F9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li Z, Suzuki Y, Huang M, et al. Comparison of reporter gene and iron particle labeling for tracking fate of human embryonic stem cells and differentiated endothelial cells in living subjects. Stem Cells. 2008;26:864–873. doi: 10.1634/stemcells.2007-0843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen IY, Greve JM, Gheysens O, et al. Comparison of optical bioluminescence reporter gene and superparamagnetic iron oxide MR contrast agent as cell markers for noninvasive imaging of cardiac cell transplantation. Mol Imaging Biol. 2009;11:178–187. doi: 10.1007/s11307-008-0182-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Raschzok N, Muecke DA, Adonopoulou MK, et al. In vitro evaluation of magnetic resonance imaging contrast agents for labeling human liver cells: implications for clinical translation. Mol Imaging Biol. 2011;13:613–622. doi: 10.1007/s11307-010-0405-y. [DOI] [PubMed] [Google Scholar]
- 29.Crabbe A, Vandeputte C, Dresselaers T, et al. Effects of MRI contrast agents on the stem cell phenotype. Cell Transplant. 2010;19:919–936. doi: 10.3727/096368910X494623. [DOI] [PubMed] [Google Scholar]
- 30.de Vries IJ, Lesterhuis WJ, Barentsz JO, et al. Magnetic resonance tracking of dendritic cells in melanoma patients for monitoring of cellular therapy. Nature biotechnology. 2005;23:1407–1413. doi: 10.1038/nbt1154. [DOI] [PubMed] [Google Scholar]
- 31.Zhu J, Zhou L, XingWu F. Tracking neural stem cells in patients with brain trauma. N Engl J Med. 2006;355:2376–2378. doi: 10.1056/NEJMc055304. [DOI] [PubMed] [Google Scholar]
- 32.Wu JC, Tseng JR, Gambhir SS. Molecular imaging of cardiovascular gene products. J Nucl Cardiol. 2004;11:491–505. doi: 10.1016/j.nuclcard.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 33.Doyle B, Kemp BJ, Chareonthaitawee P, et al. Dynamic tracking during intracoronary injection of 18F-FDG-labeled progenitor cell therapy for acute myocardial infarction. J Nucl Med. 2007;48:1708–1714. doi: 10.2967/jnumed.107.042838. [DOI] [PubMed] [Google Scholar]
- 34.Gerber TC, Gibbons RJ. Weighing the risks and benefits of cardiac imaging with ionizing radiation. JACC Cardiovasc Imaging. 2010;3:528–535. doi: 10.1016/j.jcmg.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 35.Massoud TF, Gambhir SS. Molecular imaging in living subjects: seeing fundamental biological processes in a new light. Genes Dev. 2003;17:545–580. doi: 10.1101/gad.1047403. [DOI] [PubMed] [Google Scholar]
- 36.Inubushi M, Tamaki N. Radionuclide reporter gene imaging for cardiac gene therapy. Eur J Nucl Med Mol Imaging. 2007;34(Suppl 1):S27–S33. doi: 10.1007/s00259-007-0438-x. [DOI] [PubMed] [Google Scholar]
- 37.Rodriguez-Porcel M, Gheysens O, Chen IY, Wu JC, Gambhir SS. Image-guided cardiac cell delivery using high-resolution small-animal ultrasound. Mol Ther. 2005;12:1142–1147. doi: 10.1016/j.ymthe.2005.07.532. [DOI] [PubMed] [Google Scholar]
- 38.Wu JC, Chen IY, Sundaresan G, et al. Molecular imaging of cardiac cell transplantation in living animals using optical bioluminescence and positron emission tomography. Circulation. 2003;108:1302–1305. doi: 10.1161/01.CIR.0000091252.20010.6E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang J, Zhang S, Rabinovich B, et al. Human CD34+ cells in experimental myocardial infarction: long-term survival, sustained functional improvement, and mechanism of action. Circ Res. 2010;106:1904–1911. doi: 10.1161/CIRCRESAHA.110.221762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Terrovitis J, Kwok KF, Lautamaki R, et al. Ectopic expression of the sodium-iodide symporter enables imaging of transplanted cardiac stem cells in vivo by single-photon emission computed tomography or positron emission tomography. J Am Coll Cardiol. 2008;52:1652–1660. doi: 10.1016/j.jacc.2008.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.MacLaren DC, Gambhir SS, Satyamurthy N, et al. Repetitive, non-invasive imaging of the dopamine D2 receptor as a reporter gene in living animals. Gene Ther. 1999;6:785–791. doi: 10.1038/sj.gt.3300877. [DOI] [PubMed] [Google Scholar]
- 42.Kang JH, Chung JK. Molecular-genetic imaging based on reporter gene expression. J Nucl Med. 2008;49(Suppl 2):164S–179S. doi: 10.2967/jnumed.107.045955. [DOI] [PubMed] [Google Scholar]
- 43.Cohen B, Dafni H, Meir G, Harmelin A, Neeman M. Ferritin as an endogenous MRI reporter for noninvasive imaging of gene expression in C6 glioma tumors. Neoplasia. 2005;7:109–117. doi: 10.1593/neo.04436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gilad AA, Winnard PT, Jr, van Zijl PC, Bulte JW. Developing MR reporter genes: promises and pitfalls. NMR Biomed. 2007;20:275–290. doi: 10.1002/nbm.1134. [DOI] [PubMed] [Google Scholar]
- 45.Liu G, Bulte JW, Gilad AA. CEST MRI reporter genes. Methods in molecular biology. 2011;711:271–280. doi: 10.1007/978-1-61737-992-5_13. [DOI] [PubMed] [Google Scholar]
- 46.Wu JC, Spin JM, Cao F, et al. Transcriptional profiling of reporter genes used for molecular imaging of embryonic stem cell transplantation. Physiol Genomics. 2006;25:29–38. doi: 10.1152/physiolgenomics.00254.2005. [DOI] [PubMed] [Google Scholar]
- 47.Wang FJ, Dennis JE, Awadallah A, et al. Transcriptional profiling of human mesenchymal stem cells transduced with reporter genes for imaging. Physiological Genomics. 2009;37:23–34. doi: 10.1152/physiolgenomics.00300.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Karow M, Chavez CL, Farruggio AP, et al. Site-Specific Recombinase Strategy to create iPS Cells Efficiently with Plasmid DNA. Stem Cells. 2011 Sep 6; doi: 10.1002/stem.730. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cao F, Lin S, Xie X, et al. In vivo visualization of embryonic stem cell survival, proliferation, and migration after cardiac delivery. Circulation. 2006;113:1005–1014. doi: 10.1161/CIRCULATIONAHA.105.588954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chen IY, Gheysens O, Ray S, et al. Indirect imaging of cardiac-specific transgene expression using a bidirectional two-step transcriptional amplification strategy. Gene Ther. 2010;17:827–838. doi: 10.1038/gt.2010.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Aly AA, Peterson KM, Lerman A, Lerman LO, Rodriguez-Porcel M. Role of oxidative stress in the beneficial effect of hypoxia pre-conditioning of cells transplanted to the myocardium: a molecular imaging study. J Cardiovasc Surg (Torino) 2011;52:579–585. [PMC free article] [PubMed] [Google Scholar]
- 52.Bengel FM, Anton M, Richter T, et al. Noninvasive imaging of transgene expression by use of positron emission tomography in a pig model of myocardial gene transfer. Circulation. 2003;108:2127–2133. doi: 10.1161/01.CIR.0000091401.26280.A0. [DOI] [PubMed] [Google Scholar]
- 53.Rodriguez-Porcel M, Brinton TJ, Chen IY, et al. Reporter gene imaging following percutaneous delivery in swine moving toward clinical applications. J Am Coll Cardiol. 2008;51:595–597. doi: 10.1016/j.jacc.2007.08.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gyongyosi M, Blanco J, Marian T, et al. Serial noninvasive in vivo positron emission tomographic tracking of percutaneously intramyocardially injected autologous porcine mesenchymal stem cells modified for transgene reporter gene expression. Circulation. Cardiovascular imaging. 2008;1:94–103. doi: 10.1161/CIRCIMAGING.108.797449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yaghoubi SS, Jensen MC, Satyamurthy N, et al. Noninvasive detection of therapeutic cytolytic T cells with 18F-FHBG PET in a patient with glioma. Nat Clin Pract Oncol. 2009;6:53–58. doi: 10.1038/ncponc1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Thakur ML, Lavender JP, Arnot RN, Silvester DJ, Segal AW. Indium-111-labeled autologous leukocytes in man. J Nucl Med. 1977;18:1014–1021. [PubMed] [Google Scholar]