Abstract
Purpose.
Corneal epithelial cells have large stores of glycogen, which serve as their primary energy source. Recently, we demonstrated that factor-inhibiting hypoxia-inducible factor 1 (FIH-1) diminished glycogen stores in vitro and in vivo, working through the Akt/Glycogen Synthase Kinase (GSK)-3β pathway. In this study we investigated the relationship between FIH-1 and c-kit as it pertains to limbal and corneal epithelial glycogen stores.
Methods.
Limbal and corneal epithelia from wild-type FIH-1−/− and KitW/Wv mice were stained with periodic acid Schiff (PAS) to detect glycogen. RNA samples prepared from laser-capture microdissected populations of limbal epithelium were subjected to real-time quantitative PCR to determine c-kit ligand expression. Submerged cultures of primary human corneal epithelial keratinocytes (HCEKs) transduced with FIH-1 were treated with c-kit ligand to establish further a FIH-1/c-kit interaction via Western analysis. Akt phosphorylation was assessed by Western blotting.
Results.
The limbal epithelial cells of FIH-1 null mice had an increase in glycogen levels as well as increased c-kit ligand mRNA compared with wild-type controls. Consistent with a FIH-1/c-kit association, the diminished Akt signaling observed in FIH-1-overexpressing HCEKs could be restored by the addition of c-kit ligand. Interestingly, Akt signaling and glycogen content of the corneal epithelium were significantly decreased in c-kit mutant mice.
Conclusions.
c-Kit signaling has been shown to affect glucose metabolism via the Akt/GSK-3β pathway. An inverse relationship between FIH-1 and c-kit signaling pathways accounts, in part, for differences in glycogen content between corneal and limbal epithelial cells.
Keywords: energy metabolism, Akt signaling, keratinocytes
Maintenance of limbal/corneal epithelial glycogen is essential for homeostasis as glycogen is a primary energy source. We demonstrate that FIH-1, a novel hydroxylase, alters c-kit signaling, which results in a negative regulation of glycogen metabolism.
Introduction
In order to fulfill the function of transparency, the corneal epithelium is supported by an avascular stroma. A corollary of avascularity is the longstanding recognition that corneal epithelial cells are extremely glycogen rich,1,2 with glycogen serving as the major endogenous supply of glucose.3 The corneal epithelium is also unique in that both the Embden-Meyerhof and the hexose monophosphate shunt are major pathways involved in glucose catabolism.4,5 Proper proliferation and migration of corneal epithelial cells, which are key factors involved in homeostasis and tissue regeneration, are heavily dependent on a balance between synthesis and catabolism of glycogen.6 Interestingly, limbal epithelial cells have less glycogen than their corneal epithelial counterparts.2 Such diminished glycogen could, in part, be due to the fact that the limbal epithelium is supported by a stroma that is highly vascularized, which is another source of energy. Alternatively, the limbal epithelium might inherently need less glycogen because a portion of its basal cells are putative stem cells,7–9 which may have different metabolic needs than more differentiated corneal epithelial cells. Stem cells from other systems (e.g., neuronal, hematopoietic) have been shown to exhibit different metabolic phenotypes than more differentiated cells. For example, increases in oxidation can inhibit proliferation and induce differentiation, whereas in other programs, oxidation enhances proliferation (see Ref. 10 for review). Stem cell competence in a stromal stem cell line was associated with mitochondrial clustering, high oxygen consumption, and low adenosine triphosphate (ATP) content whereas differentiation into adipocytes yielded cells with a less metabolically active phenotype.11 Knockdown of phosphoglycerate kinase, which is required for glycolysis, in myoblast precursor cells was sufficient to induce differentiation.12 Lkb1, which regulates cell growth and energy metabolism, is required for mitochondrial function and energy homeostasis in hematopoietic stem cells (HSCs), and HSCs require Lkb1 more than other hematopoietic cells.13 The paucity of comparable studies in the limbal and corneal epithelia underscores the need to understand how these tissues regulate their respective glycogen stores.
We previously demonstrated that the asparaginyl hydroxylase, factor-inhibiting hypoxia-inducible factor 1 (FIH-1), plays a key role in the maintenance of glycogen in corneal epithelial cells.14 This hydroxylase was initially found to inactivate HIF-1α in normoxic and mild hypoxic conditions by blocking the binding of the transcriptional coactivators p300 and CBP to HIF-1α.15,16 Despite the well-recognized effects that HIF has on energy production and regulation,17 we demonstrated that FIH-1's effects on corneal epithelial glycogen were HIF-1α independent.14 Furthermore, we have shown that ectopic expression of FIH-1 decreases Akt signaling and activates Glycogen Synthase Kinase (GSK)-3β. Such activation resulted in an inhibition of glycogen synthase.14 Since GSK-3β is a well-known inhibitor of glycogen synthesis,18 this partially explains how FIH-1 may negatively regulate corneal epithelial glycogen. What is not clear is what mediates the FIH-1-induced inhibition of Akt.
Akt activity can be regulated by many upstream signaling pathways, including stem cell factor (SCF)–mediated c-kit activation.19 Interaction of c-kit, a tyrosine kinase receptor, with its ligand, SCF, plays a crucial role in early hematopoiesis and also impacts the development and survival of melanocytes, mast cells, and germ cells.20 Interestingly, c-kit has recently been shown to be involved in the regulation of glucose metabolism via the Akt/GSK-3β pathway.21 In the present study, we used mice with a FIH null mutation and demonstrate that deletion of FIH-1 increases limbal epithelial glycogen stores in vivo. Such an increase is associated with an increased expression of c-kit ligand in limbal epithelial cells. In addition, KitW/Wv mice, which carry a mutation that profoundly reduces c-kit signaling,22 show a decrease of glycogen stores and Akt signaling in corneal epithelial cells. Furthermore, addition of SCF, the c-kit ligand to primary cultures of FIH-1-expressing human corneal epithelial cells (HCEKs), restored Akt signaling. Such a connection between FIH/c-kit/Akt/GSK-3β provides new insights into how FIH-1 regulates the limbal and corneal epithelial glycogen stores.
Methods
Mice
The FIH-1 null mice were generated by breeding FIHflox mouse with the Ella-Cre transgenic mouse.23 KitW/Wv mice and wild-type control littermates were kindly provided by Melissa Brown (Department of Microbiology-Immunology, Northwestern University, Chicago, IL). All experimental procedures conformed to the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research and were approved by the Northwestern University Animal Care and Use Committee (NUACUC).
Immunohistochemical and Periodic Acid Schiff Staining
Paraffin sections were processed for immunohistochemical (IHC) and periodic acid Schiff (PAS) staining as described previously.14 For FIH-1 staining, sections were incubated for 1 hour with FIH-1 rabbit polyclonal antibody (1:300; Santa Cruz Biotechnology, Dallas, TX). Sections were counterstained with hematoxylin to visualize morphology. PAS staining was performed as described previously.14 Images were taken using a Zeiss Axioplan 2 microscope system (Carl Zeiss, Thornwood, NY). Quantification was carried out using a Zeiss Axiovision program (Carl Zeiss).
Laser-Capture Microdissection
Fresh eyes from 4-week-old FIH-1 null and littermate control mice were embedded in optimal cutting temperature (OCT) compound and stored at −80°C. Limbal epithelium from 5-μm frozen sections was isolated and captured using a PALM laser-capture system (Carl Zeiss) as previously described.24
Real-Time Quantitative PCR (qPCR) Analysis
Total RNA from tissues was harvested using TRIzol (Invitrogen, Grand Island, NY). For real-time qPCR, total RNAs were purified by the RNeasy kit (Qiagen, Foster City, CA). cDNA was prepared using Superscript III Reverse transcription kit (Invitrogen). Real-time qPCR was performed on Applied Biosystems 7000 Real Time PCR Systems (Applied Biosystems, Grand Island, NY) using the Quantitative SYBR green PCR kit (Qiagen). Primer sequences used in this study were as follows: mouse SCF (c-kit ligand) forward 5′-GCC TAG TCA TTG TTG GCT AC-3′; mouse c-kit ligand reverse 5′-CCC AAG TTT GTC TAT GAT GG-3′; mouse FIH-1 forward 5′-AAG TGG GAC CTC GAA TAC CTG CAA-3′; mouse FIH-1 reverse 5′-CCC TGT TGG ACC TTG GCT TAA AGT-3.′ Mouse 18S RNA was used as the internal control.
Cell Culture
Primary HCEKs were isolated from cadaver corneas provided by Midwest Eye Banks (Ann Arbor, MI) and cultured in CnT-20 media with supplements (CellnTec, Research Triangle Park, NC) on collagen IV–coated plates (BD Biosciences, San Jose, CA) as previously described.24 For inducing c-kit signaling, HCEKs were treated with 100 ng/mL SCF (Cell Signaling Technologies, Danvers, MA) for 24 hours.
Western Blotting
HCEKs transduced with retroviral supernatants carrying either LZRS-empty vector or FIH-124 with or without SCF were lysed with radioimmunoprecipitation assay (RIPA) buffer containing 50 mM Tris-HCl (pH 7.4), 150 mM NaCl, 0.25% deoxycholic acid, 1% Tergitol Nonidet P-40 (NP-40), 1 mM EDTA, 2 mM dithiothreitol, 1 mM phenylmethylsulphonyl fluoride, phosphatase inhibitor cocktail (Thermo Scientific, West Palm Beach, FL), and protease inhibitor cocktail (Thermo Scientific), followed by centrifugation at 15,000g for 15 minutes. Mouse corneal epithelia were peeled away from the underlying stroma by incubating whole globes with PBS containing 20 mM EDTA for 1 hour at 37°C25 and subsequently were lysed with 8 μM urea sample buffer as previously described.26 Western blots were performed as described previously.24 The following antibodies were used: p-Akt (S473), p-Akt (T308), p-GSK-3β, p-glycogen synthase, p-mammalian target of rapamycin (mTOR) p-4E-BP1 (Cell Signaling Technologies), FIH-1, GSK-3β, and Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) (Santa Cruz Biotechnology).
Statistical Analysis
All experiments were repeated at least three times. Independent t-test analysis was used to conduct statistical analysis. The data are presented as means ± standard deviation (SD). The differences were considered significant for P values < 0.05.
Results and Discussion
Lack of FIH-1 Results in an Accumulation of Glycogen in Limbal Epithelium In Vivo
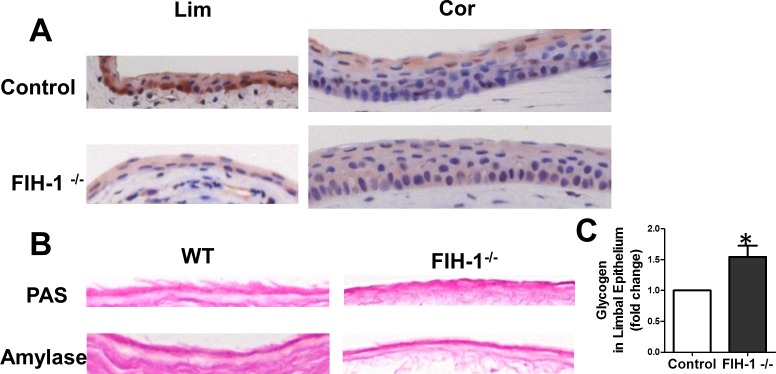
Normal corneal epithelium has large amounts of glycogen whereas glycogen stores are less in the limbal epithelium.2,14 Recently we have shown that FIH-1 is a negative regulator of glycogen stores in corneal epithelium in vitro.14 To test further the idea that FIH-1 is a negative regulator of glycogen stores, we examined mice that were null for FIH-1.23 Low levels of FIH-1 were detectable in corneal epithelial cells (Fig. 1A) in wild-type mice, whereas FIH-1 was highly expressed in mouse limbal epithelial cells (Fig. 1A). Therefore, we reasoned that depletion of FIH-1 in vivo should increase glycogen stores in limbal epithelial cells. Consistent with high levels of FIH-1 expression, we observed minimal amounts of PAS-positive amylase-sensitive material (glycogen) in the limbal epithelial cells of the wild-type mouse eyes (Fig. 1B). In contrast, depletion of FIH-1 resulted in a significant increase in glycogen in the limbal epithelium of the FIH-1−/− mice. Quantification of the staining reaction using computer-assisted image analysis (Fig. 1C) indicated that knockout of FIH-1 increased glycogen by ∼50%, which was statistically significant (P < 0.05). These findings extend our in vitro data and confirm that in vivo, FIH-1 is a negative regulator of glycogen stores.
Figure 1. .
FIH-1 negatively regulates glycogen metabolism. (A) FIH-1 immunostaining of corneal and limbal epithelia of FIH-1−/− or wild-type control mice. (B) Wild-type and FIH-1−/− mouse limbal epithelia were stained with PAS with or without amylase to digest glycogen. (C) Glycogen density was quantified using computer-assisted image analysis. Error bars: SD derived from three experiments. *P < 0.05.
FIH-1 Negatively Regulates c-Kit Ligand Expression In Vivo, Which Alters Corneal Epithelial Glycogen
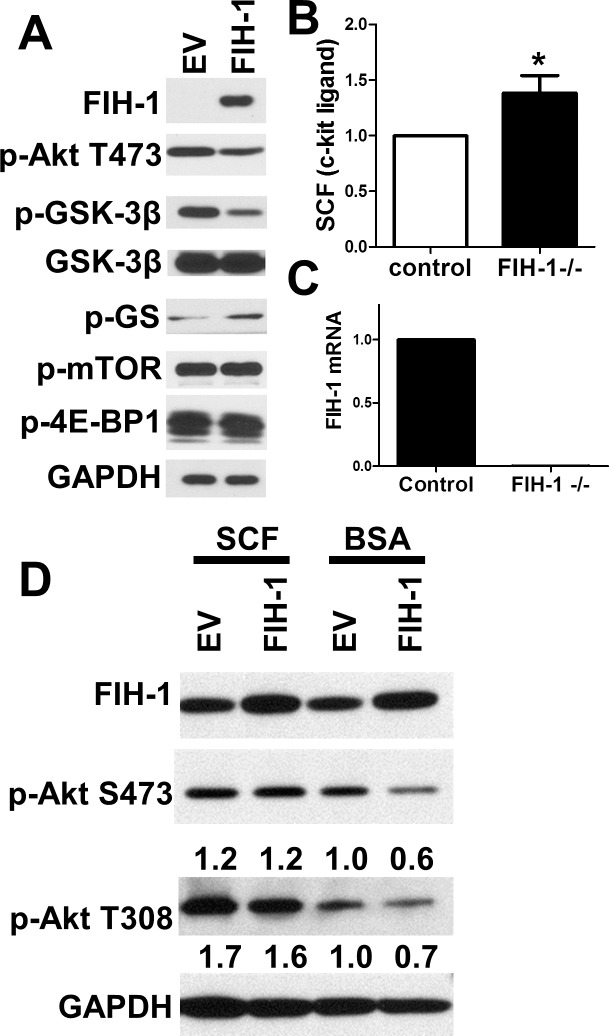
We have shown that the decrease of glycogen stores induced by ectopic expression of FIH-1 is due to a dampening of Akt activity resulting in the dephosphorylation (activation) of GSK-3β, which leads to a decrease in phospho-glycogen synthase (Fig. 2A).14 We initially hypothesized that FIH-1 might regulate Akt signaling through interactions with the mTOR complex 2, since it is well established that mTOR mediates actions of the Akt pathway.27 To test this, we transduced HCEKs with either FIH-1 or LZRS-empty vector but observed no change in the phosphorylation of mTOR or its downstream target 4E-BP1 (Fig. 2A). Recent studies have shown that c-kit plays a critical role in regulating glucose metabolism in beta cells, working through the Akt/GSK-3β pathway.21 It was observed that overexpression of c-kit in mice resulted in a quicker removal of glucose out of the blood and activation of GSK-3β.21 This suggests that c-kit may be functioning to regulate the incorporation of glucose into glycogen. Relevant to the present study, overexpression of FIH-1 inhibits c-kit ligand expression in a stromal cell line.28 Thus we posited that a diminution of FIH-1 might increase the expression of SCF, the c-kit ligand, in limbal epithelial cells, leading to the activation of Akt and inhibition of GSK3β. To test this idea, we initially determined the levels of SCF in limbal epithelia from wild-type and FIH-1−/− mice. We used laser-capture microdissection to isolate relatively pure populations of “resting” limbal epithelial cells,29 and RNA from these epithelia was subjected to real-time PCR. As predicted, we observed an increase in mRNA specifying SCF, the c-kit ligand in limbal epithelium from the FIH-1−/− mice (Figs. 2B, 2C), which suggests that FIH-1 inhibits c-kit signaling in vivo. To confirm that the observed downregulation of Akt signaling (Fig. 2A) was due to the inhibition of c-kit, we treated FIH-1-transduced HCEKs with SCF and assessed the phosphorylation status of Akt (Fig. 2D). Addition of SCF restored Akt signaling in these cells, indicating that FIH-1 regulates Akt signaling through c-kit (Fig. 2D).
Figure 2. .
FIH-1 inactivates Akt signaling via inhibition of c-kit signaling. (A) Immunoblotting of FIH-1, p-Akt (S473), p-GSK-3β, GSK-3β, p-glycogen synthase (p-GS), p-mTOR, p-4E-BP1, and GAPDH in HCEKs transduced with either FIH-1 or LZRS-empty vector. (B, C) qRT-PCR assessment of kit ligand (stem cell factor; [B]) and FIH-1 (C) mRNA expression in mouse limbal epithelium. (D) Immunoblotting of FIH-1, p-Akt(S473), p-Akt (T308), and GAPDH in HCEKs transduced with either FIH-1 or LZRS-empty vector with or without c-kit ligand (SCF). Numbers below panels represent the normalized expression signals of proteins. *P < 0.05.
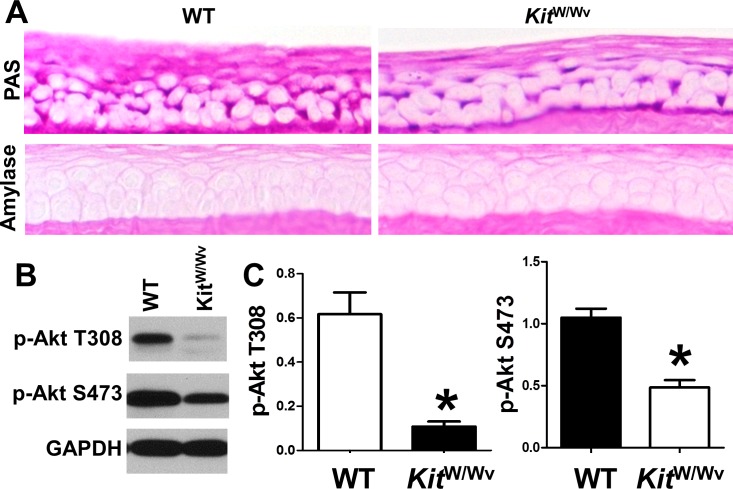
We next determined whether such a FIH-1/c-kit ligand relationship was pertinent to limbal/corneal epithelial glycogen metabolism. We utilized KitW/Wv mice that carry two mutated c-kit alleles (one is impaired and the other is null; collectively they result in an 80%–90% reduction in signaling capacity),20 and determined the glycogen levels and Akt phosphorylation status in the corneal epithelium from these mice. Consistent with the observations that FIH-1−/− mice have increased levels of SCF and also increased glycogen stores, the corneal epithelium from the mice with impaired c-kit signaling (KitW/Wv) had significantly lower amounts of PAS-positive amylase-sensitive material (glycogen) when compared with wild-type mice (Fig. 3A). In addition, Akt signaling was impaired in the corneal epithelium of the KitW/Wv mice (Figs. 3B, 3C). Collectively, these findings clearly indicate that intact c-kit signaling is required for the maintenance of corneal epithelial glycogen stores and that FIH-1's negative impact on c-kit ligand may, in part, be responsible for the diminished limbal epithelial glycogen content seen in wild-type mice.
Figure 3. .
C-kit is a positive regulator of glycogen metabolism. (A) Wild-type and KitW/Wv mouse corneal epithelia were stained with PAS with or without amylase to digest glycogen. (B) Immunoblotting of p-Akt (T308), p-Akt (S473), and GAPDH in corneal epithelia of wild-type and KitW/Wv mice. (C) Densitometry analysis of the immunoblots of p-Akt (T308) and p-Akt (S473). Values are means ± SD of three independent experiments. *P < 0.05.
It is apparent that c-kit and SCF can play other roles in corneal epithelial physiology. Recently, corneal epithelial wound repair was demonstrated to be significantly impaired in SI/SId and KitW/Wv mice that were ligand (SCF) and receptor (c-kit) deficient, respectively.30 The authors postulated that SCF/c-kit signaling may have a positive role in the attachment of corneal epithelial cells to the basement membrane during wound healing, possibly through an integrin-mediated network. Our findings provide an additional explanation for the impaired wound healing in deficient mice. It is well recognized that during wound healing, corneal epithelial cells utilize glycogen for migration1; thus, it is possible that the SCF/c-kit–deficient corneal epithelial cells fail to accumulate adequate glycogen required for energy utilization during regeneration and that this adds to an impaired wound healing response.
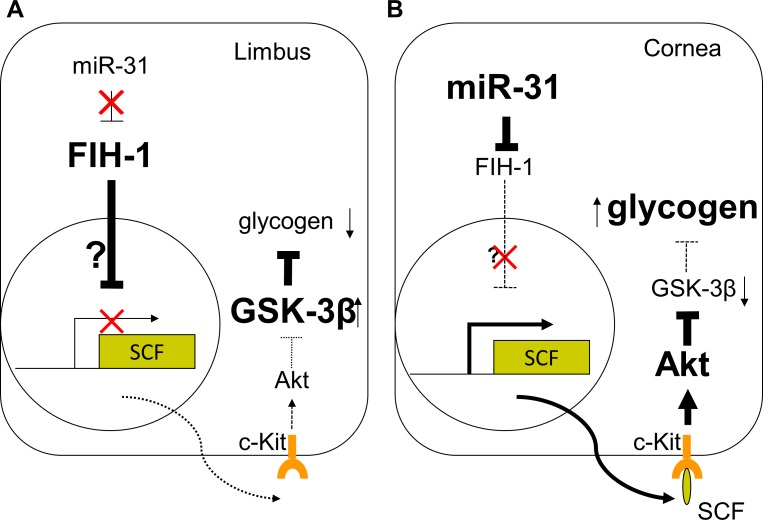
We have demonstrated in both corneal epithelium and the epidermis that FIH-1 is regulated, in part, by microRNA-31 (miR-31).14,24 Furthermore, miR-31 is preferentially expressed in corneal epithelial cells in vitro and in vivo.14,31 miR-31′s preferential corneal epithelial expression results in a significant inhibition of FIH-1 protein levels in the corneal epithelium; in contrast, in limbal epithelial cells, miR-31 is barely detectable, resulting in high levels of FIH-1.14 We now extend these findings and demonstrate that FIH-1 negatively affects c-kit ligand expression in vivo. Such an alteration in c-kit signaling most likely mediates the effect that FIH-1 has on the Akt/GSK3-β pathway, ultimately decreasing glycogen stores in limbal epithelial cells (Fig. 4). In this scenario, we propose that low amounts of miR-31 in the limbal keratinocytes (Fig. 4A) enhance FIH-1 expression, which diminishes the amount of SCF available for binding to its receptor (c-kit). This ultimately results in the activation of GSK-3β and lower amounts of glycogen. In the corneal keratinocytes (Fig. 4B), miR-31 downregulates FIH-1, which ultimately enables SCF to bind to its receptor. This in turn activates Akt signaling to enhance glycogen stores. The precise mechanism by which FIH-1 interacts with SCF is presently unclear. However, it is unlikely that the interaction is direct, since FIH-1 has been shown to exert its action via the hydroxylation of ankyrin-repeat domains (ARD) in ARD-containing proteins,15,32,33 and SCF (the c-kit ligand) does not have such motifs. A more likely scenario is that FIH-1 affects other proteins that intersect with SCF. For example, in keratinocytes, the expression of SCF is transcriptionally regulated by the transforming growth factor-β–activated kinase 1 (TAK1). The TAK1/JNK/c-Jun pathway ultimately results in SCF triggering the activation of PI3K/PKBα signaling.34 It is likely that FIH-1 directly interacts with some component(s) of these signaling cascades.
Figure 4. .
FIH-1 is highly expressed in the limbal region (A), where it diminishes glycogen stores and could contribute to lower limbal glycogen levels via inhibition of c-kit signaling. Levels of miR-31 are high in corneal epithelium (B), where it negatively regulates FIH-1, thus preserving c-kit/Akt signaling and glycogen stores.
It is important to note that all of the experiments were conducted under normoxic conditions, which is the usual state of the corneal epithelium and when FIH-1 is active.32,35 In these instances, one of miR-31's functions is to negatively regulate FIH-1, thereby maintaining glycogen stores. However, the corneal epithelium is frequently subjected to cycles of hypoxia to normoxia as a consequence of sleeping and/or contact lens wear. These periods of hypoxia/reoxygenation are a form of environmental stress that can lead to deleterious changes in corneal epithelial physiology (see Ref. 36 and references therein); one such change is glycogen depletion that when excessive can result in disruption of barrier function and inflammation and/or infection.37,38 We propose that the stored glycogen serves as a readily available source of glucose enabling the corneal epithelial cells to withstand relatively short periods of environmental stress.
Acknowledgments
Supported by US National Institutes of Health Grants EY06769, EY017536, and EY019463 (RML); NIAMS AR057216. The Northwestern University Skin Disease Research Center Pathology Core assisted in morphological analysis with support from the National Institute of Arthritis and Musculoskeletal and Skin Diseases, Grant AR057216.
Disclosure: H. Peng, None; J. Katsnelson, None; W. Yang, None; M.A. Brown, None; R.M. Lavker, None
References
- 1. Thoft RA, Friend J. Biochemical aspects of contact lens wear. Am J Ophthalmol. 1975; 80: 139–145 [DOI] [PubMed] [Google Scholar]
- 2. Thoft RA, Friend J. Biochemical transformation of regenerating ocular surface epithelium. Invest Ophthalmol Vis Sci. 1977; 16: 14–20 [PubMed] [Google Scholar]
- 3. Riley MV. Glucose and oxygen utilization by the rabbit cornea. Exp Eye Res. 1969; 8: 193–200 [DOI] [PubMed] [Google Scholar]
- 4. Kinoshita JH. Some aspects of the carbohydrate metabolism of the cornea. Invest Ophthalmol. 1962; 1: 178–186 [PubMed] [Google Scholar]
- 5. Thies RS, Mandel LJ. Role of glucose in corneal metabolism. Am J Physiol. 1985; 249: C409–C416 [DOI] [PubMed] [Google Scholar]
- 6. Lu L, Reinach PS, Kao WW. Corneal epithelial wound healing. Exp Biol Med (Maywood). 2001; 226: 653–664 [DOI] [PubMed] [Google Scholar]
- 7. Cotsarelis G, Cheng SZ, Dong G, Sun T-T, Lavker RM. Existence of slow-cycling limbal epithelial basal cells that can be preferentially stimulated to proliferate: implications on epithelial stem cells. Cell. 1989; 57: 201–209 [DOI] [PubMed] [Google Scholar]
- 8. Schermer A, Galvin S, Sun T-T. Differentiation-related expression of a major 64K corneal keratin in vivo and in culture suggests limbal location of corneal epithelial stem cells. J Cell Biol. 1986; 103: 49–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sun TT, Tseng SC, Lavker RM. Location of corneal epithelial stem cells. Nature. 2010; 463: E10–E11 [DOI] [PubMed] [Google Scholar]
- 10. Noble M, Mayer-Proschel M, Proschel C. Redox regulation of precursor cell function: insights and paradoxes. Antioxid Redox Signal. 2005; 7: 1456–1467 [DOI] [PubMed] [Google Scholar]
- 11. Lonergan T, Brenner C, Bavister B. Differentiation-related changes in mitochondrial properties as indicators of stem cell competence. J Cell Physiol. 2006; 208: 149–153 [DOI] [PubMed] [Google Scholar]
- 12. Bracha AL, Ramanathan A, Huang S, Ingber DE, Schreiber SL. Carbon metabolism-mediated myogenic differentiation. Nat Chem Biol. 2010; 6: 202–204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nakada D, Saunders TL, Morrison SJ. Lkb1 regulates cell cycle and energy metabolism in haematopoietic stem cells. Nature. 2010; 468: 653–658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Peng H, Hamanaka RB, Katsnelson J, et al. MicroRNA-31 targets FIH-1 to positively regulate corneal epithelial glycogen metabolism. FASEB J. 2012; 26: 3140–3147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mahon PC, Hirota K, Semenza GL. FIH-1: a novel protein that interacts with HIF-1alpha and VHL to mediate repression of HIF-1 transcriptional activity. Genes Dev. 2001; 15: 2675–2686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lando D, Peet DJ, Gorman JJ, et al. FIH-1 is an asparaginyl hydroxylase enzyme that regulates the transcriptional activity of hypoxia-inducible factor. Genes Dev. 2002; 16: 1466–1471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Weidemann A, Johnson RS. Biology of HIF-1alpha. Cell Death Differ. 2008; 15: 621–627 [DOI] [PubMed] [Google Scholar]
- 18. Rayasam GV, Tulasi VK, Sodhi R, Davis JA, Ray A. Glycogen synthase kinase 3: more than a namesake. Br J Pharmacol. 2009; 156: 885–898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ronnstrand L. Signal transduction via the stem cell factor receptor/c-Kit. Cell Mol Life Sci. 2004; 61: 2535–2548 [DOI] [PubMed] [Google Scholar]
- 20. Galli SJ, Kitamura Y. Genetically mast-cell-deficient W/Wv and Sl/Sld mice. Their value for the analysis of the roles of mast cells in biologic responses in vivo. Am J Pathol. 1987; 127: 191–198 [PMC free article] [PubMed] [Google Scholar]
- 21. Feng ZC, Li J, Turco BA, et al. Critical role of c-Kit in beta cell function: increased insulin secretion and protection against diabetes in a mouse model. Diabetologia. 2012; 55: 2214–2225 [DOI] [PubMed] [Google Scholar]
- 22. Kitamura Y, Go S, Hatanaka K. Decrease of mast cells in W/Wv mice and their increase by bone marrow transplantation. Blood. 1978; 52: 447–452 [PubMed] [Google Scholar]
- 23. Zhang N, Fu Z, Linke S, et al. The asparaginyl hydroxylase factor inhibiting HIF-1alpha is an essential regulator of metabolism. Cell Metab. 2010; 11: 364–378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Peng H, Kaplan N, Hamanaka RB, et al. microRNA-31/factor-inhibiting hypoxia-inducible factor 1 nexus regulates keratinocyte differentiation. Proc Natl Acad Sci U S A. 2012; 109: 14030–14034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kaplan N, Fatima A, Peng H, et al. EphA2/ephrin-A1 signaling complexes restrict corneal epithelial cell migration. Invest Ophthalmol Vis Sci. 2012; 53: 936–945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gordon K, Kochkodan JJ, Blatt H, et al. Alteration of the ephA2/ephrin-A signaling axis in psoriatic epidermis. J Invest Dermatol. 2012; 133: 712–722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hay N, Sonenberg N. Upstream and downstream of mTOR. Genes Dev. 2004; 18: 1926–1945 [DOI] [PubMed] [Google Scholar]
- 28. Nishida C, Kusubata K, Tashiro Y, et al. MT1-MMP plays a critical role in hematopoiesis by regulating HIF-mediated chemokine/cytokine gene transcription within niche cells. Blood. 2012; 119: 5405–5416 [DOI] [PubMed] [Google Scholar]
- 29. Zhou M, Li XM, Lavker RM. Transcriptional profiling of enriched populations of stem cells versus transient amplifying cells. A comparison of limbal and corneal epithelial basal cells. J Biol Chem. 2006; 281: 19600–19609 [DOI] [PubMed] [Google Scholar]
- 30. Miyamoto K, Kobayashi T, Hayashi Y, et al. Involvement of stem cell factor and c-kit in corneal wound healing in mice. Mol Vis. 2012; 18: 1505–1515 [PMC free article] [PubMed] [Google Scholar]
- 31. Ryan DG, Oliveira-Fernandes M, Lavker RM. MicroRNAs of the mammalian eye display distinct and overlapping tissue specificity. Mol Vis. 2006; 12: 1175–1184 [PubMed] [Google Scholar]
- 32. Cockman ME, Webb JD, Ratcliffe PJ. FIH-dependent asparaginyl hydroxylation of ankyrin repeat domain-containing proteins. Ann N Y Acad Sci. 2009; 1177: 9–18 [DOI] [PubMed] [Google Scholar]
- 33. Cockman ME, Webb JD, Kramer HB, Kessler BM, Ratcliffe PJ. Proteomics-based identification of novel factor inhibiting hypoxia-inducible factor (FIH) substrates indicates widespread asparaginyl hydroxylation of ankyrin repeat domain-containing proteins. Mol Cell Proteomics. 2009; 8: 535–546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lam CR, Tan MJ, Tan SH, et al. TAK1 regulates SCF expression to modulate PKBalpha activity that protects keratinocytes from ROS-induced apoptosis. Cell Death Differ. 2011; 18: 1120–1129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Coleman ML, McDonough MA, Hewitson KS, et al. Asparaginyl hydroxylation of the Notch ankyrin repeat domain by factor inhibiting hypoxia-inducible factor. J Biol Chem. 2007; 282: 24027–24038 [DOI] [PubMed] [Google Scholar]
- 36. Lu J, Wang L, Dai W, Lu L. Effect of hypoxic stress-activated Polo-like kinase 3 on corneal epithelial wound healing. Invest Ophthalmol Vis Sci. 2010; 51: 5034–5040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kimura K, Teranishi S, Kawamoto K, Nishida T. Protection of human corneal epithelial cells from hypoxia-induced disruption of barrier function by hepatocyte growth factor. Exp Eye Res. 2010; 90: 337–343 [DOI] [PubMed] [Google Scholar]
- 38. Yamamoto N, Jester JV, Petroll WM, Cavanagh HD. Prolonged hypoxia induces lipid raft formation and increases Pseudomonas internalization in vivo after contact lens wear and lid closure. Eye Contact Lens. 2006; 32: 114–120 [DOI] [PubMed] [Google Scholar]