Short abstract
Crimp morphology is believed to be related to tendon mechanical behavior. While crimp has been extensively studied at slack or nondescript load conditions in tendon, few studies have examined crimp at specific, quantifiable loading conditions. Additionally, the effect of the number of cycles of preconditioning on collagen fiber crimp behavior has not been examined. Further, the dependence of collagen fiber crimp behavior on location and developmental age has not been examined in the supraspinatus tendon. Local collagen fiber crimp frequency is quantified throughout tensile mechanical testing using a flash freezing method immediately following the designated loading protocol. Samples are analyzed quantitatively using custom software and semi-quantitatively using a previously established method to validate the quantitative software. Local collagen fiber crimp frequency values are compared throughout the mechanical test to determine where collagen fiber frequency changed. Additionally, the effect of the number of preconditioning cycles is examined compared to the preload and toe-region frequencies to determine if increasing the number of preconditioning cycles affects crimp behavior. Changes in crimp frequency with age and location are also examined. Decreases in collagen fiber crimp frequency were found at the toe-region at all ages. Significant differences in collagen fiber crimp frequency were found between the preload and after preconditioning points at 28 days. No changes in collagen fiber crimp frequency were found between locations or between 10 and 28 days old. Local collagen fiber crimp frequency throughout mechanical testing in a postnatal developmental mouse SST model was measured. Results confirmed that the uncrimping of collagen fibers occurs primarily in the toe-region and may contribute to the tendon’s nonlinear behavior. Additionally, results identified changes in collagen fiber crimp frequency with an increasing number of preconditioning cycles at 28 days, which may have implications on the measurement of mechanical properties and identifying a proper reference configuration.
1. Introduction
Consistently and repeatedly characterizing the mechanical response of soft tissues can be challenging given their time and history dependence [1,2]. While it is commonly accepted that preconditioning is an important component of mechanical testing protocols, the mechanisms of preconditioning are poorly understood. Recent studies have identified correlations between collagen fiber realignment and preconditioning [3,4]. Additionally, a recent study in rat tail tendon fascicle showed that preconditioning was accompanied by a decrease in the crimp period and a shift of the toe-region of the stress-strain curve to higher strains [5]. Crimp morphology is believed to be related to tendon mechanical behavior. While crimp has been extensively studied at slack or nondescript load conditions [6–10], few studies have examined crimp at specific, quantifiable loads. Additionally, the effect of number of preconditioning cycles on collagen fiber crimp frequency has not yet been examined. Further research is necessary to examine the effect of preconditioning on tendon structural response to load and to determine how the structure of tendon affects its mechanical properties. Additional information on the effect of preconditioning will improve the repeatability and consistency of experimental results and aid interpretation of results across studies.
In addition, the effect of developing structure on the tendon's ability to respond to mechanical load through the uncrimping of collagen fibers has not yet been examined. A recent study demonstrated that where collagen fiber realignment occurred throughout the mechanical testing protocol may depend on developmental age and the maturity of the collagen fiber matrix [11]. It is possible that the ability of collagen fibers to uncrimp in response to mechanical load may also depend on the tendon's underlying structure and may affect tendon mechanical properties. Studies have speculated that collagen fiber crimp behavior changes throughout development [12–14]. However, the ability of collagen fibers to uncrimp in response to load has not been extensively studied throughout postnatal development nor has it been examined in the supraspinatus tendon (SST). A higher collagen fiber crimp frequency at younger ages may explain the elongated toe-region seen throughout postnatal development [11,15] as increased crimp has been shown to affect the toe-region of the stress-strain curve [10].
Additionally, it has been suggested that collagen fiber crimp patterns vary by location [16,17]. Compared to the tendon midsubstance, the supraspinatus tendon-to-bone insertion site experiences higher strains, demonstrates a more disorganized collagen fiber distribution, weaker mechanics, and changes in fiber realignment behavior [3,11,18,19]. Studies in ligament have also shown a “preferential” uncrimping of the central third of the ligament as well as near the bone [16]. This suggests that the insertion site and midsubstance locations in the SST may display differences in crimp behavior, although neither local collagen fiber crimp frequency nor the potential dependence of collagen fiber uncrimping on location have yet been quantified.
Therefore, the objective of this study was to quantify local changes in collagen fiber crimp frequency in a developmental mouse SST model throughout a mechanical testing protocol to determine (1) where fiber uncrimping occurs throughout the mechanical test, (2) if collagen fiber crimp behavior is dependent on the number of preconditioning cycles, (3) if local collagen fiber crimp frequency changes with developmental age, and (4) if collagen fiber crimp behavior is dependent on tendon location. We hypothesize that (1) Collagen fiber uncrimping will occur primarily in the toe-region of the mechanical test regardless of age; (2) Collagen fiber crimp frequency will decrease with increased number of preconditioning cycles; (3) Crimp frequency will decrease with developmental age; (4) The insertion site will demonstrate an increased crimp frequency compared to the tendon midsubstance.
2. Methods
2.1. Sample Preparation.
This study was approved by the University of Pennsylvania IACUC. Postnatal mice in a C57/BL/6 (Jackson Laboratory) background were bred on site. All litters were reduced to six pups within 1 day of birth to reduce variance from litter size [20]. Pups were weaned at 21 days after birth and separated by sex. In order to compare changes in crimp frequency throughout the mechanical test, a litter was defined as a single sample [15] and mechanical testing points were randomly assigned to shoulders within each litter. SSTs from male and female postnatal mice were removed at 4, 10, and 28 days old (N = 9–11 for each age and mechanical testing point). Under a stereomicroscope, SSTs were dissected out and excess tissue was removed with the tendon still attached to the humeral head. As described previously, the humeral head was trimmed to a small bone chip and then both ends of the tendon were secured with cyanoacrylate adhesive between pieces of sandpaper [11]. Grip-to-grip gauge length of the samples was 1.5 mm for 4 and 10 days old and 2.5 mm for 28 days old [11]. Tendons were secured in the grips and a grip holder was used to ensure the tendons were not loaded or damaged during handling [11,15].
2.2. Mechanical Testing and Histology.
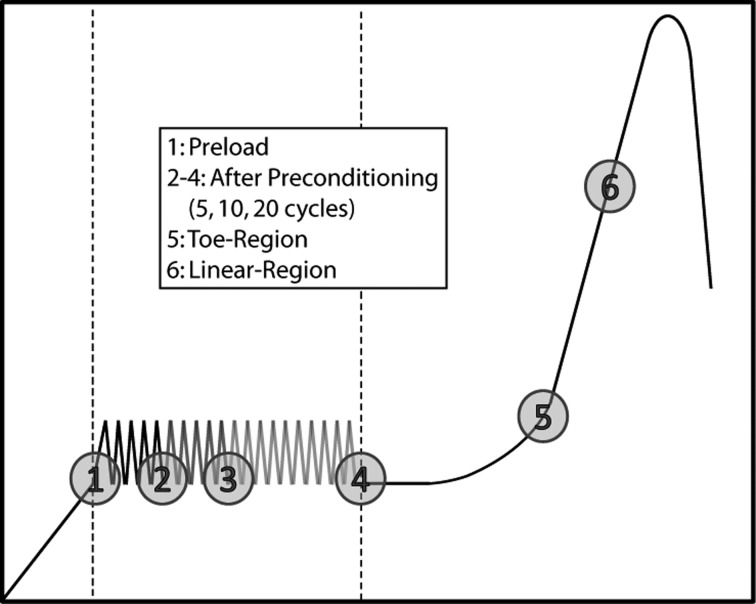
Samples were placed in a tank and loaded into a tensile testing system (Instron, Norwood, MA). Samples were kept moist with phosphate buffered saline spray throughout the testing process, and a 10 N load cell was used for all tests. Collagen fiber crimp was assessed at six different points during the mechanical test: at the preload (0.005 N for 4 and 10 day tendons, 0.02 N for 28 day tendons), after 5, 10, 20 cycles of preconditioning (0.005–0.008 N for 4 and 10 day tendons and 0.002–0.04 N for 28 day tendons), and at the toe- and linear-regions (Fig. 1) [11]. Preconditioning was performed at load control but represented average strains of 0.5 and 1% for 4 days, 1 and 2% for 10 days and 28 days at an average rate of 0.07 Hz. Strains representing the toe- and linear-regions were determined using a structural fiber recruitment model at 50% fiber recruitment to represent the transition strain (intersection of toe- and linear-regions) and 75% to represent the linear region as described in previous studies [11,21]. Immediately following the designated tensile loading protocol, tendons were snap-frozen (sprayed for 20 s with flash freezing spray (Decon Laboratories, King of Prussia, PA)) while mounted in the testing device to obtain a “snapshot” of crimp at the desired point in the mechanical test [22]. Samples were sharply detached at the insertion site and top grip and quickly embedded in tissue freezing medium (Triangle Biomedical Sciences, Durham, NC). All samples were cut into 8 μm sections and were stained with Picrosirius Red and Hematoxylin.
Fig. 1.
A schematic of the testing protocol with six points at which crimp was assessed
2.3. Data Analysis.
For each sample, two sections were analyzed using quantitative and semi-quantitative methods at the midsubstance and insertion sites for 10 and 28 days and at one location (encompassing the majority of the tendon) at 4 days due to the decreased size of collagen fibers and resolution limitations during analysis. Sections were examined using polarized light microscopy and custom software in a blinded manner.
2.3.1. Quantitative Analysis.
Software (MATLAB, Natwick, MA) was developed to quantitatively analyze collagen crimp frequency. Blinded users selected a region for analysis, and the pixel intensity variation was determined along the length of the collagen fibers. After normalizing the image, average crimp frequency was determined by pixel fluctuations. For each location analyzed, the results from the two sections selected were averaged and crimp frequency in is reported.
2.3.2. Software Validation.
To validate the software, preload, toe, and linear-region samples from the 28 day insertion site were analyzed for percent crimp area by a previously established semi-quantitative method [22]. The same regions selected for quantitative analysis were assessed by two blinded graders to determine percent crimped area. Briefly, for each region selected, a grid was drawn over the selection, dividing it into six sub-regions. Each sub-region was given a grade of I-III as defined previously [22]. Samples were classified as Type I (substantial crimp), Type II (intermediate crimp), or Type III (minimal crimp) [22]. Representative images for Types I-III crimp were identified for the SST based on the criteria previously described [21] (Fig. 2). Grader scores were compiled and total percent crimped area was calculated for each sample at each location [21]. Quantitative data was transformed to a I-III scale to compare to the semi-quantitative grading results. To determine intra-user reliability, one researcher repeated the quantitative analysis three times for five samples. To determine inter-user reliability, two independent researchers performed the quantitative analysis on a subset of samples and their results were compared.
Fig. 2.
Representative samples of type I (left), type II (middle), and type III (right) crimp in the supraspinatus tendon
2.4. Statistical Analysis
2.4.1. Software Validation.
Intra-class correlation coefficient (ICC) was used to determine the proportion of variance due to repeated-user and between-user variability. A high ICC indicates little variation between the frequencies of each sample determined by the users. A Bland-Altman analysis was used to examine consistency between quantitative and semi-quantitative methods.
2.4.2. Crimp Frequency.
Imputation, using the predictive-mean matching method, was performed to represent missing crimp frequency values within each litter [23–25]. A three-way (region of test, location, and age) repeated measures ANOVA was used and a Bonferroni correction was applied if interactions were significant. Post hoc t-tests were used to evaluate changes in crimp frequency and corrections for multiple statistical comparisons were made for each hypothesis. To address hypothesis 1, post hoc paired t-tests were used to compare to changes in crimp frequency throughout adjacent points in the mechanical test. For example, preload and five cycles of preconditioning data were examined to determine if uncrimping occurred during the five cycles of preconditioning. Similar methods were used to identify changes in crimp frequency during 10 and 20 cycles in addition to at the toe- and linear-regions of the ramp-to-failure. Additionally, paired t-tests with a Bonferroni correction were applied to evaluate changes during the toe-region with each preconditioning protocol. To address hypothesis 2, paired t-tests with a Bonferroni correction were made to evaluate changes during each of the preconditioning protocols compared to the preload. To address hypothesis 3, post hoc t-tests were used to compare local changes in crimp frequency with developmental age. Crimp frequency is presented as mean ± standard deviation.
3. Results
ICC indicated that frequency measurements were consistent and repeatable (0.921 for intra-user and 0.857 inter-users). Bland-Altman plots demonstrated a normal distribution of error between the quantitative and semi-quantitative methods. The repeated measures three-way ANOVA identified that the effects of time and age were significant. The ANOVA demonstrated that no changes in crimp frequency were identified between locations. No interactions between time, age, or location were found to be significant. Results, as well as statistical corrections for multiple comparisons, are presented by hypothesis. A table of p-values is also provided for completeness and to aid in interpretation of these results (Tables 1 and 2).
Table 1.
p-values for changes in crimp frequency for adjacent points throughout the mechanical testing protocol. * Indicates significant difference at p < 0.016, compares distributions at 4 days and at 10 and 28 days at the insertion site and midsubstance locations throughout the mechanical test. Also examines changes between all points after preconditioning and the toe-region.
| Age and location | Preload versus 5 cycles | 5 versus 10 cycles | 10 versus 20 cycles | 5 cycles versus Toe | 10 cycles versus Toe | 20 cycles versus Toe | Toe versus linear |
|---|---|---|---|---|---|---|---|
| 4 days | 0.7 | 0.09 | 0.3 | 0.03 | 0.01* | 0.02 | 0.1 |
| 10 day Ins | 0.3 | 0.4 | 0.08 | <0.0001* | <0.0001* | <0.0001* | 0.5 |
| 10 day Mid | 0.8 | 0.4 | 0.6 | 0.007* | 0.002* | 0.0005* | 0.4 |
| 28 day Ins | 0.1 | 0.6 | 0.4 | 0.01* | 0.0003* | 0.2 | 0.4 |
| 28 day Mid | 0.02 | 0.2 | 0.09 | 0.002* | 0.0007* | 0.01* | 0.5 |
Table 2.
p-values for changes in crimp frequency with increasing number of preconditioning cycles compared to the preload. *Indicates significant difference at p < 0.016, compares distributions at 4 days and at 10 and 28 days at the insertion site and midsubstance locations with increasing number of preconditioning cycles compared to the preload.
| Age and location | Preload versus 5 cycles | Preload versus 10 cycles | Preload versus 20 cycles |
|---|---|---|---|
| 4 days | 0.7 | 0.3 | 0.4 |
| 10 day Ins | 0.3 | 0.5 | 0.07 |
| 10 day Mid | 0.8 | 0.7 | 0.4 |
| 28 day Ins | 0.1 | 0.01* | 0.1 |
| 28 day Mid | 0.02 | 0.0002* | 0.02 |
To address hypothesis 1, comparisons between adjacent points throughout the test were made. Additional comparisons between points analyzed after five cycles and ten cycles of preconditioning compared to the toe-region were also made. For this hypothesis, significance was set at p < 0.016. At 4 and 10 days old, the uncrimping of collagen fibers was confined to the toe-region of the mechanical test (Fig. 3 and Table 1). A significant decrease in crimp frequency was found following ten cycles of preconditioning compared to the toe-region at 4 days (Fig. 3 and Table 1). At 10 days, significant decreases in crimp frequency were identified at the toe-region compared to all preconditioning protocols at both locations (Fig. 3 and Table 1). At 28 days, significant decreases in collagen fiber crimp frequency were present between five and ten cycles of preconditioning and during the toe-region of the mechanical test at the insertion site (Fig. 4 and Table 1). Significant decreases in collagen fiber crimp frequency were found during the toe-region at the midsubstance for all preconditioning protocols (Fig. 4 and Table 1). Average changes in crimp frequency throughout the mechanical test for the 28 day insertion site and midsubstance location were (ins:mid): between preload and 5 cycles (−4:−11), 5 cycles and 10 cycles (3:8), 10 and 20 cycles (5:9), 20 cycles and the toe-region (6:12), toe- and linear-regions (5:2).
Fig. 3.
Crimp frequency at 10 days old demonstrates that crimp frequency decreases during the toe-region regardless of the mechanical testing protocol
Fig. 4.
Crimp frequency demonstrates that crimp frequency decreases during the toe-region and increases from the preload to ten cycles of preconditioning at both locations
To address hypothesis 2, collagen fiber crimp frequency was examined at the preload compared to each preconditioning protocol. For this hypothesis, significance was set at p < 0.016. At 4 and 10 days, the increasing number of preconditioning cycles did not affect collagen fiber crimp behavior (Table 2). At the 28 day insertion site, an increase in crimp frequency was found after ten cycles of preconditioning compared to the preload. At the midsubstance location, a significant increase in crimp frequency was found between the preload and ten cycles of preconditioning (Fig. 4 and Table 2). Additionally, while not defined as significant after correction, (p = 0.02 for both comparisons) an increase in crimp frequency was found after five cycles of preconditioning compared to the preload while a decrease in crimp frequency was found after 20 cycles (Fig. 5 and Table 2). Average changes in crimp frequency after preconditioning compared to the preload for the 28 day insertion site and midsubstance location were (ins:mid): between preload and 5 cycles (−4, −11), preload and 10 cycles (−1, −3), and preload and 20 cycles (4,4).
Fig. 5.
Representative images from the 28 day insertion site throughout the mechanical test demonstrate an increase in crimp frequency between the preload and after ten cycles of preconditioning and a decrease in crimp frequency between after five and ten cycles of preconditioning and the toe-region
To address hypothesis 3, no changes in crimp frequency were found between 10 and 28 days with the quantitative analysis. Unfortunately, due to resolution limitations, comparisons were not made with the 4 day time points. However, histology indicates that the 4 days tendons demonstrated a smaller, less developed hierarchical structure compared to the 10 and 28 day tendons (Fig. 6).
Fig. 6.
Representative images for 4, 10, and 28 days at the preload, toe- and linear-region. Histology demonstrates the smaller collagen fibers and scale of crimp present at 4 days compared to 28 days.
To address hypothesis 4, no changes in crimp frequency with location were found at either 10 or 28 days. Due to resolution limitations, only one region was analyzed at 4 days.
4. Discussion
This study quantified collagen fiber crimp frequency throughout a mechanical testing protocol in a postnatal developmental mouse SST model. As expected, the majority of collagen fiber uncrimping was confined to the toe-region for all ages, supporting the concept that the uncrimping of collagen fibers occurs during the toe-region of a mechanical test. Tendons from 10 day old mice were found to function as a continuous unit, with the insertion site and midsubstance locations demonstrating similar crimp responses to mechanical load. A recent study also demonstrated that the insertion site and midsubstance locations of 10 day tendons exhibit similar collagen fiber realignment behavior in response to load [11]. These results support the concept that tendon fibrils have linearly fused by 10 days to form long, continuous fibers, which can transmit load along the length of the tendon [11,26–29]. Interestingly, at 28 days, the insertion site and midsubstance locations demonstrated slightly different crimp behaviors. At the 28 day insertion site, significant decreases in collagen fiber crimp frequency were present between five and ten cycles of preconditioning and the toe-region, while no significant decrease in crimp frequency were found between 20 cycles of preconditioning and the toe-region (p = 0.2) (Figs. 4 and 5). At the 28 day midsubstance, significant decreases in collagen fiber crimp frequency were found during the toe-region for all preconditioning protocols (Fig. 4 and Table 1). Overall, these findings support previous results and speculation that the uncrimping of collagen fibers may explain the toe-region of the mechanical test [5,30–35].
While similar crimp behavior was found during the toe-region at all ages, it should be noted that the actual strain levels at which the samples were flash-frozen for each age were different. A structural fiber recruitment model was used to determine toe- and linear-region strains [11,21]. Grip-to-grip strains representing the toe- and linear-regions calculated from the structural model were as follows: 17 and 22% for 4 days, 22 and 29% strain for 10 days, and 5 and 7% strain for 28 days. While the overall crimp behavior demonstrated at each age was similar, the early developmental tendons may require a prolonged exposure to load and higher strains in order to uncrimp and transition to the linear-region. This supports previous speculation that 4 and 10 day tendons demonstrate a delayed structural response to mechanical load explained by the immature networks of collagen fibrils present at early postnatal development [11,28]. Additionally, a previous study in rat tail tendon defined linear region strains as higher than 6% [5] indicating that the 28 day collagen fibril network may be approaching maturity. Further, the extent of the toe-region is believed to be dependent on crimp angle and tendon function [34].
Interestingly, results from this study at 28 days suggest collagen fiber crimp frequency following preconditioning may be dependent upon the number of cycles applied. Determining a reference configuration before measuring mechanical properties is very important for consistent mechanical testing and interpretation of experimental results. Additionally, it has been suggested that the chosen reference configuration and protocol may have strong implications for the transition strain and nonlinear region properties [30]. While increasing the number of preconditioning cycles did not affect collagen fiber crimp behavior at 4 and 10 days, significant increases in collagen fiber crimp frequency were found following ten cycles of preconditioning compared to the preload at both locations at 28 days (Fig. 4). In addition, while not significant after Bonferroni correction for multiple comparisons, an increase in crimp frequency was found after five cycles of preconditioning compared to the preload, while a decrease in crimp frequency was found after 20 cycles at the midsubstance location (Table 2). These results suggest that the preconditioning protocol applied may affect collagen fiber crimp behavior and, therefore, may influence mechanical properties measured later in the mechanical testing process. A previous study in rat tail tendon fascicles found an increase in crimp each time a new strain was reached, which they termed preconditioning [5]. They suggest that preconditioning may be associated with a change in the stress-free configuration explained primarily by the sliding of microstructures inside the fascicle leading to a shift in the toe-region of the stress-strain curve [5]. In support, our current study also identified an increase in collagen fiber crimp frequency following five and ten cycles of preconditioning compared to the preload. However, this study also identified a pattern of decreasing crimp frequency with an increasing number of preconditioning cycles at the same load (Fig. 4 and Table 2). This indicates that crimp behavior in response to mechanical load may possess viscoelastic and elastic components, which merit further study. Additionally, the literature is currently unclear on whether the effects of preconditioning are reversible and further studies are necessary to examine the effect and subsequent recovery of structural changes during preconditioning [5,33]. It has been suggested that relaxation and recovery are governed by separate mechanisms and may involve a different time and length scale depending on the tissue age, function, and applied protocol [5,36]. Additionally, previous studies suggest that collagen fiber realignment is also correlated with preconditioning [3,4]. These results indicate that collagen fiber uncrimping may also be affected by preconditioning and may be correlated with other structural changes seen during preconditioning [5,33,37].
This study also examined changes in crimp frequency with developmental age. No significant changes in crimp frequency were found between 10 and 28 days old. Unfortunately, given the small scale of crimp at 4 days, we were not able to compare crimp frequency between 4 and 10 days nor 4 and 28 days due to resolution limitations. However, in agreement with previous work, histology suggests that 4 day tendon crimp exists on a much smaller scale in that it is compromised of an immature fibril network with small fibril diameters (Fig. 6) [11,26–28]. Previous literature suggests that collagen fiber crimp frequency decreases with age while total number of crimp stays constant [12–14]. The lack of changes between 10 and 28 days suggests the decrease in transition strain previously reported between 10 and 28 days may be explained by other factors such as increases in fiber-fiber and fiber-matrix connections throughout maturity [11]. Previous studies also suggest the uncrimping of collagen fibers may not be the only mechanism driving the toe-region; realignment of collagen fibers, fascicle rotation, and fiber recruitment may account for the differences in transition strain noted with developmental age [34]. In addition, previous work in our laboratory suggests that 4 and 10 day tendons require a longer exposure to mechanical load before structurally responding through the realignment of collagen fibrils, which may explain the longer toe-region demonstrated throughout development [11].
Finally, while no differences in crimp frequency were found in this study between locations, differences in crimp behavior were identified at 28 days. Comparing average changes in crimp frequency for each set of paired data demonstrated that the midsubstance and insertion site locations displayed similar patterns of crimp behavior, but the effect of preconditioning on collagen fiber crimp frequency was more pronounced at the midsubstance location. Interestingly, no decrease in collagen fiber crimp frequency was found between 20 cycles and the toe-region at the insertion site (p = 0.2), suggesting that collagen fiber crimp steadily decreased with an increasing number of preconditioning cycles (Fig. 4 and Table 2). Significant decreases in crimp frequency were also found at the toe-region compared to all post-preconditioning time points at the midsubstance location (Fig. 4). This result is supported by the average changes in crimp frequency per paired sample. Changes in crimp frequency were smaller at the insertion site compared to the midsubstance, which indicates the overall response to load may be decreased at the insertion site. Previous work in heart valve soft tissue biomaterials found that loading perpendicular to the preferred collagen fiber orientation resulted in fiber reorientation but not fiber uncrimping [38]. While tendon is primarily composed of collagen fibers aligned in the direction of loading, the insertion site displays a more disorganized collagen fiber distribution [11,18,19]. It is possible that this modest display of collagen fiber uncrimping at the insertion site could be explained by the more disorganized collagen fiber distribution, where the fiber must first reorient in the direction of loading before uncrimping. Additionally, previous studies suggest that changes in collagen fiber crimp frequency are nonuniform along the length of the tendon, and some have indicated a preferential uncrimping near the bone [17,30].
This study is not without limitations. First, the small size of the collagen network at the 4 day time point did not allow for quantitative comparisons across ages or location within the 4 days. All images were taken using a 20x objective to allow for comparisons of equal resolution across groups; however, given the small fibril size at 4 days old, the fluctuations in pixel intensity were very close together and may have been oversampled in the analysis process. For this reason, comparisons were not made between 4 day tendons and additional time points. However, comparisons were made across 4 day points throughout the mechanical test because the same bias existed across all groups. Therefore, we are confident in our analysis of 4 day tendon behavior throughout the mechanical test, which was also confirmed by semi-quantitative analysis (data not shown). Additionally, the histology strongly supports the theory that 4 day tendons are composed primarily of small collagen fibril intermediates.
Secondly, the histology from this project was stained in multiple batches, though analysis methods performed were consistent and repeatable. Care was taken to ensure that the custom quantitative software normalized each image by the average pixel intensity and established exclusion criteria based on standard deviation of pixel fluctuations to exclude artifact and only include fluctuations representing collagen fiber crimp. This method minimizes the potential effect of different batches of stain on the histological analyses. In addition, this study examined collagen fiber crimp behavior throughout one mechanical testing protocol. Future studies are necessary to further examine the effects of collagen fiber crimp behavior in response to different preconditioning protocols and different magnitudes of preconditioning. Additionally, future studies are needed to examine additional points throughout the ramp-to-failure test, which will enable examination of earlier loads in the toe-region to further document the progress of local fiber uncrimping with increasing age. Finally, this study did not examine the ability of collagen fibers to return to initial collagen fiber crimp frequency. However, this study provides valuable insight into tendon structural response to load in the presence of preconditioning cycles. Future studies are needed to examine the conditions necessary for collagen fiber crimp to “recover” or return to a specified reference state.
In conclusion, this study utilized a technique for examining local collagen fiber crimp frequency throughout mechanical testing in a postnatal developmental mouse SST model. Results confirm that uncrimping of collagen fibers contributes to the toe-region of the mechanical test. Results also indicate that other factors, such as collagen fiber cross-links and maturity of the collagen matrix may also contribute its nonlinear behavior. Additionally, this study identified changes in collagen fiber crimp frequency with increasing number of preconditioning cycles, which may have implications on the measurement of mechanical properties and identifying a proper reference configuration. Future studies will examine collagen fiber crimp frequency and re-alignment behavior in a mature mouse model for comparison with the developmental time points and correlations between collagen fiber alignment, collagen fiber crimp frequency, and mechanical properties such as toe-region modulus and transition strain.
Acknowledgment
The authors would like to acknowledge Joseph Sarver, Nicholas Trasolini, and Brian Lee for assistance and the NIH/NIAMS supported Penn Center for Musculoskeletal Disorders for financial support.
References
- [1]. Cheng, S. , Clarke, E. C. , and Bilston, L. E. , 2009, “The Effects of Preconditioning Strain on Measured Tissue Properties,” J. Biomech., 42(9), pp. 1360–136210.1016/j.jbiomech.2009.03.023 [DOI] [PubMed] [Google Scholar]
- [2]. Woo, S. L. , 1982, “Mechanical Properties of Tendons and Ligaments. I. Quasi-Static and Nonlinear Viscoelastic Properties,” Biorheology, 19(3), pp. 385–396 [DOI] [PubMed] [Google Scholar]
- [3]. Miller, K. S. , Edelstein, L. , Connizzo, B. K. , and Soslowsky, L. J. , 2012, “Effect of Preconditioning and Stress Relaxation on Local Collagen Fiber Re-Alignment: Inhomogeneous Properties of Rat Supraspinatus Tendon,” J. Biomech. Eng., in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4]. Quinn, K. P. , and Winkelstein, B. A. , 2011, “Preconditioning is Correlated With Altered Collagen Fiber Alignment in Ligament,” J. Biomech. Eng., 133(6), p. 064506.10.1115/1.4004205 [DOI] [PubMed] [Google Scholar]
- [5]. Houssen, Y. G. , Gusachenko, I. , Schanne-Klein, M. C. , and Allain, J. M. , 2011, “Monitoring Micrometer-Scale Collagen Organization in Rat-Tail Tendon Upon Mechanical Strain Using Second Harmonic Microscopy,” J. Biomech., 44(11), pp. 2047–205210.1016/j.jbiomech.2011.05.009 [DOI] [PubMed] [Google Scholar]
- [6]. Franchi, M. , Raspanti, M. , Dell'Orbo, C. , Quaranta, M. , De Pasquale, V. , Ottani, V. , and Ruggeri, A. , 2008, “Different Crimp Patterns in Collagen Fibrils Relate to the Subfibrillar Arrangement,” Connect. Tissue Res., 49(2), pp. 85–9110.1080/03008200801913635 [DOI] [PubMed] [Google Scholar]
- [7]. Franchi, M. , Fini, M. , Quaranta, M. , De Pasquale, V. , Raspanti, M. , Giavaresi, G. , Ottani, V. , and Ruggeri, A. , 2007, “Crimp Morphology in Relaxed and Stretched Rat Achilles Tendon,” J. Anat., 210(1), pp. 1–710.1111/j.1469-7580.2006.00666.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8]. Franchi, M. , Ottani, V. , Stagni, R. , and Ruggeri, A. , 2010, “Tendon and Ligament Fibrillar Crimps Give Rise to Left-Handed Helices of Collagen Fibrils in Both Planar and Helical Crimps,” J. Anat., 216(3), pp. 301–30910.1111/j.1469-7580.2009.01188.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9]. Franchi, M. , Quaranta, M. , Macciocca, M. , Leonardi, L. , Ottani, V. , Bianchini, P. , Diaspro, A. , and Ruggeri, A. , 2010, “Collagen Fibre Arrangement and Functional Crimping Pattern of the Medial Collateral Ligament in the Rat Knee,” Knee Surg. Sports Traumatol. Arthrosc., 18(12), pp. 1671–167810.1007/s00167-010-1084-6 [DOI] [PubMed] [Google Scholar]
- [10]. Hurschler, C. , Provenzano, P. P. , and Vanderby, R., Jr. , 2003, “Scanning Electron Microscopic Characterization of Healing and Normal Rat Ligament Microstructure Under Slack and Loaded Conditions,” Connect. Tissue Res., 44(2), pp. 59–68 [PubMed] [Google Scholar]
- [11]. Miller, K. S. , Connizzo, B. K. , and Soslowsky, L. J. , 2011, “Collagen Fiber Re-Alignment in a Neonatal Developmental Mouse Supraspinatus Tendon Model,” Ann. Biomed. Eng., in press10.1007/s10439-011-0490-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12]. Diamant, J. , Keller, A. , Baer, E. , Litt, M. , and Arridge, R. G. , 1972, “Collagen; Ultrastructure and its Relation to Mechanical Properties as a Function of Ageing,” Proc. R. Soc. London, Ser. B, 180(60), pp. 293–31510.1098/rspb.1972.0019 [DOI] [PubMed] [Google Scholar]
- [13]. Gathercole, L. J. , and Keller, A. , 1991, “Crimp Morphology in the Fibre-Forming Collagens,” Matrix, 11(3), pp. 214–234 [DOI] [PubMed] [Google Scholar]
- [14]. Shah, J. S. , Palacios, E. , and Palacios, L. , 1982, “Development of Crimp Morphology and Cellular Changes in Chick Tendons,” Dev. Biol., 94(2), pp. 499–50410.1016/0012-1606(82)90366-9 [DOI] [PubMed] [Google Scholar]
- [15]. Ansorge, H. L. , Adams, S. , Birk, D. E. , and Soslowsky, L. J. , 2011, “Mechanical, Compositional, and Structural Properties of the Post-Natal Mouse Achilles Tendon,” Ann. Biomed. Eng., 39(7), pp. 1904–191310.1007/s10439-011-0299-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16]. Boorman, R. S. , Norman, T. , Matsen, F. A., III , and Clark, J. M. , 2006, “Using a Freeze Substitution Fixation Technique and Histological Crimp Analysis for Characterizing Regions of Strain in Ligaments Loaded in Situ,” J. Orthop. Res., 24(4), pp. 793–79910.1002/jor.20081 [DOI] [PubMed] [Google Scholar]
- [17]. Stouffer, D. C. , Butler, D. L. , and Hosny, D. , 1985, “The Relationship Between Crimp Pattern and Mechanical Response of Human Patellar Tendon-Bone Units,” J. Biomech. Eng., 107(2), pp. 158–16510.1115/1.3138536 [DOI] [PubMed] [Google Scholar]
- [18]. Lake, S. P. , Miller, K. S. , Elliott, D. M. , and Soslowsky, L. J. , 2009, “Effect of Fiber Distribution and Realignment on the Nonlinear and Inhomogeneous Mechanical Properties of Human Supraspinatus Tendon Under Longitudinal Tensile Loading,” J. Orthop. Res., 27(12), pp. 1596–160210.1002/jor.20938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19]. Thomopoulos, S. , Williams, G. R. , Gimbel, J. A. , Favata, M. , and Soslowsky, L. J. , 2003, “Variation of Biomechanical, Structural, and Compositional Properties Along the Tendon to Bone Insertion Site,” J. Orthop. Res., 21(3), pp. 413–41910.1016/S0736-0266(03)0057-3 [DOI] [PubMed] [Google Scholar]
- [20]. Festing, M. F. , 2006, “Design and Statistical Methods in Studies Using Animal Models of Development,” ILAR J., 47(1), pp. 5–14 [DOI] [PubMed] [Google Scholar]
- [21]. Peltz, C. D. , Sarver, J. J. , Dourte, L. M. , Wurgler-Hauri, C. C. , Williams, G. R. , and Soslowsky, L. J. , 2010, “Exercise Following a Short Immobilization Period is Detrimental to Tendon Properties and Joint Mechanics in a Rat Rotator Cuff Injury Model,” J. Orthop. Res., 28(7), pp. 841–84510.1002/jor.21059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22]. Thornton, G. M. , Shrive, N. G. , and Frank, C. B. , 2002, “Ligament Creep Recruits Fibres at Low Stresses and can Lead to Modulus-Reducing Fibre Damage at Higher Creep Stresses: A Study in Rabbit Medial Collateral Ligament Model,” J. Orthop. Res., 20(5), pp. 967–97410.1016/S0736-0266(02)00028-1 [DOI] [PubMed] [Google Scholar]
- [23]. Landerman, L. R. , Land, K. C. , and Pieper, C. F. , 1997, “An Empirical Evaluation of the Predictive Mean Matching Method for Imputing Missing Values,” Sociolog. Methods Res., 26(1), pp. 3–3310.1177/0049124197026001001 [Google Scholar]
- [24]. Little, R. J. A. , 1988, “Missing-Data Adjustments in Large Surveys,” J. Bus. Econ. Stat., 6(3), pp. 287–29610.2307/1391878 [Google Scholar]
- [25]. Yuan, Y. , 2011, “Multiple Imputation Using SAS Software,” J. Stat. Software, 45(6), pp. 1–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26]. Birk, D. E. , Nurminskaya, M. V. , and Zycband, E. I. , 1995, “Collagen Fibrillogenesis in Situ: Fibril Segments Undergo Post-Depositional Modifications Resulting in Linear and Lateral Growth During Matrix Development,” Dev. Dyn., 202(3), pp. 229–24310.1002/aja.1002020303 [DOI] [PubMed] [Google Scholar]
- [27]. Birk, D. E. , Zycband, E. I. , Woodruff, S. , Winkelmann, D. A. , and Trelstad, R. L. , 1997, “Collagen Fibrillogenesis in Situ: Fibril Segments Become Long Fibrils as the Developing Tendon Matures,” Dev. Dyn., 208(3), pp. 291–29810.1002/(SICI)1097-0177(199703)208:3<291::AID-AJA1>3.0.CO;2-D [DOI] [PubMed] [Google Scholar]
- [28]. Zhang, G. , Young, B. B. , Ezura, Y. , Favata, M. , Soslowsky, L. J. , Chakravarti, S. , and Birk, D. E. , 2005, “Development of Tendon Structure and Function: Regulation of Collagen Fibrillogenesis,” J. Musculoskeletal and Neuronal Interact., 5(1), pp. 5–21 [PubMed] [Google Scholar]
- [29]. Provenzano, P. P. , and Vanderby, R., Jr. , 2006, “Collagen Fibril Morphology and Organization: Implications for Force Transmission in Ligament and Tendon,” Matrix Biol., 25(2), pp. 71–8410.1016/j.matbio.2005.09.005 [DOI] [PubMed] [Google Scholar]
- [30]. Hansen, K. A. , Weiss, J. A. , and Barton, J. K. , 2002, “Recruitment of Tendon Crimp With Applied Tensile Strain,” J. Biomech. Eng., 124(1), pp. 72–7710.1115/1.1427698 [DOI] [PubMed] [Google Scholar]
- [31]. Woo, S. L. , Debski, R. E. , Zeminski, J. , Abramowitch, S. D. , Saw, S. S. , and Fenwick, J. A. , 2000, “Injury and Repair of Ligaments and Tendons,” Annu. Rev. Biomed. Eng., 2, pp. 83–11810.1146/annurev.bioeng.2.1.83 [DOI] [PubMed] [Google Scholar]
- [32]. Rigby, B. J. , 1964, “Effect of Cyclic Extension on the Physical Properties of Tendon Collagen and Its Possible Relation to Biological Ageing of Collagen,” Nature, 202, pp. 1072–107410.1038/2021072a0 [DOI] [PubMed] [Google Scholar]
- [33]. Rigby, B. J. , Hirai, N. , Spikes, J. D. , and Eyring, H. , 1959, “The Mechanical Properties of Rat Tail Tendon,” J. Gen. Physiol., 43(2), pp. 265–28310.1085/jgp.43.2.265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34]. Screen, H. R. , Lee, D. A. , Bader, D. L. , and Shelton, J. C. , 2004, “An Investigation Into the Effects of the Hierarchical Structure of Tendon Fascicles on Micromechanical Properties,” Proc. Inst. Mech. Eng., Part H: J. Eng. Med., 218(2), pp. 109–11910.1243/095441104322984004 [DOI] [PubMed] [Google Scholar]
- [35]. Viidik, A. , 1972, “Simultaneous Mechanical and Light Microscopic Studies of Collagen Fibers,” Z. Anat. Entwicklungsgesch, 136(2), pp. 204–21210.1007/BF00519178 [DOI] [PubMed] [Google Scholar]
- [36]. Duenwald, S. E. , Vanderby, R., Jr. , and Lakes, R. S. , 2010, “Stress Relaxation and Recovery in Tendon and Ligament: Experiment and Modeling,” Biorheology, 47(1), pp. 1–14 [DOI] [PubMed] [Google Scholar]
- [37]. Lokshin, O. , and Lanir, Y. , 2009, “Viscoelasticity and Preconditioning of Rat Skin Under Uniaxial Stretch: Microstructural Constitutive Characterization,” J. Biomech. Eng., 131(3), p. 031009.10.1115/1.3049479 [DOI] [PubMed] [Google Scholar]
- [38]. Sellaro, T. L. , Hildebrand, D. , Lu, Q. , Vyavahare, N. , Scott, M. , and Sacks, M. S. , 2007, “Effects of Collagen Fiber Orientation on the Response of Biologically Derived Soft Tissue Biomaterials to Cyclic Loading,” J. Biomed. Mater. Res. Part A, 80(1), pp. 194–20510.1002/jbm.a.30871 [DOI] [PubMed] [Google Scholar]