Abstract
The high prevalence of diabetes in African-American (AA) women has been widely assumed to be related to the greater prevalence of obesity in this group. Catecholamine release acting on central adipose tissue has been proposed to be a contributing factor. The aim of this article was to examine the interaction of plasma catecholamines and central adiposity on fasting and nonfasting glucose levels in two separate samples. In both studies, the women were healthy, nondiabetic of similar age. In addition, both studies assessed plasma epinephrine (EPI) and norepinephrine (NOREPI) levels collected at three time points. In study 1, catecholamines were measured during a standardized laboratory mental stress task and in study 2, they were measured during the initial phase (10 min) of an intravenous glucose tolerance test (IVGTT). Results from both studies revealed significant effects of EPI on fasting glucose in the obese women. In study 1, mean EPI levels were significantly related to fasting glucose in AA women with high trunk fat (β = 0.60, P < 0.001). Because high BMI was associated with high trunk fat in women, we used BMI >30 as a proxy for high trunk fat (>32%) in study 2. In study 2, EPI response to the glucose bolus was a strong predictor of fasting glucose in AA women with BMI >30 (β = 0.75, P < 0.003). We conclude that the effect of central adiposity on fasting glucose may be moderated by plasma EPI. This suggests that adrenal medullary activity could play a role in the pathophysiology of type 2 diabetes.
Introduction
African Americans (AAs) have twice the prevalence of type 2 diabetes compared to non-Hispanic white women (1). The greater prevalence of diabetes in AA women has been widely assumed to be related to the greater prevalence of obesity in this group (2). Central adiposity appears to play a special role in the negative effects of excessive body fat, and has been related to hyperinsulinemia, diabetes, and increased risk of cardiovascular disease (3). The reasons for this are not entirely clear but may be related to the fact that abdominal adipose tissue exhibits enhanced lipolysis compared with gluteofemoral fat (4). However, even central adiposity may not be sufficient to explain abnormalities in glucose metabolism. Stress has been implicated in the pathophysiology of glucose dysregulation (5,6) and the metabolic syndrome (7). The effects of stress on glucose metabolism are hypothesized to be mediated by counter-regulatory hormones that are released in response to stress resulting in elevated blood glucose levels and decreased insulin action. There is animal work to support the notion that stress reliably produces hyperglycemia in type 2 diabetes, as well as evidence that the autonomic nervous system plays a role in the pathophysiology of this condition in both animals and humans (8). Our group has previously shown that diet-induced obesity enhances glycemic response to epinephrine (EPI) in mice (9). It has been suggested that augmented catecholamine release acting on central adipose tissue may be an important contributing factor to abnormalities in glucose metabolism in humans (10). Catecholamines are potent regulators of lipolysis in human adipocytes through stimulatory b1- and b2-adrenoreceptors or inhibitory a2-adrenoreceptors (11). Increased lipolysis could contribute to metabolic dysregulation due to lipotoxic effects on muscle, liver, and pancreas (12). In addition, EPI has been shown to produce a significant time delay in the ability of insulin to inhibit hepatic glucose production after a glucose load (13). Therefore, increased circulating catecholamines as well as increased central obesity may be necessary for the development of abnormal glucose metabolism. We now report results from two separate studies examining the interaction of plasma catecholamines and central adiposity on fasting and nonfasting glucose levels in AA women.
Methods and Procedures
Study 1
Subjects
Sixty healthy AA women of age 33 ± 9 years participated in the study. They all signed informed consent approved by the Duke University School of Medicine Institutional Review Board.
Protocol
After an overnight fast of 10–12 h, participants arrived to the Duke Clinical Research Unit. A venous catheter was placed between 8:00 and 9:00 am, and a blood sample was drawn to assess fasting glucose. Participants were then given a liquid meal (Boost) (55% carbohydrate, 25% protein, and 20% fat) to drink within 10 min. Postprandial glucose and insulin levels were collected 2 h after administration of the meal. Later on the same day, plasma EPI and norepinephrine (NOREPI) were measured at three time points during a standardized laboratory mental stress protocol as previously described (14). The mental stress protocol started with a 20-min resting baseline period, after which the first blood draw was performed. The second blood draw was taken after a 5-min anger recall task, where the participants were told to recall and talk about an event when someone made them really angry. The last blood draw was done after a recovery period of 15 min.
Finally, participants underwent whole-body dual-energy X-ray absorptiometry scanning using a Hologic Discovery A scanner 82364 (s/w version 12.6.3; Hologic, Bedford, MA). Analysis of fat mass, lean mass, and % fat mass was acquired for the entire body, and percent body fat was established for several body regions including trunk fat, which was a measure of central adiposity.
Study 2
Subjects
Twenty-eight healthy AA women of age 34 ± 5 years participated in the study. They all signed informed consent approved by the Duke University School of Medicine Institutional Review Board.
Protocol
Screening oral GTT
After an overnight fast of 10–12 h, participants arrived to the Duke Clinical Research Unit. A venous catheter was placed between 8:00 and 9:00 am, and a blood sample was drawn to assess fasting glucose and insulin. Participants were then given a liquid meal (Boost) (55% carbohydrate, 25% protein, and 20% fat) to drink within 10 min. A postprandial glucose level was collected 2 h later.
IVGTT
On a separate day within a 2-week period, participants arrived to the Duke Clinical Research Unit after an overnight fast of 10–12 h for an intravenous glucose tolerance test (IVGTT) assessment. A venous catheter was placed between 8:00 and 9:00 am. A 0.3 g/kg body weight bolus of glucose (maximum of 35 g) was then injected intravenously. Blood samples were obtained for plasma glucose and insulin at 0, 2, 3, 4, 5, 6, 8, 10, 12, 15, 18, 20, 25, 30, 40, 50, 60, 70, 80, 90, 100, 110, 120, 140, 150, 160, 180, 210, and 240 min. Fasting plasma EPI and NOREPI were assessed before the IVGTT. Plasma catecholamines were also measured at 5 and 10 min into the IVGTT. Change scores for EPI and NOREPI levels (difference between baseline and the 10-min level) were calculated in order to establish EPI change score estimate for this neuroendocrine parameter. The blood was collected into tubes containing heparin and glutathione. Blood samples were collected and centrifuged in the cold, and plasma samples are stored frozen at −80 °C until analysis.
Glucose assays
Plasma glucose was assayed by the glucose oxidase method using a Glucose Analyzer II (Beckman Instruments, Fullerton, CA).
Catecholamine assays
Five cubic centimeters of blood were collected into purple top tubes (EDTA) at three separate time points over an hour. Plasma levels of NOREPI and EPI were later assessed by high performance liquid chromatography with electrochemical detection (15).
Central adiposity determination in study 2
Although there was no dual-energy X-ray absorptiometry assessment in study 2, in one of our sample consisting of 334 AA and white women, BMI levels >30 predicted a high trunk fat level (>32%) in 100% of all women assessed. We therefore used the BMI cutoff of >30 as a proxy for high trunk fat in study 2.
Statistics
Statistical analyses were performed using SPSS (version 17.0 for Windows; SPSS, Chicago, IL). A natural logarithmic transformation (ln) was computed for EPI and NOREPI mean values to correct for positive skewness in these variables. In study 1, the mean levels of EPI and NOREPI over the three measurements were used in the regression analysis. In study 2, change in EPI and NOREPI from baseline to 10 min after the glucose bolus was calculated and used in the regression analysis.
Regression analysis tested for interaction effects of catecholamine × adiposity group on fasting and nonfasting glucose. In addition, general linear models were applied to test relationships of EPI and NOREPI levels and change scores to fasting and nonfasting glucose in women with high vs. low levels of central adiposity.
Results
Study 1
Fasting glucose level (means ± s.d.) was 87.5 ± 8.5 mg/dl and postprandial glucose level at 2 h after the meal was 92.0 ± 17.5. Fasting insulin level was 16.0 ± 8.4 mg/dl and postprandial insulin level at 2 h after the meal was 79.2 ± 67.5 mg/dl. The mean EPI level was 23.4 ± 17.7 pg/ml at baseline, 27.4 ± 17.3 pg/ml during anger recall, and 29.5 ± 20.9 pg/ml after recovery, with the mean EPI level over the three time points being 26.7 ± 16.5 pg/ml and the median EPI level = 23 pg/ml. The mean NOREPI level was 302.1 ± 164.7 pg/ml at baseline, 327.0 ± 175.2 pg/ml during anger recall, and 277.0 ± 138.2 pg/ml after recovery, with the mean NOREPI level for the three time points being 302.1 ± 151.1 pg/ml and the median NOREPI level = 302 pg/ml.
Mean percent trunk fat was 31.6 ± 10.3 and median trunk fat was 32%. The associations of EPI and NOREPI levels over the three measurements were significant, with correlation coefficients ranging from Spearman’s ρ = 0.68, P < 0.001 to Spearman’s ρ = 0.72, P < 0.001 for EPI and Spearman’s ρ = 0.85, P < 0.001 to Spearman’s ρ = 0.87, P < 0.001 for NOREPI. Study variables (means ± s.d.) by obesity group are presented in Table 1.
Table 1.
Descriptive statistics (means ± s.d.) of study variables in the study 1 obesity groups
| Low trunk fat | High trunk fat | |
|---|---|---|
| N | 31 | 29 |
| Age (years) | 28.0 ± 8.1 | 32.5 ± 8.3* |
| Trunk fat (%) | 22.9 ± 5.3 | 39.4 ± 4.7*** |
| Waist | 69.4 ± 8.4 | 87.7 ± 12.1*** |
| BMI | 23.6 ± 3.1 | 33.4 ± 4.6*** |
| Waist/hip ratio | 0.73 ± 0.12 | 0.78 ± 0.10** |
| Fasting glucose (mg/dl) | 86.0 ± 7.1 | 89.1 ± 8.0 |
| Postprandial glucose (mg/dl) | 84.4 ± 6.7 | 97.0 ± 18.4** |
| Fasting insulin (μU/ml) | 11.9 ± 7.1 | 18.8 ± 7.7** |
| Postprandial insulin (μU/ml) | 62.1 ± 42.8 | 89.1 ± 77.0** |
| Mean EPI (pg/ml) | 31.2 ± 14.5 | 22.3 ± 17.3 |
| Mean NOREPI (pg/ml) | 293.3 ± 167.7 | 311.5 ± 124.7 |
EPI, epinephrine; NOREPI, norepinephrine.
P < 0.01,
P < 0.01,
P < 0.001.
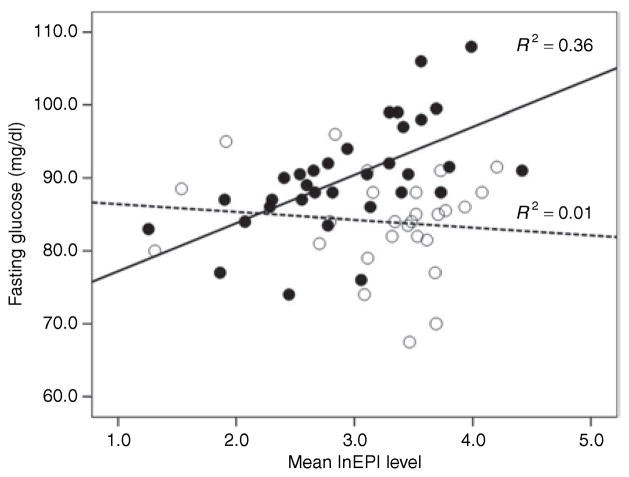
Regression analysis revealed a significant interaction of lnEPI levels and trunk fat group (high, low) for fasting glucose (P = 0.03), but not for 2-h postprandial glucose (P = 0.70). In the women with high trunk fat (>32%), the association of lnEPI mean level to fasting glucose was significant (β = 0.60, P < 0.001) (Figure 1). There was no association between lnEPI and fasting glucose in the low trunk fat group (Figure 1). There was no significant interaction of NOREPI and trunk fat on fasting (P = 0.96) or nonfasting glucose (P = 0.73).
Figure 1.
Association of EPI to fasting glucose in African-American women with high (solid black circles) vs. low levels (white circles) of trunk fat. Lines are fitted separately for the high trunk fat (solid line) and low trunk fat (dashed line) group. EPI, epinephrine.
A median split analysis of the EPI levels revealed a significant interaction effect of trunk fat × EPI group (P = 0.003), where the high trunk fat/high EPI group had significantly higher fasting glucose compared to the other groups (Figure 2).
Figure 2.
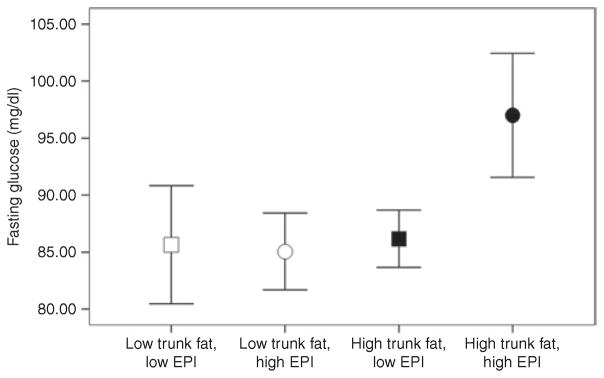
Mean levels of fasting glucose are displayed by trunk fat group (white = low trunk fat (≤32%), black = high trunk fat (>32%)) and EPI group (squares = low EPI (<25 pg/ml), circles = high EPI (≥25 pg/ml)) in African-American women. Bars represent 95% confidence intervals. EPI, epinephrine.
Study 2
Fasting glucose level (means ± s.d.) was 86.5 ± 8.4 mg/dl and postprandial glucose level at 2 h after the meal was 90.1 ± 15.5. Fasting insulin level was 18.7 ± 10.9 mg/dl and postprandial insulin level at 2 h after the meal was 96.1 ± 63.9 mg/dl. The baseline EPI level was 36.4 ± 30.0 pg/ml; at 5 min of the IVGTT, it was 34.8 ± 21.3 pg/ml; and at 10 min of the IVGTT, it was 31.1 ± 25.0. The mean baseline NOREPI level was 255.3 ± 115.3 pg/ml; at 5 min, 316.9 ± 140.1 pg/ml; and at 10 min, 324.6 ± 139.2 pg/ml.
Study variables (means ± s.d.) by obesity group are presented in Table 2.
Table 2.
Descriptive statistics (means ± s.d.) of study variables in the study 2 obesity groups
| Low BMI | High BMI | |
|---|---|---|
| N | 14 | 14 |
| Age (years) | 33.8 ± 5.3 | 35.2 ± 5.3 |
| BMI | 25.2 ± 2.0 | 37.3 ± 3.6*** |
| Waist (cm) | 86.0 ± 7.1 | 107.2 ± 9.4*** |
| Fasting glucose (mg/dl) | 82.6 ± 6.8 | 91.8 ± 7.4** |
| Postprandial glucose (mg/dl) | 84.4 ± 14.3 | 96.7 ± 14.4* |
| Fasting insulin (μU/ml) | 15.1 ± 11.0 | 22.3 ± 10.1 |
| Postprandial insulin (μU/ml) | 69.6 ± 32.4 | 124.1 ± 80.6* |
| SI ((mU/liter)−1·min−1) | 5.4 ± 6.0 | 2.7 ± 2.5 |
| AIRg (mU/liter−1·min) | 739 ± 514 | 1,236.1 ± 943 |
| DI | 3,058 ± 2,504 | 1,930 ± 1,000 |
| Sg (100 min−1) | 2.5 ± 1.5 | 1.8 ± 1.1 |
| Mean EPI (pg/ml) | 37.8 ± 28.2 | 32.1 ± 17.9 |
| Mean NOREPI (pg/ml) | 313.7 ± 112.0 | 286.4 ± 144.2 |
AIRg, acute insulin response; DI, disposition index; EPI, epinephrine; NOREPI, norepinephrine; Sg, glucose effectiveness; SI, insulin sensitivity.
P < 0.01,
P < 0.01,
P < 0.001.
Regression analysis revealed a strong trend toward a significant interaction of change in EPI to the glucose bolus central adiposity group for fasting glucose (P = 0.08), but not for 2-h postprandial glucose (P = 0.27). In the women with high central adiposity as estimated by BMI (>30), the association of EPI change between baseline and 10 min to fasting glucose was significant (β = 0.75, P = 0.003) (Figure 3), whereas there was no association between EPI changes and fasting glucose in the low BMI group (Figure 3). We conducted a median split of the EPI change score and BMI. As can be observed in Figure 4, the high BMI/high EPI change group showed higher fasting glucose compared to all other groups (Figure 4). There was no significant association between NOREPI change and obesity group on fasting (P = 0.60) or nonfasting glucose (P = 0.21).
Figure 3.
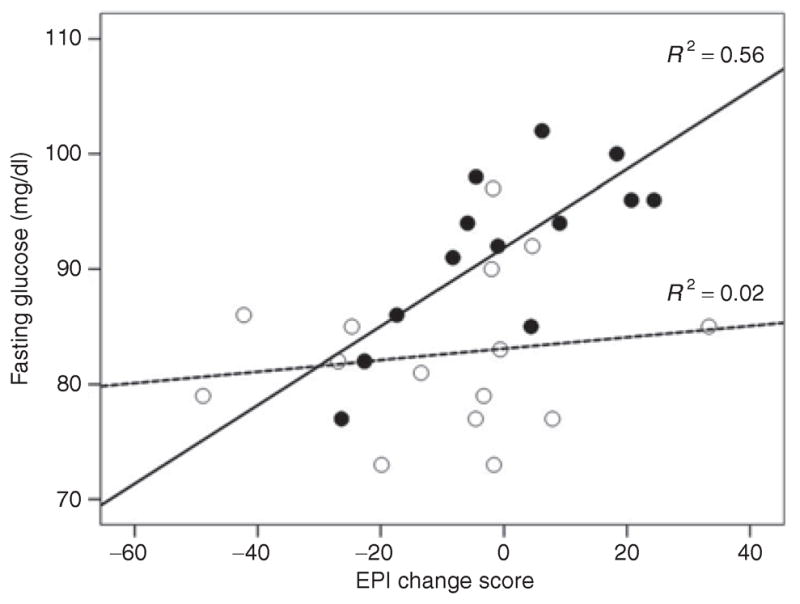
Association of EPI change score (change baseline to 10 min) to fasting glucose in African-American women with high (black solid circles) vs. low levels (white circles) of BMI. Lines are fitted separately for the high BMI (solid line) and low BMI (dashed line) group. EPI, epinephrine.
Figure 4.
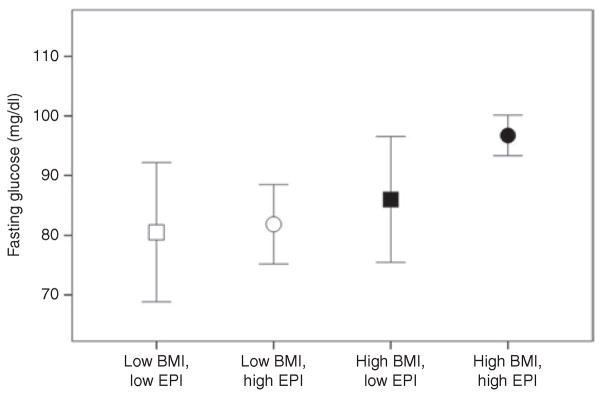
Mean levels of fasting glucose are displayed by BMI group (white = low BMI (≤30%), black = high BMI (>30%)) and EPI change group (squares = EPI reduction, circles = EPI increase, controlling for baseline EPI levels) in African-American women. Bars represent 95% confidence intervals. EPI, epinephrine.
Discussion
It has long been known that an accumulation of central, and in particular, visceral adipose tissue is related to increased risk of diabetes and cardiovascular disease (3,12,16). It has been suggested that catecholamine release acting on visceral adipose tissue will increase nonesterified fatty acids (NEFA) that will promote insulin resistance and increased fasting glucose levels (10). In our two samples of healthy AA women, individual differences in EPI interact with differences in central adiposity to determine fasting glucose. Our results from study 1 show that high levels of EPI in the presence of high central adiposity as measured by dual-energy X-ray absorptiometry were associated with fasting glucose at levels indicative of impaired glucose metabolism (17). However, high central adiposity in the absence of high EPI levels is not associated with higher fasting glucose. We did not find an effect of NOREPI on fasting glucose in the centrally obese women. This is consistent with a recent finding by de Glisezinski et al. who showed that EPI but not NOREPI was responsible for exercise-induced lipid mobilization (18). That EPI was associated with fasting and not 2-h postprandial glucose suggests that this effect may involve hepatic glucose production, possibly mediated by EPI-induced changes in NEFA.
Our second study showed that an EPI increase in response to the glucose bolus during an IVGTT predicted fasting glucose in AA women with high BMI, but not in the women with low BMI. Our findings extend our previous report, where we showed that there are individuals who show an initial increase in EPI in response to a glucose load (19). Because administration of a glucose load should evoke an anabolic response and a decrease in counter-regulatory hormones in healthy individuals (20), these results suggest that an abnormal catabolic neuroendocrine response after a glucose load, i.e., an inability to reduce EPI levels, appears to interact with obesity in determining fasting glucose in AA women. This finding suggests that obesity per se is not sufficient to explain impaired glucose metabolism. A catabolic sympathoadrenal response to a glucose load must also be present for obesity to be associated with impaired fasting glucose.
A major weakness of these studies is that they were not designed to properly explore potential mechanisms for this effect. Because increases in NEFA have been proposed to lead to impaired glucose metabolism (21), it would have been of importance to examine the impact of EPI on NEFA levels measured simultaneously. Also, EPI levels were collected at two different times after fasting glucose, so the temporal relationship between changes in EPI, glucose and NEFA remains to be determined. Future studies in which glucose, EPI and NEFA are measured simultaneously overnight as well as after an IV glucose challenge will be necessary to properly interpret our current observations.
Another weakness of the present study is that we cannot definitively state that our observed interaction of central adiposity and EPI on fasting glucose is restricted to AA women. There are studies showing that high levels of adiposity are more common among AA women as compared to white women (22), potentially making the observed phenomenon more prevalent in AA women. A recent study also showed that AA women have a higher density of β-receptors in omental adipose tissue (a component of visceral fat) as compared to white women (23), possibly making AA women more susceptible to the effects of adrenergic stimulation (4) on central adipose tissue.
Finally, the dual-energy X-ray absorptiometry measurement cannot discriminate between visceral and subcutaneous tissue. Visceral adipose tissue has been shown to be more metabolically active than other adipose tissue sites (24). However, in AA women, subcutaneous tissue may also be of importance because increased truncal subcutaneous adipose tissue has been associated with insulin resistance, particularly in minority groups (25,26).
The results reported here suggest when high central adiposity is present, adrenal medullary activity may be implicated in the development of abnormalities in glucose metabolism. This observation confirms and extends earlier speculation by our group and others on the potential role of adrenergic activity in the etiology of diabetes (5,9,10,27).
Acknowledgments
This research was supported by NHLBI grants P01-HL36587 and R01- HL076020.
Footnotes
Disclosure
The authors declared no conflict of interest.
References
- 1.Harris MI, Eastman RC, Cowie CC, Flegal KM, Eberhardt MS. Racial and ethnic differences in glycemic control of adults with type 2 diabetes. Diabetes Care. 1999;22:403–408. doi: 10.2337/diacare.22.3.403. [DOI] [PubMed] [Google Scholar]
- 2.Okosun IS. Racial differences in rates of type 2 diabetes in American women: how much is due to differences in overall adiposity? Ethn Health. 2001;6:27–34. doi: 10.1080/13557850120040379. [DOI] [PubMed] [Google Scholar]
- 3.Stern MP, Haffner SM. Body fat distribution and hyperinsulinemia as risk factors for diabetes and cardiovascular disease. Arteriosclerosis. 1986;6:123–130. doi: 10.1161/01.atv.6.2.123. [DOI] [PubMed] [Google Scholar]
- 4.Rebuffé-Scrive M, Anderson B, Olbe L, Björntorp P. Metabolism of adipose tissue in intraabdominal depots in severely obese men and women. Metabolism. 1990;39:1021–1025. doi: 10.1016/0026-0495(90)90160-e. [DOI] [PubMed] [Google Scholar]
- 5.Surwit RS, Schneider MS, Feinglos MN. Stress and diabetes mellitus. Diabetes Care. 1992;15:1413–1422. doi: 10.2337/diacare.15.10.1413. [DOI] [PubMed] [Google Scholar]
- 6.Surwit RS, Schneider MS. Role of stress in the etiology and treatment of diabetes mellitus. Psychosom Med. 1993;55:380–393. doi: 10.1097/00006842-199307000-00005. [DOI] [PubMed] [Google Scholar]
- 7.Björntorp P. Visceral fat accumulation: the missing link between psychosocial factors and cardiovascular disease? J Intern Med. 1991;230:195–201. doi: 10.1111/j.1365-2796.1991.tb00431.x. [DOI] [PubMed] [Google Scholar]
- 8.Surwit RS, Williams PG. Animal models provide insight into psychosomatic factors in diabetes. Psychosom Med. 1996;58:582–589. doi: 10.1097/00006842-199611000-00006. [DOI] [PubMed] [Google Scholar]
- 9.Surwit RS, Kuhn CM, Cochrane C, McCubbin JA, Feinglos MN. Dietinduced type II diabetes in C57BL/6J mice. Diabetes. 1988;37:1163–1167. doi: 10.2337/diab.37.9.1163. [DOI] [PubMed] [Google Scholar]
- 10.Bergman RN. Orchestration of glucose homeostasis: from a small acorn to the California oak. Diabetes. 2007;56:1489–1501. doi: 10.2337/db07-9903. [DOI] [PubMed] [Google Scholar]
- 11.Fain JN, Garcia-Sáinz JA. Adrenergic regulation of adipocyte metabolism. J Lipid Res. 1983;24:945–966. [PubMed] [Google Scholar]
- 12.Bays HE, González-Campoy JM, Bray GA, et al. Pathogenic potential of adipose tissue and metabolic consequences of adipocyte hypertrophy and increased visceral adiposity. Expert Rev Cardiovasc Ther. 2008;6:343–368. doi: 10.1586/14779072.6.3.343. [DOI] [PubMed] [Google Scholar]
- 13.Vicini P, Avogaro A, Spilker ME, Gallo A, Cobelli C. Epinephrine effects on insulin-glucose dynamics: the labeled IVGTT two-compartment minimal model approach. Am J Physiol Endocrinol Metab. 2002;283:E78–E84. doi: 10.1152/ajpendo.00530.2001. [DOI] [PubMed] [Google Scholar]
- 14.Brummett BH, Boyle SH, Kuhn CM, Siegler IC, Williams RB. Positive affect is associated with cardiovascular reactivity, norepinephrine level, and morning rise in salivary cortisol. Psychophysiology. 2009;46:862–869. doi: 10.1111/j.1469-8986.2009.00829.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kilts CD, Gooch MD, Knopes KD. Quantitation of plasma catecholamines by on-line trace enrichment high performance liquid chromatography with electrochemical detection. J Neurosci Methods. 1984;11:257–273. doi: 10.1016/0165-0270(84)90088-8. [DOI] [PubMed] [Google Scholar]
- 16.Bergman RN, Kim SP, Hsu IR, et al. Abdominal obesity: role in the pathophysiology of metabolic disease and cardiovascular risk. Am J Med. 2007;120:S3–8. doi: 10.1016/j.amjmed.2006.11.012. discussion S29. [DOI] [PubMed] [Google Scholar]
- 17.Nichols GA, Hillier TA, Brown JB. Normal fasting plasma glucose and risk of type 2 diabetes diagnosis. Am J Med. 2008;121:519–524. doi: 10.1016/j.amjmed.2008.02.026. [DOI] [PubMed] [Google Scholar]
- 18.de Glisezinski I, Larrouy D, Bajzova M, et al. Adrenaline but not noradrenaline is a determinant of exercise-induced lipid mobilization in human subcutaneous adipose tissue. J Physiol (Lond) 2009;587:3393–3404. doi: 10.1113/jphysiol.2009.168906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Surwit RS, Lane JD, Millington DS, et al. Hostility and minimal model of glucose kinetics in African American women. Psychosom Med. 2009;71:646–651. doi: 10.1097/PSY.0b013e3181acee4c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Penev P, Spiegel K, Marcinkowski T, Van Cauter E. Impact of carbohydraterich meals on plasma epinephrine levels: dysregulation with aging. J Clin Endocrinol Metab. 2005;90:6198–6206. doi: 10.1210/jc.2005-0415. [DOI] [PubMed] [Google Scholar]
- 21.Duez H, Lewis GF. Fat metabolism in insulin resistance and type 2 diabetes. In: Feinglos MN, Bethel MA, editors. Type 2 Diabetes Mellitus: An Evidence- Based Approach to Practical Management. Vol. 1. Humana; Durham, NC: 2008. pp. 49–73. [Google Scholar]
- 22.Brancati FL, Kao WH, Folsom AR, Watson RL, Szklo M. Incident type 2 diabetes mellitus in African American and white adults: the Atherosclerosis Risk in Communities Study. JAMA. 2000;283:2253–2259. doi: 10.1001/jama.283.17.2253. [DOI] [PubMed] [Google Scholar]
- 23.McConnaughey MM, Sheets KA, Davis J, et al. Differences in betaadrenergic receptor densities in omental and subcutaneous adipose tissue from obese African American and Caucasian women. Metabolism. 2004;53:247–251. doi: 10.1016/j.metabol.2003.09.014. [DOI] [PubMed] [Google Scholar]
- 24.Jensen MD. Adipose tissue and fatty acid metabolism in humans. J R Soc Med. 2002;95 (Suppl 42):3–7. [PMC free article] [PubMed] [Google Scholar]
- 25.Lovejoy JC, de la Bretonne JA, Klemperer M, Tulley R. Abdominal fat distribution and metabolic risk factors: effects of race. Metabolism. 1996;45:1119–1124. doi: 10.1016/s0026-0495(96)90011-6. [DOI] [PubMed] [Google Scholar]
- 26.Wagenknecht LE, Langefeld CD, Scherzinger AL, et al. Insulin sensitivity, insulin secretion, and abdominal fat: the Insulin Resistance Atherosclerosis Study (IRAS) Family Study. Diabetes. 2003;52:2490–2496. doi: 10.2337/diabetes.52.10.2490. [DOI] [PubMed] [Google Scholar]
- 27.Surwit RS, Feinglos MN. Stress and autonomic nervous system in type II diabetes. A hypothesis. Diabetes Care. 1988;11:83–85. doi: 10.2337/diacare.11.1.83. [DOI] [PubMed] [Google Scholar]