Abstract
Transient low-level viremia of 50–400 HIV RNA copies/mL (TLLV) is common during antiretroviral therapy, but its pathogenesis, consequences and optimal management are unclear. Heightened immune activation is associated with detrimental outcomes, including impaired CD4+ T-cell reconstitution. Using CD38/HLA-DR expression on CD8+ T-cells measured in two large studies, we determined associations between TLLV and immune activation levels before, during, and after TLLV. We found that TLLV does not significantly change CD8+ T-cell activation, and that higher CD8+ T-cell activation during viral suppression <50 copies/mL is associated with a modest increase in the risk of a subsequent TLLV.
Keywords: low-level, viremia, blip, immune, activation, CD8+
INTRODUCTION
Transient low-level viremia of 50–400 copies/mL (TLLV) is common after plasma HIV RNA suppression to <50 copies/mL during antiretroviral therapy (ART) [1–4], but its pathogenesis, consequences and optimal management are unclear. Occurrence of TLLV is sometimes linked to lapses in adherence [5]. It is uncertain whether TLLV reflects release of archived viruses from reservoirs and/or bursts of viral replication. Random HIV RNA variation around a mean value <50 copies/mL [3] and laboratory assay artifacts [6] are other potential explanations. Detection of HIV RNA below 50 copies/mL may also be a risk factor for TLLV since detection of HIV RNA below 50 copies/mL correlates with subsequent virologic rebound [7]. Compared to transient viremia above 400 copies/mL, TLLV is less likely to increase the risk of virologic failure or antiretroviral drug resistance [1, 3, 4, 8], although some studies suggest TTLV may be important clinically [7, 9, 10].
The dominant source of immune activation in untreated HIV infection is antigenic stimulation, whether by HIV, co-pathogens, or products of bacterial translocation from the gut [11–13]. Markers of immune activation decline during ART; however, the levels remain abnormally elevated after ART-mediated viral suppression [14–16]. It is unknown whether TLLV causes recrudescence of HIV antigenic stimulation and immune activation, or whether elevated immune activation causes TLLV. Understanding these issues is important because heightened immune activation has been linked to detrimental outcomes such as blunted CD4+ T-cell recovery [15, 17]. If TLLV elevates immune activation, one would expect that an episode of TLLV would precede an increase in activation. If, alternatively, heightened immune activation triggers transient release of HIV from reservoirs and results in TLLV, then an episode of TLLV would be preceded by an increase in immune activation.
To dissect these issues, we determined within-subject changes in immune activation, as measured by CD38/HLA-DR expression on CD8+ T-cells, before, during and after TLLV. We also conducted between-groups analyses, comparing immune activation levels during viral suppression <50 copies/mL between subjects who subsequently experienced TLLV versus those who maintained HIV RNA <50 copies/mL. In an additional between-groups analysis, we explored whether having detectable HIV RNA below 50 copies/mL at a pre-TLLV time point is predictive of a subsequent TLLV
METHODS
We analyzed data from AIDS Clinical Trials Group (ACTG) study A384, a randomized trial in treatment-naïve HIV-infected patients [18, 19] and ACTG Longitudinal Linked Randomized Trials (ALLRT), an observational cohort of HIV-infected patients who initiated ART in randomized clinical trials [20]. CD38/HLA-DR expression on CD8+ T-cells was measured on fresh PBMC samples using ACTG consensus methods every 16–24 weeks in 623 subjects in ACTG A384 [16, 17] and in 2391 subjects during 2000–2003 in ALLRT (every 16 weeks 2000–2002, every 48 weeks 2002–2003). HIV RNA quantification in ACTG A384 and ALLRT was performed using the Roche Amplicor assay v1.0 or v1.5 (Roche Diagnostic Systems, Branchburg, New Jersey, USA). HIV RNA from 1997–2012 were examined for TLLV (measured every 8 weeks after week 24 in A384; every 16 weeks in ALLRT through 2006, then twice every 48 weeks). Samples with <50 HIV RNA copies/mL were qualitatively sub-classified into HIV RNA undetected or detected based on optical density readings <0.2 or ≥0.2 in the Roche assay [21]. Institutional Review Boards at participating sites approved the protocols; subjects provided written informed consent.
We defined TLLV as an isolated HIV RNA 50–400 copies/mL that was preceded and followed by values <50 copies/mL with no more than 6 months between measurements and no change in the ART regimen. We included subjects who 1) experienced TLLV after at least 6 months of HIV RNA <50 copies/mL, and 2) had immune activation data available before, during and after an episode of TLLV (Aim 1), and at one timepoint of interest for Aim 2 (pre-TLLV). In subjects who had more than one episode of TLLV, we only analyzed the first occurrence (along with immune activation data around the time of that event).
For Aim 1 (longitudinal within-subject analysis), each eligible subject served as his/her own control. CD8+ T-cell activation was compared between the TLLV time point and the pre- and post-TLLV time points using repeated measures analysis (generalized estimating equations with identity link). In supplemental analyses, we compared just the pre-TLLV and TLLV time points, and the TLLV and post-TLLV time points. Sensitivity analyses examined the subset of subjects whose TLLV amplitudes were 50–200 copies/mL vs. 201–400 copies/mL. Variability (standard deviation, SD) in the change in CD8+ T-cell activation over short time frames during viral suppression was estimated to be 8.5% (percentage-points) over 48 weeks in ACTG 384. Using SD=8.5%, a sample size of 66 subjects was estimated to provide statistical power >80% to detect a 3.0 percentage-point within-subject increase in immune activation.
For Aim 2 (cross-sectional between-groups analysis), we employed a case-control design and conditional logistic regression to compare CD8+ T-cell activation during viral suppression (HIV RNA <50 copies/mL) between subjects who subsequently experienced TLLV (cases) and those whose HIV RNA remained <50 copies/mL (controls). Case and control subjects were matched by parent study, sex, age (± 5 years), duration of HIV RNA suppression (± 6 months) and duration of ART (± 6 months).
Finally, in an exploratory analysis we focused on subjects with information on whether HIV RNA was qualitatively detected or undetected when HIV RNA was <50 copies/mL; conditional logistic regression evaluated whether detection versus non-detection of HIV RNA at the pre-TLLV time point with HIV RNA <50 copies/mL was predictive of a subsequent TLLV.
RESULTS
Population with TLLV in A384 and ALLRT
A total of 1861/5042 (37%) subjects experienced TLLV. For those with TLLV, there was a median of 6.0 person-years of follow-up and a median of 31 HIV RNA measurements per subject.
No significant within-subject change in CD8+ T-cell activation pre-, during and post-TLLV
There were 64 subjects who had TLLV and CD8+ T cell activation data available at the pre-TLLV, TLLV and post-TLLV time points; these subjects formed the population for the within-subject longitudinal analysis.. They were 88% male with a median age of 39 years pre-TLLV. The median HIV RNA during TLLV was 94 (range, 50–400) copies/mL. The median (25th–75th percentiles) CD4+ T-cell count prior to TLLV was 539 (348–759) cells/mm3. Median (25th–75th) CD8+ T-cell activation before, during and after TTLV was 21% (10–33), 22% (12–33%), and 20% (12–31%), respectively. The pre-TLLV time point was a median (25th–75th) of 3.7 (3.4–3.9) months prior to the TLLV event, and the post-TLLV time point was 3.7 (3.6–4.0) months after the TLLV event. We did not detect a significant association between TLLV and CD8+ T-cell activation (95% CI: −1.4, 1.6 percentage-points, comparing pre- and post-TLLV to the TLLV time point; p=0.88), nor in CD8+ T-cell activation changes between the pre-TLLV and TLLV timepoints (p=0.65) or between the TLLV and post-TLLV timepoints (p=0.86). Results were similar in a sensitivity analysis restricted to 58 subjects with HIV RNA of 50–200 copies/ml at TLLV (95% CI: −1.8, 1.5 percentage-points; p=0.84).
Higher CD8+ T-cell activation during viral suppression is associated with increased risk of subsequent TLLV
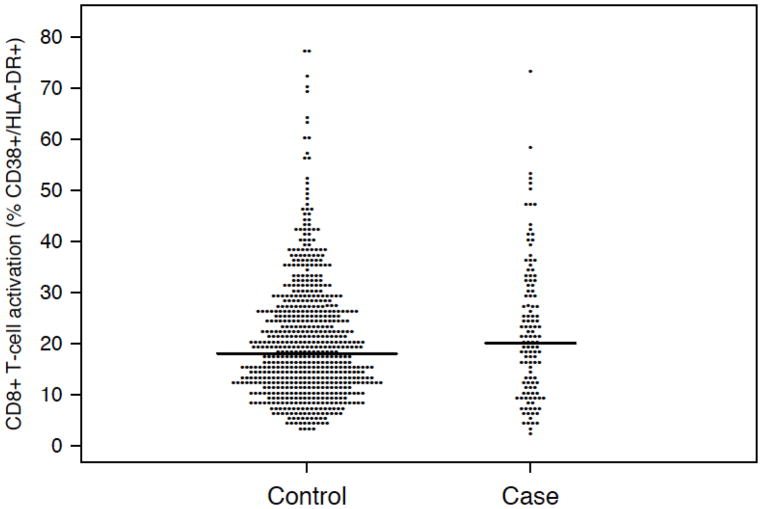
In a cross-sectional between-groups analysis to examine whether CD8+ T-cell activation was associated with a subsequent episode of TLLV, cases were 123 subjects with subsequent TLLV (89% male; median age 42 years; median CD4 count=547 cells/mm3; median CD8+ T-cell activation=20%). CD8+ T-cell activation was measured a median (25th–75th) 1.9 (1.8–3.5) months prior to TLLV. There were 629 matched controls who maintained HIV RNA <50 copies/mL (88% male; median age 40 years; median CD4 count=490 cells/mm3; median CD8+ T-cell activation=18%). Figure 1 shows slightly higher CD8+ T activation in cases vs. controls. CD8+ T-cell activation at the time of viral suppression was significantly associated with increased risk of a subsequent TLLV (odds ratio=1.19 per 10 percentage points higher CD8+ T-cell activation; 95% CI: 1.01, 1.41; p=0.034). Neither CD4 cell count at the time of viral suppression nor nadir CD4 count was associated with risk of TLLV (p=0.20 and 0.27, respectively).
Figure 1.
CD8+ T-cell activation for 123 cases with subsequent transient low-level viremia and 629 matched controls who maintained HIV RNA <50 copies/mL. Horizontal bars display medians.
We conducted a similar case-control analysis using 118 case and 569 control subjects with information on whether HIV RNA was qualitatively detected when HIV RNA was <50 copies/mL. HIV RNA was detected in 10.2% of samples with <50 HIV RNA copies/mL. A trend towards increased risk of TLLV in the group with detected HIV RNA was found, but this did not reach significance (odds ratio=1.79; 95% CI: 0.96, 3.35, p=0.07).
DISCUSSION
We investigated associations between TLLV and immune activation using CD38/HLA-DR expression on CD8+ T-cells measured as part of two ACTG studies. In a longitudinal within-subject analysis, with each patient serving as his/her own control, we found no significant change in CD8+ T-cell activation during TLLV, consistent with results of a smaller study (N=15) that used a broader definition of low-level viremia (50–1000 HIV RNA copies/mL) [22]. However another study reported increased T-cell activation during transient viremia above 200 copies/mL, although no association was found when viremia was under 200 copies/mL [23]. In cross-sectional between-groups case-control analyses, higher CD8+ T-cell activation during viral suppression (HIV RNA <50 copies/mL) was associated with an increased risk of a subsequent TLLV, though this effect appears to be of limited magnitude.
The clinical ramifications of TLLV are unknown and most clinicians manage these episodes with adherence reinforcement. There have been signals, however, that TLLV may not be innocuous. Each episode lasts approximately 22 days [2] and increased TLLV frequency is associated with slower decay of latently infected cells [24]. Since heightened immune activation during ART may be detrimental, the absence of a significant increase in immune activation with TLLV in our study provides reassurance that TLLV is unlikely to cause clinical harm through this mechanism.
Although we found an association between higher CD8+ T-cell activation during viral suppression and increased risk of subsequent TLLV, there is no proof that higher immune activation is the cause of TLLV. Another potential explanation for the observed association is that increased CD8+ T-cell activation is related to as yet unidentified factors that drive TLLV. Alternatively, CD8+ T-cell activation and TLLV may be driven by common factors, such as incomplete adherence or larger virus reservoirs. \
In an exploratory analysis, we found a trend towards an association between qualitative detection of HIV RNA below 50 copies/mL and subsequent TLLV, but this did not reach statistical significance, perhaps due to small sample size. The fact that only 10% of subjects with HIV RNA <50 copies/mL in our study had detectable HIV RNA raises questions about the sensitivity of the test; approximately two-thirds have detectable virus using single-copy assays [25]. Other studies have reported a relationship between HIV detection below 50 copies/mL and subsequent confirmed viral rebound [7. 9]
Our study has a number of limitations. First, some antiretroviral drugs used in ALLRT and A384 (such as unboosted protease inhibitors) are no longer recommended due partly to inferior virologic efficacy. We addressed this by focusing on patients who attained HIV RNA <50 copies/mL. Second, only one marker of immune activation (i.e., CD38/HLA-DR expression on CD8+ T-cells) was evaluated, constraining on our ability to address the full breath of immune activation and inflammation associated with HIV and ART. Potential co-infections on which we lacked information (such as herpesviruses) may have confounded our within-patient analysis. Hypothetically, co-infections can trigger immune activation, increase target T-cell availability and cause transient viremia [26]. The follow-up time points were over three months after the TLLV episodes and it is possible for an increase in T-cell activation due to the TLLV to have occurred sooner and waned by the follow-up time point. Finally, the relatively small number of patients in the analysis may limit the generalizability of our results.
In conclusion, TLLV during suppressive ART (HIV RNA <50 copies/mL) is unlikely to worsen CD8+ T-cell activation while higher CD8+ T-cell activation during viral suppression is associated with a modest increase in the risk of a subsequent TLLV. Future studies should further characterize the association between CD8+ T-cell activation milieu during viral suppression and subsequent TLLV, and probe the role of potential non-immunologic precursors of TLLV including residual viremia measured with single copy HIV RNA assay.
Acknowledgments
Funding and other support
The project described was supported by Award Number U01AI068636 and U01AI68634 from the National Institute of Allergy and Infectious Diseases. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Allergy and Infectious Diseases or the National Institutes of Health. The authors would like to thank the chairs and teams of the contributing ACTG studies and the study volunteers for their time and effort.
Footnotes
Conflicts of Interest
Authors report no conflicts of interest.
Presented in part at the 19th Conference of Retroviruses and Opportunistic Infections. Washington, March 5–8, 2012. Abstract 275
References
- 1.Sungkanuparph S, Overton ET, Seyfried W, Groger RK, Fraser VJ, Powderly WG. Intermittent episodes of detectable HIV viremia in patients receiving nonnucleoside reverse-transcriptase inhibitor-based or protease inhibitor-based highly active antiretroviral therapy regimens are equivalent in incidence and prognosis. Clin Infect Dis. 2005;41(9):1326–32. doi: 10.1086/496985. [DOI] [PubMed] [Google Scholar]
- 2.Di Mascio M, Markowitz M, Louie M, et al. Dynamics of intermittent viremia during highly active antiretroviral therapy in patients who initiate therapy during chronic versus acute and early HIV type 1 infection. J Virol. 2004;78:10566–73. doi: 10.1128/JVI.78.19.10566-10573.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nettles RE, Kieffer TL, Kwon P, et al. Intermittent HIV-1 viremia (Blips) and drug resistance in patients receiving HAART. JAMA. 2005;293:817–29. doi: 10.1001/jama.293.7.817. [DOI] [PubMed] [Google Scholar]
- 4.Grennan JT, Loutfy MR, DeSheng Su, et al. The magnitude of virologic blips is associated with a higher risk for virologic rebound in HIV-infected individuals: a recurrent events analysis. Journal Infect Dis. 2012;205(8):1230–8. doi: 10.1093/infdis/jis104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miller LG, Golin CE, Liu H, Hays RD, Hua J, Wenger NS, Kaplan AH. No evidence of an association between transient HIV viremia (blips) and lower adherence to the antiretroviral medication regimen. Journal Infect Dis. 2004;189:1487–96. doi: 10.1086/382895. [DOI] [PubMed] [Google Scholar]
- 6.Stosor V, Palella FJ, Berzins B, et al. Transient viremia in HIV infected patients and use of plasma preparation tubes. Clin Infect Dis. 2005;41:1671–4. doi: 10.1086/498025. [DOI] [PubMed] [Google Scholar]
- 7.Doyle T, Smith C, Vitiello P, et al. Plasma HIV-1 RNA detection below 50 copies/mL and risk of virologic rebound in patients receiving highly active antiretroviral therapy. Clin Infect Dis. 2012;54(5):729–37. doi: 10.1093/cid/cir936. [DOI] [PubMed] [Google Scholar]
- 8.Easterbrook PJ, Ives N, Waters A, et al. The natural history and clinical significance of intermittent viraemia in patients with initial viral suppression to < 400 copies/ml. AIDS. 2002;16:1521–7. doi: 10.1097/00002030-200207260-00009. [DOI] [PubMed] [Google Scholar]
- 9.Maggiolo F, Callegaro A, Cologni G, et al. Ultrasensitive assessment of residual low-level HIV viremia in HAART-treated patients and risk of virological failure. J Acquir Immune Defic Syndr. 2012;60(5):473–82. doi: 10.1097/QAI.0b013e3182567a57. [DOI] [PubMed] [Google Scholar]
- 10.Li JZ, Gallien S, Do TD, et al. Prevalence and significance of HIV-1 drug resistance mutations among patients on antiretroviral therapy with detectable low-level viremia. Antimicrob Agents Chemother. 2012;56(11):5998–6000. doi: 10.1128/AAC.01217-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cohen Stuart JWT, Hazebergh MD, Hamman D, et al. The dominant source of CD4+ and CD8+ T-cell activation in HIV infection is antigenic stimulation. J Acquire Immune Defic Syndr. 2000;25:203–11. doi: 10.1097/00126334-200011010-00001. [DOI] [PubMed] [Google Scholar]
- 12.Plaeger SF, Collins BS, Musib R, Deeks SG, Read S, Embry A. Immune activation in the pathogenesis of treated chronic HIV disease: a workshop summary. AIDS Res Hum Retroviruses. 2012;28(5):469–77. doi: 10.1089/aid.2011.0213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sandler NG, Douek DC. Microbial translocation in HIV infection: causes, consequences and treatment opportunities. Nat Rev Microbiol. 2012;10(9):655–66. doi: 10.1038/nrmicro2848. [DOI] [PubMed] [Google Scholar]
- 14.French MA, King MS, Tschampa JM, da Silva BA, Landay AL. Serum immune activation markers are persistently increased in patients with HIV infection after 6 years of antiretroviral therapy despite suppression of viral replication and reconstitution of CD4+ T-cells. J Infect Dis. 2009;200:1212–5. doi: 10.1086/605890. [DOI] [PubMed] [Google Scholar]
- 15.Hunt PW, Martin JN, Sinclair E, et al. T-cell activation is associated with lower CD4+ T-cell gains in human immunodeficiency virus-infected patients with sustained viral suppression during antiretroviral therapy. J infect Dis. 2003;187(10):1534–43. doi: 10.1086/374786. [DOI] [PubMed] [Google Scholar]
- 16.Robbins GK, Spritzler JG, Chan ES, et al. Incomplete reconstitution of T-cell subsets on combination antiretroviral therapy in the AIDS Clinical Trials Group protocol 384. Clin Infect Dis. 2009;48(3):350–61. doi: 10.1086/595888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gandhi RT, Spritzler J, Chan E, et al. Effect of baseline- and treatment-related factors on immunologic recovery after initiation of antiretroviral therapy in HIV-1–positive subjects: results from ACTG 384. J Acquir Immune Defic Syndr. 2006;42:426–34. doi: 10.1097/01.qai.0000226789.51992.3f. [DOI] [PubMed] [Google Scholar]
- 18.Robbins GK, De Gruttola V, Shafer RW, et al. Comparison of sequential three-drug regimens as initial therapy for HIV-1 infection. N Engl J Med. 2003;349(24):2304–15. doi: 10.1056/NEJMoa030265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shafer RW, Smeaton LM, Robbins GK, et al. Comparisons of four-drug regimens and pairs of sequential three-drug regimens as initial therapy for HIV-1 infection. N Engl J Med. 2003;349(24):2304–15. doi: 10.1056/NEJMoa030265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smurzynski M, Collier AC, Koletar SL, et al. AIDS clinical trials group longitudinal linked randomized trials (ALLRT): rationale, design, and baseline characteristics. HIV Clin Trials. 2008;9(4):269–82. doi: 10.1310/hct0904-269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Flynn PM, Rudy BJ, Douglas SD, et al. Virologic and immunologic outcomes after 24 weeks in HIV type-1 infected adolescents receiving highly active antiretroviral therapy. J Infect Dis. 2004;190(2):271–9. doi: 10.1086/421521. [DOI] [PubMed] [Google Scholar]
- 22.Karlsson AC, Younger SR, Martin JN, et al. Immunologic and virologic evolution during periods of intermittent and persistent low-level viremia. AIDS. 2004;18:981–9. doi: 10.1097/00002030-200404300-00005. [DOI] [PubMed] [Google Scholar]
- 23.Castro P, Plana M, Gonzalez R, et al. Influence of episodes of intermittent viremia)blips) on immune responses and viral load rebound in successfully treated HIV-infected patients. AIDS Research and Hum Retroviruses. 2013;29(1):68–76. doi: 10.1089/aid.2012.0145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ramratnam B, Mittler JE, Zhang L, et al. The decay of the latent reservoir of replication-competent HIV-1 is inversely correlated with the extent of residual viral replication during prolonged anti-retroviral therapy. Nat Med. 2000;6(1):82–5. doi: 10.1038/71577. [DOI] [PubMed] [Google Scholar]
- 25.Chun T, Murray D, Justement JS, Hallahan CW, Moir S, Kovacs C, Fauci AS. Relationship between residual plasma viremia and the size of HIV proviral DNA reservoirs in infected individuals receiving effective antiretroviral therapy. J Infect Dis. 2011;204:135–8. doi: 10.1093/infdis/jir208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jones LE, Parelson AS. Transient viremia, plasma viral load and reservoir replenishment in HIV-infected patients on antiretroviral therapy. J Acquir Immune Defic Syndr. 2007;45(5):483–93. doi: 10.1097/QAI.0b013e3180654836. [DOI] [PMC free article] [PubMed] [Google Scholar]