Abstract
PURPOSE
To determine patterns of diffusion of diagnostic tests and therapeutic interventions in the United States through 2010 for patients with newly diagnosed exudative macular degeneration (AMD).
DESIGN
Retrospective longitudinal cohort analysis.
METHODS
SETTING AND PATIENT POPULATION
A total of 23 941 Medicare beneficiaries with exudative AMD newly diagnosed during 1992–2009.
OBSERVATION PROCEDURES
Current Procedural Technology (CPT-4) billing codes were used to identify use of diagnostic tests (optical coherence tomography, fluorescein angiography, and fundus photography) and therapeutic interventions (argon laser photocoagulation, photodynamic therapy, intravitreal corticosteroids, and anti–vascular endothelial growth factor [VEGF] agents) used by these beneficiaries during the first year following diagnosis.
MAIN OUTCOME MEASURES
Rates of use of study diagnostic and therapeutic procedures.
RESULTS
Diffusion was rapid for each successive new diagnostic and treatment modality, with use of newer procedures quickly replacing existing ones. The number of beneficiaries treated with anti-VEGF agents for exudative AMD was considerably greater than for prior innovations, rising from use in 4.0% of beneficiaries in 2004–05 to 62.7% in 2009–10. In each year from first diagnosis years 2006–2009 and in different practice settings, use of bevacizumab exceeded that of ranibizumab (60%-78% vs 33%-47%, respectively). Rates of diffusion of the various therapies were relatively similar in communities throughout the United States irrespective of presence of a major teaching hospital in the vicinity.
CONCLUSIONS
Newer, more effective therapeutic interventions for exudative AMD diffused rapidly throughout the United States, quickly replacing older, less effective interventions. Although improving patient outcomes, rapid diffusion raises important public policy issues for Medicare and other payers to consider.
Age-related macular degeneration (amd) is a common cause of legal blindness among older Americans.1 Exudative AMD is far less prevalent than nonexudative AMD but carries a worse visual prognosis.2 Although the therapeutic options for managing patients with exudative AMD were limited until recently, new therapies for this sight-threatening condition have emerged, improving the prognosis for patients with this condition.
Beginning in the 1980s, argon laser photocoagulation therapy was the most common, and essentially only, treatment option for exudative AMD.3–5 This treatment, although effective for extrafoveal lesions, was associated with iatrogenic vision loss in certain patients.6,7 With the approval of photodynamic therapy (PDT) by the US Food and Drug Administration (FDA) in 2000,8 ophthalmologists had a safer option for treating subfoveal choroidal neovascularization with reduced risk of vision loss from macular scarring; yet the cost-effectiveness of PDT was soon found to be marginal at best.9 In the mid-2000s, intravitreal corticosteroids, which can inhibit progression of choroidal neovascularization,10,11 emerged as another treatment alternative; however, the treatment’s side effects included development or worsening of cataract and glaucoma, and its effectiveness was questionable.12,13 Most recently, use of vascular endothelial growth factor (VEGF) inhibitors (anti-VEGFs)—typically involving intravitreal injection in an office setting under local anesthesia—became a viable treatment alternative for exudative AMD. The first of these agents, pegaptanib (Macugen), which reduces visual acuity loss from exudative AMD but produces limited improvement in best-corrected visual acuity (BCVA),14,15 received FDA approval in December 2004, followed by ranibizumab (Lucentis) in June 2006. Around the same time, bevacizumab (Avastin), an anti-VEGF with an effectiveness similar to ranibizumab but at much lower cost,16 became a popular, although off-label, treatment option. Approximately one-third of patients receiving ranibizumab or bevacizumab recover significant BCVA,17–19 and ranibizumab therapy is generally more effective than PDT alone.15 Serious adverse events associated with anti-VEGF agents, although rare, include endophthalmitis, retinal detachment, and intraocular hemorrhage.15,20 Furthermore, frequent reinjections are commonly required.
These newer technologies, although not cures for the disease, do improve the clinical course for many patients, yet at a substantial cost to Medicare.21 This relatively high expense of the newer therapies during a time of overall budgetary constraints raises 2 issues: (1) Should the effectiveness of new therapeutic products be tested in the general population, instead of trials involving a limited population, before coverage is approved by insurance in general and Medicare in particular? (2) To what extent should coverage be limited to those therapies with demonstrated superiority in cost-effectiveness comparisons? To date, the policy choice has been to cover many promising technologies before outcomes and complications are well documented. Coverage of technologies for treating exudative AMD is a case in point.
While it is widely appreciated that there have been technological advances in diagnostic testing and therapeutic interventions for exudative AMD in recent years, what is not yet well documented is how diffusion of new technology has affected use of older technologies. The primary aim of this analysis is to quantify changes in utilization of these different technologies over time among Medicare beneficiaries with newly diagnosed exudative AMD. This study also seeks to better understand whether rates of adoption of technology vary by location relative to academic health centers.
METHODS
The duke university institutional review board approved this study prospectively. The IRB approved this study as a secondary analysis of data collected by someone else.
We used a 5% sample of the Medicare claims with dates of service from 1991 to 2010 to identify a national random sample of Medicare beneficiaries whose first diagnosis of exudative AMD (International Classification of Diseases, Ninth Revision [ICD-9-CM] code 362.52) appeared in these claims during 1992–2009 (N = 106,481). Beneficiaries with a diagnosis of diabetes mellitus (ICD-9-CM 250.xx), vein occlusions (ICD-9-CM 362.3x), cystoid macular edema (ICD-9-CM 362.53), histoplasmosis retinitis unspecified (ICD-9-CM 115.92), chorioretinitis due to toxoplasmosis (ICD-9-CM 130.2), progressive high myopia (ICD-9-CM 360.21), retinal neovascularization not otherwise specified (ICD-9-CM 362.16), retinal edema (ICD-9-CM 362.83), angioid streaks of choroid (ICD-9-CM 363.43), rubeosis iridis (ICD-9-CM 364.42), and glaucoma associated with vascular disorders (ICD-9-CM 365.63) were excluded (N = 62,561) to reduce the possibility of misattributing the use of diagnostic and therapeutic interventions for other retinal conditions besides exudative AMD. To ensure that the first diagnosis of exudative AMD in the claims was the beneficiaries’ first actual diagnosis, beneficiaries with diagnoses in 1991 were excluded (N = 2057). We also excluded beneficiaries who enrolled in a Medicare Advantage HMO (N = 4150), moved outside the United States (N = 131), died (N = 3410), or ceased to be listed in the Medicare enrollment file for another reason (N = 4121) within 1 year of the first exudative AMD diagnosis. Finally, we excluded beneficiaries who did not have a second diagnosis of exudative AMD in the 6 months following the first diagnosis (N = 6110), yielding a total sample of 23 941 enrollees with newly diagnosed exudative AMD. The Duke University Institutional Review Board determined that studies based on these data are exempt from requiring approval since the data are de-identified to the investigators.
We examined diffusion patterns of 4 therapeutic interventions for exudative AMD: argon laser photocoagulation (Current Procedure Terminology [CPT]-4 codes 67210 or 67220); PDT (CPT-4 code 67221 or in the year 2000, code 67299); corticosteroids (CPT-4 codes J1870, J1880, J3300, or J3301 administered by intravitreal injection CPT-4 code 67028 on the same date); and intravitreal anti-VEGF agent injections (CPT-4 codes J2503, J3490, J3590, J9035, Q2024, C9233, or C9399 administered by intravitreal injection CPT-4 code 67028). We required each claim for one of these therapies to also list a diagnosis code of exudative AMD. We measured diffusion in terms of (1) percent of sample persons receiving treatment from the date of first diagnosis of exudative AMD through the following calendar year; and (2) percent of sample persons receiving particular therapeutic procedures in calendar year 2010 by year of first claim with an exudative AMD diagnosis: 2009, 2008, 2007, and 2006.
To assess relationships between use of diagnostic technologies, number of beneficiaries diagnosed with exudative AMD, and use of therapeutic technologies, we examined trends in the use of 3 diagnostic technologies within 7 days of the first exudative AMD diagnosis (including the date of first diagnosis): optical coherence tomography (OCT; CPT-4 code 92135); intravenous fluorescein angiography (IVFA; CPT-4 codes 92230 or 92235), and fundus photography (CPT-4 code 92250). We excluded beneficiaries with any glaucoma diagnosis (ICD-9-CM 365.xx) in our analysis of trends in the use of diagnostic tests since some of these tests could be used to also evaluate glaucoma.
Many technologies are first adopted by major teaching hospitals and later diffuse to the community providers. We sought to ascertain whether this pattern also applies to therapies for exudative AMD. To determine relationships between trends in the fraction of beneficiaries who received each of the 4 therapies after first being diagnosed with exudative AMD and proximity to a major teaching hospital, we stratified beneficiaries into 3 groups: (1) those who lived in a county with a major teaching hospital; (2) those who lived in the Hospital Referral Region containing a major teaching hospital but in a county other than where the teaching hospital was located22; and (3) those living in Hospital Referral Regions containing no major teaching hospitals.23 Locations where care was received were based on zip codes of the place of residence of each Medicare beneficiary. Hospital Referral Regions are designed to be health care markets for tertiary medical care.23 Tertiary care facilities typically offer a full range of specialty and subspecialty services and have residency programs in these fields. This geographic unit is widely used in health services research and health policy. There are 306 Hospital Referral Regions in the United States.23
We defined “major teaching hospital” as follows. First, we used addresses of members of the American Association of Medical Colleges (AAMC) as of 201024 to designate counties with and without AAMC members. Second, we deleted AAMC members that did not have an accredited ophthalmology residency training program.25 Of the 103 Hospital Referral Regions with AAMC members, 27 did not have an accredited ophthalmology residency program, leaving 76 Hospital Referral Regions with a major teaching hospital as we defined it. The remaining 230 Hospital Referral Regions did not have a major teaching hospital.
Comparisons of the proportion of enrollees who received the different technologies from one year to the next were performed using t tests, as were t tests performed to determine whether differences exist in the use of the different diagnostic tests and therapies among enrollees who received care in practices located in each of the 3 settings. Since the use of t tests was only for assessing whether or not there is a statistical difference between a proportion receiving a diagnostic test or intervention in one year from the proportion for the next year and not to assess for causal relations among variables, there was no need to adjust for multiple comparisons.
RESULTS
TRENDS IN NUMBER OF NEW DIAGNOSES OF EXUDATIVE MACULAR DEGENERATION
The number of beneficiaries with newly diagnosed exudative AMD in the Medicare 5% sample in a given year rose from 3641 in the baseline year, 1992, to 7490 in the year 2009, a 106% increase (Figure). The peak number of newly diagnosed beneficiaries with exudative AMD was in the year 2006 (N = 10 473 from 5% sample of claims), about a year after the introduction of anti-VEGF therapy. After exclusions, the analysis sample varied from 908 in 1992 to 2338 in 2009. On average, newly diagnosed beneficiaries in our analysis sample were 79.5 years old (standard deviation: 7.8); 30.1% were male; 96.5% were white, 1.1% African American, 0.6% Asian, 1.3% other race, and 0.5% Hispanic ethnicity. The demographic characteristics of persons in the study sample remained relatively constant over time (data not shown). All of the study therapeutic procedures were more likely to be administered soon after diagnosis than subsequently. Nearly forty-seven percent (46.9%) of beneficiaries who were newly diagnosed with exudative AMD in 2009 received anti-VEGF therapies in 2010, whereas only 36.8% of those newly diagnosed in 2008 and only 32.8% in 2007 received any anti-VEGF treatment in 2010.
FIGURE.
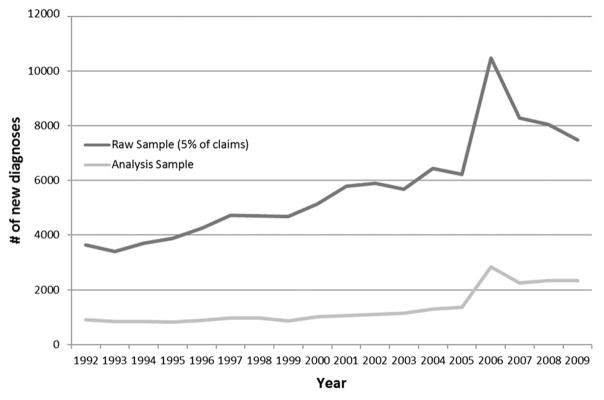
First diagnoses of exudative age-related macular degeneration by year.
DIFFUSION OF ANCILLARY TESTS TO DIAGNOSE EXUDATIVE MACULAR DEGENERATION
In 1992 (before the existence of OCT) IVFA and fundus photography were performed on the day of or just prior to the date of diagnosis for 53.0% and 43.9% of new cases of exudative AMD, respectively (Table 1). By 2002, the proportions of newly diagnosed exudative AMD patients receiving IVFA and fundus photography were 62.5% and 55.1%, respectively, and only 0.7% of these patients underwent OCT testing. From 2002 to 2008 there was a rapid diffusion of OCT utilization and by 2009, 51.6% of those with incident exudative AMD underwent OCT testing. During the period of rapid diffusion of OCT, proportions of individuals with newly diagnosed exudative AMD who underwent IVFA declined somewhat. For this analysis, use of the 3 diagnostic technologies was not mutually exclusive; thus, some patients may have undergone more than 1 of these tests at the time of diagnosis.
TABLE 1.
Beneficiaries With Exudative Age-Related Macular Degeneration Receiving a Testa at Time of First Diagnosis, by Test Type (%)
| Year of First Diagnosis | Intravenous Fluorescein Angiography | Fundus Photography | Optical Coherence Tomography |
|---|---|---|---|
| 1992 | 53.0 | 43.9 | 0 |
| 1993 | 54.8 | 47.8 | 0 |
| 1994 | 56.1 | 51.0 | 0 |
| 1995 | 58.0 | 47.7 | 0 |
| 1996 | 55.7 | 51.1 | 0 |
| 1997 | 56.0 | 51.9 | 0 |
| 1998 | 57.7 | 54.6 | 0 |
| 1999 | 64.7b | 58.1 | 0 |
| 2000 | 63.4 | 55.0 | 0 |
| 2001 | 63.1 | 54.4 | 0 |
| 2002 | 62.5 | 55.1 | 0.7b |
| 2003 | 67.9b | 62.6c | 2.7c |
| 2004 | 63.2b | 58.5 | 7.2c |
| 2005 | 65.5 | 54.4 | 15.1c |
| 2006 | 52.8c | 45.2c | 24.9c |
| 2007 | 55.7 | 40.9c | 39.7c |
| 2008 | 52.0b | 37.7 | 44.2c |
| 2009 | 48.5b | 32.1c | 51.6c |
Tests conducted within a week of date of first diagnosis.
Compared with previous year (t test); P < .05.
Compared with previous year (t test); P < .01.
DIFFUSION OF THERAPIES FOR EXUDATIVE MACULAR DEGENERATION
Argon laser photocoagulation was already a mature technology at baseline. The proportion of beneficiaries with newly diagnosed exudative AMD receiving argon laser photocoagulation peaked in 1994 and 1999 at 22.2% (Table 2). Proportions of patients undergoing argon laser photocoagulation remained stable from 1992 to 2000 but soon thereafter rapidly declined to a level of only 2.4% by the year 2009.
TABLE 2.
Beneficiaries With Exudative Age-Related Macular Degeneration Receiving Therapy Within 1 Year of First Diagnosis, by Therapy Type (%)
| Year of First Diagnosis | Argon Laser Photocoagulation Therapy | Photodynamic Therapy | Steroids | Anti-VEGF |
|---|---|---|---|---|
| 1992 | 17.6 | 0 | 0 | 0 |
| 1993 | 20.0 | 0 | 0 | 0 |
| 1994 | 22.2 | 0 | 0 | 0 |
| 1995 | 20.7 | 0 | 0 | 0 |
| 1996 | 18.1 | 0 | 0 | 0 |
| 1997 | 17.9 | 0 | 0 | 0 |
| 1998 | 18.1 | 0 | 0 | 0 |
| 1999 | 22.2a | 1.4b | 0 | 0 |
| 2000 | 21.0 | 16.2b | 0 | 0 |
| 2001 | 11.2b | 25.9b | 0.1 | 0 |
| 2002 | 11.7 | 28.1 | 0.8a | 0 |
| 2003 | 11.0 | 29.6 | 3.0b | 0 |
| 2004 | 9.5 | 37.5b | 7.7b | 4.0b |
| 2005 | 7.7 | 29.5b | 8.0 | 26.0b |
| 2006 | 3.9b | 10.6b | 3.1b | 47.1b |
| 2007 | 2.8a | 5.7b | 1.2b | 58.4b |
| 2008 | 1.8a | 4.8 | 0.7 | 60.3 |
| 2009 | 2.4 | 2.7b | 1.0 | 62.7 |
VEGF = vascular endothelial growth factor.
Compared with previous year (t test); P < .05.
Compared with previous year (t test); P < .01.
First introduced into the mainstream in 2000, PDT diffused rapidly, with the proportion of patients with newly diagnosed exudative AMD undergoing PDT increasing 26-fold, from 1.4% in 1999 (patients who received their diagnosis in 1999) to 37.5% in 2004. From 2005 to 2009, there was a rapid decline in PDT use, down to 2.7%. Use of intravitreal corticosteroids began in 2001. The proportion of patients with newly diagnosed exudative AMD who received intravitreal corticosteroids was low (0.1%), increased to 8.0% in 2005, and then declined to only 1.0% in 2009.
Anti-VEGF therapies were introduced in 2004. That year, 4.0% of newly diagnosed patients with exudative AMD received these agents. By 2005, the proportion undergoing anti-VEGF injections increased 6-fold from the prior year, to 26.0%. The rapid diffusion of this therapy continued from 2005 to 2009, more than doubling to 62.7%. In 2009, use of anti-VEGFs was 10 times greater than all 3 of the other interventions combined for exudative AMD.
PROXIMITY TO A MAJOR TEACHING HOSPITAL AND DIFFUSION
Both increases and decreases in use of different therapeutic procedures were quite similar irrespective of whether or not the beneficiary with exudative AMD lived in a county with a major teaching hospital, in a Hospital Referral Region with a major teaching hospital but not in the county in which the practice was located, or in a Hospital Referral Region without a major teaching hospital (Table 3). There were higher utilization levels of anti-VEGFs each year for patients receiving care in practices located away from major teaching hospitals relative to those residing in communities close to major teaching facilities in 2005, 2006, 2008, and 2009.
TABLE 3.
Beneficiaries With Exudative Age-Related Macular Degeneration Receiving Therapy Within 1 Year of First Diagnosis, by Proximity to a Major Teaching Hospital by Therapy Type (%)
| Year of First Diagnosis | Argon Laser Photocoagulation Therapy
|
Photodynamic Therapy
|
Steroids
|
Anti-VEGF
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MTH County | MTH HRR | Other HRR | MTH County | MTH HRR | Other HRR | MTH County | MTH HRR | Other HRR | MTH County | MTH HRR | Other HRR | |
| 1992 | 19.3 | 16.3 | 17.6 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 1993 | 16.7 | 18.5 | 22.5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 1994 | 15.8b | 19.7 | 25.9 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 1995 | 19.3 | 18.8 | 22.5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 1996 | 18.2 | 14.9 | 20.0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 1997 | 17.8 | 18.4 | 17.6 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 1998 | 14.4 | 18.1 | 19.4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 1999 | 15.0b | 24.4 | 23.9 | 1.7 | 0.8 | 1.6 | 0 | 0 | 0 | 0 | 0 | 0 |
| 2000 | 22.0 | 20.7 | 20.8 | 12.9 | 17.3 | 16.6 | 0 | 0 | 0 | 0 | 0 | 0 |
| 2001 | 11.3 | 12.1 | 10.6 | 26.8 | 22.5 | 27.4 | 0 | 0 | 0.2 | 0 | 0 | 0 |
| 2002 | 7.9a | 10.5 | 13.7 | 29.6 | 24.0 | 29.6 | 0.5 | 1.0 | 0.9 | 0 | 0 | 0 |
| 2003 | 6.4a | 12.7 | 11.7 | 26.5 | 30.9 | 30.1 | 2.7 | 1.2b | 4.0 | 0 | 0 | 0 |
| 2004 | 8.0 | 9.1 | 10.2 | 31.9 | 38.9 | 38.7 | 6.7 | 8.3 | 7.8 | 3.8 | 2.1b | 5.1 |
| 2005 | 5.2 | 9.4 | 7.8 | 26.5 | 31.9 | 29.5 | 6.2 | 8.2 | 8.7 | 22.0a | 25.0 | 28.3 |
| 2006 | 2.5b | 2.8b | 5.1 | 11.5 | 10.3 | 10.4 | 3.5 | 2.0a | 3.6 | 40.2b | 45.0b | 50.9 |
| 2007 | 2.4 | 2.7 | 3.0 | 5.2 | 5.5 | 5.9 | 1.5 | 0.9 | 1.3 | 55.3 | 58.2 | 59.8 |
| 2008 | 0.9a | 2.1 | 2.1 | 3.7a | 3.5b | 6.1 | 0.6 | 0.6 | 0.8 | 55.6a | 60.8 | 61.9 |
| 2009 | 3.1 | 2.6 | 2.1 | 2.9 | 2.2 | 3.1 | 1.0 | 0.5 | 1.4 | 55.6b | 62.6 | 65.3 |
HRR = Hospital Referral Region; MTH = major teaching hospital; VEGF = vascular endothelial growth factor.
Area compared with Other HRR (t test); P < .05.
Area compared with Other HRR (t test); P < .01.
Likewise, increases and decreases in use of specific anti-VEGF agents were also similar by proximity to major teaching hospitals (Table 4). Among the 3 anti-VEGF agents, bevacizumab dominated the other 2 agents in all 3 practice locations during 2006–2009. Pegaptanib had high use rates in 2005 but decreased considerably by 2006 and was rarely used in 2007, in all 3 practice areas. By 2009, 70.3% or higher of newly diagnosed patients with exudative AMD that were treated with anti-VEGF injections, received bevacizumab in all 3 practice areas.
TABLE 4.
Beneficiaries With Exudative Age-Related Macular Degeneration Receiving Bevacizumab, Ranibizumab, or Pegaptanib Within 1 Year of First Diagnosis Conditional on Receipt of Anti-VEGF Therapy, by Proximity to a Major Teaching Hospital (%)
| Year of First Diagnosis | Bevacizumab
|
Ranibizumab
|
Pegaptanib
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| MTH County | MTH HRR | Other HRR | MTH County | MTH HRR | Other HRR | MTH County | MTH HRR | Other HRR | |
| 2005 | 39.1 | 45.9 | 38.5 | 10.9 | 17.3 | 13.3 | 68.8 | 62.2a | 73.8 |
| 2006 | 60.8 | 66.0 | 61.1 | 42.7 | 43.8 | 43.1 | 22.0 | 16.0a | 22.3 |
| 2007 | 69.7 | 70.1 | 65.2 | 41.6 | 41.8 | 46.5 | 1.2 | 2.2 | 1.7 |
| 2008 | 72.9 | 78.3a | 72.6 | 39.1 | 33.1 | 36.7 | 0.4 | 0.9 | 0.3 |
| 2009 | 70.3 | 73.1 | 73.4 | 41.4 | 38.8 | 39.0 | 0.9 | 0.4 | 1.3 |
HRR = Hospital Referral Region; MTH = major teaching hospital; VEGF = vascular endothelial growth factor.
Area compared with Other HRR (t test); P < .01.
Area compared with Other HRR (t test); P < .05.
DISCUSSION
There has been substantial innovation in diagnosis and treatment of exudative AMD during the past 2 decades. Diffusion has been rapid for each successive new treatment modality, with utilization of newer treatments quickly replacing existing treatment options. The number of individuals treated with anti-VEGF agents for exudative AMD has been considerably greater than prior innovations. Both FDA-approved ranibizumab and the off-label agent bevacizumab have been highly utilized. Rates of diffusion occurred in communities throughout the United States irrespective of presence of a major teaching hospital in the vicinity.
A key factor driving introduction of new technologies is market size, which reflects patient and provider demand for such technologies.26 In the case of exudative AMD treatments, in the past, many newly diagnosed patients were told that, given the natural history of the disease, severe visual impairment would result. The treatments only tended to slow down the visual decline at best, or create an immediate iatrogenic scotoma at worst. Compared with newer interventions like anti-VEGF injections, the limited number of patients eligible for earlier treatments may have influenced whether some ophthalmologists chose to invest in these technologies and incorporate them into their practices. Given the high incidence of newly diagnosed patients and the limited effectiveness of therapeutic options, demand for more effective therapies continued, as evidenced by the rapid diffusion of each new technology into the marketplace. If providers and patients had been satisfied with the performance of existing technologies—whether it was argon laser photocoagulation, PDT, or intravitreal corticosteroids—we would have expected to have observed a different pattern of diffusion: that is, more gradual increased use as providers would have initially been skeptical to try the new treatment modality and only over time, having observed success in a limited number of their own patients and patients of other providers, become sufficiently convinced to integrate the newer technology into their practices. Dissatisfaction with outcomes of older therapeutic options accounts for the rapid rates of decline in use of the older therapeutic options we are observing.
Adoption of a new technology, in part, depends on fixed costs, which must be incurred prior to adoption, such as for training in the new techniques and investments in new equipment. Holding other factors constant, higher fixed costs impede technology adoption. Fee-for-service payment does not explicitly cover such fixed costs. Rather, total payment depends on units of service provided and payment per unit. Higher payment per unit may encourage adoption of technologies with high fixed cost, but to the extent unit payment exceeds marginal cost, it may lead to overprovision of services and decreased rates of decline in use. Since patients would typically receive only 1 of the therapies at a time—that is, the therapies are substitutes—for there to be an adequate financial incentive to perform a particular therapy, not only must payment cover the marginal cost of the therapy, but the profit margins of each of the substitute therapies are also important. For example, even if PDT has a positive profit margin, if the margin for anti-VEGF therapy is higher, there is a financial incentive to substitute anti-VEGF for PDT.
Use of the older therapeutic technologies, some of which involve investments in equipment, fell dramatically with the introduction of the anti-VEGF therapies. The fixed cost of providing anti-VEGF, such as bevacizumab, is low, although the cost of keeping a supply of ranibizumab has been a concern for smaller practices. The low fixed costs of anti-VEGF therapies probably contributed to their rapid growth relative to the pattern observed for PDT after it was initially introduced.
Our study shows that as of 2009, ophthalmologists treating Medicare enrollees with exudative AMD were using anti-VEGF agents more often than the other treatment options. In addition to the impact of studies that demonstrate that anti-VEGF agents are more effective than alternative treatment modalities on practice patterns,27 there are additional incentives to providers that may influence their choice of therapy. For ranibizumab, there is a Medicare inventory fee benefit, financial benefits through Genentech for volume purchases, and further rebates when the drugs are purchased with a credit card. Among the anti-VEGF agents, we found each year that the more expensive anti-VEGF agent, ranibizumab, was used less often than the cheaper alternative, bevacizumab. This may be attributable to several factors. The out-of-pocket cost for a Medicare patient who does not have supplemental insurance for ranibizumab is approximately $400 per treatment, but for bevacizumab it is only approximately $8. Considering that many patients require multiple injections, the difference in costs that patients must pay for these interventions can be substantial, and that may affect the decision of which agent to use. Also, the cost to purchase and store large quantities of ranibizumab is considerably greater than that of bevicizumab, and this may influence which agent some providers opt to use.
Although one might expect that a very high proportion of enrollees with newly diagnosed exudative AMD would receive anti-VEGF agents, there are several reasons why only approximately two-thirds of those who received a diagnosis of exudative AMD received these agents. Potential reasons why beneficiaries may not have received these agents include a lack of access to retina specialists or ophthalmologists who are comfortable administering these repeated injections, a lack of knowledge by some eye care providers about the benefits of treating exudative AMD with these agents as compared with some of the more traditional prior approaches, and determination by the provider that the visual acuity from exudative AMD is at end stage. Patient refusal of treatment or opting not to follow up with a retina specialist after receiving the diagnosis by a nonspecialist who does not perform these injections would also affect utilization rates.
One pattern of technological diffusion originates at academic medical centers and, over time, gradually diffuses to surrounding communities. This pattern is not observed for new therapies for exudative AMD, possibly because providers could quickly learn how to perform interventions such as PDT and anti-VEGF injections from attending conferences and interacting with colleagues. Also, a combination of lack of effective alternatives, pressure from patients who were desperate to try something that might effectively reduce the loss in BCVA, and Medicare coverage for the new technologies may have accelerated adoption in areas more distant from major teaching hospitals. Examples of other technological innovations in the field of ophthalmology that were first developed in the private practice setting include phacoemulsification, keratorefractive surgery, and surgery for macular holes.
Although not the main focus of the paper, an interesting finding from our analysis was the large rise over time in the number of enrollees each year who were diagnosed with exudative AMD. We suspect that this rise in number of enrollees diagnosed with exudative AMD is likely the result of technological advances in ocular imaging devices such as OCT, which made it easier for clinicians to detect abnormalities in the macula relative to earlier years, when providers relied much more upon clinical examination and IVFA to diagnose this condition. The majority of these patients were receiving care from non–retina specialists (comprehensive ophthalmologists or optometrists) at the time they were first diagnosed with exudative AMD, and technological advances such as OCT that became commercially available more recently likely substantially aided nonspecialists in diagnosing this condition. Also, the increased availability of more effective interventions such as anti-VEGF agents that could restore vision for persons with exudative AMD may have offered providers a greater incentive to diagnose and treat these patients in the latter years relative to the earlier years, when treatments were less successful.
There are a variety of potential explanations as to why utilization of fluorescein angiography and fundus photography rose during the pre-OCT era (1992–2002). This may be attributable, in part, to changes in provider practice patterns, for example, as older ophthalmologists and optometrists leave the workforce and are replaced with younger clinicians who are more accustomed to using these ancillary tests in practice. Second, in the early 1990s, equipment for fluorescein angiography and fundus photography may have been expensive, and fewer providers may have had access to the equipment. Although we have no access to transaction prices for large medical equipment, which are usually kept secret between manufacturers and purchasers, such prices typically decrease as a technology diffuses,27 in the health field often as a discount and incentive to purchase.28 Assuming this phenomenon also occurred with this study’s imaging technologies, as equipment prices decreased, more providers may have had access to the equipment to be able to perform these tests. Finally, changes in utilization of these tests may be partly driven by financial factors. Providers have incentives to order more tests to first recoup the costs of the purchase of the equipment and then to make financial gains.
There are several implications for Medicare payment policy. A major issue concerns whether payers (and employers) should be more proactive in sponsoring rigorous evaluations of comparative effectiveness, particularly when the price differential is as great as it has been for the different anti-VEGF therapies. A second issue concerns the high rate of decline in use of older therapies for exudative AMD. Do high rates of decline in use indicate previous waste of public funds? Should Medicare have waited for more conclusive evidence on effectiveness before covering some of these newer procedures? On the one hand, imposing more regulatory hurdles would impede the rate of innovation, which could be beneficial or detrimental to patients. Reductions in use following replacement by a superior technology are common in other sectors, such as telegraph by telephone and passenger railroad by alternative forms of passenger transport.29 However, the purchases are not typically covered by insurance. For a public program, such as Medicare, society must be willing to pay for false starts with the introduction of newer technology, with the benefit being more rapid development of new technologies.
Strengths of this analysis include use of a nationally representative sample of Medicare beneficiaries to capture trends in the use of diagnostic and therapeutic procedures over a time period spanning nearly 2 decades. Unlike studies based on a few sites of care, our analysis represents care as delivered in many types of settings and geographic areas.
We also acknowledge several limitations. First, we excluded beneficiaries in Medicare Advantage plans and persons receiving care in some sites, such as Veterans Affairs medical centers. Thus, our findings may not be applicable to these groups. Second, claims data have specific limitations. There are no clinical findings except as expressed in diagnostic codes. There is a risk of miscoding and misdiagnosis. Requiring a confirmatory diagnosis of exudative AMD helped minimize such errors from affecting our findings. Furthermore, the codes do not distinguish extrafoveal pathology from juxtafoveal or subfoveal pathology; and certain interventions, such as argon laser photocoagulation, may not be appropriate for patients with juxtafoveal or subfoveal disease. Third, although we could document changes in procedure volume, we could not document appropriateness of use. We calculated trends in payment per unit of services, but not over- or under-payment for specific procedures. Fourth, while the data imply that diffusion of new technologies was rapid throughout the United States, not only in the proximity of major teaching hospitals, we examined diffusion only over broad geographic categories. A more detailed analysis of diffusion by geographic area may identify specific areas in which the new technologies are not available to Medicare beneficiaries with exudative AMD. Finally, the database did not contain information on which Medicare enrollees had supplemental insurance. Since the out-of-pocket cost for some of these therapies for those who do not have supplemental insurance can be considerable, we wish we could have considered that factor in the analysis.
In sum, during 1992–2009, newer, more effective therapeutic interventions for exudative AMD diffused rapidly throughout the United States, quickly replacing older, less effective interventions. Although promising in terms of improved clinical outcomes, rapid diffusion raises important public policy issues, which Medicare and other third-party payers will have to address.
Acknowledgments
The authors indicate the following funding support and financial disclosures: National Eye Institute K23 Mentored Clinician Scientist Award (J.D.S.;1K23EY019511-01); Blue Cross Blue Shield of Michigan Foundation (J.D.S.); National Institute on Aging (5R01AG017473-11). The funding organizations had no role in the design or conduct of this research.
Biographies

Joshua D. Stein is an Assistant Professor of Ophthalmology and Visual Sciences at the University of Michigan, Ann Arbor, Michigan. He is a health services researcher whose primary research interest involves using large health care claims databases to study utilization patterns and outcomes of eye care throughout the United States.

Frank A. Sloan is the J. Alexander McMahon Professor of Health Policy and Management and Professor of Economics at Duke University Durham, North Carolina, since 1993. Sloan’s primary research interest is health economics. He has studied many facets of medical malpractice, hospitals, physicians’ services, specialty courts, families’ decisions about long-term care, pharmaceuticals, drinking and driving, drug and alcohol use prevention, and cost effectiveness analysis of medical technologies.
Footnotes
ALL AUTHORS HAVE COMPLETED AND SUBMITTED THE ICMJE FORM FOR DISCLOSURE OF POTENTIAL CONFLICTS OF INTEREST. No conflicting relationship exists for any author. Contributions of authors: involved in design and conduct of the study (F.S., J.S., B.H.); collection, management, analysis, and interpretation of the data (F.S., J.S., B.H.); and preparation, review, or approval of the manuscript (F.S., J.S., B.H., G.C.).
References
- 1.Klein R, Wang Q, Klein BEK, Moss SE, Meuer SM. The relationship of age-related maculopathy, cataract, and glaucoma to visual-acuity. Invest Ophthalmol. 1995;36(1):182–191. [PubMed] [Google Scholar]
- 2.Ferris FL, Fine SL, Hyman L. Age-related macular degeneration and blindness due to neovascular maculopathy. Arch Ophthalmol. 1984;102(11):1640–1642. doi: 10.1001/archopht.1984.01040031330019. [DOI] [PubMed] [Google Scholar]
- 3.Macular Photocoagulation Study Group. Argon laser photocoagulation for neovascular maculopathy three-year results from randomized clinical trials. Arch Ophthalmol. 1986;104(5):694–701. [PubMed] [Google Scholar]
- 4.Macular Photocoagulation Study Group. Laser photocoagulation of subfoveal neovascular lesions in age-related macular degeneration: Results of a randomized clinical trial. Arch Ophthalmol. 1991;109(9):1220–1231. doi: 10.1001/archopht.1991.01080090044025. [DOI] [PubMed] [Google Scholar]
- 5.Macular Photocoagulation Study Group. Argon laser photo coagulation for idiopathic neovascularization results of a randomized clinical trial. Arch Ophthalmol. 1983;101(9):1358–1361. doi: 10.1001/archopht.1983.01040020360003. [DOI] [PubMed] [Google Scholar]
- 6.Kaiser PK. Antivascular endothelial growth factor agents and their development: Therapeutic implications in ocular diseases. Am J Ophthalmol. 2006;142(4):660–668. doi: 10.1016/j.ajo.2006.05.061. [DOI] [PubMed] [Google Scholar]
- 7.Giansanti F, Virgili G, Bini A, et al. Intravitreal bevacizumab therapy for choroidal neovascularization secondary to age-related macular degeneration: 6-month results of an open-label uncontrolled clinical study. Eur J Ophthalmol. 2007;17(2):230–237. doi: 10.1177/112067210701700213. [DOI] [PubMed] [Google Scholar]
- 8.Bressler NM. Photodynamic therapy of subfoveal choroidal neovascularization in age-related macular degeneration with verteporfin: Two-year results of two randomized clinical trials—TAP report 2. Arch Ophthalmol. 2001;119(2):198–207. [PubMed] [Google Scholar]
- 9.Sharma S, Brown GC, Brown MM, Hollands H, Shah GK. The cost-effectiveness of photodynamic therapy for fellow eyes with subfoveal choroidal neovascularization secondary to age-related macular degeneration. Ophthalmology. 2001;108(11):2051–2059. doi: 10.1016/s0161-6420(01)00764-3. [DOI] [PubMed] [Google Scholar]
- 10.Becerra EM, Morescalchi F, Gandolfo F, et al. Clinical evidence of intravitreal triamcinolone acetonide in the management of age-related macular degeneration. Curr Drug Targets. 2011;12(2):149–172. doi: 10.2174/138945011794182746. [DOI] [PubMed] [Google Scholar]
- 11.Jonas JB, Kreissig I, Hugger P, Sauder G, Panda-Jonas S, Degenring R. Intravitreal triamcinolone acetonide for exudative age related macular degeneration. Br J Ophthalmol. 2003;87(4):462–468. doi: 10.1136/bjo.87.4.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gillies MC, Larsson J. The effect of intravitreal triamcinolone on foveal edema in exudative macular degeneration. Am J Ophthalmol. 2007;144(1):134–136. doi: 10.1016/j.ajo.2007.03.019. [DOI] [PubMed] [Google Scholar]
- 13.Gillies MC, Simpson JM, Luo W, et al. A randomized clinical trial of a single dose of intravitreal triamcinolone acetonide for neovascular age-related macular degeneration - one year results. Arch Ophthalmol. 2003;121(5):667–673. doi: 10.1001/archopht.121.5.667. [DOI] [PubMed] [Google Scholar]
- 14.Gragoudas ES, Adamis AP, Cunningham ET, Feinsod M, Guyer DR, Neova VISO. Pegaptanib for neovascular age-related macular degeneration. N Engl J Med. 2004;351(27):2805–2816. doi: 10.1056/NEJMoa042760. [DOI] [PubMed] [Google Scholar]
- 15.Heier JS. Ranibizumab combined with verteporfin photodynamic therapy in neovascular age-related macular degeneration. Arch Ophthalmol. 2007;125(1):138. doi: 10.1001/archopht.124.11.1532. [DOI] [PubMed] [Google Scholar]
- 16.Martin DF, Maguire MG, Ying G-S, et al. CATT Research Group. Ranibizumab and bevacizumab for neovascular age-related macular degeneration. N Engl J Med. 2011;364(20):1897–1908. doi: 10.1056/NEJMoa1102673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Avery RL, Pieramici DJ, Rabena MD, Castellarin AA, Nasir MA, Giust MJ. Intravitreal bevacizumab (avastin) for neovascular age-related macular degeneration. Ophthalmology. 2006;113(3):363–372. doi: 10.1016/j.ophtha.2005.11.019. [DOI] [PubMed] [Google Scholar]
- 18.Mantel I, Zografos L, Ambresin A. Early clinical experience with ranibizumab for occult and minimally classic neovascular membranes in age-related macular degeneration. Ophthalmologica. 2008;222(5):321–323. doi: 10.1159/000144075. [DOI] [PubMed] [Google Scholar]
- 19.Rich RM, Rosenfeld PJ, Puliafito CA, et al. Short-term safety and efficacy of intravitreal bevacizumab (avastin) for neovascular agerelated macular degeneration. Retina. 2006;26(5):495–511. doi: 10.1097/01.iae.0000225766.75009.3a. [DOI] [PubMed] [Google Scholar]
- 20.Wu L, Martinez-Castellanos MA, Quiroz-Mercado H, et al. Twelve-month safety of intravitreal injections of bevacizumab (Avastin): Results of the Pan-American Collaborative Retina Study Group (PACORES) Graefes Arch Clin Exp Ophthalmol. 2008;246(1):81–87. doi: 10.1007/s00417-007-0660-z. [DOI] [PubMed] [Google Scholar]
- 21.Day S, Acquah K, Lee PP, Mruthyunjaya P, Sloan FA. Medicare costs for neovascular age-related macular degeneration, 1994–2007. Am J Ophthalmol. 2011;152(6):1014–1020. doi: 10.1016/j.ajo.2011.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wennberg DE, Lucas FL, Birkmeyer JD, Bredenberg CE, Fisher ES. Variation in carotid endarterectomy mortality in the Medicare population - trial hospitals, volume, and patient characteristics. JAMA. 1998;279(16):1278–1281. doi: 10.1001/jama.279.16.1278. [DOI] [PubMed] [Google Scholar]
- 23.The Trustees of Dartmouth College. [Accessed: May 19, 2012.];The Dartmouth atlas of health care: Data by region. 2012 Available at: http://www.dartmouthatlas.org/data/region/
- 24.Association of American Medical Colleges. [Accessed: September 12, 2012.];Medical school member directory. 2010 Available at: https://members.aamc.org/eweb/DynamicPage.aspx?site=AAMC&webcode=AAMCOrgSearchResult&orgtype=Medical%20School.
- 25.Accreditation Council for Graduate Medical Education. [Accessed: September 12, 2012.];Accredited ophthalmology specialty programs. 2012 Available at: http://www.acgme.org/adspublic/
- 26.Finkelstein A. The aggregate effects of health insurance: evidence from the introduction of medicare. Q J Econ. 2007;122(1):1–37. [Google Scholar]
- 27.Bagchi K, Kirs P, Lopez F. The impact of price decreases on telephone and cell phone diffusion. Inf Manage. 2008;45(3):183–193. [Google Scholar]
- 28.Oh EH, Imanaka Y, Evans E. Determinants of the diffusion of computed tomography and magnetic resonance imaging. Int J Technol Assess Health Care. 2005;21(1):73–80. doi: 10.1017/s0266462305050099. [DOI] [PubMed] [Google Scholar]
- 29.Comin D, Hobijn B. An Exploration of Technology Diffusion. Am Econ Rev. 2010;100(5):2031–2059. [Google Scholar]


