Abstract
Nicotinamide adenine dinucleotide (NAD)+, a coenzyme involved in redox activities in the mitochondrial electron transport chain, has been identified as a key regulator of the lifespan-extending effects, and the activation of NAD+ expression has been linked with a decrease in beta-amyloid (Aβ) toxicity in Alzheimer’s disease (AD). Nicotinamide riboside (NR) is a NAD+ precursor, it promotes peroxisome proliferator-activated receptor-γ coactivator 1 (PGC)-1α expression in the brain. Evidence has shown that PGC-1α is a crucial regulator of Aβ generation because it affects β-secretase (BACE1) degradation. In this study we tested the hypothesis that NR treatment in an AD mouse model could attenuate Aβ toxicity through the activation of PGC-1α-mediated BACE1 degradation. Using the Tg2576 AD mouse model, using in vivo behavioral analyses, biochemistry assays, small hairpin RNA (shRNA) gene silencing and electrophysiological recording, we found (1) dietary treatment of Tg2576 mice with 250 mg/kg/day of NR for 3 months significantly attenuates cognitive deterioration in Tg2576 mice and coincides with an increase in the steady-state levels of NAD+ in the cerebral cortex; (2) application of NR to hippocampal slices (10 µM) for 4 hours abolishes the deficits in long-term potentiation recorded in the CA1 region of Tg2576 mice; (3) NR treatment promotes PGC-1α expression in the brain coinciding with enhanced degradation of BACE1 and the reduction of Aβ production in Tg2576 mice. Further in vitro studies confirmed that BACE1 protein content is decreased by NR treatment in primary neuronal cultures derived from Tg2576 embryos, in which BACE1 degradation was prevented by PGC-1α-shRNA gene silencing; and (4) NR treatment and PGC-1α overexpression enhance BACE1 ubiquitination and proteasomal degradation. Our studies suggest that dietary treatment with NR might benefit AD cognitive function and synaptic plasticity, inpart by promoting PGC-1α-mediated BACE1 ubiquitination and degradation, thus preventing Aβ production in the brain.
Keywords: Nicotinamide riboside, Alzheimer’s disease, β-secretase (BACE1), Promotes peroxisome proliferator-activated, receptor (PPAR)-γ coactivator 1 (PGC)-1α, Ubiquitin–proteasome system, Mitochondrial metabolism, Synaptic plasticity, Long-term potentiation
1. Introduction
Nicotinamide adenine dinucleotide (NAD)+ has been identified as a key regulator in the lifespan-extending effects of calorie restriction in a number of species. Numerous studies have suggested that NAD+ mediates multiple major biological processes, including calcium homeostasis, energy metabolism, mitochondrial functions, cell death, and aging in various tissues including brain. Increasing evidence has suggested that NAD+ might play important roles in metabolic processes in the brain, and has effects on brain functioning such as neurotransmission, learning, and memory. Recent studies have shown that the activation of NAD expression has been linked with a decrease in the amyloid toxicity in Alzheimer’s disease (AD) animal models (Kim et al., 2007; Qin et al., 2006), in which it might relate to the interactions with the expression of peroxisome proliferator-activated receptor-γ coactivator 1 (PGC)-1α (Nemoto et al., 2005) and through the activation of neuronal NAD-dependent deacetylase sirtuin-1 (SIRT1) activation (Qin et al., 2006; Rodgers et al., 2005). It has been shown that during metabolic stress conditions such as the fasting state, hypoxia, NAD+ levels, and SIRT1 protein levels are increased, leading to deacetylation of PGC-1α, subsequently increasing the expression of PGC-1α, promoting gluconeogenic transcriptional program (Rodgers et al., 2005), consequently protecting the mitochondrial energy metabolism. Thus, the benefits of the NAD+ stimulating cell survival have raised the hope that using pharmacological agents to increase NAD+ concentrations might provide therapeutic benefits in delaying the onset and slowing the progression of AD dementia.
Nicotinamide riboside (NR) is a NAD precursor, which is converted to NAD through action of human NrK1 and NrK2 genes in the de novo fashion (Bieganowski and Brenner, 2004; Bieganowski et al., 2003). Evidence shows that NR treatment increases intracellular NAD+ concentration and improves NAD+-dependent activities in the cell by increasing silent mating-type information regulation 2 (Sir2)-dependent gene silencing and longevity via nicotinamide riboside kinase (NRK) 1-dependent NAD+ synthesis (Belenky et al., 2007). Thus it is possible that the exogenous application of NR is capable of promoting the biosynthesis of NAD, thus promoting the beneficial effects of NAD (Braidy et al., 2008). Excitingly, it has been reported that treatment with nicotinamide prevents cognition in AD transgenic mice via a mechanism involving sirtuin inhibition and reduction of tau phosphorylation (Green et al., 2008). However, the role of NR in the beta-amyloid (Aβ) deposition in AD brain is still not clear.
It has been shown that PGC-1α also plays an important role in energy metabolism by regulating mitochondrial function in different tissues. The expression of PGC-1 has been found significantly decreased in Alzheimer’s brains, and it is involved in the Aβ pathological generation by affecting the processing of amyloid precursor protein (APP), at least partially through enhancing the α-secretase activity (Qin et al., 2009; Wu et al., 2006). Recently, our group and others reported that 1 of the mechanisms in which the PGC-1 decreases the Aβ burden is also involved in the regulation of the F-Box (FbX)2-E3-ligase-mediated β-secretase (BACE1) degradation (Gong et al., 2010; Katsouri et al., 2011) as it does in other E3 ligases in the ubiquitin system in other tissues. Encouraged by the effects of NAD on promoting the PGC-1 expression, in this study, we tested the hypothesis that exogenous treatment of NR might reduce the Aβ burden in AD brain via enhancing PGC-1α expression, which increases BACE1 ubiquitination, degradation, and improves mitochondrial metabolism. Our study provides a novel therapeutic strategy for the treatment of AD.
2. Methods
2.1. Animals
Tg2576 mice were crossed with PGC-1α−/− mice (Qin et al., 2009) and generated PGC-1α−/−/Tg2576 mice. Animals were backcrossed at least 10 generations onto normal C57BL/6 mice (Jackson Laboratories, Bar Harbor, ME, USA). All experiments were approved by the Mount Sinai School of Medicine Animal Care committees.
2.2. Primary neuronal cell culture
Tg2576 mouse primary neuronal cell cultures were prepared from the brains of 14.5-day-old embryos bred from wild type C57BL/6 females crossed with Tg2576 males, as described previously (Gong et al., 2010). Briefly, after isolation, cerebral hemispheres were placed into Dulbecco’s modified Eagle’s medium supplemented with 10% fetal bovine serum (FBS) and 1× penicillin-streptomycin. Brain tissue was dissociated and the single cells were suspended in Dulbecco’s modified Eagle’s medium supplemented with 10% FBS and 1× penicillin-streptomycin. Cells were seeded into precoated 12-well plates (BD Biosciences) at a concentration of 8 × 105 cells per well. After 30 minutes of incubation in a tissue culture incubator, the cells were changed to neural basal medium (Invitrogen) supplemented with 0.5 mM L-glutamine (Cellgro), 1 × B-27 (Invitrogen), and 1× penicillin-streptomycin (Invitrogen). The cells were kept in an atmosphere of 95% air and 5% CO2. After 7 more days of incubation, cells were used for the treatment.
2.3. BACE1 activity measurements and quantification of amyloid peptides by enzyme-linked immunosorbent assay
The measurement of the Aβ levels has been described previously (Gong et al., 2004). Briefly, levels of Aβ1–40 and Aβ1–42 in primary cultured Tg2576 neurons infected with various adenoviral vectors were determined using sandwich type enzyme-linked immunosorbent assay (ELISA) (Biosource International, Camarillo, CA, USA). The background from control medium (transfected with the adenogreen fluorescent protein [GFP] vector) was subtracted from the sample values.
2.4. NR treatment and behavioral assessment
Seven- to 8-month-old Tg2576 mice were treated with 250 mg/kg/day NR; control Tg2576 mice were treated with saline. Treatments started at approximately 5–6 months of age, and lasted until 10–11 months of age. Mice had their cognitive functions assessed by the object recognition protocol. Mice were first placed in an apparatus and allowed to explore an object. After a certain interval, the mouse was returned to the apparatus, which contained the familiar object and a novel object (Bevins and Besheer, 2006). The time which the mouse spent on the novel object was calculated and was compared between treated and control groups. After behavioral assessment, mice were sacrificed, and their brains were dissected. One hemisphere was snap frozen for subsequent assessment of Aβ-specific ELISA as discussed above. The other hemisphere was fixed in formaldehyde for subsequent stereological quantitative assessments of neuritic plaque pathology, as previously described (Green et al., 2008; Wang et al., 2008). In parallel, control studies using age-, sex-, and strain-matched wild type (WT) mice, were conducted to evaluate the potential effect of NR treatment on cognitive function in the absence of Aβ neuropathology.
2.5. Western blot
Cells and tissues were lysed in either radioimmunoprecipitation assay (RIPA) buffer lysis buffer or Cell Signaling Lysis Buffer supplemented with protease inhibitors. Fifty to 100 µg of protein lysate was then run on sodium dodecyl sulfate polyacrylamide gel electrophoresis. Proteins were transferred to a nitrocellulose transfer membrane (Whatman). The membranes were blocked in 5% fat-free milk for 1 hour, then incubated in primary antibody for 1 hour, horseradish peroxidase (HRP)-conjugated secondary antibody for 1 hour, and developed in enhanced chemiluminescence (ECL) substrate.
2.6. Hippocampal slice preparation and electrophysiology recording
We cut four hundred µm brain slices from Tg2576 mice, and WT littermates, and maintained them in an interface chamber at 29 °C for 90 minutes before recording, as previously reported (Gong et al., 2006). The bath solution consisted of 124.0 mM NaCl, 4.4 mM KCl, 1.0 mM Na2HPO4, 25.0 mM NaHCO3, 2.0 mM CaCl2, 2.0 mM MgSO4, and 10.0 mM glucose. The stimulating electrode, a bipolar tungsten electrode, was placed at the level of the Schaeffer collateral fibers, whereas the recording electrode, a glass electrode filled with bath solution, was placed at the level of the CA1 stratum radiatum. Basal synaptic transmission was assayed by plotting the stimulus voltages against slopes of field excitatory postsynaptic potentials. For the long-term potentiation (LTP) experiments, a 15-minute baseline was recorded every minute at an intensity that evoked a response of approximately 35% of the maximum evoked response. LTP was induced using ø-burst stimulation (4 pulses at 100 Hz, with the bursts repeated at 5 Hz and each tetanus, including three 10-burst trains, separated by 15 seconds). Two-way analysis of variance followed by Bonferroni’s test was used for statistical analysis. Data are mean ± standard error of the mean (error bar) of the results from 2 independent experiments.
2.7. Reverse transcription (RT)-polymerase chain reaction on mitochondrial gene expression in the brains of Tg2576 mice
Five-month-old Tg2576 mice were treated with 250 mg/kg/day of NR for approximately 3 months, and total RNA from the cerebral cortex was extracted using RNeasy Mini Kit (Qiagen) 24 hours after behavioral testing. Complementary DNA was synthesized using Superscript III First-Strand Synthesis SuperMix for qRT-polymerase chain reaction (PCR) (Invitrogen) with 1 µg of total RNA. Quantitative RT-PCR was performed using Maxima SYBR Green Master Mix (Fermentas).
3. Results
3.1. NR treatment promotes cognition coincided with induction of PGC-1α
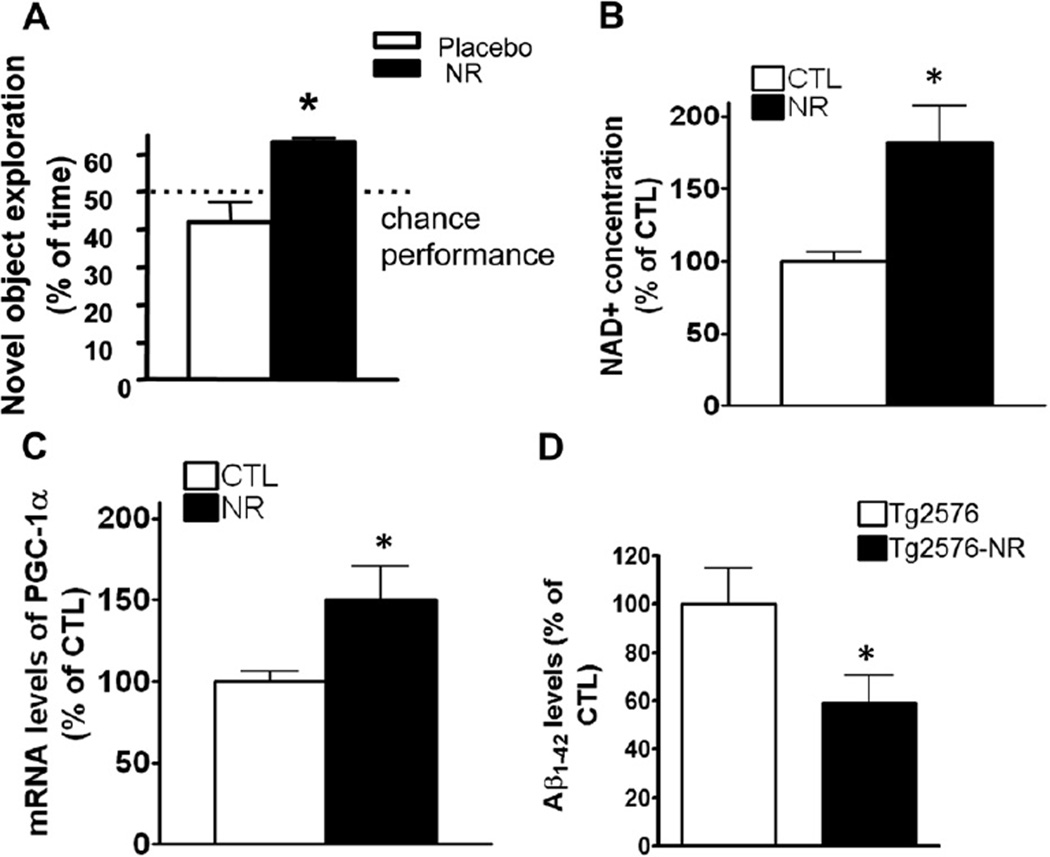
To assess if NR has any protective effects on cognitive function as we proposed, we first treated Tg2576 AD transgenic (APP) mice (Hsiao et al., 1996) (7–8-month-old) with 250 mg/kg/day of NR (equivalent to 1300 mg/kg/day in the human) for 3 months via drinking water. We found that the NR treatment significantly improved cognitive performance of these mice in an object recognition test, which is a cognitive task to examine hippocampal- and cortical-dependent learning. This cognition function is compromised by AD pathology in this mouse model (Oddo et al., 2003). In particular, nontreated control Tg2576 mice performed at the chance level (42.0 ± 9.2%), and the chance of NR-treated Tg2576 mice recognizing a novel level object was significantly better (63.2 ± 1.7%; p < 0.05) (Fig. 1A). To confirm the improvement of the behavior in Tg2576 mice is because of the effects of the NR treatment and its link with NAD, next we tested the bioavailability of NR in the brain in this treatment protocol. We found that this NR treatment significantly increased the steady-state levels of NAD+ in the cerebral cortex (Fig. 1B). Our data implicates NR as a potential feasible therapy for treating energy metabolism defects in the AD brain through enhancing NAD+ levels in brain.
Fig. 1.
NR improves cognitive function in Tg2576 mice via a promotion of NAD+ and PGC-1α levels. (A) Treatment with NR 250 mg/kg/day in Tg2576 mice for 3 months improves cognitive function. Object recognition memory test, performed as described by Bevins and Besheer (2006). Values are expressed as mean ± standard error of the mean, n = 10 mice per group. * p < 0.05, 2-tailed Student t test. (B) The NR treatment significantly increased the levels of NAD+ levels measured by NAD/NADH Assay Kit (Abcam). (C) PGC-1α mRNA levels in brain. Values are expressed as mean ± standard error of the mean, n = 8 mice per group. * p < 0.05, n = 5 mice per group, 2-tailed Student t test. (D) Enzyme-linked immunosorbent assay showed the levels of Aβ1–42 levels in brains treated with NR comparing with placebo-treated brains in Tg2576 mice. n = 8 mice. Abbreviations: Aβ, beta-amyloid; CTL, control; mRNA, messenger RNA; NAD, nicotinamide adenine dinucleotide; NR, nicotinamide riboside; PGC-1α, peroxisome proliferator-activated receptor-γ coactivator 1α.
3.2. NR treatment enhances NAD+ and PGC-1α expression
Because the increase in NAD+ has been associated with the promotion of PGC-1α expression in various tissues, we further explored whether the treatment with NR could have any effects on PGC-1α expression and further affect the Aβ levels. Indeed, our quantitative RT-PCR analysis in the brain samples from the mice of NR-treated and control groups showed NR significantly increased the PGC-1α gene expression compared with the untreated group, p < 0.05 (Fig. 1C). One of the important roles of PGC-1α has been reported to be reduction of Aβ burden, thus we further tested the Aβ levels in these mice. Consistent with the increase in the PGC-1α expression, the Aβ levels are significantly decreased in the groups treated with NR (Fig. 1D).
3.3. NR improves synaptic plasticity in Tg2576 mice
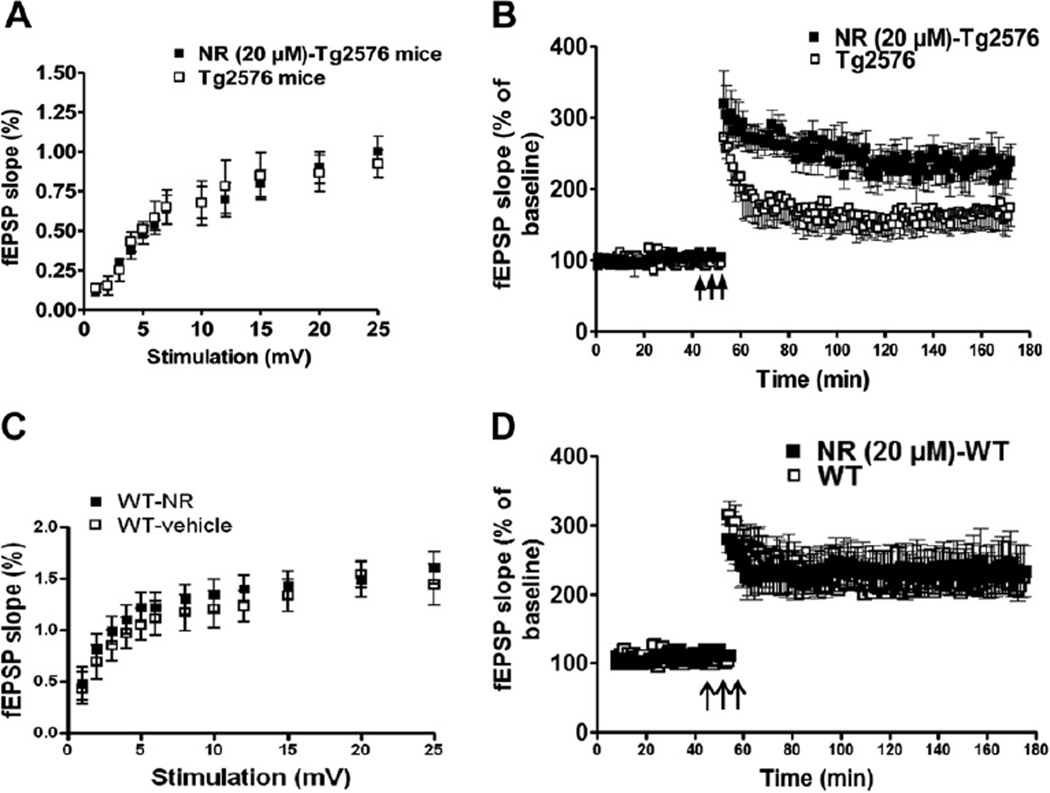
Because NR treatment improves cognitive function, we next used electrophysiological recording to study if NR has effects on synaptic plasticity in hippocampal CA1 region, reflected by LTP. We found there was no difference in the input-output curves generated using the field excitatory postsynaptic potential versus stimulus intensity between the NR treatment and control Tg2576 mice (Fig. 2A). For the LTP, after a 60-minute NR treatment (20 µM), the responses were significantly increased, and were 224 ± 15% at 120 minutes of the theta-burst stimuli, and in the control mice, the response were 164 ± 12% (Fig. 2B) (n = 8 slices; p < 0.05). The NR perfusion in this condition does not affect the input-output curve, nor the LTP levels in WT mice (Fig. 2C and D); p > 0.05.
Fig. 2.
NR-treated slices from Tg2576 mice improves synaptic function but has no effect on WT mice. (A) Application of NR at 20 µM for 4 hours in hippocampal slices from Tg2576 mice (12–14-month-old) has no significantly effects on basal synaptic transmission recorded in CA1 region (A) (p > 0.05), however, the deficits of LTP in the Tg2576 mice was greatly rescued, n = 8 slices, 2-way analysis of variance; p < 0.01 (B). Perfusion of NR has no effects on the basal synaptic transmission (C) nor on the LTP (D) in hippocampal slices from WT littermates (n = 8, 2-way analysis of variance; p > 0.05). Abbreviations: fEPSP, field excitatory postsynaptic potentials; LTP, long-term potentiation; NR, nicotinamide riboside; WT, wild type.
3.4. PGC-1α deficiency promotes the generation of Aβ peptide in AD models
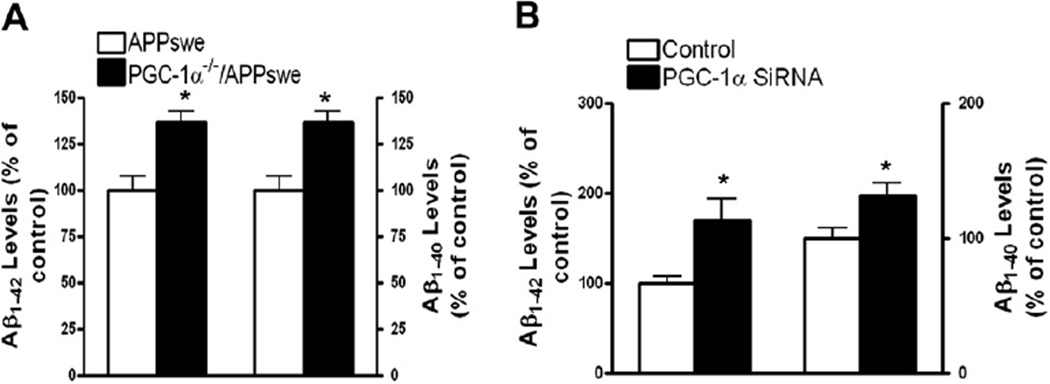
It has been suggested that reduced PGC-1α expression in the AD brain strongly correlates with the progression of clinical dementia and neuropathology (Qin et al., 2009), based on the data showing that NR treatment increases PGC-1α expression. Thus it is interesting to know if PGC-1α deficiency might causally promote AD type β-amyloidosis in in vitro and in vivo experimental models. First, we crossed Tg2576 mice with PGC-1α null (PGC-1α−/−) mice (Leone et al., 2005) to generate PGC-1α+/−/APP double transgenic mice, followed by crossing PGC-1α+/−/APP F1 mice with PGC-1α null mice to generate PGC-1α−/−/APP (PGC-1α null/APP) mice. Assessed by ELISA, we found that the homozygous knockout of PGC-1α in PGC-1α−/−/APP mice significantly promoted Aβ neuropathology (reflected by Aβ1–40 and Aβ1–42 levels in the cerebral cortex and hippocampus) by approximately 40%, in comparison with age- and sex-matched APP (Tg2576) mice (Fig. 3A) (n = 3; p < 0.05). Further, in in vitro feasibility studies using primary cortical-hippocampal neuron cultures derived from Tg2576 mice, we found that reducing the cellular levels of PGC-1α by approximately 1.5-fold (data not shown) by infecting cultured cells with an adenoviral PGC-1α silencing shRNA significantly increased the accumulation of β-amyloid Aβ1–40 and Aβ1–42 peptides in the conditioned medium by 1.2-fold or more (Fig. 3B). Collectively, these studies support our working hypothesis that downregulation of PGC-1α in the brain might causally promote amyloid neuropathology, and NR treatment improves the PGC-1α expression, thus reducing the Aβ burden in AD mouse models.
Fig. 3.
PGC-1α deficiency promotes the generation of Aβ peptides in vivo and in vitro. (A) Increased Aβ1–40 or Aβ1–42 concentrations in approximately 4-month-old PGC-1α−/−/APP mice relative to age- and sex-matched control APP (Tg2576) mice as assessed by enzyme-linked immunosorbent assay. (B) Silencing PGC-1α in primary corticohippocampal neurons derived from Tg2576 embryos resulted in elevation of Alzheimer’s disease-type Aβ levels in the cultures with conditioned medium relative to control neuron cultures. PGC-1α silencing was achieved by infecting neurons with a PGC-1α shRNA adenovirus (a gift from Dr Puigserver) at 10 MOI. Control cells were infected with a GFP adenovirus. Aβ accumulation in cultured media was assessed 24 hours after adenoviral infection. Values are expressed as mean ± standard error of the mean from 2–3 independent experiments, with n = 3–4 per group; * p < 0.05; ** p < 0.01; 2-tailed Student t test. Abbreviations: Aβ, beta-amyloid; GFP, green fluorescent protein; MOI, multiplicity of infection; PGC-1α, peroxisome proliferator-activated receptor-γ coactivator 1α; shRNA, small hairpin RNA.
3.5. NR reduces BACE1 levels through promotion of PGC-1α expression
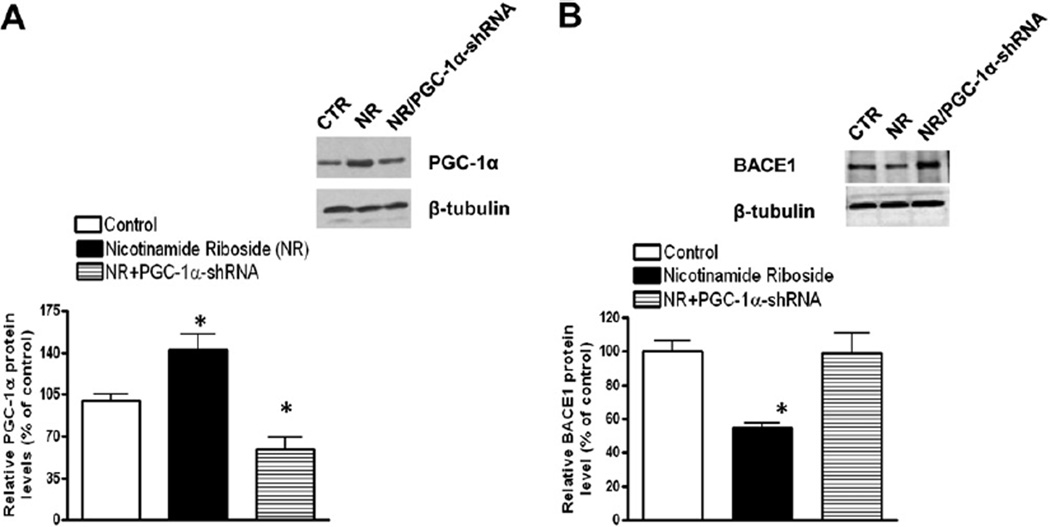
Because NR treatment reducing Aβ levels is coincident with the enhanced expression of PGC-1α, which has been associated with degradation of BACE1 (Gong et al., 2010), next we tested if the effects of NR on the Aβ levels is also via a regulation on BACE1 degradation.
We tested the protein levels of BACE1 and PGC-1α in primary cultured cortical neurons from brains of Tg2576 mice treated with NR. We first confirmed the effects of NR on the promotion of PGC-1α expression by Western blot analysis, though this increase was abolished by the infection of adenoviral PGC-1α shRNA (Fig. 4A). Next, we probed the levels of BACE1 protein and found that BACE1 protein levels were significantly decreased by NR treatment, and this decrease was largely abolished by PGC-1α silencing by adenoviral PGC-1α shRNA (Fig. 4B), suggesting that NR affects the Aβ levels through regulation of PGC-1α expression.
Fig. 4.
NR promotes PGC-1α responses and BACE1 degradation in the brain. (A) NR-treated primary neuron cultures derived from Tg2576 mice showed increased PGC-1α protein levels probed by Western blot analysis with anti-PGC-1α antibody (Sigma). Values are expressed as mean ± standard error of the mean; n = 3; * p < 0.05, Student t test. (B) BACE1 protein levels are decreased by NR treatment and the decrease was attenuated by PGC-1α-shRNA probed by Western blot analysis with anti-BACE1 antibody (Sigma). Values are expressed as mean ± standard error of the mean; n = 3; * p < 0.05, Student t test. Inset shows the protein of adeno-PGC-1α and PGC-1α-shRNA after infected in neurons. Abbreviations: BACE1, β-secretase; CTR, control; NR, nicotinamide riboside; PGC-1α, peroxisome proliferator-activated receptor-γ coactivator 1α; shRNA, small hairpin RNA.
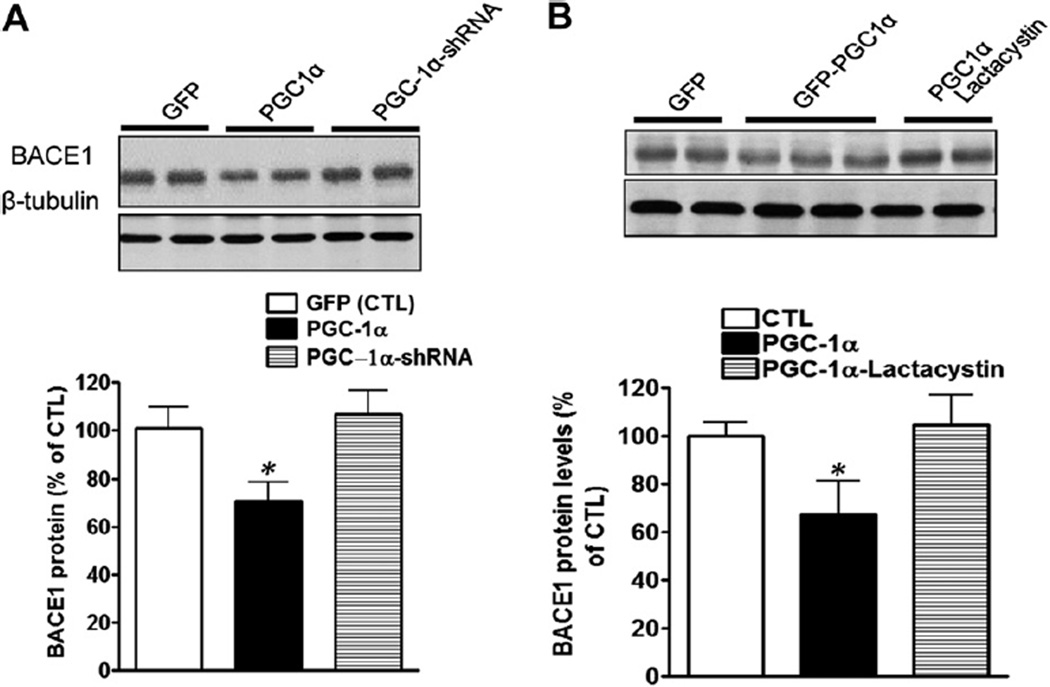
To further mechanistically explore the association between PGC-1α and BACE1, we overexpressed PGC-1α in cortical-hippocampal neuron cultures derived from Tg2576 mice. We found that the BACE1 levels were decreased by the PGC-1α expression, and this decrease was abolished by the PGC-1α gene silencing assessed by Western blot analysis (Fig. 5A). Because the ubiquitin-proteasome system (UPS) has been reported to be linked with BACE1 degradation, we then treated the adenoviral PGC-1α-infected neurons with lactacystin (5 µM), a specific proteasome inhibitor, for 4 hours. Consistent with our previous report, lactacystin significantly inhibited BACE1 degradation, suggesting that PGC-1α might exert influence on BACE1 via UPS-mediated degradation (Fig. 5B).
Fig. 5.
PGC-1α expression promotes BACE1 degradation. (A) Primary hippocampal-cortical neurons derived from Tg2576 embryos at 14 days in vitro were infected by adenoviral-GFP PGC-1α, scramble PGC-1α-shRNA, or PGC-1α-shRNA, respectively, 72 hours after infection, cell lysates were collected and analyzed via Western blot using anti-BACE1 antibodies. BACE1 levels were quantified and normalized against the level of β-tubulin and plotted as percentage of CTL. Data are expressed as mean±standard error of the mean (n=5). * p<0.05 compared with CTL group. Inset represents PGC-1α immunoreactive signals. (B) The degradation of BACE1 caused by PGC-1α expression on was blocked by lactacystin treatment (5 µM). Data are expressed as mean ± standard error of the mean (n = 5). * p < 0.05 compared with CTL group. Inset represents BACE1 immunoreactive signals. Abbreviations: BACE1, β-secretase; CTL, control; GFP, green fluorescent protein; PGC-1α, peroxisome proliferator-activated receptor-γ coactivator 1α; shRNA, small hairpin RNA.
3.6. NR promotes BACE1 degradation through an increase in the BACE1 ubiquitination
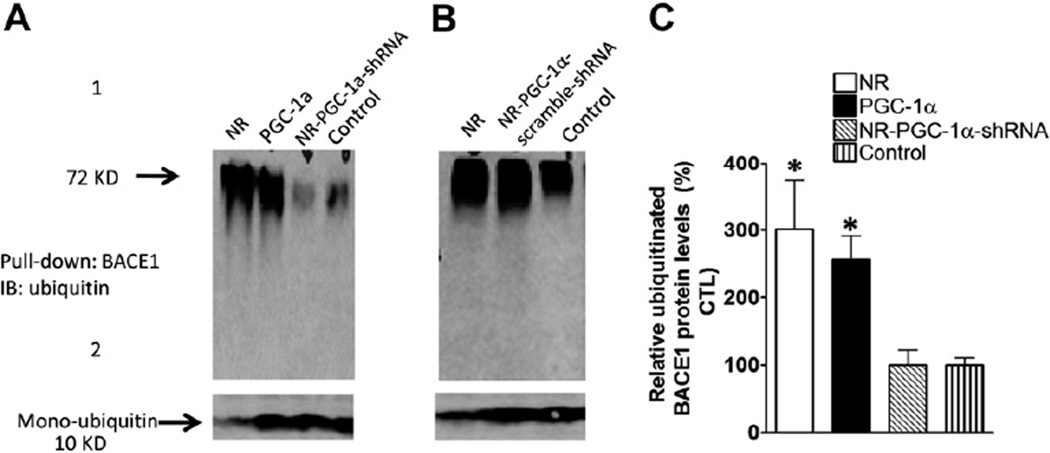
Because protein ubiquitination is an essential step for most proteins degraded by UPS, we further explored whether NR treatment increased the BACE1 ubiquitination. HEK293 cells stably transfected with BACE1 vector with Myc-tag (Myc-BACE1) were treated with NR, and infected with adenoviral PGC-1α, and adenoviral PGC-1α shRNA. The protein extracts were probed by Western blot with anti-ubiquitin antibody. We found that the ubiquitinated BACE1 protein levels markedly increased in NR-treated and PGC-1α overexpressing HEK293 cells relative to control cells, suggesting that PGC-1α promotes BACE1 degradation in UPS through BACE1 ubiquitination (Fig. 6, upper panel). Consistent with the increase in ubiquitinated BACE1 levels, the levels of monoubiquitin levels were decreased in the cells with NR treatment or PGC-1α expression (Fig. 6, lower panel).
Fig. 6.
NR promotes BACE1 ubiquitination and degradation is linked with PGC-1α. (A) BACE1 stable HEK293 cells were infected with adeno-PGC-1α or adeno-PGC-1α-shRNA and scramble shRNA (B). The adeno-GFP viral constructs were used as control. Cells were treated with NR 200 µg and 5 µM lactacystin. After 48 hours of transfection, cell lysate was collected and BACE1 immunoprecipitated using anti-BACE1 antibody, and probed with anti-ubiquitin antibodies (Sigma). (C) Quantification of the square area in the left panel by ImageJ and represented in the graph. This area represents BACE1 proteins at different ubiquitinated levels. Data are mean ± SEM (error bar) of the results from 2 independent experiments. Student t test was performed; * p < 0.05 compared with GFP-lactacystin treated cells. Abbreviations: BACE1, β-secretase; CTL, control; GFP, green fluorescent protein; NR, nicotinamide riboside; PGC-1α, peroxisome proliferator-activated receptor-γ coactivator 1α; SEM, standard error of the mean; WB, Western blot analysis.
3.7. NR induces PGC-1α-associated energy metabolism genes
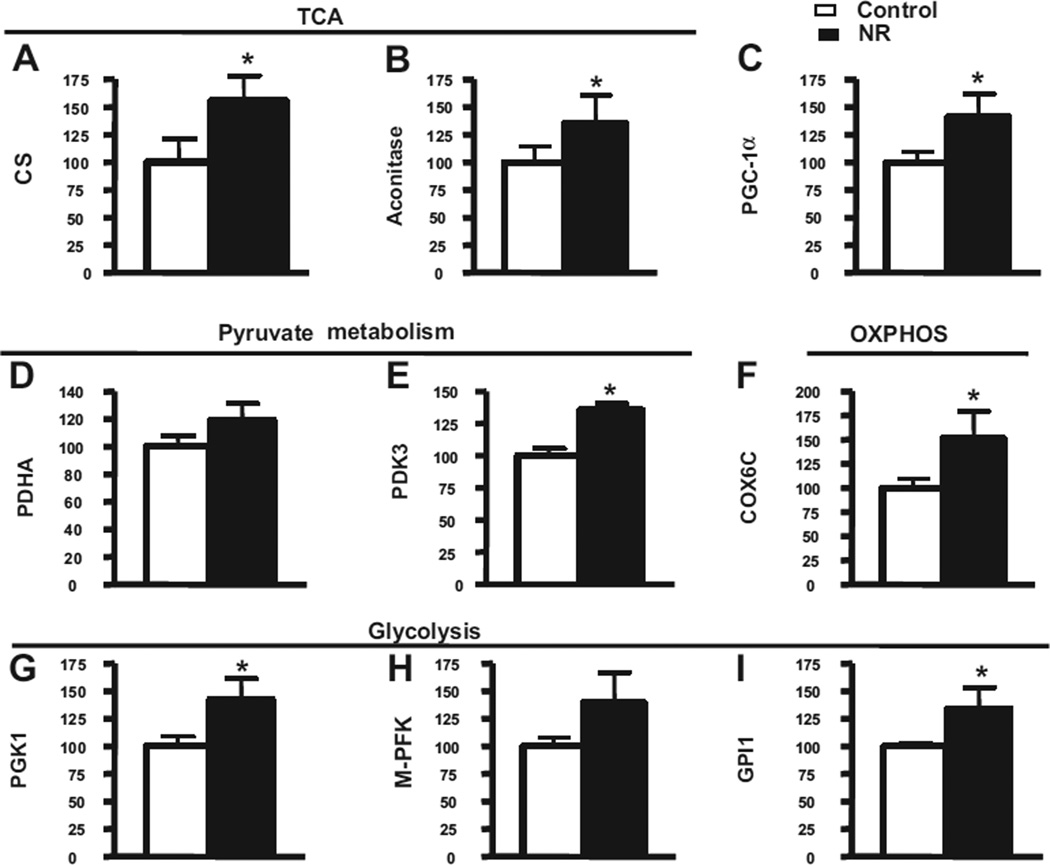
Because the treatment of NR promotes PGC-1α expression and increases intracellular NAD+ levels, it is interesting to know whether the PGC-1α associated energy metabolism genes were affected by the NR treatments. Because it has been reported that some of these genes were downregulated in the AD brain, we tested the messenger RNA (mRNA) levels of these genes in the brain extract from Tg2576 mice. We found that treating Tg2576 mice with NR for 3 months significantly promoted the expression of gene products involved in a wide range of mitochondrial metabolism, such as citrate synthase (Fig. 7A) and aconitase genes (Fig. 7B) in the tricarboxylic acid cycle; pyruvate dehydrogenase kinase (Fig. 7E) in pyruvate metabolism, cytochrome c subunit Vic in mitochondrial oxidative-phosphorylation (Fig. 7F); the human phosphoglycerate kinase and glucose phosphate isomerase 1 genes in glycolysis (Fig. 7G), assessed by quantitative RT-PCR suggesting that NR might promote cognitive performance, in part, by inducing expression of gene products involved in mitochondrial energy metabolism and linked with PGC-1α expression.
Fig. 7.
Energy metabolism gene products are induced in response to short-term NR treatment in the brains of Tg2576 mice. In this study, 5-month-old Tg2576 mice were treated with 250 mg/kg/day of NR for approximately 3 months, and total RNA from the cerebral cortex was extracted using RNeasy Mini Kit (Qiagen) 24 hours after behavioral testing. Complementary DNA was synthesized using Superscript III First-Strand Synthesis SuperMix for quantitative RT-PCR (Invitrogen) with 1 µg of total RNA. Quantitative RT-PCR was performed using Maxima SYBR Green Master Mix (Fermentas). Gene targets examined here included (A) CS, (B) aconitase, (C) PGC-1α, (D) PDHA, (E) PDK3, (F) COX6C, (G) PGK1, (H) M-PFK, and (I) GPI1. Fold changes between groups were calculated using the ΔCt method. Statistical analysis was done in GraphPad Prism 4. Abbreviations: COX6C, cytochrome c subunit Vic; CS, citrate synthase; GPI1, glucose phosphate isomerase 1; M-PFK, muscle phosphofructokinase; NR, nicotinamide riboside; OXPHOS, oxidative phosphorylation system; PCR, polymerase chain reaction; PDHA, pyruvate dehydrogenase; PDK3, pyruvate dehydrogenase kinase 3; PGC-1α, peroxisome proliferator-activated receptor-γ coactivator 1α; PGK1, phosphoglycerate kinase; RT, reverse transcription; TCA, tricarboxylic acid cycle.
4. Discussion
This study for the first time mechanistically explored the effects of NR on the attenuation of amyloid toxicity, improves cognitive function, and synaptic plasticity in AD mouse models. We for the first time demonstrated that the effects of NR are associated with the promotion of PGC-1α function and the ubiquitin proteasome system. The latter 2 have been indicated to play important roles in AD pathogenesis, and PGC-1α has been especially indicated in diabetes-related metabolism deterioration and aging-related dementia.
It has been shown that the effects of NAD on increasing life span have been linked with the activation of Sir1, Sir2, and further activation of PGC-1α expression, consequently affecting mitochondrial metabolism. NR is a NAD precursor. Evidence shows that extra-cellular NR application could increase intracellular NAD levels through glutamine-dependent NAD(+) synthetase (Qns) 1-independent and Nrk1-dependent pathways (Bieganowski and Brenner, 2004). Our data shows that treatment with NR in Tg2576 mice improves synaptic plasticity and behavioral function and is coincident with the increase in the NAD+ and PGC-1α levels, suggesting that the NR protective effects might be linked to the PGC-1α-regulated reduction of Aβ. Silencing PGC-1α abolished the effects of NR on the BACE1 degradation, further supporting this hypothesis.
Recently, evidence showed that BACE1 posttranslational degradation is a potential novel link in PGC-1α mediated protection against AD amyloid neuropathology (Gong et al., 2010; Katsouri et al., 2011; Kwak et al., 2011). BACE1 is a key secretase involved in the processing of APP, ultimately resulting in generation of amyloidogenic Aβ peptides. Here, we found that PGC-1α plays a important role in enhancing BACE1 degradation through mechanisms influencing UPS-mediated responses as NR does, suggesting that NR might also affect the UPS system through the regulation of PGC-1α. Thus, our study for the first time demonstrates this novel feature that NR promotes PGC-1α expression and BACE1 degradation in mechanisms associated with APP processing and AD β-amyloidosis in the brain.
Cerebral glucose hypometabolism has been associated with progression of AD dementia (Henneberg and Hoyer, 1995; Herholz et al., 2002) and positron emission tomography studies demonstrated that glucose utilization is reduced markedly in the brain of mild cognitive impairment and early stage AD (Jagust et al., 1991; Minoshima et al., 1995), which is associated with abnormal mitochondrial function. In recent genome-wide DNA microarray studies from our laboratory (Qin et al., 2009), we found that, among other genes involved in energy metabolism in the brain and other peripheral organs, PGC-1α expression is decreased significantly in the AD brain as part of the progression of AD dementia. Consistent with this evidence, we reported that PGC-1α expression is decreased in AD brain (Gong et al., 2010; Qin et al., 2009), as we recently found in the brains of the Tg2576 mouse model of AD neuropathology and cognitive deterioration. Based on this evidence, in this study, we continued exploring mechanistically the potential role of PGC-1α in mechanisms associated with energy metabolism in AD pathogenesis. Coincidently, the mitochondrial metabolism-related genes such as the Citrate synthase gene in tricarboxylic acid cycle genes (Srivastava et al., 2007), aconitase (Marmolino et al., 2010), pyruvate metabolism gene, pyruvate dehydrogenase kinase 3 (Sugden and Holness, 2003), cytochrome c subunit Vic (Szuplewski and Terracol, 2001), phosphoglycerate kinase (Fan et al., 2012), and glucose phosphate isomerase 1 (Morizot and Siciliano, 1982) were affected by the treatment of NR, which is possibly through the regulation of PGC-1α expression. On the other hand, NR has direct effects on the mitochondrial membrane, to prevent membrane depolarization during oxidation stress conditions, because the membrane potential is essential for the adenosine triphosphate (ATP) synthesis (Halestrap et al., 1997; La Piana et al., 2003; Woodfield et al., 1997).
In summary, our study suggested that the effects of NR on the reduction of Aβ toxicity through the alterations of PGC-1α expression, and the activation of UPS-regulated BACE1 degradation, also have indirect protective effects on mitochondrial metabolism through promotion of PGC-1α-mediated mitochondrial gene expression besides the reported NR direct effects on mitochondrial membrane protection. Our study provides a novel aspect of a potential application of NR in AD treatment.
Acknowledgements
The studies described here were supported in part from a grant from the Veterans Administration by the US National Institutes of Health grants to G.M.P. and by grant from Alzheimer’s Association (IIRG-08-89354) to B.G. The authors thank Dr Ottavio Arancio (Columbia University) for his advice on the reported electrophysiological recording and thank Ms. Amanda Bilski for assisting in preparation of the manuscript.
Footnotes
Disclosure statement
All authors have no potential conflicts of interest.
All experiments were approved by the Mount Sinai School of Medicine Animal Care committees.
References
- Belenky P, Racette FG, Bogan KL, McClure JM, Smith JS, Brenner C. Nicotinamide Riboside Promotes Sir2 Silencing and Extends Lifespan via Nrk and Urh1/Pnp1/Meu1 Pathways to NAD+ Cell. 2007;129:473–484. doi: 10.1016/j.cell.2007.03.024. [DOI] [PubMed] [Google Scholar]
- Bevins RA, Besheer J. Object recognition in rats and mice: a one-trial non-matching-to-sample learning task to study ‘recognition memory’. Nat. Protoc. 2006;1:1306–1311. doi: 10.1038/nprot.2006.205. [DOI] [PubMed] [Google Scholar]
- Bieganowski P, Brenner C. Discoveries of nicotinamide riboside as a nutrient and conserved NRK genes establish a Preiss-Handler independent route to NAD+ in fungi and humans. Cell. 2004;117:495–502. doi: 10.1016/s0092-8674(04)00416-7. [DOI] [PubMed] [Google Scholar]
- Bieganowski P, Pace HC, Brenner C. Eukaryotic NAD+ synthetase Qns1 contains an essential, obligate intramolecular thiol glutamine amidotransferase domain related to nitrilase. J. Biol. Chem. 2003;278:33049–33055. doi: 10.1074/jbc.M302257200. [DOI] [PubMed] [Google Scholar]
- Braidy N, Guillemin G, Grant R. Promotion of cellular NAD(+) anabolism: therapeutic potential for oxidative stress in ageing and Alzheimer’s disease. Neurotox. Res. 2008;13:173–184. doi: 10.1007/BF03033501. [DOI] [PubMed] [Google Scholar]
- Fan X, Sha LN, Zeng J, Kang HY, Zhang HQ, Wang XL, Zhang L, Yang RW, Ding CB, Zheng YL, Zhou YH. Evolutionary dynamics of the Pgk1 gene in the polyploid genus Kengyilia (Triticeae: Poaceae) and its diploid relatives. PLoS One. 2012;7:e31122. doi: 10.1371/journal.pone.0031122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong B, Cao Z, Zheng P, Vitolo OV, Liu S, Staniszewski A, Moolman D, Zhang H, Shelanski M, Arancio O. Ubiquitin hydrolase Uch-L1 rescues beta-amyloid-induced decreases in synaptic function and contextual memory. Cell. 2006;126:775–788. doi: 10.1016/j.cell.2006.06.046. [DOI] [PubMed] [Google Scholar]
- Gong B, Chen F, Pan Y, Arrieta-Cruz I, Yoshida Y, Haroutunian V, Pasinetti GM. SCFFbx2-E3-ligase-mediated degradation of BACE1 attenuates Alzheimer’s disease amyloidosis and improves synaptic function. Aging Cell. 2010;9:1018–1031. doi: 10.1111/j.1474-9726.2010.00632.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong B, Vitolo OV, Trinchese F, Liu S, Shelanski M, Arancio O. Persistent improvement in synaptic and cognitive functions in an Alzheimer mouse model after rolipram treatment. J. Clin. Invest. 2004;114:1624–1634. doi: 10.1172/JCI22831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green KN, Steffan JS, Martinez-Coria H, Sun X, Schreiber SS, Thompson LM, LaFerla FM. Nicotinamide restores cognition in Alzheimer’s disease transgenic mice via a mechanism involving sirtuin inhibition and selective reduction of Thr231-phosphotau. J. Neurosci. 2008;28:11500–11510. doi: 10.1523/JNEUROSCI.3203-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halestrap AP, Woodfield KY, Connern CP. Oxidative stress, thiol reagents, and membrane potential modulate the mitochondrial permeability transition by affecting nucleotide binding to the adenine nucleotide translocase. J. Biol. Chem. 1997;272:3346–3354. doi: 10.1074/jbc.272.6.3346. [DOI] [PubMed] [Google Scholar]
- Henneberg N, Hoyer S. Desensitization of the neuronal insulin receptor: a new approach in the etiopathogenesis of late-onset sporadic dementia of the Alzheimer type (SDAT)? Arch. Gerontol. Geriatr. 1995;21:63–74. doi: 10.1016/0167-4943(95)00646-3. [DOI] [PubMed] [Google Scholar]
- Herholz K, Salmon E, Perani D, Baron JC, Holthoff V, Frolich L, Schonknecht P, Ito K, Mielke R, Kalbe E, Zundorf G, Delbeuck X, Pelati O, Anchisi D, Fazio F, Kerrouche N, Desgranges B, Eustache F, Beuthien-Baumann B, Menzel C, Schroder J, Kato T, Arahata Y, Henze M, Heiss WD. Discrimination between Alzheimer dementia and controls by automated analysis of multicenter FDG PET. Neuroimage. 2002;17:302–316. doi: 10.1006/nimg.2002.1208. [DOI] [PubMed] [Google Scholar]
- Hsiao K, Chapman P, Nilsen S, Eckman C, Harigaya Y, Younkin S, Yang F, Cole G. Correlative memory deficits, Abeta elevation, and amyloid plaques in transgenic mice. Science. 1996;274:99–103. doi: 10.1126/science.274.5284.99. [DOI] [PubMed] [Google Scholar]
- Jagust WJ, Seab JP, Huesman RH, Valk PE, Mathis CA, Reed BR, Coxson PG, Budinger TF. Diminished glucose transport in Alzheimer’s disease: dynamic PET studies. J. Cereb. Blood Flow Metab. 1991;11:323–330. doi: 10.1038/jcbfm.1991.65. [DOI] [PubMed] [Google Scholar]
- Katsouri L, Parr C, Bogdanovic N, Willem M, Sastre M. PPARgamma co-activator-1alpha (PGC-1alpha) reduces amyloid-beta generation through a PPARgamma-dependent mechanism. J. Alzheimers Dis. 2011;25:151–162. doi: 10.3233/JAD-2011-101356. [DOI] [PubMed] [Google Scholar]
- Kim D, Nguyen MD, Dobbin MM, Fischer A, Sananbenesi F, Rodgers JT, Delalle I, Baur JA, Sui G, Armour SM, Puigserver P, Sinclair DA, Tsai LH. SIRT1 deacetylase protects against neurodegeneration in models for Alzheimer’s disease and amyotrophic lateral sclerosis. EMBO J. 2007;26:3169–3179. doi: 10.1038/sj.emboj.7601758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwak YD, Wang R, Li JJ, Zhang YW, Xu H, Liao FF. Differential regulation of BACE1 expression by oxidative and nitrosative signals. Mol. Neurodegener. 2011;6:17. doi: 10.1186/1750-1326-6-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Piana G, Marzulli D, Consalvo MI, Lofrumento NE. Cytochrome c-induced cytosolic nicotinamide adenine dinucleotide oxidation, mitochondrial permeability transition, and apoptosis. Arch. Biochem. Biophys. 2003;410:201–211. doi: 10.1016/s0003-9861(02)00687-2. [DOI] [PubMed] [Google Scholar]
- Leone TC, Lehman JJ, Finck BN, Schaeffer PJ, Wende AR, Boudina S, Courtois M, Wozniak DF, Sambandam N, Bernal-Mizrachi C, Chen Z, Holloszy JO, Medeiros DM, Schmidt RE, Saffitz JE, Abel ED, Semenkovich CF, Kelly DP. PGC-1alpha deficiency causes multi-system energy metabolic derangements: muscle dysfunction, abnormal weight control and hepatic steatosis. PLoS Biol. 2005;3:e101. doi: 10.1371/journal.pbio.0030101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marmolino D, Manto M, Acquaviva F, Vergara P, Ravella A, Monticelli A, Pandolfo M. PGC-1alpha down-regulation affects the antioxidant response in Friedreich’s ataxia. PLoS One. 2010;5:e10025. doi: 10.1371/journal.pone.0010025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minoshima S, Frey KA, Foster NL, Kuhl DE. Preserved pontine glucose metabolism in Alzheimer disease: a reference region for functional brain image (PET) analysis. J. Comput. Assist. Tomogr. 1995;19:541–547. doi: 10.1097/00004728-199507000-00006. [DOI] [PubMed] [Google Scholar]
- Morizot DC, Siciliano MJ. Linkage group IV of fish of the genus Xiphophorus (Poeciliidae): assignment of loci coding for pyruvate kinase-1, glucosephosphate isomerase-1, and isocitrate dehydrogenase-1. Biochem. Genet. 1982;20:505–518. doi: 10.1007/BF00484701. [DOI] [PubMed] [Google Scholar]
- Nemoto S, Fergusson MM, Finkel T. SIRT1 functionally interacts with the metabolic regulator and transcriptional coactivator PGC-1{alpha} J. Biol. Chem. 2005;280:16456–16460. doi: 10.1074/jbc.M501485200. [DOI] [PubMed] [Google Scholar]
- Oddo S, Caccamo A, Kitazawa M, Tseng BP, LaFerla FM. Amyloid deposition precedes tangle formation in a triple transgenic model of Alzheimer’s disease. Neurobiology of Aging. 2003;24:1063–1070. doi: 10.1016/j.neurobiolaging.2003.08.012. [DOI] [PubMed] [Google Scholar]
- Qin W, Haroutunian V, Katsel P, Cardozo CP, Ho L, Buxbaum JD, Pasinetti GM. PGC-1{alpha} expression decreases in the Alzheimer disease brain as a function of dementia. Arch. Neurol. 2009;66:352–361. doi: 10.1001/archneurol.2008.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin W, Yang T, Ho L, Zhao Z, Wang J, Chen L, Zhao W, Thiyagarajan M, MacGrogan D, Rodgers JT, Puigserver P, Sadoshima J, Deng H, Pedrini S, Gandy S, Sauve AA, Pasinetti GM. Neuronal SIRT1 activation as a novel mechanism underlying the prevention of Alzheimer disease amyloid neuropathology by calorie restriction. J. Biol. Chem. 2006;281:21745–21754. doi: 10.1074/jbc.M602909200. [DOI] [PubMed] [Google Scholar]
- Rodgers JT, Lerin C, Haas W, Gygi SP, Spiegelman BM, Puigserver P. Nutrient control of glucose homeostasis through a complex of PGC-1alpha and SIRT1. Nature. 2005;434:113–118. doi: 10.1038/nature03354. [DOI] [PubMed] [Google Scholar]
- Srivastava S, Barrett JN, Moraes CT. PGC-1alpha/beta upregulation is associated with improved oxidative phosphorylation in cells harboring nonsense mtDNA mutations. Hum. Mol. Genet. 2007;16:993–1005. doi: 10.1093/hmg/ddm045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugden MC, Holness MJ. Recent advances in mechanisms regulating glucose oxidation at the level of the pyruvate dehydrogenase complex by PDKs. Am. J. Physiol. Endocrinol. Metab. 2003;284:E855–E862. doi: 10.1152/ajpendo.00526.2002. [DOI] [PubMed] [Google Scholar]
- Szuplewski S, Terracol R. The cyclope gene of Drosophila encodes a cytochrome c oxidase subunit VIc homolog. Genetics. 2001;158:1629–1643. doi: 10.1093/genetics/158.4.1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Ho L, Zhao W, Ono K, Rosensweig C, Chen L, Humala N, Teplow DB, Pasinetti GM. Grape-derived polyphenolics prevent Abeta oligomerization and attenuate cognitive deterioration in a mouse model of Alzheimer’s disease. J. Neurosci. 2008;28:6388–6392. doi: 10.1523/JNEUROSCI.0364-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodfield KY, Price NT, Halestrap AP. cDNA cloning of rat mitochondrial cyclophilin. Biochim. Biophys. Acta. 1997;1351:27–30. doi: 10.1016/s0167-4781(97)00017-1. [DOI] [PubMed] [Google Scholar]
- Wu Z, Huang X, Feng Y, Handschin C, Feng Y, Gullicksen PS, Bare O, Labow M, Spiegelman B, Stevenson SC. Transducer of regulated CREB-binding proteins (TORCs) induce PGC-1alpha transcription and mitochondrial biogenesis in muscle cells. Proc. Natl. Acad. Sci. U. S. A. 2006;103:14379–14384. doi: 10.1073/pnas.0606714103. [DOI] [PMC free article] [PubMed] [Google Scholar]