Abstract
A hallmark of hypertension is an increase in arterial myocyte voltage-dependent Ca2+ (CaV1.2) currents that induces pathological vasoconstriction. CaV1.2 channels are heteromeric complexes comprising a pore forming CaV1.2α1 with auxiliary α2δ and β subunits. Molecular mechanisms that elevate CaV1.2 currents during hypertension and the potential contribution of CaV1.2 auxiliary subunits are unclear. Here, we investigated the pathological significance of α2δ subunits in vasoconstriction associated with hypertension.
Age-dependent development of hypertension in spontaneously hypertensive rats (SHR) was associated with an unequal elevation in α2δ-1 and CaV1.2α1 mRNA and protein in cerebral artery myocytes, with α2δ-1 increasing more than CaV1.2α1. Other α2δ isoforms did not emerge in hypertension. Myocytes and arteries of hypertensive SHR displayed higher surface-localized α2δ-1 and CaV1.2α1 proteins, surface α2δ-1 to CaV1.2α1 ratio (α2δ-1:CaV1.2α1), CaV1.2 current-density and non-inactivating current, and pressure- and - depolarization-induced vasoconstriction than those of Wistar-Kyoto controls. Pregabalin, an α2δ-1 ligand, did not alter α2δ-1 or CaV1.2α1 total protein, but normalized α2δ-1 and CaV1.2α1 surface expression, surface α2δ-1:CaV1.2α1, CaV1.2 current-density and inactivation, and vasoconstriction in myocytes and arteries of hypertensive rats to control levels.
Genetic hypertension is associated with an elevation in α2δ-1 expression that promotes surface trafficking of CaV1.2 channels in cerebral artery myocytes. This leads to an increase in CaV1.2 current-density and a reduction in current inactivation that induces vasoconstriction. Data also suggest that α2δ-1 targeting is a novel strategy that may be used to reverse pathological CaV1.2 channel trafficking to induce cerebrovascular dilation in hypertension.
Keywords: Calcium channels, Genetic Hypertension, Vasodilation, Vasoconstriction
Introduction
Hypertension is associated with an elevation in arterial contractility that increases systemic blood pressure and restricts organ blood flow, leading to end-organ damage.1 Hypertension is also a major predictor for a variety of cerebral diseases, including stroke, Alzheimer’s disease, and dementia. One characteristic pathological alteration that occurs in hypertension is an elevation in vascular smooth muscle cell (myocyte) voltage-dependent Ca2+ influx.2, 3 Voltage-dependent L-type Ca2+ (CaV1.2) channels are the primary Ca2+ entry pathway in arterial myocytes and are essential for contractility regulation by a wide variety of stimuli, including intravascular pressure, membrane potential, and vasoconstrictors.4–8 A hypertension-associated elevation in CaV1.2 currents leads to an increase in intracellular Ca2+ concentration ([Ca2+]i) and vasoconstriction.9–11 However, molecular mechanisms that elevate arterial myocyte CaV1.2 currents in hypertension, leading to vasoconstriction, are unclear.
CaV1.2 channels are heteromeric complexes comprising a pore forming α1 with auxiliary α2δ and β subunits.12 Four α2δ (1 through 4) subunit isoforms have been identified that are each encoded by different genes.13, 14 α2δ subunits undergo post-translational cleavage into a highly glycosylated extracellular α2 and a smaller δ subunit, which are subsequently coupled by a disulfide bond to form a single functional protein.14, 15 α2δ subunits are membrane-bound by the bilayer-spanning δ subunit. Recently, α2δ-1 was identified as being critical for functional trafficking of CaV1.2α1 subunits to the plasma membrane (surface) in arterial myocytes.16 To date, no studies have investigated pathological or disease-associated molecular changes in CaV1.2 auxiliary subunits, including α2δ subunits, in myocytes of resistance-size arteries. In addition, it is unclear whether the subunit composition of arterial myocyte surface CaV1.2 channels is altered in disease. Given that arterial myocyte CaV1.2 currents are elevated during hypertension, leading to vasoconstriction, we determined the subunit composition of CaV1.2 channels and investigated the involvement of α2δ subunits in this pathological alteration.9–11 Elucidating molecular mechanisms governing α2δ subunit regulation of CaV1.2 channels in hypertension could lead to the development of novel approaches to treat cardiovascular diseases.
Here, we used a genetic model of hypertension, the spontaneously hypertensive rat (SHR), to investigate the pathological significance of arterial myocyte α2δ subunits in hypertension. We show that that during hypertension, an elevation in α2δ-1 expression increases plasma membrane CaV1.2 currents in arterial myocytes, leading to vasoconstriction. We also identify α2δ-1 as a novel therapeutic target to induce cerebrovascular dilation in hypertension.
Methods
Cell isolation and tissue preparation
All animal protocols used were reviewed and approved by the Animal Care and Use Committee at the University of Tennessee Health Science Center. Male 6 or 12 week old SHR and Wistar Kyoto (WKY) rats were euthanized by intraperitoneal injection of sodium pentobarbital (150 mg/Kg body weight, Vortech Pharmaceuticals, Dearborn, MI). Middle cerebral, posterior cerebral, and cerebellar arteries (~100–200 μm diameter) were studied. Myocytes were enzymatically dissociated from dissected cerebral arteries, as previously described.4
Blood pressure measurements
Diastolic and systolic blood pressures were measured in conscious rats using a tail cuff sphygmomanometer (Kent Scientific, Torrington, Conn).
RT-PCR
RT-PCR was performed on myocytes individually collected under a microscope using an enlarged patch-clamp pipette to prevent contamination from other arterial wall cell types, as previously described.4
Quantitative real-time PCR
Total RNA was isolated from cerebral arteries using Trizol (Invitrogen, Grand Island, NY). cDNA was transcribed using Affinity Script Multiple temperature reverse transcriptase (Stratagene, Clara, CA). Gene specific primers and probes were designed using the Universal Probe Library (UPL). Sequences of primers and probes used and PCR reaction efficiencies are given in Table S1.
Protein analysis and biochemistry
Proteins were separated on SDS-PAGE gels and analyzed by Western blotting. Blots were cut at the 75 kDa marker to allow simultaneous probing of the upper section for α2δ-1 and lower section for actin. The upper portion of the blot was then re-probed for CaV1.2α1. Protein band intensities were determined using Quantity One (BioRad, Hercules, CA) software. For quantification, protein band intensities were first normalized to actin and then to appropriate control samples.
Artery surface biotinylation
To determine the distribution of α2δ-1 and CaV1.2α1 subunit proteins between surface and intracellular compartments, artery surface biotinylation was used, as previously described.16
Patch-clamp electrophysiology
Whole cell CaV1.2 currents were recorded in isolated myocytes using the whole cell patch-clamp configuration, as previously described.16
Pressurized artery myography
Endothelium-denuded artery diameter was measured over a range of intravascular pressures (20–100 mmHg) in the presence and absence of nimodipine (1 μmol/L) using edge-detection myography, as previously described.17 Diameter responses to elevating extracellular K+ from 6 to between 20 and 60 mmol/L at 10 mmHg in the presence of pinacidil (10 μmol/L), a KATP channel opener, were also recorded. Arteries treated with pregabalin for 24 h were also maintained in pregabalin throughout these experiments to inhibit CaV1.2 subunit membrane re-insertion.
Statistical analysis
Summary data are presented as mean ± SEM. Significance was determined using paired or unpaired t-tests with Welsh correction, or ANOVA followed by Student-Newman Keuls for multiple groups. P<0.05 was considered significant. Power analysis was carried out where P>0.05 to verify that sample size was sufficient to give a value of >0.8.
An expanded Methods section is available as Supplemental Documentation.
Results
Age-dependent development of genetic hypertension is associated with an elevation in arterial myocyte α2δ-1 and CaV1.2α1 subunit expression
The pathological involvement of arterial myocyte CaV1.2 subunits was studied using a rat genetic model of hypertension. At 6 weeks of age, WKY and SHR rat diastolic, systolic, and mean arterial blood pressures were similar (Fig. S1). In contrast, at 12 weeks of age, diastolic, systolic, and mean arterial pressures were ~63, 65, and 72 mmHg higher in SHR than WKY rats, respectively (Fig S1).
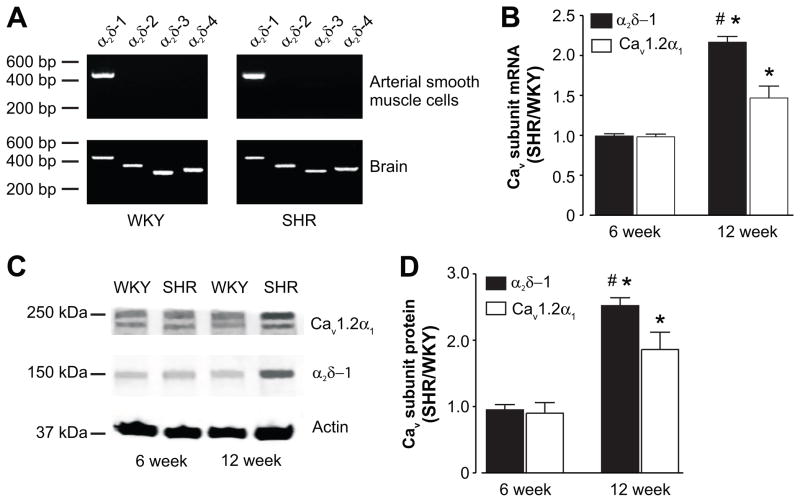
Four different α2δ isoforms have been described, with α2δ-1 the only isoform expressed in normotensive Sprague-Dawley (SD) rat cerebral artery myocytes.14, 16 We tested the hypothesis that hypertension is associated with a shift in α2δ isoform expression in myocytes of resistance-size arteries. RT-PCR detected only α2δ-1 in pure cerebral artery myocytes from 12 week old WKY and hypertensive SHR rats (Fig. 1A). In contrast, the same primers amplified transcript for all α2δ isoforms in WKY and SHR whole brain (Fig. 1A).
Figure 1.
α2δ-1 and CaV1.2α1 subunit mRNA and protein are elevated in hypertension. A, Representative gel (of 3 experiments) illustrating RT-PCR amplification of transcripts for α2δ-1 through -4 in isolated arterial myocytes and whole brain of 12 week old WKY and SHR rats. B, Mean quantitative PCR data for α2δ-1 and CaV1.2α1 mRNA in 6 (n=8 for each) and 12 (n=6 for each) week old SHR rat arteries normalized to Rps5 and then to age-matched WKY controls. C, Exemplar Western blot illustrating α2δ-1 and CaV1.2α1 protein from 6 and 12 week old WKY and SHR rat whole arterial lysate. Blots were physically cut at 75 kDa to allow probing for actin and α2δ-1/CaV1.2α1. D, Mean data illustrating that α2δ-1 and CaV1.2α1 proteins are elevated in hypertension. * indicates P<0.05 when compared with the respective mRNA/protein at 6 weeks, # indicates P<0.05 when compared with CaV1.2α1 mRNA/protein at 12 weeks (n=8 for each protein at each age).
Quantitative PCR was performed to compare α2δ-1 and CaV1.2α1 message levels in 6 and 12 week old WKY and SHR cerebral arteries. Eight different reference genes were screened to identify those with similar mRNA levels in cerebral arteries of WKY and SHR (Table S1). Rps5 mRNA levels were similar in WKY and SHR arteries and thus, Rps5 was used as the reference gene for these experiments (Table S2). Quantitative PCR indicated that mean α2δ-1 and CaV1.2α1 mRNA levels were similar in 6 week old WKY and SHR arteries (Fig. 1B). In contrast, α2δ-1 and CaV1.2 mRNAs were ~2.1- and 1.5-fold higher, respectively, in 12 week old SHR than WKY arteries (Fig. 1B). Age-dependent development of hypertension was also associated with a larger increase in α2δ-1 than CaV1.2α1 mRNA (Fig. 1B). These data indicate that hypertension is associated with an elevation in α2δ-1 and CaV1.2α1 subunit mRNA, but not with the appearance of other α2δ isoforms, in arterial myocytes.
Next, we investigated whether age-dependent development of genetic hypertension is associated with upregulation of α2δ-1 and CaV1.2α1 proteins in cerebral arteries. α2δ-1 and CaV1.2α1 protein levels were similar in 6 week old WKY and SHR arteries (Fig. 1C, D). Aging between 6 and 12 weeks did not alter α2δ-1 and CaV1.2α1 protein in WKY rat arteries, but increased these proteins ~2.1- and 1.4-fold in SHR arteries (Fig. S2). At 12 weeks of age, α2δ-1 and CaV1.2α1 proteins were ~2.5- and 1.7-fold higher in SHR than age-matched WKY arteries (Fig. 1C, D). In agreement with message levels, age-dependent development of hypertension also increased α2δ-1 more than CaV1.2α1 protein (Fig. 1C, D, S2).
In summary, these data indicate that genetic hypertension is associated with transcriptional upregulation of both α2δ-1 and CaV1.2α1 in cerebral artery myocytes. α2δ-1 and CaV1.2α1 proteins are elevated more than their respective mRNAs (Fig. 1B–D, S2), suggesting that hypertension-associated changes in post-translational events also contribute to increased CaV1.2 channel subunit expression during hypertension. Furthermore, during hypertension there is a larger increase in mRNA and protein for α2δ-1 than for CaV1.2α1.
Hypertension is associated with an elevation in surface α2δ-1 and CaV1.2α1 proteins in arteries
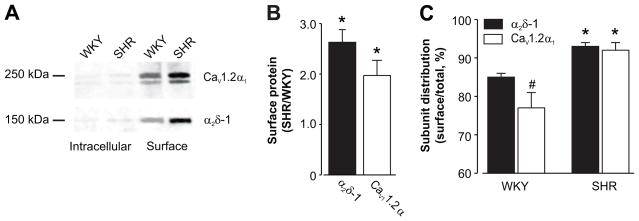
α2δ-1 induces membrane trafficking of CaV1.2α1 subunits in SD rat arterial myocytes.16 Therefore, we tested the hypothesis that an increase in α2δ-1 contributes to elevated surface CaV1.2 expression in hypertension. Surface (plasma membrane) and intracellular α2δ-1 and CaV1.2α1 proteins were measured in age-matched WKY and hypertensive SHR cerebral arteries using biotinylation. Surface-localized α2δ-1 and CaV1.2α1 proteins were ~2.6- and 2-fold higher, respectively, in SHR than WKY rat arteries (Fig. 2A, B). A larger percentage of total α2δ-1 and CaV1.2α1 was located at the plasma membrane in SHR than WKY arteries (Fig. 2A, C). In WKY arteries, more of the total amount of α2δ-1 (~85 %) than CaV1.2α1 (~77%) was located at the surface. In contrast, in SHR arteries the percentage of total α2δ-1 (~93 %) and CaV1.2α1 (~92 %) located at the surface were similar (Fig. 2A, C). These data indicate that during hypertension, an elevation in α2δ-1 and CaV1.2α1 total protein translates to an increase in surface expression of these subunits in arterial myocytes. Furthermore, hypertension is associated with an alteration in the distribution of α2δ-1 and CaV1.2α1 proteins between intracellular and surface compartments.
Figure 2.
Arterial surface α2δ-1 and CaV1.2α1 subunits are elevated in hypertension. A, Representative Western blot illustrating increased surface expression of both α2δ-1 and CaV1.2α1 proteins in 12 week old WKY and SHR arteries. Blot was physically cut at 75 kDa to allow probing for α2δ-1 and CaV1.2α1. B, Mean data illustrate that surface levels of α2δ-1 and CaV1.2α1 subunits are higher in arteries during hypertension. C, Mean data illustrating the percentage of total (surface + intracellular) α2δ-1 and CaV1.2α1 proteins located at the surface * indicates P<0.05 versus same protein in age-matched WKY rat arteries, # indicates P<0.05 when compared with WKY α2δ-1 (n=5 – 9 each for WKY and SHR).
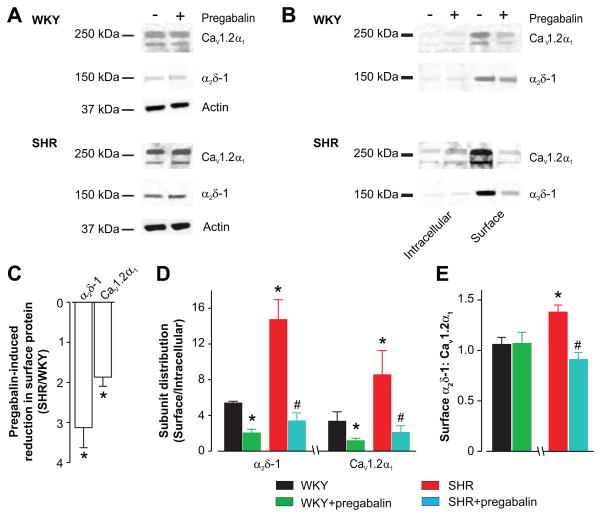
Pregabalin reduces surface trafficking of CaV1.2 channel subunits more effectively in hypertensive than normotensive rat arteries
Pregabalin, an α2δ-1/2 ligand, reduces surface trafficking of CaV1.2, 2.1, and 2.2 channels in neurons and arterial myocytes.14, 16, 18–20 Next, we studied pregabalin regulation of α2δ-1 and CaV1.2α1 subunit surface expression and subunit cellular distribution in WKY and SHR cerebral arteries. For these experiments, arteries were incubated for 24 h with or without pregabalin. Pregabalin (24 h) did not alter total protein of α2δ-1 (% control: WKY, 115 ± 9; SHR, 118 ± 20) or CaV1.2α1 (% control: WKY, 116 ± 10; SHR, 109 ± 14) (Fig. 3A, WKY n= 4–5, SHR n= 5, P>0.05 for each). In contrast, pregabalin reduced surface α2δ-1 and CaV1.2 and increased intracellular levels of these proteins in both WKY and SHR arteries (Fig. 3A, B, C, S3). Pregabalin reduced plasma membrane α2δ-1 and CaV1.2α1 ~3.1 and 1.9-fold more, respectively, in hypertensive SHR than WKY control arteries (Fig. 3C). To evaluate pregabalin regulation of α2δ-1 and CaV1.2 cellular distribution, surface:intracellular protein ratios were calculated. Consistent with data shown in figure 2C, a larger proportion of α2δ-1 and CaV1.2 subunits were present at the plasma membrane in SHR than WKY arteries (Fig. 3D). Pregabalin induced a larger reduction in surface:intracellular α2δ-1 and CaV1.2 in SHR than in WKY arteries (Fig. 3D).
Figure 3.
Pregabalin reduces surface expression of α2δ-1 and CaV1.2α1 channel proteins more effectively in arteries of hypertensive rats than in controls. A, Representative Western blot illustrating that pregabalin does not change total (whole arterial) α2δ-1 and CaV1.2α1 proteins in WKY and SHR arteries. B, Representative Western blots illustrating pregabalin (24 h)-induced changes in surface and intracellular α2δ-1 and CaV1.2α1 proteins. Blots were cut at 75 kDa to allow probing for α2δ-1 and CaV1.2α1. C, Pregabalin reduced surface α2δ-1 and CaV1.2α1 proteins more in SHR than WKY arteries. D, Mean data illustrating α2δ-1 and CaV1.2α1 subunit distribution in WKY and SHR arteries and regulation by pregabalin. E, Surface α2δ-1 to CaV1.2α1 and modulation by pregabalin. Pregabalin concentration in all figures was 100 μmol/L. * indicates P<0.05 compared with untreated WKY and # indicates P<0.05 versus untreated SHR rat arteries (n=4–5 each for untreated and pregabalin-treated WKY and SHR).
Hypertension was associated with a larger increase in surface α2δ-1 than CaV1.2α1 protein in arteries (Fig. 2A, B). We calculated the band intensity ratio of surface α2δ-1 to CaV1.2α1 and regulation by pregabalin. While this methodology cannot determine subunit stoichiometry, total protein loaded in each lane is identical, allowing comparison of this ratio in SHR and WKY arteries from the same blot. The mean surface α2δ-1:CaV1.2α1 band intensity ratio was ~1.38 in SHR arteries and ~1.06 in WKY arteries, or ~1.3-fold higher in SHR (Fig. 3E). Pregabalin reduced the surface α2δ-1 to CaV1.2α1 band intensity ratio to ~0.91 in SHR rat arteries, but did not change the ratio in WKY rat arteries (Fig. 3E).
Collectively, these data indicate that pregabalin blocks surface expression of α2δ-1 and CaV1.2α1 subunits more effectively in hypertensive than normotensive rat arteries. During hypertension, surface α2δ-1 protein is elevated more so than CaV1.2α1 protein, leading to an increase in the ratio of plasma membrane α2δ-1 to CaV1.2α1 subunits. Pregabalin reverses this elevation in surface α2δ-1 to CaV1.2α1 subunits. These data also indicate that α2δ-1 is essential for upregulation of surface CaV1.2 channels in arterial myocytes during genetic hypertension.
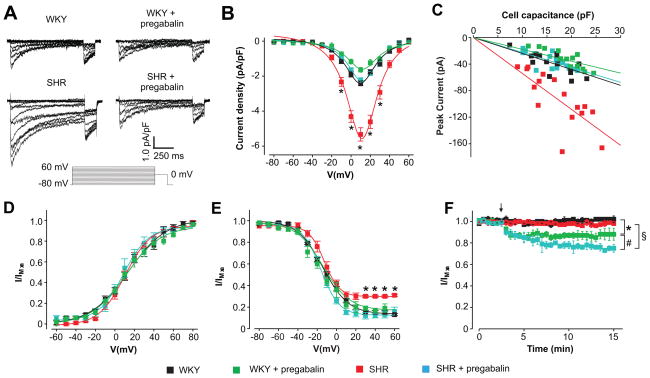
α2δ-1 targeting reverses hypertension-associated modifications in CaV1.2 current density and inactivation in arterial myocytes
To investigate the functional impact of elevated α2δ-1 expression and effects of α2δ-1 targeting, CaV1.2 currents were measured in age-matched WKY and hypertensive SHR cerebral artery myocytes. Mean peak CaV1.2 current density (Ba2+ as charge carrier) was ~5.3 pA/pF in hypertensive SHR compared with ~2.4 pA/pF in WKY cells, or ~2.2-fold larger (Fig. 4A, B and Table 1). Pregabalin (24 h) reduced peak CaV1.2 current density in SHR cells to ~2.2 pA/pF, or by ~59 %, and to ~1.6 pA/pF in WKY cells, or by ~32 % (Fig. 4A, B and Table 1). Pregabalin reduced peak CaV1.2 current density in SHR myocytes to the current density of untreated WKY cells (Fig 4A, B and Table 1). The relationship between cell capacitance and peak CaV1.2 current was investigated (Fig. 4C). When data were fit with a linear function, the slope was −5.41 for SHR and −2.40 for WKY cells, or 2.3-fold higher (Fig. 4C). Pregabalin reduced slopes by ~58 and 25 % in SHR and WKY cells, respectively (Fig. 4C). Slopes were similar for untreated WKY and pregabalin-treated SHR cells (Fig. 4C, P>0.05). Mean cell capacitance for WKY (16.3 ± 0.8 pF) and SHR (SHR 17.1 ± 1.3 pF) cells were similar and were not altered by pregabalin (WKY, 18.8 ± 1 pF; SHR 15.8 ± 0.8 pF; P>0.05 when comparing all), indicating that current density and slope increased due to changes in CaV1.2 channels (Fig. 4B, C).
Figure 4.
Pregabalin reverses elevated CaV1.2 currents in hypertensive rat arterial smooth muscle cells. A, Representative CaV1.2 current density recordings from control and pregabalin-treated WKY and SHR arterial smooth muscle cells (10 mmol/L Ba2+ as charge carrier). B, Mean current density-voltage relationships of WKY (n=17), pregabalin-treated WKY (n=13), SHR (n=16) and pregabalin-treated SHR (n=18) cells. C, Scatter plot with linear fit for peak CaV1.2 current versus cell capacitance in WKY (n=17), pregabalin-treated WKY (n=13), SHR (n=16) and pregabalin-treated SHR (n=18) cells. WKY: slope=−2.40, r=−0.76, p=3.3×10-4. WKY+pregabalin: slope=−1.79, r=−0.90, p=7.5×10-4. SHR: slope=−5.41, r=−0.77, p=3.5×10-4. SHR+pregabalin: slope=−2.25, r=−0.72, p=1.1×10-4. D, Voltage-dependent CaV1.2 current activation in WKY (n=13), pregabalin-treated WKY (n=9), SHR (n=12) and pregabalin-treated SHR (n=6) cells. E, Voltage-dependent current inactivation of WKY (n=17), pregabalin-treated WKY (n=13), SHR (n=16) and pregabalin-treated SHR (n=18) cells. *indicates significance from WKY at indicated potentials (P<0.05). F, Graph illustrating the time course of CaV1.2 currents (at +20 mV) and inhibition by acute pregabalin (100 μmol/L). WKY (control n=8, pregabalin n=9), SHR (control n=14, pregabalin n=6) cells. The arrow (not applicable for controls) indicates where pregabalin was added. Pregabalin concentration in all figures was 100 μmol/L. * indicates P<0.05 when compared to untreated WKY, # indicates P<0.05 when compared to pregabalin-treated WKY and § indicates P<0.05 when compared to untreated SHR.
Table 1.
Properties of arterial myocyte CaV1.2 currents. Numbers in parentheses indicate experimental number.
| WKY | WKY + pregabalin | SHR | SHR + pregabalin | |
|---|---|---|---|---|
| IV relationship | ||||
| Peak current density (pA/pF) | 2.40±0.15 (17) | 1.65±0.13 (13)* | 5.33±0.38 (16)*,# | 2.23±0.14 (16) |
| Peak voltage (mV) | 10.25±0.74 (17) | 12.31±1.45 (13) | 11.09±1.03 (16) | 11.26±0.89 (18) |
| Voltage-dependent activation | ||||
| V1/2act (mV) | 9.24±1.88 (12) | 10.70±3.72 (9) | 11.62±1.80 (12) | 11.18±3.29 (5) |
| Slope | 13.09±1.55 (12) | 14.56±1.66 (9) | 12.74±2.84 (12) | 10.51±1.09 (5) |
| Voltage-dependent inactivation | ||||
| V1/2inact (mV) | −14.23±1.57 (8) | −17.20±1.28 (13) | −11.33±1.90 (16) | −14.71±1.15 (11) |
| Slope | 6.44±0.76 (8) | 8.58±1.16 (13) | 7.86±0.70 (16) | 8.09±0.69 (11) |
indicates P<0.05 compared to WKY and
indicates P<0.05 versus SHR + pregabalin.
The voltage-dependence of half-maximal CaV1.2 current activation (V1/2act) and slope (k) were similar in untreated control and pregabalin-treated WKY and SHR arterial myocytes (Fig. 4D and Table 1). The voltage-dependence of half-maximal inactivation (V1/2inact) and k were also similar in untreated control and pregabalin-treated WKY and SHR cells (Fig. 4E and Table 1). In contrast, untreated SHR cells displayed a non-inactivating CaV1.2 current that was ~2-fold larger than in WKY cells (Fig. 4A, E). Pregabalin (24 h) reduced the non-inactivating current in SHR cells such that it was similar to WKY cells (Fig. 4A, E). CaV1.2 current inactivation rates (τ) were similar in control and pregabalin-treated WKY and SHR cells (Fig. S4).
In addition to acting as an inhibitor of α2δ-1-induced CaV1.2 channel trafficking, pregabalin is a weak CaV1.2 channel pore blocker that does not directly alter CaV1.2 current voltage-dependence in normotensive SD rat arterial myocytes.16 To determine whether the reduction in CaV1.2 current amplitude in pregabalin-treated WKY and SHR rat myocytes was due to CaV1.2 pore block, we measured CaV1.2 current regulation in untreated cells by acute bath application of pregabalin. Acute pregabalin reduced CaV1.2 currents in WKY cells by ~12 % (Fig. 4F). In contrast, pregabalin reduced CaV1.2 currents in SHR myocytes by ~23 %, or ~1.9-fold more than in WKY cells (Fig. 4F). Acute pregabalin-induced CaV1.2 current inhibition was significantly smaller than that induced by 24 h pregabalin treatment in both WKY (~32 % inhibition) and SHR (~59 % inhibition) cells (Figs. 4B, C, F). When combined with the biochemical data illustrated in Figure 3, these data indicate that acute and chronic pregabalin inhibit CaV1.2 currents through distinct mechanisms in arterial myocytes.
Collectively, data indicate that genetic hypertension is associated with an elevation in α2δ-1 expression that stimulates surface expression of CaV1.2α1 subunits, leading to a CaV1.2 current elevation and an increase in non-inactivating current. α2δ-1 targeting reduces the hypertension-associated α2δ-1-induced elevation in CaV1.2α1 surface expression, leading to a reduction in CaV1.2 current density. α2δ-1 targeting also restores CaV1.2 current inactivation.
α2δ-1 targeting reverses elevated pressure- and depolarization-induced vasoconstriction in hypertension
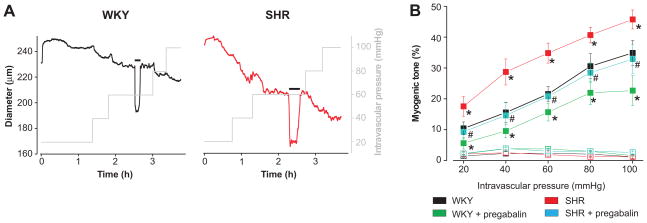
The functional significance of hypertension-associated alterations in α2δ-1 signaling was studied by measuring arterial contractility. Diameter regulation by intravascular pressure (20–100 mmHg) was measured in WKY and SHR cerebral arteries that had been incubated for 24 h with or without pregabalin. SHR arteries developed more myogenic tone than WKY arteries over the entire pressure range (Fig. 5A, B). Pregabalin reduced myogenic tone in WKY and SHR arteries, decreased tone more in SHR than in WKY arteries (e.g., % reduction in myogenic tone at 60 mmHg: SHR, ~41 %; WKY, ~28 %), and reduced tone in SHR arteries to levels in untreated WKY arteries (Fig. 5A, B). Nimodipine (1 μmol/L), a voltage-dependent Ca2+ channel blocker, fully dilated control and pregabalin-treated WKY and SHR arteries at all pressures (20–100 mmHg) studied, indicating that myogenic tone occurred due to CaV1.2 channel activation (Fig. 5B). Passive arterial diameters were similar for WKY (249 ± 11 μm) and hypertensive SHR (242 ± 9μm) cerebral arteries (values given at 60 mmHg, n=10 for each, P>0.05).
Figure 5.
Pregabalin reverses elevated pressure-induced vasoconstriction in hypertension. A, Representative traces illustrating steady-state myogenic tone in response to increasing intravascular pressure in a WKY (black) and SHR (red) artery. Horizontal black bars indicate an increase in bath K+ from 6 to 60 mmol/L. B Pregabalin (24 h, 100 μmol/L) reduced pressure (20 – 100 mmHg)-induced myogenic tone (filled symbols) more so in arteries from hypertensive rats than in controls. Myogenic tone was abolished by nimodipine (1 μmol/L, empty symbols). Mean data (n: WKY, 6–10; WKY + pregabalin, 6–9; SHR, 6–10; SHR+pregabalin, 6–7). * indicates P<0.05 when compared with untreated WKY and # indicates P<0.05 for SHR+pregabalin when compared with untreated SHR.
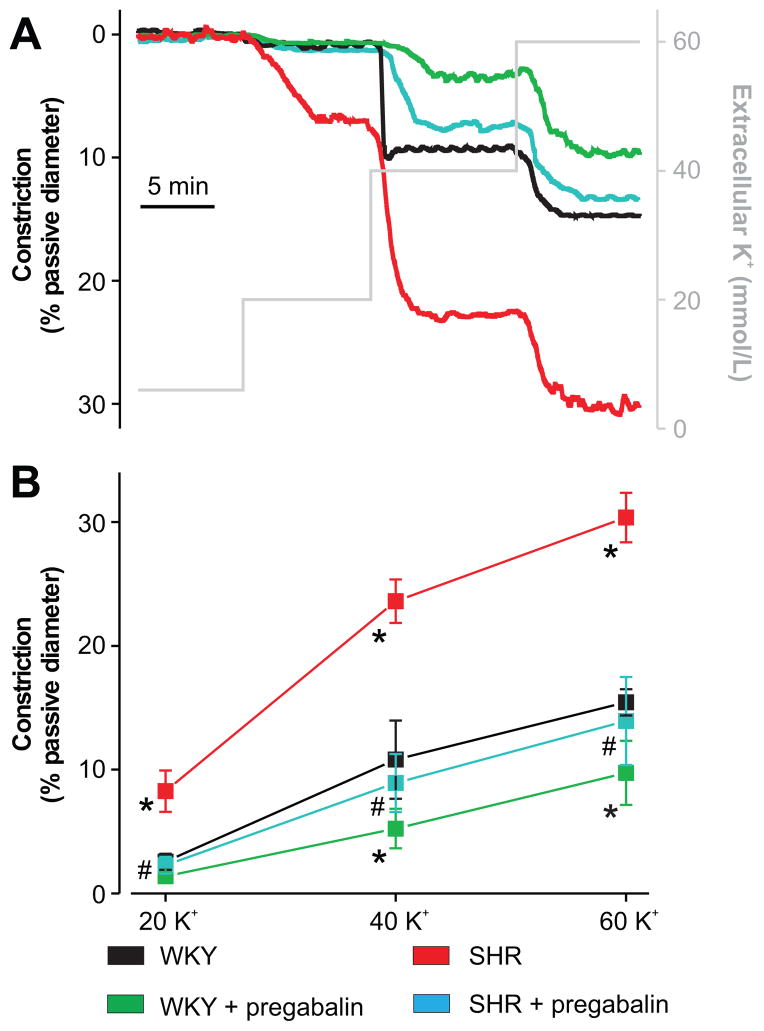
Elevating extracellular K+ induces depolarization, activation of voltage-gated Ca2+ channels, Ca2+ influx and vasoconstriction.4 As an alternative approach to investigate the functional impact of α2δ-1 targeting, we studied K+-induced vasoconstriction in WKY and SHR arteries. Increasing extracellular K+ from 6 to 20, 40, or 60 mmol/L induced graded vasoconstriction that was larger in SHR than WKY cerebral arteries (Fig. 6A, B). Pregabalin reduced K+-induced vasoconstriction more in SHR than WKY arteries (Fig. 6A, B). For example, pregabalin reduced the mean 60 mmol/L K+-induced constriction by ~54 % in SHR and ~37 % in WKY (Fig. 6B). These data indicate that α2δ-1 targeting reduces pressure- and depolarization-induced vasoconstriction more effectively in hypertensive SHR than in control WKY rat arteries.
Figure 6.
Pregabalin reverses elevated depolarization-induced vasoconstriction in hypertension. A, Representative traces illustrating diameter responses to increasing extracellular K+. B, Pregabalin (24 h, 100 μmol/L) reduced depolarization (20, 40, and 60 mmol/L K+)-induced vasoconstriction more so in arteries from hypertensive rats than controls. Mean data (n: WKY, 6; WKY + pregabalin, 6; SHR, 6; SHR + pregabalin, 6). * indicates P<0.05 when compared with untreated WKY, and # indicates P<0.05 for SHR + pregabalin when compared with untreated SHR.
Discussion
To date, no studies have investigated involvement of CaV1.2 channel auxiliary subunits in the pathological elevation of arterial myocyte CaV1.2 currents and vasoconstriction in hypertension. Here, we demonstrate for the first time that genetic hypertension is associated with transcriptional and post-translational upregulation of α2δ-1 subunits in myocytes of resistance-size arteries. The additional α2δ-1 subunits increase surface trafficking of CaV1.2α1 subunits, which are also elevated in hypertension. The consequent increase in surface α2δ-1 and CaV1.2α1 proteins elevates CaV1.2 current density and generates a non-inactivating current, leading to vasoconstriction. We also demonstrate that α2δ-1 targeting normalizes myocyte α2δ-1 and CaV1.2α1 surface expression, re-establishes CaV1.2 current density and inactivation, and reduces hypertensive rat artery contractility to levels in controls. These data indicate that α2δ-1 elevates CaV1.2 currents and CaV1.2-dependent vasoconstriction during hypertension and demonstrate that α2δ-1 targeting is a viable therapeutic strategy to reverse these pathological alterations and induce cerebrovascular dilation.
Our data indicate that the development of genetic hypertension is associated with a transcriptional and post-translational increase in α2δ-1 and CaV1.2α1 in arterial myocytes. In contrast, other α2δ isoforms did not emerge during hypertension, an alteration that could have contributed to pathological CaV1.2 current modifications. Previous studies have described that CaV1.2α1 mRNA and protein is higher in mesenteric arteries and aorta of hypertensive SHR than WKY rat controls.21, 22 In contrast, angiotensin II- and hypoxia-induced hypertension did not alter CaV1.2α1 mRNA, but elevated CaV1.2α1 protein in cultured mesenteric arteries and neonatal piglet pulmonary arteries.23, 24 These findings lead to the proposal that hypertension may not be associated with an increase in CaV1.2α1 message, but post-translational upregulation of CaV1.2α1 protein.21–24 Here, we used both age-dependent development of hypertension in SHR and comparison to WKY rat controls to investigate relative changes in α2δ-1 and CaV1.2α1 mRNA and protein. Our data indicate that the increase in α2δ-1 (~2.1-fold) and CaV1.2α1 (~1.5-fold) mRNA cannot fully account for the elevation in α2δ-1 (~2.5-fold) and CaV1.2α1 (~1.7-fold) proteins during hypertension. These data indicate that both transcriptional and post-translational mechanisms elevate α2δ-1 and CaV1.2α1 proteins in cerebral artery myocytes during hypertension.
Using a novel application of biotinylation, we recently determined the surface to intracellular distribution of arterial α2δ-1 and CaV1.2α1 proteins in normotensive rats.16 Essentially all (>95 %) α2δ-1 and CaV1.2α1 proteins locate to the surface in cerebral artery myocytes of normotensive SD rats.16 Here, a smaller percentage of total α2δ-1 (~85%) and CaV1.2α1 (~77 %) was located in the plasma membrane in WKY rat arteries. Explanations for slight differences in α2δ-1 and CaV1.2α1 distribution between SD and WKY rats include the different rat strains and animal age (7 weeks in ref. 16 vs 12 weeks here). To determine the cellular distribution of α2δ-1 and CaV1.2α1 subunit proteins in SHR and WKY cerebral arteries, we compared the percentage of total protein expressed at the surface and the surface:intracellular protein ratio in both SHR and WKY cerebral arteries. Both of these analysis methods indicate that a higher proportion of α2δ-1 and CaV1.2α1 is located at the plasma membrane in hypertensive rat arteries than in controls. The net result of both the transcription and translational increase in α2δ-1 and CaV1.2α1 protein and higher relative surface expression elevates plasma membrane levels of these proteins. Our data also indicate that there is a fractional shift in surface α2δ-1:CaV1.2α1 during hypertension, a change that occurs due to a larger elevation in surface α2δ-1 than CaV1.2α1. These results provide evidence that an elevation in α2δ-1 to CaV1.2α1 subunit ratio can modify native CaV1.2 current properties and that there may not be rigid α2δ-1:CaV1.2α1 subunit stoichiometry in arterial myocytes. Also possible is that in normotension, a proportion of arterial myocyte CaV1.2 channel complexes may not contain α2δ-1 subunits. During hypertension, the higher elevation in surface α2δ-1 than CaV1.2α1 may increase the proportion of channels that contain α2δ-1. Future studies should be designed to further investigate native CaV1.2 channel stoichiometry in arterial myocytes and changes that occur in cardiovascular disease. Collectively, these results indicate that α2δ-1 increases surface expression and functionality of CaV1.2α1 subunits in arterial myocytes during hypertension.
Pregabalin is a gabapentinoid drug used to treat neuropathic pain, fibromyalgia, and epileptic seizures.25, 26 Of all four α2δ isoforms, only α2δ-1 and -2 contain complete metal ion adhesion site and RRR motifs which are required for gabapentanoid drug binding.14, 20 Gabapanetin reduced α2δ subunit recycling from Rab-11-positive recycling endosomes.27 Pregabalin also reduced surface expression of both α2δ-1 and CaV1.2α1 proteins in cerebral artery myocytes of normotensive SD rats.16 Here, pregabalin did not alter total α2δ-1 or CaV1.2α1 protein. Rather, pregabalin reduced surface α2δ-1 and CaV1.2α1 more in hypertensive arteries than in control rat arteries, essentially normalizing surface levels of these proteins to those in WKY. Pregabalin also reduced the α2δ-1 to CaV1.2α1 subunit ratio in hypertensive rat arteries to that in WKY controls. Given that pregabalin normalized elevated CaV1.2 current density and the proportion of non-inactivating current to those in WKY cells, our data indicate that upregulated α2δ-1 functionality contributes to the increase in CaV1.2 currents in arterial myocytes in hypertension.
Voltage-dependent Ca2+ currents are elevated in myocytes from vasculature including renal, cerebral and mesenteric arteries, when studying a variety of different hypertension models such as SHR, angiotensin II-induced, aortic banding, stroke-prone SHR, hypoxia-induced pulmonary hypertension, and Osborne-Mendel rats on a high fat diet.2, 3, 7, 9, 21, 23, 24, 28–30 CaV1.2 current density measured here is consistent with that previously reported in cerebral artery myocytes when using WKY and SHR rat models.2, 30 Our data indicate that CaV1.2 current density was ~2.2-fold larger in hypertensive than control rat arterial myocytes. In contrast, CaV1.2 current V1/2act and V1/2inact were similar in WKY and SHR cells, consistent with previous reports.2, 9, 28, 30 A non-inactivating CaV1.2 current in myocytes of hypertensive rats was double that in controls, a modification that would significantly increase Ca2+ influx at steady-state membrane potentials, thereby stimulating vasoconstriction. Pregabalin (24 h) reduced elevated CaV1.2 current density and non-inactivating current to levels in controls, suggesting that these pathological modifications occurred due to an increase in α2δ-1 surface expression. Our data are consistent with pregabalin acting as both a weak CaV1.2 channel pore blocker and an effective chronic inhibitor of α2δ-1 surface expression in hypertensive rat arterial myocytes. In our previous study, acute pregabalin reduced CaV1.2 currents by ~33 % in SD rat cerebral artery myocytes.16 Here, the same acute pregabalin concentration reduced CaV1.2 currents by ~12 % in WKY rat myocytes. Our previous study used 7 week old SD rats, whereas here acute pregabalin effects were measured in 12 week old WKY rat myocytes. Our data indicate that CaV1.2 channel properties in 12 week old WKY myocytes are not identical to those in 7 week old SD rat myocytes, including the percentage of CaV1.2α1 protein that is located at the surface. Data here indicate that acute pregabalin is a more effective inhibitor of CaV1.2 currents in SHR than WKY myocytes. This may be due to the higher number of surface α2δ-1 subunits and the higher α2δ-1:CaV1.2 ratio in SHR myocytes. Acute gabapentin also inhibited voltage-dependent Ca2+ currents in pyramidal neocortical cells, but did not alter currents generated by recombinant CaV2.1 channels or endogenous Ca2+ channels in dorsal root ganglia neurons.31, 32 In a model of neuropathic pain in which α2δ-1 is upregulated, chronic pregabalin inhibited α2δ-1 trafficking to pre-synaptic terminals, thereby inhibiting Ca2+ channel function.19 Our data indicate that chronic pregabalin inhibits α2δ-1-induced trafficking of CaV1.2α1 channel subunits, thereby reducing CaV1.2 currents in arterial myocytes during hypertension.
Intravascular pressure and depolarization both stimulated a larger vasoconstriction in arteries of hypertensive rats than in controls. Consistent with our findings, pressure-induced Ca2+ influx and associated vasoconstriction were larger in arteries from animal models with both genetic- and induced-hypertension.7, 21, 23, 24 We show that nimodipine abolished myogenic tone at all pressures, indicating that CaV1.2 channel activity was essential to generate tone in hypertensive and control rat arteries. Chronic pregabalin (24 h) was a more effective vasodilator of hypertensive than control rat arteries, effectively reversing the pathological vasoconstriction. Pregabalin is also a weak CaV1.2 channel pore blocker, which induces a small vasodilation.16 Thus, pregabalin-induced CaV1.2 pore block may also have contributed to vasodilation in both WKY and SHR arteries. Although unlikely, pregabalin could have caused vasodilation through additional mechanisms, including through inducing membrane hyperpolarization. Our data are inconsistent with this possibility as pregabalin similarly reduced surface CaV1.2 subunits, CaV1.2 currents, myogenic tone and K+-induced constriction and inhibition of pressure- and depolarization-induced vasoconstriction were equivalent. Thus, data demonstrate that pregabalin dilates hypertensive rat arteries primarily by reducing surface expression of CaV1.2 subunits in myocytes.
Hypertension is associated with increased risk for cerebral diseases, including stroke, Alzheimer’s disease, and dementia. Cerebral blood flow is reduced in hypertensive humans and 12 week old SHR rats, when compared to normotensive controls.33, 34 Voltage-dependent Ca2+ channel blockers have been used for over two decades to treat hypertension.35 However, Ca2+ channel blockers inhibit CaV1.2 channels in multiple cell types in vivo and induce multiple side effects, including sweating, edema, and nausea.35, 36 Therefore, the development of alternative approaches to target CaV1.2 channels in arterial myocytes could provide significant benefits over current inhibitors. Here, we used pregabalin, as an in vitro tool to test the concept that α2δ-1 targeting induces vasodilation in cerebral arteries of hypertensive animals. Data here provide a foundation for future studies aimed at developing novel approaches to target α2δ-1 in arterial myocytes. All data in our study were obtained by studying cerebral arteries that regulate brain regional blood flow but do not control systemic blood pressure. Clinical pregabalin does not appear to modify systemic blood pressure in normotensive humans at doses used to treat neuropathic pain, fibromyalgia, and epileptic seizures.26 There are several explanations for this observation. First, there are a large number of distinct mechanisms that control cerebral and systemic artery contractility. To date, no studies have examined the molecular identity or physiological functions of α2δ subunits in systemic artery myocytes that regulate diastolic and systolic blood pressure. α2δ-1 may not be the principal α2δ isoform, or α2δ subunits may not regulate CaV1.2 channel activity in systemic artery myocytes. Pregabalin is an α2δ-1/2 ligand. If α2δ-1 or α2δ-2 are not expressed or do not regulate CaV1.2 channels in systemic artery myocytes, pregabalin should not induce systemic vasodilation or alter blood pressure. Second, clinical doses of pregabalin that are used to treat neuropathic pain, fibromyalgia, and epileptic seizures may be insufficient to induce vasodilation in vivo. Gabapentin, a lower affinity pregabalin analogue, enters cells through system-L neutral amino acid transporters.32 Arterial myocytes may not uptake pregabalin as effectively as neurons. In vivo, intracellular myocyte pregabalin concentrations may be less than those obtained in vitro that alter CaV1.2 function. Third, many in vivo mechanisms, including those mediated by baroreceptors or the renin-angiotensin sytem, may compensate for pregabalin-induced systemic vasodilation, leading to no net change in blood pressure. Fourth, our data indicate that pregabalin is more effective at inhibiting CaV1.2α1 subunit trafficking in cerebral artery myocytes of hypertensive than normotensive rats. In vivo, pregabalin may be a more effective vasodilator in hypertensive subjects and have a smaller effect in normotensive subjects in which clinical systemic blood pressure measurements have been obtained. Our study provides the first evidence that arterial myocyte α2δ-1 functionality is upregulated in hypertension and that α2δ-1 targeting is a novel approach for reducing pathological vasoconstriction in hypertension. Data also indicate that α2δ-1 targeting can modify cerebral artery contractility, setting the stage for future studies to use a variety of other α2δ-1 targeting strategies, including RNA interference and genetic models, to investigate physiological and pathological involvement of α2δ subunits in arteries of other vascular beds and in vivo.
In summary, we identify for the first time that a hypertension-associated increase in α2δ-1 elevates CaV1.2α1 surface expression in arterial myocytes leading to pressure- and depolarization-induced vasoconstriction. Our data also indicate that α2δ-1 targeting is a novel approach to reverse elevated CaV1.2 channel surface expression in arterial myocytes and vasoconstriction in hypertension.
Supplementary Material
Perspectives.
A hallmark of hypertension is an increase in voltage-dependent CaV1.2 currents in arterial myocytes that induces vasoconstriction. 1–3 Molecular mechanisms that elevate arterial myocyte CaV1.2 currents in hypertension and the significance of auxiliary subunits in this pathological alteration are unclear. We show that the development of genetic hypertension is associated with a transcriptional and post-translational upregulation of α2δ-1 subunits in cerebral artery myocytes. This increase in α2δ-1 subunits elevates CaV1.2 channel surface expression, CaV1.2 current density, and vasoconstriction. α2δ-1 targeting using pregabalin, an α2δ-1 ligand, reduced α2δ-1 and CaV1.2 surface expression and CaV1.2 current density in myocytes. Pregabalin also dilated cerebral arteries of hypertensive rats. Our study provides the first evidence that α2δ-1 subunits are upregulated in cerebral artery myocytes during hypertension and contribute to the pathological elevation in myocyte CaV1.2 currents and vasoconstriction. We also identify α2δ-1 as a potential novel therapeutic target for inducing cerebrovascular dilation in hypertension.
Novelty and Significance.
1) What is new?
We demonstrate for the first time that genetic hypertension is associated with a transcriptional and post-translational upregulation of α2δ-1 subunits in myocytes of resistance-size cerebral arteries that increase CaV1.2α1 subunit surface trafficking, thereby elevating CaV1.2 current density and arterial contractility.
Pharmacological targeting of α2δ-1 inhibits the pathological increase in CaV1.2 current density and cerebral artery contractility during hypertension. This study identifies α2δ-1 as a novel therapeutic target for inducing cerebrovascular vasodilation in hypertension.
2) What Is Relevant?
Upregulation of α2δ-1 subunits is essential for the elevation in CaV1.2 current density and cerebrovascular tone in genetic hypertension.
Pharmacological targeting of α2δ-1 can reverse the pathological elevation in surface CaV1.2 channels, CaV1.2 current density and vasoconstriction in cerebral artery myocytes.
3) Summary
Upregulation of α2δ-1 subunits during genetic hypertension increases CaV1.2 channel surface expression and CaV1.2 current density, leading to vasoconstriction. α2δ-1 targeting reverses this pathological increase in CaV1.2 channel surface expression, CaV1.2 current density and contractility in cerebral arteries.
Acknowledgments
We thank Dr. Marie Dennis Leo for critical reading of the manuscript.
Funding Sources
This work was supported by NIH grants to J.H.J.
Non-standard Abbreviations and Acronyms
- None
Footnotes
Conflict of Interest/Disclosures
None.
References
- 1.Schmieder RE. End organ damage in hypertension. Dtsch Arztebl Int. 2010;107:866–873. doi: 10.3238/arztebl.2010.0866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wilde DW, Furspan PB, Szocik JF. Calcium current in smooth muscle cells from normotensive and genetically hypertensive rats. Hypertension. 1994;24:739–746. doi: 10.1161/01.hyp.24.6.739. [DOI] [PubMed] [Google Scholar]
- 3.Cox RH, Lozinskaya IM. Augmented calcium currents in mesenteric artery branches of the spontaneously hypertensive rat. Hypertension. 1995;26:1060–1064. doi: 10.1161/01.hyp.26.6.1060. [DOI] [PubMed] [Google Scholar]
- 4.Jaggar JH. Intravascular pressure regulates local and global Ca2+ signaling in cerebral artery smooth muscle cells. Am J Physiol. 2001;281:C439–C448. doi: 10.1152/ajpcell.2001.281.2.C439. [DOI] [PubMed] [Google Scholar]
- 5.Gollasch M, Nelson MT. Voltage-dependent Ca2+ channels in arterial smooth muscle cells. Kidney Blood Press Res. 2000;20:355–371. doi: 10.1159/000174250. [DOI] [PubMed] [Google Scholar]
- 6.Navedo MF, Amberg GC, Westenbroek RE, Sinnegger-Brauns MJ, Catterall WA, Striessnig J, Santana LF. CaV1.3 channels produce persistent calcium sparklets, but CaV1.2 channels are responsible for sparklets in mouse arterial smooth muscle. Am J Physiol Heart Circ Physiol. 2007;293:H1359–H1370. doi: 10.1152/ajpheart.00450.2007. [DOI] [PubMed] [Google Scholar]
- 7.Sonkusare S, Palade PT, Marsh JD, Telemaque S, Pesic A, Rusch NJ. Vascular calcium channels and high blood pressure: pathophysiology and therapeutic implications. Vascul Pharmacol. 2006;44:131–142. doi: 10.1016/j.vph.2005.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wamhoff BR, Bowles DK, Owens GK. Excitation-transcription coupling in arterial smooth muscle. Circ Res. 2006;98:868–878. doi: 10.1161/01.RES.0000216596.73005.3c. [DOI] [PubMed] [Google Scholar]
- 9.Pesic A, Madden JA, Pesic M, Rusch NJ. High blood pressure upregulates arterial L-type Ca2+ channels: is membrane depolarization the signal? Circ Res. 2004;94:e97–104. doi: 10.1161/01.RES.0000131495.93500.3c. [DOI] [PubMed] [Google Scholar]
- 10.Sonkusare S, Fraer M, Marsh JD, Rusch NJ. Disrupting calcium channel expression to lower blood pressure: new targeting of a well-known channel. Mol Interv. 2006;6:304–310. doi: 10.1124/mi.6.6.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Falcone JC, Granger HJ, Meininger GA. Enhanced myogenic activation in skeletal muscle arterioles from spontaneously hypertensive rats. Am J Physiol. 1993;265:H1847–H1855. doi: 10.1152/ajpheart.1993.265.6.H1847. [DOI] [PubMed] [Google Scholar]
- 12.Catterall WA. Structure and regulation of voltage-gated Ca2+ channels. Annu Rev Cell Dev Biol. 2000;16:521–555. doi: 10.1146/annurev.cellbio.16.1.521. [DOI] [PubMed] [Google Scholar]
- 13.Cole RL, Lechner SM, Williams ME, Prodanovich P, Bleicher L, Varney MA, Gu G. Differential distribution of voltage-gated calcium channel alpha-2 delta (α2δ) subunit mRNA-containing cells in the rat central nervous system and the dorsal root ganglia. J Comp Neurol. 2005;491:246–269. doi: 10.1002/cne.20693. [DOI] [PubMed] [Google Scholar]
- 14.Davies A, Hendrich J, Van Minh AT, Wratten J, Douglas L, Dolphin AC. Functional biology of the α2δ subunits of voltage-gated calcium channels. Trends Pharmacol Sci. 2007;28:220–228. doi: 10.1016/j.tips.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 15.Andrade A, Sandoval A, Oviedo N, De WM, Elias D, Felix R. Proteolytic cleavage of the voltage-gated Ca2+ channel α2δ subunit: structural and functional features. Eur J Neurosci. 2007;25:1705–1710. doi: 10.1111/j.1460-9568.2007.05454.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bannister JP, Adebiyi A, Zhao G, Narayanan D, Thomas CM, Feng JY, Jaggar JH. Smooth muscle cell α2δ-1 subunits are essential for vasoregulation by CaV1.2 channels. Circ Res. 2009;105:948–955. doi: 10.1161/CIRCRESAHA.109.203620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Adebiyi A, McNally EM, Jaggar JH. Sulfonylurea receptor-dependent and -independent pathways mediate vasodilation induced by KATP channel openers. Mol Pharmacol. 2008;74:736–743. doi: 10.1124/mol.108.048165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Taylor CP, Angelotti T, Fauman E. Pharmacology and mechanism of action of pregabalin: the calcium channel α2-δ (alpha2-delta) subunit as a target for antiepileptic drug discovery. Epilepsy Res. 2007;73:137–150. doi: 10.1016/j.eplepsyres.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 19.Bauer CS, Nieto-Rostro M, Rahman W, Tran-Van-Minh A, Ferron L, Douglas L, Kadurin I, Sri RY, Fernandez-Alacid L, Millar NS, Dickenson AH, Lujan R, Dolphin AC. The increased trafficking of the calcium channel subunit α2δ-1 to presynaptic terminals in neuropathic pain is inhibited by the α2δ ligand pregabalin. J Neurosci. 2009;29:4076–4088. doi: 10.1523/JNEUROSCI.0356-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dooley DJ, Taylor CP, Donevan S, Feltner D. Ca2+ channel α2δ ligands: novel modulators of neurotransmission. Trends Pharmacol Sci. 2007;28:75–82. doi: 10.1016/j.tips.2006.12.006. [DOI] [PubMed] [Google Scholar]
- 21.Pratt PF, Bonnet S, Ludwig LM, Bonnet P, Rusch NJ. Upregulation of L-type Ca2+ channels in mesenteric and skeletal arteries of SHR. Hypertension. 2002;40:214–219. doi: 10.1161/01.hyp.0000025877.23309.36. [DOI] [PubMed] [Google Scholar]
- 22.Wang WZ, Saada N, Dai B, Pang L, Palade P. Vascular-specific increase in exon 1B-encoded CaV1.2 channels in spontaneously hypertensive rats. Am J Hypertens. 2006;19:823–831. doi: 10.1016/j.amjhyper.2006.01.020. [DOI] [PubMed] [Google Scholar]
- 23.Wang WZ, Pang L, Palade P. Angiotensin II causes endothelial-dependent increase in expression of CaV1.2 protein in cultured arteries. Eur J Pharmacol. 2008;599:117–120. doi: 10.1016/j.ejphar.2008.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hirenallur-S DK, Haworth ST, Leming JT, Chang J, Hernandez G, Gordon JB, Rusch NJ. Upregulation of vascular calcium channels in neonatal piglets with hypoxia-induced pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol. 2008;295:L915–L924. doi: 10.1152/ajplung.90286.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jensen TS, Madsen CS, Finnerup NB. Pharmacology and treatment of neuropathic pains. Curr Opin Neurol. 2009;22:467–474. doi: 10.1097/WCO.0b013e3283311e13. [DOI] [PubMed] [Google Scholar]
- 26.Gajraj NM. Pregabalin: its pharmacology and use in pain management. Anesth Analg. 2007;105:1805–1815. doi: 10.1213/01.ane.0000287643.13410.5e. [DOI] [PubMed] [Google Scholar]
- 27.Tran-Van-Minh A, Dolphin AC. The α2δ ligand gabapentin inhibits the Rab11-dependent recycling of the calcium channel subunit α2δ-2. J Neurosci. 2010;30:12856–12867. doi: 10.1523/JNEUROSCI.2700-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Simard JM, Li X, Tewari K. Increase in functional Ca2+ channels in cerebral smooth muscle with renal hypertension. Circ Res. 1998;82:1330–1337. doi: 10.1161/01.res.82.12.1330. [DOI] [PubMed] [Google Scholar]
- 29.Wilde DW, Massey KD, Walker GK, Vollmer A, Grekin RJ. High-fat diet elevates blood pressure and cerebrovascular muscle Ca2+ current. Hypertension. 2000;35:832–837. doi: 10.1161/01.hyp.35.3.832. [DOI] [PubMed] [Google Scholar]
- 30.Xie MJ, Zhang LF, Ma J, Cheng HW. Functional alterations in cerebrovascular K+ and Ca2+ channels are comparable between simulated microgravity rat and SHR. Am J Physiol Heart Circ Physiol. 2005;289:H1265–H1276. doi: 10.1152/ajpheart.00074.2005. [DOI] [PubMed] [Google Scholar]
- 31.Stefani A, Spadoni F, Bernardi G. Gabapentin inhibits calcium currents in isolated rat brain neurons. Neuropharmacology. 1998;37:83–91. doi: 10.1016/s0028-3908(97)00189-5. [DOI] [PubMed] [Google Scholar]
- 32.Hendrich J, Van Minh AT, Heblich F, Nieto-Rostro M, Watschinger K, Striessnig J, Wratten J, Davies A, Dolphin AC. Pharmacological disruption of calcium channel trafficking by the α2δ ligand gabapentin. Proc Natl Acad Sci U S A. 2008;105:3628–3633. doi: 10.1073/pnas.0708930105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fujishima M, Ibayashi S, Fujii K, Mori S. Cerebral blood flow and brain function in hypertension. Hypertens Res. 1995;18:111–117. doi: 10.1291/hypres.18.111. [DOI] [PubMed] [Google Scholar]
- 34.Katsuta T. Decreased local cerebral blood flow in young and aged spontaneously hypertensive rats. Fukuoka Igaku Zasshi. 1997;88:65–74. [PubMed] [Google Scholar]
- 35.Grossman E, Messerli FH. Calcium antagonists. Prog Cardiovasc Dis. 2004;47:34–57. doi: 10.1016/j.pcad.2004.04.006. [DOI] [PubMed] [Google Scholar]
- 36.Meka N, Katragadda S, Cherian B, Arora RR. Combination therapy in hypertension: A focus on angiotensin receptor blockers and calcium channel blockers. Am J Ther. 2010;17:61–67. doi: 10.1097/MJT.0b013e31815db6c0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.