Abstract
Electrical stimulation of the cervical vagus nerve reduces infarct size by approximately 50% after cerebral ischemia in rats. The mechanism of ischemic protection by vagus nerve stimulation (VNS) is not known. In this study, we investigated whether the infarct reducing effect of VNS was mediated by activation of the parasympathetic vasodilator fibers that originate from the sphenopalatine ganglion (SPG) and innervate the anterior cerebral circulation. We examined the effects of electrical stimulation of the cervical vagus nerve in two groups of rats: one with and one without SPG ablation. Electrical stimulation was initiated 30 min after induction of ischemia, and lasted for 1h. Measurement of infarct size 24h later revealed that the volume of ischemic damage was smaller in those animals that received VNS treatment (41.32 ± 2.07% vs. 24.19 ± 2.62% of the contralateral hemispheric volume, n=6 in both; p<0.05). SPG ablation did not abolish this effect; the reduction in infarct volume following VNS was 58% in SPG-damaged animals, 41% in SPG-intact animals (p>0.05). In both SPG-intact and SPG-damaged animals VNS treatment resulted in better motor outcome (p<0.05 vs. corresponding controls for both). Our findings show that VNS can protect the brain against acute ischemic injury, and that this effect is not mediated by SPG projections.
Keywords: Cerebral ischemia, vagus nerve, sphenopalatine ganglion, cerebral blood flow, electrical stimulation, rat
Introduction
Electrical stimulation of the right cervical vagus nerve reduces infarct volume by 50% after ipsilateral middle cerebral artery (MCA) occlusion in rats (Ay et al., 2009; Ay et al., 2011; Sun et al., 2011). Understanding the mechanism by which VNS exerts ischemic protection requires careful consideration of the anatomical connections of the vagus nerve in the central nervous system. Afferent fibers of the vagus nerve synapse in the nucleus tractus solitarius (NTS) within the medulla, from which projections synapse with key structures such as the fastigial nucleus, locus ceruleus, raphe nucleus, thalamus, and superior salivatory nucleus (Agassandian et al., 2003; Kalia et al., 1980a; Kalia et al., 1980b; Li et al., 2010; Morgane et al., 1979). Among these structures, the superior salivatory nucleus appears to have particular importance. The parasympathetic fibers arising from the superior salivatory nucleus in the pons run along the facial nerve until they synapse in the sphenopalatine ganglion (SPG), from which projections innervate the anterior cerebral circulation via the greater superficial petrosal nerve (Edvinsson et al., 2002; Walters et al., 1986). Electrical stimulation of the SPG and the superficial petrosal nerve leads to a 10–20% increase in vessel diameter in experimental animals, and in turn, improved CBF (Toda et al., 2000a; Toda et al., 2000b). Surgical ablation of the SPG is associated with reduced CBF and larger infarct size after MCA occlusion in rats (Boysen et al., 2009; Kano et al., 1991; Koketsu et al., 1992). In contrast, electrical stimulation of the nerve bundle that projects from the SPG is associated with smaller infarct size in animals with permanent MCAO (Henninger et al., 2007). Given the known anatomical connections between afferent vagal fibers and the superior salivatory nucleus, we sought to determine whether VNS-induced protection after focal cerebral ischemia was mediated by activation of the SPG. To do so, we examined the effect of VNS on infarct size after transient MCA occlusion in rats with and without bilateral SPG ablation.
Material and Methods
All experiments were performed in accordance with the NIH Guide for the Care and Use of Laboratory Animals, and were approved by the Massachusetts General Hospital Subcommittee on Research Animal Care. Adult male Wistar rats (350–400 g, Charles River Laboratories, Wilmington, MA) were used. Animals were randomly selected for VNS treatment and SPG ablation, and assigned to one of four experimental groups: txSPGi (SPG-intact VNS; n=6), txSPGd (SPG-damaged VNS; n=6), shamSPGi (SPG-intact no VNS; n=6), and shamSPGd (SPG-damaged no VNS; n=6). All surgical procedures were performed under isoflurane anesthesia (4–5% in 30% oxygen and 70% nitrous oxide for induction, 1–2% in room air for maintanance). Rectal temperature was measured intermittently throughout surgery, and maintained at 37.5 °C by a homeothermic blanket. To alleviate pain, 0.25% bupivacaine was applied topically to the wounds, and buprenorphine HCl (0.05 mg/kg) was injected subcutaneously at the end of the surgical procedures.
SPG ablation
Animals were first assigned to one of two groups for the SPG ablation (SPG-damaged group; SPGd) or a sham surgical procedure (SPG-intact group; SPGi). A sagittal scalp incision was made near the right and left upper orbit, and the medial orbital wall was exposed to visualize the ethmoidal foramen (Hara et al., 1986; Suzuki et al., 1990). Intraorbital structures and retroorbital fat were retracted laterally (Suzuki et al., 1988). In the SPGd animals, all structures traversing the ethmoidal foramen were transected, including parasympathetic nerve fibers from the SPG and the nasociliary nerve. (Suzuki et al., 1989). Because perivascular fibers from the SPG innervate cerebral arteries bilaterally (Hara et al., 1986), transection was performed on both sides. In the SPGi animals, the medial orbital wall was exposed bilaterally, and the nerve fibers traversing the ethmoidal foramen were visualized but left intact.
MCA occlusion
Ischemia surgery was carried out one week after SPG ablation. The right femoral artery was cannulated to monitor arterial blood pressure (ABP), heart rate (HR), arterial blood gases, and pH. A burr hole was drilled over the right parietal cortex, 5 mm lateral and 1 mm posterior to bregma, to position the laser Doppler flowmeter probe, and regional CBF (rCBF) was measured continuously, prior to the start of ischemia until early reperfusion (Blood FlowMeter, ADInstruments, Colorado Springs, CO). To ensure that the laser Doppler signals received were not affected by the surgery, the rat was placed supine after drilling burr hole for Doppler probe. All the surgical procedures (including preparation of the vagus nerve for electrode implantation) were performed except the common carotid artery (CCA) clamping and the intraarterial filament placement into the internal carotid artery (ICA). Then, animal’s head was extended and immobilized in a stereotaxic frame in supine position, and the laser Doppler probe was placed with the help of a micromanipulator and a magnifying mirror. With this experimental setup it is possible to access the cervical area and perform minor procedures (e.g., CCA clamping, inserting the occluding filament into the ICA, and implanting the stimulation electrodes into the vagus nerve) without affecting laser Doppler signal (Hungerhuber et al., 2006; Lumenta et al., 2006; Schmid-Elsaesser et al., 1998). To induce cerebral ischemia, a 4-0 nylon monofilament coated with silicone (diameter: 0.39 ± 0.02 mm; Doccol Corporation, Redlands, CA) was introduced into the right ICA, to a depth of about 19 mm, until it occluded the origin of the MCA (Longa et al., 1989). The filament was left in place for two hours, and then withdrawn to allow reperfusion. Since laser Doppler flowmetry does not provide absolute flow values, we used a conservative definition for ischemia (reduction in the laser Doppler signal of ≥60% of baseline) based on STAIR criteria (1999).
VNS
The SPGi and SPGd groups were subdivided for VNS treatment as tx (VNS) and sham (no VNS). Self-constructed VNS electrodes (Ay et al., 2009; Smith et al., 2005) were implanted on the right cervical vagus nerve. In txSPGi and txSPGd groups, VNS treatment was initiated 30 min after ischemia. A 30 sec train of 0.5 msec square pulses at an amplitude of 0.5 mA and frequency of 20 Hz was delivered at 5 min intervals, for one hour (Grass Model S48 stimulator and constant current unit, Grass Instruments, West Warwick, RI). These procedures (including electrode implantation into the vagus nerve) were duplicated for the sham stimulation paradigm (shamSPGi and shamSPGd), although the animals were not exposed to electrical stimulation.
Neurological evaluation
At the end of the experimental period (20 min after reperfusion), the electrodes and arterial catheters were removed, incisions were sutured, and animals were allowed to awaken from anesthesia. Neurological deficit was evaluated on a five-point scale (0=no deficit to 4=no spontaneous walking) (Bederson et al., 1986; Longa et al., 1989) 3h and 24h after ischemia.
Histology
Animals were euthanized 24h after ischemia by potassium chloride injection under isoflurane anesthesia, and the brain was rapidly removed. Starting from the frontal, the brain was immediately sliced into seven 2 mm-thick sections using a brain matrix. These sections were incubated with 2,3,5-triphenyltetrazolium chloride at room temperature for 30 min, and then transferred into 10% formalin and kept at 4 °C for 48h. Images of these sections were then obtained using a digital camera. The infarct area as well as the ipsilateral non-infarct area and controlateral hemispheric area were outlined manually in all of the sections using Image J (NIH), in a blinded fashion. The infarct volume was calculated by multiplying the infarct area (contralateral hemispheric area minus ipsilateral non-infarct area) by slice thickness and expressed as a percentage of the contralateral hemispheric volume.
Sample size and data analysis
Sample size calculation (n=6) was based on a hypothesized reduction of 40% in mean infarct volume in the treatment group compared with the control group, using an alpha of 0.05 (two-tailed) with 95% power. Animals that did not show significant neurological deficit (i.e., functional score < 2) 3h after ischemia, as well as those that developed infarction limited to the striatum or exhibited intracranial bleeding were considered technical failures. In such cases, the animals were replaced to reach the calculated sample size.
With the exception of neurological deficit measures, which are expressed as median ± interquartile range (IQR), the analyzed data are expressed as mean ± SEM. Laser Doppler flowmeter measurements are expressed as a percentage of baseline measurements. Physiological measurements (ABP, HR, blood gases and pH, and temperature) were analyzed by repeated measures ANOVA, and when needed, by the Student-Newman-Keuls test. Infarct volumes were compared using two-way ANOVA, and when needed, by the Student-Newman-Keuls test. Neurological scores were compared using repeated measures ANOVA, and as needed, followed by the Mann-Whitney U test. A p value of <0.05 was considered statistically significant.
Results
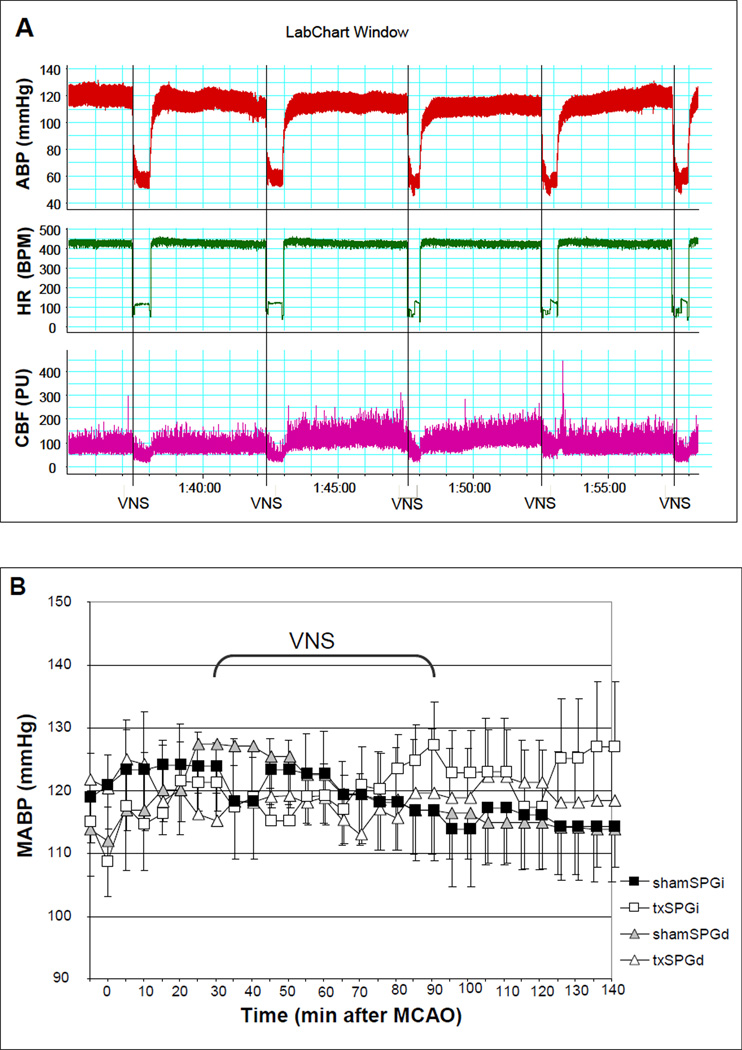
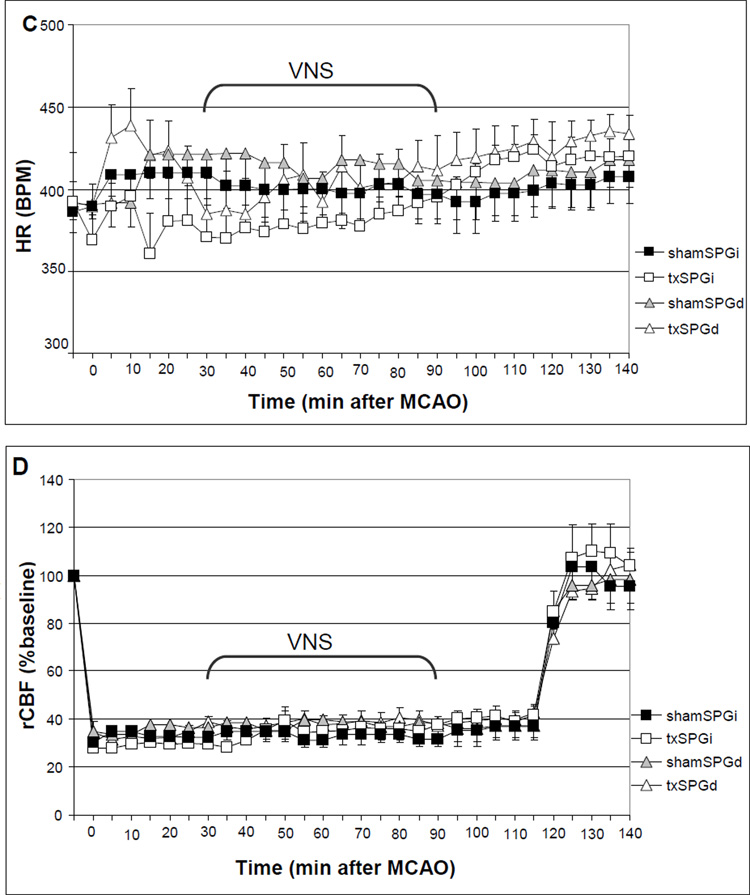
We excluded four animals that met the predefined exclusion criteria; one animal in the shamSPGd group developed subarachnoid hemorrhage due to ICA perforation, and two animals in txSPGi and one animal in txSPGd group did not develop cortical and subcortical infarct within the MCA territory due to filament occlusion failure. There was no statistically significant difference in body temperature, arterial blood gas and pH among the four experimental groups. VNS led to an immediate and transient 30 sec decrease in ABP and HR, both of which returned to prestimulation levels as soon as stimulation was stopped (Fig.1A). The amplitude of decrease in mean ABP (MABP) was 35.47 ± 8.6 mm Hg (29% of baseline; number of measurements=78) in the txSPGi animals, and 27.41 ± 5.85 mm Hg (23% of baseline; number of measurements=78) in the txSPGd animals (repeated measures ANOVA: F(1,10)=1.67, p=0.22). The amplitude of decrease in HR was 186.29 ± 44.92 beats/min (46% of baseline; n=78) in the txSPGi animals, and 176.59 ± 31.46 beats/min (39% of baseline; n=78) in the txSPGd animals (repeated measures ANOVA: F(1,10)=0.33, p=0.57). There was no difference in MABP (Fig.1B) and HR (Fig.1C) across all four groups during the entire treatment period (repeated measures ANOVA: F(3,20) = 0.008, p=0.99 for MABP, F(3,20) = 0.43, p=0.73 for HR).
Figure 1.
(A) ABP, HR, and rCBF decreased transiently during electrical stimulation, as seen in the representative recordings. However, VNS had no overall effect on MABP (B), HR (C), and rCBF (D), regardless of SPG ablation. Repeated measures of ANOVA: F(3,20) = 0.008, p=0.99 for MABP, ANOVA: F(3,20) = 0.43, p=0.73 for HR, and F(3,20) = 0.16, p=0.92 for rCBF.
Occlusion of the MCA caused a decrease of more than 60% in the laser Doppler signal, which was completely reversed by reperfusion in all animals (Fig.1D). VNS caused a drop in rCBF during each 30 sec train of stimulation (Fig.1A). The mean reduction in rCBF was marginally responsive to SPG ablation, measuring 33.12 ± 8.06 % baseline (n=78) in the txSPGi animals, and 20.14 ± 5.67 % baseline (n=78) in the txSPGd animals (repeated measures ANOVA: F(1,10)=3.48, p=0.09). Throughout the experimental period there was no difference in rCBF across the four groups (Fig.1D) (repeated measures of ANOVA: F(3,20) = 0.16, p=0.92).
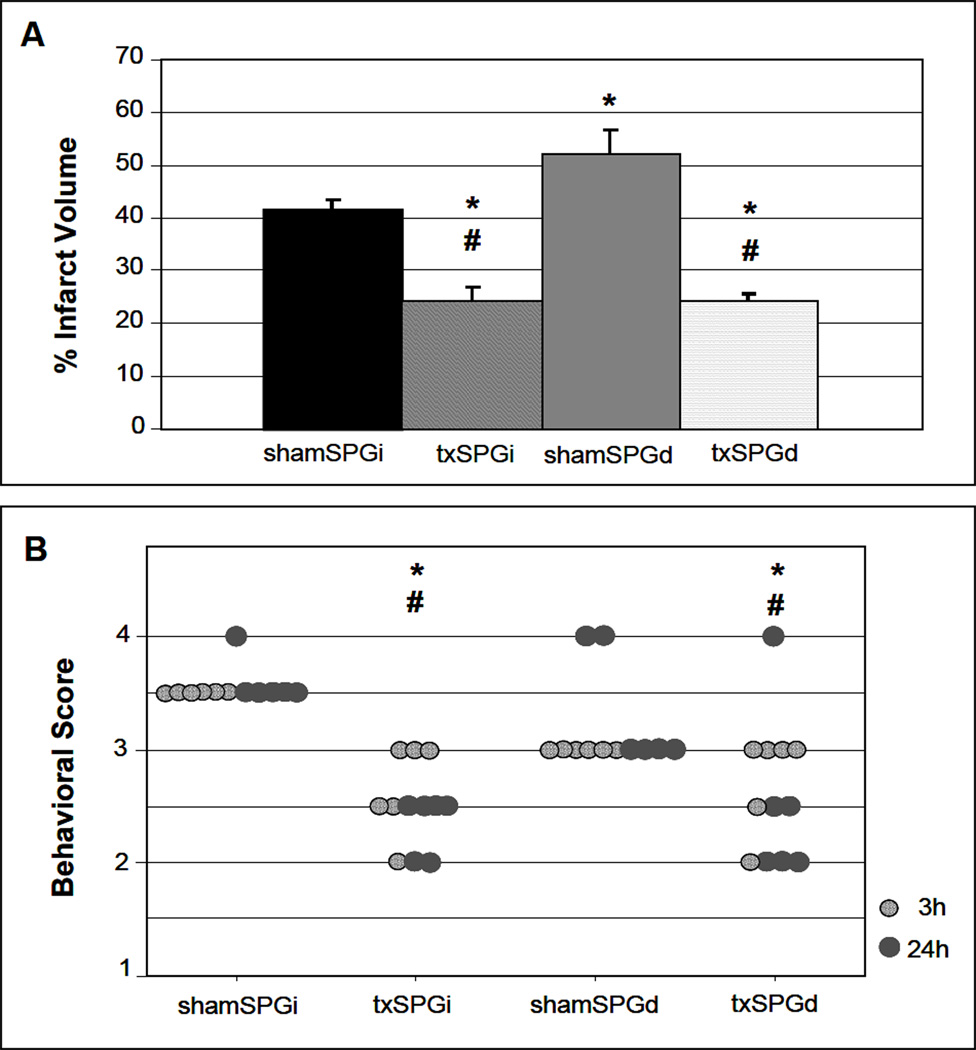
In shamSPGi animals, occlusion of the right MCA for 2h caused a consistent infarct in the ipsilateral cerebral cortex and in the underlying striatum. In contrast, infarct volume was altered significantly by SPG ablation and VNS treatment (ANOVA: F(3,20)=22.04, p<0.0001). SPG ablation resulted in larger infarct size in sham stimulation group; the mean infarct volume and SEM in shamSPGi and shamSPGd animals measured 41.32 ± 2.07 (n=6) and 52.34 ± 4.59 (n=6) % of the contralateral hemispheric volume, respectively (p<0.05; Fig.2A). VNS caused a significant reduction in infarct volume; the mean infarct volume and SEM in txSPGi and txSPGd animals measured 24.19 ± 2.62 (n=6) and 24.32 ± 1.26 (n=6) % of the contralateral hemispheric volume, respectively (p<0.05 for both txSPGi and txSPGd animals vs. shamSPGi and shamSPGd animals; Fig.2A). Comparison between the txSPGi and txSPGd animals revealed no difference in infarct volume as a result of VNS treatment (p>0.05). Inclusion of the 4 animals that were initially excluded due to experimental failure did not change the overall results; the respective mean infarct volume and SEM in txSPGi and txSPGd animals were 20.27 ± 3.2 (n=8) and 22.56 ± 2.1 (n=7) % of the contralateral hemispheric volume (p<0.05 for both txSPGi and txSPGd animals vs. shamSPGi and shamSPGd animals). VNS treatment did not lead to difference in infarct volume between txSPGi and txSPGd animals (p>0.05).
Figure 2.
The effect of VNS on infarct size (A) and neurological deficit (B) after MCA occlusion. (A) VNS is associated with reduced infarct size in both SPGi and SPGd animals. Among sham stimulation animals, the infarct volume was larger in shamSPGd than shamSPGi. ANOVA F(3,20)=22.04, p<0.0001; Student-Newman-Keuls test: * p<0.05 vs. shamSPGi, +p<0.05 vs. shamSPGd. (B) VNS is associated with better functional outcome in both SPGi and SPGd animals 24 h after ischemia. Repeated measures ANOVA: F(3,20)=6.14, p=0.0039; Mann-Whitney U test: * p<0.05 vs. shamSPGi, +p<0.05 vs. shamSPGd.
In both txSPGi and txSPGd animals, VNS treatment was associated with better motor outcome (repeated measures ANOVA: F(3,20)=6.14, p=0.004; Fig.2B). At 24 h the median neurological scores and IQR in the shamSPGi and shamSPGd groups were 3.0 ± 0.0 and 3.0 ± 1.0, respectively (p>0.05). Corresponding scores for VNS treatment were 2.5 ± 0.5 in the txSPGi animals and 2.25 ± 0.5 in txSPGd animals (p<0.05 for both vs. no VNS groups; Fig.2B).
Discussion
This study demonstrates that electrical stimulation of the cervical vagus nerve is associated with improved tissue and functional outcome following MCA occlusion in rats, regardless of whether the SPG is intact. This finding indicates that the vagus nerve, which provides parasympathetic innervation to the entire intracranial vascular system, does not offer ischemic protection via parasympathetic SPG projections that innervate the cerebral circulation. This study also shows that VNS reduces infarct volume by approximately 50%, and thereby confirms previous findings of VNS in cerebral ischemia (Ay et al., 2009; Ay et al., 2011; Sun et al., 2011). We have previously shown that stimulation of the right vagus nerve for 1 h, starting 30 min after occlusion of the right MCA, leads to 50% reduction in infarct size in rats (Ay et al., 2009). Our later studies have also shown that stimulation of the left vagus nerve elicits an effect on infarct size comparable that seen with right side stimulation (Ay et al., 2011). Recently, Sun et al. have reported a 56% reduction in infarct size in response to VNS, using the same experimental design and stimulation parameters used in this study (Sun et al., 2011). The exact mechanism of VNS-mediated ischemic protection is not known. The observation that infarct reducing effect of VNS was not attenuated by SPG ablation suggests that parasympathetic system-mediated vasodilation and resultant CBF increase is not the primary mechanism of protection by VNS. In accordance with this, we found that VNS was not associated with improved rCBF within the ischemic tissue. In contrast, confirming one of our earlier reports (Ay et al., 2011), we did observe a temporary reduction in rCBF during each individual train of stimulation; however, these temporary reductions were followed by immediate recovery of rCBF, and resulted in no overall change during the VNS treatment period. In light of previous reports and the findings we present here, it is important to investigate potential CBF-independent mechanisms of VNS-mediated neuroprotection. Such mechanisms may include inhibition of neuronal excitability (Zagon et al., 2000), activation of anti-inflammatory pathways (Tracey, 2007), and inhibition of excitotoxicity (Miyamoto et al., 2003). The role of each of these mechanisms remains to be elucitated in future studies.
Our findings confirm previously published data showing that SPG ablation potentiates conversion of ischemic tissue into infarction, and increases infarct volume following MCA occlusion in rats (Kano et al., 1991; Koketsu et al., 1992). The present study extends these prior reports by showing that VNS is associated with greater reduction in infarct size in SPG-damaged animals compared to SPG-intact animals (53% versus 41%). The mechanism by which VNS reverses the infarct promoting effect of SPG ablation is not known. We speculate that the vagal system and the SPG converge upon similar protective pathways at the tissue level (e.g., anti-inflammatory effect), where reduced function in one system is rapidly compensated by the other.
In addition to the SPG (Henninger et al., 2007) and vagus nerve (Ay et al., 2009), there are other neural structures such as the cerebellar fastigial nucleus (Berger et al., 1990), periaqueductal gray matter (Glickstein et al., 2003), and subthalamic vasodilator area (Glickstein et al., 2001) in which stimulation is known to reduce infarct size in experimental animals. However, at present, only the SPG and vagus nerve can be stimulated using minimally-invasive means. The safety and efficacy of a neurostimulator that can be implanted into the soft palate to deliver stimulation to the SPG is currently being evaluated in patients with acute ischemic stroke (ImpACT-24: Implant for Augmentation of CBF Trial) (Khurana et al., 2009). Minimally invasive VNS techniques such as transauricular vagus nerve stimulation via an external ear electrode are also currently under development for human application (Busch et al.; Stefan et al.). We demonstrate that although anatomical connections exist between the systemic parasympathetic pathway (activated by VNS) and the cerebrovascular parasympathetic pathway (activated by SPG stimulation), these two pathways leverage the brain’s own intrinsic systems, which render it more tolerant to ischemia via independent mechanisms. The existence of two competing pathways that operate independently of each other warrants future studies to investigate whether VNS and SPG stimulation have different safety and efficacy profiles. Future studies are also needed to show whether concurrent VNS and SPG stimulation potentiates the infarct reducing effects of each.
Acknowledgements
I.A. was supported by the American Heart Association (10SDG2600218). H.A. was supported by NIH-NINDS (R01NS059710). This research was carried out in whole at the Athinoula A. Martinos Center for Biomedical Imaging at the Massachusetts General Hospital, using resources provided by the Center for Functional Neuroimaging Technologies, P41RR14075, a P41 Regional Resource supported by the Biomedical Technology Program of the National Center for Research Resources (NCRR), National Institutes of Health. We thank Sinan Ozer for his help with the data analysis and Nichole L. Eusemann for proofreading the manuscript.
Abbreviations
- SPG
sphenopalatine ganglion
- CBF
cerebral blood flow
- MCA
middle cerebral artery
- VNS
vagus nerve stimulation
- HR
heart rate
- ABP
arterial blood pressure
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest: The authors report no conflicts of interest.
Literature References
- Recommendations for standards regarding preclinical neuroprotective and restorative drug development. Stroke. 1999;30:2752–2758. doi: 10.1161/01.str.30.12.2752. [DOI] [PubMed] [Google Scholar]
- Agassandian K, Fazan VP, Margaryan N, Dragon DN, Riley J, Talman WT. A novel central pathway links arterial baroreceptors and pontine parasympathetic neurons in cerebrovascular control. Cell Mol Neurobiol. 2003;23:463–478. doi: 10.1023/A:1025059710382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ay I, Lu J, Ay H, Gregory Sorensen A. Vagus nerve stimulation reduces infarct size in rat focal cerebral ischemia. Neurosci Lett. 2009;459:147–151. doi: 10.1016/j.neulet.2009.05.018. [DOI] [PubMed] [Google Scholar]
- Ay I, Sorensen AG, Ay H. Vagus nerve stimulation reduces infarct size in rat focal cerebral ischemia: An unlikely role for cerebral blood flow. Brain Res. 2011;1392:110–115. doi: 10.1016/j.brainres.2011.03.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bederson JB, Pitts LH, Tsuji M, Nishimura MC, Davis RL, Bartkowski H. Rat middle cerebral artery occlusion: evaluation of the model and development of a neurologic examination. Stroke. 1986;17:472–476. doi: 10.1161/01.str.17.3.472. [DOI] [PubMed] [Google Scholar]
- Berger SB, Ballon D, Graham M, Underwood MD, Khayata M, Leggiero RD, Koutcher JA, Reis DJ. Magnetic resonance imaging demonstrates that electric stimulation of cerebellar fastigial nucleus reduces cerebral infarction in rats. Stroke. 1990;21:III172–III176. [PubMed] [Google Scholar]
- Boysen NC, Dragon DN, Talman WT. Parasympathetic tonic dilatory influences on cerebral vessels. Auton Neurosci. 2009;147:101–104. doi: 10.1016/j.autneu.2009.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busch V, Zeman F, Heckel A, Menne F, Ellrich J, Eichhammer P. The effect of transcutaneous vagus nerve stimulation on pain perception - An experimental study. Brain Stimul. doi: 10.1016/j.brs.2012.04.006. [DOI] [PubMed] [Google Scholar]
- Edvinsson L, Hamel E. Perivascular nerve in brain vessels. In: Edvinsson L, Krause D, editors. Cerebral blood flow and metabolism. Philadelphia: Lippincott, Williams & Wilkins; 2002. pp. 43–67. [Google Scholar]
- Glickstein SB, Ilch CP, Golanov EV. Electrical stimulation of the dorsal periaqueductal gray decreases volume of the brain infarction independently of accompanying hypertension and cerebrovasodilation. Brain Res. 2003;994:135–145. doi: 10.1016/j.brainres.2003.08.001. [DOI] [PubMed] [Google Scholar]
- Glickstein SB, Ilch CP, Reis DJ, Golanov EV. Stimulation of the subthalamic vasodilator area and fastigial nucleus independently protects the brain against focal ischemia. Brain Res. 2001;912:47–59. doi: 10.1016/s0006-8993(01)02602-6. [DOI] [PubMed] [Google Scholar]
- Hara H, Weir B. Pathway of acetylcholinesterase containing nerves to the major cerebral arteries in rats. J Comp Neurol. 1986;250:245–252. doi: 10.1002/cne.902500211. [DOI] [PubMed] [Google Scholar]
- Henninger N, Fisher M. Stimulating circle of Willis nerve fibers preserves the diffusion-perfusion mismatch in experimental stroke. Stroke. 2007;38:2779–2786. doi: 10.1161/STROKEAHA.107.485581. [DOI] [PubMed] [Google Scholar]
- Hungerhuber E, Zausinger S, Westermaier T, Plesnila N, Schmid-Elsaesser R. Simultaneous bilateral laser Doppler fluxmetry and electrophysiological recording during middle cerebral artery occlusion in rats. J Neurosci Methods. 2006;154:109–115. doi: 10.1016/j.jneumeth.2005.12.004. [DOI] [PubMed] [Google Scholar]
- Kalia M, Mesulam MM. Brain stem projections of sensory and motor components of the vagus complex in the cat: I. The cervical vagus and nodose ganglion. J Comp Neurol. 1980a;193:435–465. doi: 10.1002/cne.901930210. [DOI] [PubMed] [Google Scholar]
- Kalia M, Mesulam MM. Brain stem projections of sensory and motor components of the vagus complex in the cat: II. Laryngeal, tracheobronchial, pulmonary, cardiac, and gastrointestinal branches. J Comp Neurol. 1980b;193:467–508. doi: 10.1002/cne.901930211. [DOI] [PubMed] [Google Scholar]
- Kano M, Moskowitz MA, Yokota M. Parasympathetic denervation of rat pial vessels significantly increases infarction volume following middle cerebral artery occlusion. J Cereb Blood Flow Metab. 1991;11:628–637. doi: 10.1038/jcbfm.1991.114. [DOI] [PubMed] [Google Scholar]
- Khurana D, Kaul S, Bornstein NM. Implant for augmentation of cerebral blood flow trial 1: a pilot study evaluating the safety and effectiveness of the Ischaemic Stroke System for treatment of acute ischaemic stroke. Int J Stroke. 2009;4:480–485. doi: 10.1111/j.1747-4949.2009.00385.x. [DOI] [PubMed] [Google Scholar]
- Koketsu N, Moskowitz MA, Kontos HA, Yokota M, Shimizu T. Chronic parasympathetic sectioning decreases regional cerebral blood flow during hemorrhagic hypotension and increases infarct size after middle cerebral artery occlusion in spontaneously hypertensive rats. J Cereb Blood Flow Metab. 1992;12:613–620. doi: 10.1038/jcbfm.1992.85. [DOI] [PubMed] [Google Scholar]
- Li C, Fitzgerald ME, Ledoux MS, Gong S, Ryan P, Del Mar N, Reiner A. Projections from the hypothalamic paraventricular nucleus and the nucleus of the solitary tract to prechoroidal neurons in the superior salivatory nucleus: Pathways controlling rodent choroidal blood flow. Brain Res. 2010;1358:123–139. doi: 10.1016/j.brainres.2010.08.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longa EZ, Weinstein PR, Carlson S, Cummins R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke. 1989;20:84–91. doi: 10.1161/01.str.20.1.84. [DOI] [PubMed] [Google Scholar]
- Lumenta DB, Plesnila N, Klasner B, Baethmann A, Pruneau D, Schmid-Elsaesser R, Zausinger S. Neuroprotective effects of a postischemic treatment with a bradykinin B2 receptor antagonist in a rat model of temporary focal cerebral ischemia. Brain Res. 2006;1069:227–234. doi: 10.1016/j.brainres.2005.11.043. [DOI] [PubMed] [Google Scholar]
- Miyamoto O, Pang J, Sumitani K, Negi T, Hayashida Y, Itano T. Mechanisms of the anti-ischemic effect of vagus nerve stimulation in the gerbil hippocampus. Neuroreport. 2003;14:1971–1974. doi: 10.1097/00001756-200310270-00018. [DOI] [PubMed] [Google Scholar]
- Morgane PJ, Jacobs MS. Raphe projections to the locus coeruleus in the rat. Brain Res Bull. 1979;4:519–534. doi: 10.1016/0361-9230(79)90037-6. [DOI] [PubMed] [Google Scholar]
- Schmid-Elsaesser R, Zausinger S, Hungerhuber E, Baethmann A, Reulen HJ. A critical reevaluation of the intraluminal thread model of focal cerebral ischemia: evidence of inadvertent premature reperfusion and subarachnoid hemorrhage in rats by laser-Doppler flowmetry. Stroke. 1998;29:2162–2170. doi: 10.1161/01.str.29.10.2162. [DOI] [PubMed] [Google Scholar]
- Smith DC, Modglin AA, Roosevelt RW, Neese SL, Jensen RA, Browning RA, Clough RW. Electrical stimulation of the vagus nerve enhances cognitive and motor recovery following moderate fluid percussion injury in the rat. J Neurotrauma. 2005;22:1485–1502. doi: 10.1089/neu.2005.22.1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefan H, Kreiselmeyer G, Kerling F, Kurzbuch K, Rauch C, Heers M, Kasper BS, Hammen T, Rzonsa M, Pauli E, Ellrich J, Graf W, Hopfengartner R. Transcutaneous vagus nerve stimulation (t-VNS) in pharmacoresistant epilepsies: A proof of concept trial. Epilepsia. doi: 10.1111/j.1528-1167.2012.03492.x. [DOI] [PubMed] [Google Scholar]
- Sun Z, Baker W, Hiraki T, Greenberg JH. The effect of right vagus nerve stimulation on focal cerebral ischemia: an experimental study in the rat. Brain Stimul. 2011;5:1–10. doi: 10.1016/j.brs.2011.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki N, Hardebo JE, Owman C. Origins and pathways of cerebrovascular vasoactive intestinal polypeptide-positive nerves in rat. J Cereb Blood Flow Metab. 1988;8:697–712. doi: 10.1038/jcbfm.1988.117. [DOI] [PubMed] [Google Scholar]
- Suzuki N, Hardebo JE, Owman C. Origins and pathways of cerebrovascular nerves storing substance P and calcitonin gene-related peptide in rat. Neuroscience. 1989;31:427–438. doi: 10.1016/0306-4522(89)90385-0. [DOI] [PubMed] [Google Scholar]
- Suzuki N, Hardebo JE, Owman C. Origins and pathways of choline acetyltransferase-positive parasympathetic nerve fibers to cerebral vessels in rat. J Cereb Blood Flow Metab. 1990;10:399–408. doi: 10.1038/jcbfm.1990.70. [DOI] [PubMed] [Google Scholar]
- Toda N, Ayajiki K, Tanaka T, Okamura T. Preganglionic and postganglionic neurons responsible for cerebral vasodilation mediated by nitric oxide in anesthetized dogs. J Cereb Blood Flow Metab. 2000a;20:700–708. doi: 10.1097/00004647-200004000-00007. [DOI] [PubMed] [Google Scholar]
- Toda N, Tanaka T, Ayajiki K, Okamura T. Cerebral vasodilatation induced by stimulation of the pterygopalatine ganglion and greater petrosal nerve in anesthetized monkeys. Neuroscience. 2000b;96:393–398. doi: 10.1016/s0306-4522(99)00557-6. [DOI] [PubMed] [Google Scholar]
- Tracey KJ. Physiology and immunology of the cholinergic antiinflammatory pathway. J Clin Invest. 2007;117:289–296. doi: 10.1172/JCI30555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walters BB, Gillespie SA, Moskowitz MA. Cerebrovascular projections from the sphenopalatine and otic ganglia to the middle cerebral artery of the cat. Stroke. 1986;17:488–494. doi: 10.1161/01.str.17.3.488. [DOI] [PubMed] [Google Scholar]
- Zagon A, Kemeny AA. Slow hyperpolarization in cortical neurons: a possible mechanism behind vagus nerve simulation therapy for refractory epilepsy? Epilepsia. 2000;41:1382–1389. doi: 10.1111/j.1528-1157.2000.tb00113.x. [DOI] [PubMed] [Google Scholar]