Abstract
Objective
Soluble fms-like tyrosine kinase 1 (sFlt1) is an antiangiogenic protein that is associated with a number of disorders of placental angiogenesis. It has been hypothesized that disruption of placental angiogenesis may contribute to the pathophysiology of preterm delivery (PTD). However, the relationship of PTD risk to variation in sFlt1 levels is not well known. We investigate the relationship between longitudinal variation in maternal serum concentrations of sFlt1 and risk of PTD.
Methods
Data were collected in a longitudinal cohort study involving 278 pregnant women. Maternal serum sFlt1 concentrations were measured at 6–10, 10–14, 16–20, 22–26, and 32–36 weeks gestation. Data analyses used longitudinal regression models using repeated measures that allow robust inferences from our modest sample size. The outcome was birth prior to 37 weeks gestation.
Results
sFlt1 concentrations were higher in first trimester for preterm compared to term deliveries. This relationship reversed in second trimester because sFlt1 concentrations increased more rapidly across gestation for term deliveries. In Cox proportional hazards analyses, a 2 ng higher sFlt1 concentration across gestation was associated with a hazard ratio of 1.3 (95% CI: 1.1, 1.5) for PTD suggesting the importance of levels in early pregnancy.
Conclusion
Elevated maternal serum sFlt1 concentration during pregnancy is associated with increased risk of PTD.
Keywords: angiogenic factors, Cox regression, Flt1 protein, longitudinal analysis, placental hormones, pregnancy
INTRODUCTION
Preterm delivery (PTD) contributes to 28 percent of neonatal deaths worldwide and remains a major global health challenge1. Despite advances in our understanding of gestational biology, the proportion of infants born preterm has continued to rise over the last two decades even in developed countries such as the United States2. Our failure to reduce the incidence of PTD stems, in part, from limited knowledge about the basic pathophysiological mechanisms that lead to alterations in the length of gestation.
The placental vascular system plays a vital role in normal gestation, and its contribution to risk of PTD remains understudied3. Disruption of important angiogenic and vasculogenic processes underlying placentation contributes to the pathophysiology of many adverse perinatal outcomes4–6. Accordingly, spontaneous preterm delivery is associated with histopathological evidence of disordered placentation7–12. Placentas from pregnancies complicated by PTD are found to have a greater degree of failure of physiologic transformation of the spiral arteries in the myometria and decidua of the placental bed than normal pregnant women at term. These abnormalities may compromise placental blood flow and nutrient delivery to the developing fetus. Accordingly, recent data suggests that pregnancies with either a higher uterine or umbilical artery resistance are more likely to be affected by PTD13.
Growing evidence suggests that disordered placentation involved in adverse perinatal outcomes may be associated with dysregulation of factors involved in angiogenesis14,15. Soluble fms-like tyrosine kinase 1 (sFlt1) is a splice variant consisting only of the six extracellular ligand-binding domains of the vascular endothelial growth factor (VEGF) receptor Flt1. sFlt1, secreted primarily by syncytiotrophoblasts into the maternal circulation15, is an antiangiogenic protein that antagonizes the effects of the proangiogenic proteins placental growth factor (PLGF) and VEGF. It has been strongly associated with disorders of placentation15.
There has been a single cross-sectional study estimating the relationship between maternal serum levels of sFlt1 in late first trimester with the risk of subsequent adverse outcome16. However, no prior studies have documented the relationship between variation in time-dependent measures of factors involved in placental angiogenesis and the risk of PTD. The goal of this analysis was to investigate the influence of longitudinal variation in maternal serum concentrations of sFlt1 measured at five time points beginning in early first trimester (6–10 weeks gestation) on variation in the risk of PTD.
METHODS
Data Collection and Study Variables
Data were collected as part of a prospective pilot study of pregnant women who presented for prenatal care in early first trimester to the University of Michigan Health System. The Institutional Review Board of the University of Michigan Medical School approved the study protocols. All women who were 18 to 45 years of age, between 6–10 weeks gestation with a singleton pregnancy, and intended to deliver at the study hospital were eligible for the study. Participants were seen at five study visits at 6–10, 10–14, 16–20, 22–26, and 32–36 weeks gestation. Informed consent was obtained at the initial visit. Subjects with major structural fetal abnormalities or prenatally diagnosed aneuploidy were excluded from the analysis.
Data were analyzed for a cohort of 278 subjects who delivered a live infant and were measured for all relevant variables during the first two years of the study. Maternal data were obtained at each visit from an interview, questionnaire, and a maternal blood draw, as well as by review of medical records. Infant data, including date of delivery, type of delivery, birth weight, and sex, were collected at delivery and abstracted from the medical record. Gestational age (GA) was estimated from ultrasound measures between 6–10 weeks gestation. Less than 3% of data were missing for any variable. Pregnancy induced hypertension was classified according to the criteria of Davey and MacGillivray, endorsed by the International Society for the Study of Hypertension in Pregnancy 17.
At each visit, maternal blood samples were collected in a serum separator tube and allowed to clot for 30 minutes before centrifugation for 15 minutes at 1000 × g. The serum samples were aliquoted and stored at −86° C until analysis. Serum concentration of maternal sFlt1 and PLGF were determined using solid-phase enzyme-linked immunosorbent assay (ELISA) conducted in duplicate using commercially available kits (R&D Systems, Inc., Minneapolis, MN). Personnel who were blinded to all patient identifiers and pregnancy outcomes performed assays.
Statistical Analysis
All statistical analyses were performed using SAS version 9.1 (SAS Institute, Cary, NC). Descriptive analyses were conducted to ascertain demographic characteristics of the study population and to document variations in sFlt1 and PlGF levels across pregnancy. The PlGF concentrations were transformed to the logarithm of concentration (log(PlGF)) due to the skewed PlGF distributions across pregnancy. The chi-square test was used to assess statistical significance of categorical variables, while the Student’s t-test was used for continuous variables. A p-value of less than 0.05 was considered statistically significant. For all analyses, preterm delivery (PTD) was defined as gestation lasting less than 37 completed weeks.
Two complementary longitudinal data methods were used to analyze our data. First, we used SAS PROC MIXED to implement a mixed linear regression model to analyze the time dependence of the repeated measures of maternal serum sFlt1 and PLGF levels measured across pregnancy for term and preterm deliveries. These models account for the correlations between repeated measures of each factor over gestation. A significant advantage of these models is that they use all available data in the analysis and do not exclude participants with missing data in discrete intervals. In addition, the repeated measures design used here decreases between person variation resulting in increased statistical power when compared to other study designs18. As a result, robust inferences could be made with a modest sample size.
Second, the Cox proportional hazards regression model was implemented in SAS PROC PHREG. Hazard ratios (HR) and 95% confidence intervals (CI) were generated to estimate the risk of PTD in relation to concentrations of angiogenic factors (sFlt1 and PlGF) over pregnancy. In this model, the outcome event for our study was birth prior to 37 completed weeks gestation. Time zero was the date of conception (2 weeks post-LMP) calculated using the first trimester ultrasound and birth events were censored at 37 weeks (258 days) post-LMP using gestational age in days as the time scale. By definition, women are not at risk of PTD after 37 weeks post-LMP. Thus, the Cox model evaluates the hazard of a PTD. Ties were handled using the method of Efron. In each analysis, the predictor variable (maternal serum concentration of each angiogenic factor) was modeled as a time-dependent covariate in the Cox regression. The time dependence of the sFlt1 and PLGF concentrations was described by a step-function starting at the time of conception with a value of 0 ng/mL. The concentration takes the value of the measurement at each visit and extends from the midpoint between the prior visit (or conception for the first visit) and the current visit to the midpoint between the current visit and the following visit (or to delivery for the last measure). Mode of delivery was defined as a spontaneous or indicated, where the latter includes medically indicated cesarean deliveries. Mode of delivery was incorporated into the Cox regression model using stratification. A stratified analysis was performed to ensure that mode of delivery was accounted for in our results. Common etiological factors, including placental vascular dysfunction likely contribute to indicated and spontaneous PTD though with different proportional hazards. The stratified analysis was meant to account for the possibility that the shape of the hazard function for PTD may not be identical between these two strata. Inspection of the Schoenfeld residuals did not demonstrate any consistent deviations to invalidate the proportional hazard assumption.
RESULTS
Table 1 presents the sociodemographic and health characteristics of study participants and their newborn infants included in our analysis. Characteristics are stratified on preterm status at delivery. Of the 278 participants, nineteen women delivered preterm for an overall PTD rate of about 7%, with a gestational age range from 25 to 36 5/7 weeks. Nearly all of our preterm cases occurred after 32 weeks gestation (84%, 16 of 19). Our sample was very homogeneous with regard to measures of socioeconomic status and race. Approximately 81% of our participants were White, 6% were African-American, and 95% were of non-Hispanic in origin. Approximately 86% of our sample was married at the time of enrollment and 44% of the study participants had a post-graduate education. Nearly half of our sample reported a total household income of $80,000 or greater. About 65% of our sample was multiparous and few participants smoked during pregnancy (8%). As expected for this homogeneous sample with little variation in sociodemographic risk factors, ethnicity, maternal age, parity, marital status, education, income, pre-pregnancy BMI, chronic illness, and smoking did not significantly predict risk of PTD. Of note, there were only three cases of pregnancy induced hypertension as defined by Davey and MacGillivray divided between term and preterm deliveries17. As such, our findings are very unlikely to result simply from pregnancy-induced hypertension and separate subgroup analyses support this conclusion. Similarly, there was a low prevalence of other chronic and pregnancy-related conditions.
Table 1.
Maternal and infant characteristics of the study sample1
| All Deliveries | Term Deliveries | Preterm Deliveries | ||||
|---|---|---|---|---|---|---|
| N=278 | % | N=259 | % | N=19 | % | |
| Race | ||||||
| White | 226 | 81.3 | 211 | 81.5 | 15 | 79.0 |
| Black | 17 | 6.1 | 14 | 5.4 | 3 | 15.8 |
| Asian | 18 | 6.5 | 17 | 6.6 | 1 | 5.3 |
| Other | 15 | 5.4 | 15 | 5.8 | 0 | 0.0 |
| Unknown | 2 | 0.7 | 2 | 0.8 | 0 | 0.0 |
| Hispanic ethnicity | ||||||
| Yes | 12 | 4.3 | 12 | 4.6 | 0 | 0.0 |
| No | 266 | 95.7 | 247 | 95.4 | 19 | 100.0 |
| Maternal age | ||||||
| ≤30 | 109 | 39.2 | 103 | 39.8 | 6 | 31.6 |
| >30 | 169 | 60.8 | 156 | 60.2 | 13 | 68.4 |
| Prenatal smoking | ||||||
| Yes | 23 | 8.3 | 23 | 8.9 | 0 | 0 |
| No | 249 | 89.6 | 230 | 88.8 | 19 | 100.0 |
| Unknown | 6 | 2.2 | 6 | 2.3 | 0 | 0 |
| Education level | ||||||
| College or less | 157 | 56.5 | 145 | 56.0 | 12 | 63.2 |
| Post-graduate | 121 | 43.5 | 114 | 44.0 | 7 | 36.8 |
| Annual household income | ||||||
| ≤$80,000 | 135 | 48.6 | 123 | 47.5 | 12 | 63.2 |
| >$80,000 | 135 | 48.6 | 129 | 49.8 | 6 | 31.6 |
| Unknown | 8 | 2.9 | 7 | 2.7 | 1 | 5.3 |
| Marital status | ||||||
| Married | 238 | 85.6 | 224 | 86.5 | 14 | 73.7 |
| Unmarried | 40 | 14.4 | 35 | 13.5 | 5 | 26.3 |
| Parity | ||||||
| Primipara | 97 | 34.9 | 88 | 34.0 | 9 | 47.4 |
| Multipara | 181 | 65.1 | 171 | 66.0 | 10 | 52.6 |
| Infant gender | ||||||
| Male | 141 | 50.7 | 128 | 49.4 | 13 | 68.4 |
| Female | 137 | 49.3 | 131 | 50.6 | 6 | 31.6 |
| Mode of delivery | ||||||
| Spontaneous | 194 | 69.8 | 184 | 71.0 | 10 | 52.6 |
| Indicated | 84 | 30.2 | 75 | 29.0 | 9 | 47.4 |
| Mean | SD2 | Mean | SD | Mean | SD | |
| Gestational age at birth | 39.1 | 1.9 | 39.4 | 1.1 | 34.4 | 3.5 |
| (weeks)* | ||||||
| Birth weight (grams)* | 3421.9 | 508.3 | 3480.2 | 433.9 | 2583.4 | 736.5 |
percentages have been rounded and may not total 100
SD=standard deviation
p<0.0001
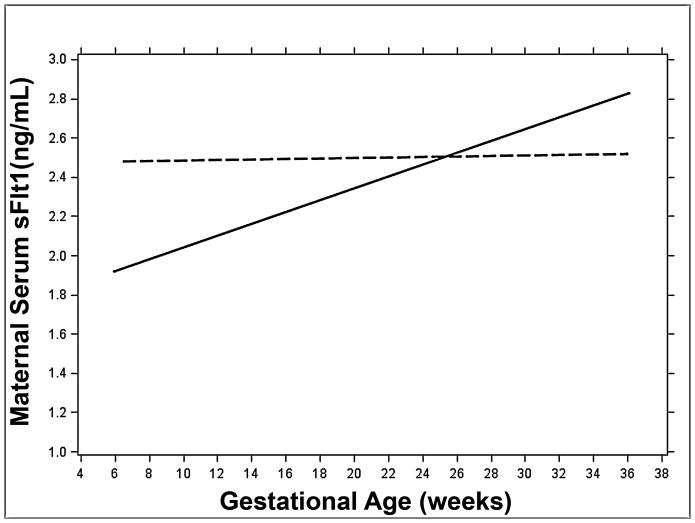
Figure 1 contains a plot of the mixed linear regression of gestational age on sFlt1 concentrations for term and preterm deliveries. We found that concentrations of sFlt1 were higher at early visits for mothers who delivered preterm compared to term. However, sFlt1 concentrations did not significantly change across gestation for PTD. The mixed linear regression coefficient for preterm deliveries was 0.0002 (95% CI:−0.01, 0.01). In contrast, concentrations of sFlt1 significantly increased during pregnancy for mothers who delivered term infants (β=0.004; 95% CI: 0.003, 0.006). As a result, the difference in sFlt1 concentrations for the two groups decreases as gestation approaches about 180 days at which point the trajectories cross and sFlt1 becomes higher for mothers who delivered term.
FIGURE 1.
The gestational age dependence of the maternal serum sFlt1 concentration (ng/mL). Regression lines calculated using mixed linear models are shown for term (solid line) and preterm (dashed line) deliveries.
We found that the logarithm of the PlGF concentration significantly increased across gestation for both mothers who delivered preterm (β=0.029; 95% CI: 0.025, 0.033) and term (β=0.022; 95% CI: 0.021, 0.023). In contrast, to our results for sFlt1, there was no statistically significant difference in the trajectory of log(PlGF) between preterm and term deliveries. Similarly, there was no statistically significant difference in the sFlt1/PlGF ratio across gestation for both mothers who delivered preterm and term.
A proportional hazard regression analysis was used to model the longitudinal influence of sFlt1 concentration on the hazard of PTD. Of the 19 preterm deliveries in our cohort, 9 were born by an indicated delivery (47%). The reasons for the indicated PTD were varied with no particular patterns evident. Indications included suspected infection, fibroids, bleeding, IUGR, failure to progress following preterm labor or PPROM, and maternal obesity. Among the term deliveries, 75 of the 184 deliveries were indicated (29%). Although this difference was not statistically significant (p=0.12) mode of delivery (spontaneous or medically indicated) was incorporated into a stratified Cox regression. Although these categories are likely to share common etiological factors, including placental vascular dysfunction, that contribute to risk of PTD, this model accounts for the possibility that the proportionality of the hazard for PTD may not be identical between these two strata. In this model, the assumption is that we are fitting separate models for each mode of delivery under the constraint that the coefficients are equal but the baseline hazard functions are not equal. Using this model, we found that a 2 ng higher sFlt1 concentration across gestation was significantly (p = 0.004) associated with a 1.3 (95% CI: 1.1, 1.5) hazard ratio for PTD. Similar results were found when the strata variable (mode of delivery) was excluded (HR=1.2, 95%CI: 1.1, 1.4), suggesting that our results were robust.
In contrast to our results for sFlt1, the proportional hazard regression analysis modeling the longitudinal influence of log(PlGF) concentration on the hazard of PTD did not show any significant effect (HR=0.83, 95% CI: 0.36, 1.91). We then investigated the predictive ability of the sFlt1/PlGF ratio for PTD. The proportional hazard regression analysis showed a significant but small increase in the hazard for PTD (HR=1.013; 95% CI: 1.003, 1.023) associated with increases in sFlt1/PlGF.
DISCUSSION
We used complementary longitudinal methods to investigate the effect of longitudinal variation in maternal serum sFlt1 concentration on risk of PTD in a population-based prospective cohort of women with a low prevalence of sociodemographic risk factors. Our study design allowed us to document the relationship between the trajectory of sFlt1 concentration across pregnancy and PTD status with a modest sample size starting very early in first trimester. We found that maternal serum concentrations of sFlt1 were higher in early pregnancy for mothers who delivered preterm, but increased significantly more rapidly across gestation for mothers who delivered term (Figure 1). We were then able to assess the contribution of longitudinal variation in sFlt1 levels to the risk of PTD. We found that pregnancies with a higher sFlt1 concentration were more likely to be affected by PTD.
Our findings suggest the timing and pattern of changes in maternal sFlt1 may be important in predicting PTD such that elevated levels in early in pregnancy may be an important predictor of PTD. The observed trajectories of maternal sFlt1 may explain an apparently contradictory prior report showing a small decrease in PTD risk associated with higher levels of sFlt1 measured between 10 and 14 weeks gestation16. Variation in the crossover point of the regression lines in different population samples may have resulted in the appearance of a protective effect of sFlt1 later in gestation.
By studying a low-risk population, many confounding factors, both measured and unmeasured, are potentially accounted for by our sampling. As expected, none of the maternal sociodemographic covariates were significant risk factors for PTD in our sample, most likely because of their low prevalence. Moreover, of particular importance to our analysis, there were only three cases of pregnancy-induced hypertension in this low-risk population, so that our findings are not simply the result of pregnancy-induced hypertension in PTD. Similarly, there was a low prevalence of other chronic and pregnancy-related conditions. The identification and recruitment of study participants early in pregnancy inherently limits study participation to a group of women who present early for prenatal care and are able to attend multiple study visits. Women without prenatal care or with late, interrupted, or sporadic care are less likely to have been included.
Because of its design, our study included a modest sample size with only nineteen cases of PTD. However, we collected multiple measures of our covariates, Our longitudinal models using these repeated measures had substantially more statistical power compared to comparable studies measuring sFlt1 at a single time point with a similar sample size18. As a result, robust inferences could be made even with a modest sample size. In future studies, the relative contribution of variation in sFlt1 concentration to variation in risk of PTD in other populations and the contribution of modifying factors can be determined in larger sample sets.
Our results support a biological relationship between PTD, placental vascular dysfunction, and disordered placentation. Placental angiogenesis is influenced by the balance between a number of anti-angiogenic factors and a number of proangiogenic factors. Dysregulation of factors in the vascular endothelial growth factor (VEGF) family plays a particularly important role in the pathophysiology of disordered placentation. These potent angiogenic factors, including placental growth factors, are abundantly expressed by placental tissues15. The biological functions of the VEGFs are mediated by a family of cognate protein tyrosine kinase receptors, including Flt1 and Flk115. The soluble receptor, sFlt1, is an antiangiogenic factor that is capable of sequestering circulating ligand or dimerizing with full-length receptor and preventing signal transduction. Abnormal placentation involving other perinatal outcomes is associated with increased placental production of the soluble antiangiogenic factors such as sFlt115.
Our study is among the first to suggest that elevated maternal serum sFlt1 concentration during pregnancy is associated with increased risk of PTD. In contrast, concentrations of PlGF were not associated with risk of PTD. The latter finding is consistent with prior reports and in contrast to findings for the hypertensive disorders of pregnancy16,19. The interplay among these factors will have to be considered more fully in larger studies to appreciate the role of this system in the etiology of PTD.
Markers such as maternal serum sFlt1 concentration suggest a role for abnormal placentation in the pathogenesis of PTD. A variety of clinical conditions including acute infection, chronic infection, abnormal inflammatory responses, altered homocysteine metabolism, dyslipidemia, and insulin resistance, can influence placental vascular development. Thus, abnormal placentation may mediate the effects of a range of environmental, genetic, and physiological factors on the risk of PTD. That is to say, abnormal placental vascular development may represent a final common pathway for a number of pathologic processes that are associated with PTD 5. As a potential etiologic mechanism, placental vascular dysfunction may offer novel preventative and therapeutic possibilities for PTD in the future.
Acknowledgments
We thank Dr. Greg Dyson and Mr. Ray Lowery for their assistance with data analyses. We thank Dr. Marjorie Treadwell for help with collection of ultrasound data and Dr. Dawn P. Misra for her critical review of the manuscript.
FUNDING
VKM was supported by a Doris Duke Clinical Scientist Development Award (Grant 2007092); a NIH Mentored Scientist Award (K08-HD045609), and a NIH Pediatric Child Health Research Center (K12-HD028820). PK was supported by University of Michigan’s Head and Neck Cancer Specialized Programs of Research Excellence Grant (5-P50-CA097248). The General Clinical Research Center at the University of Michigan Health System funded by grant number M01-RR000042 from the National Institutes of Health; the Michigan Clinical Research Unit which is supported by a Clinical and Translational Science Award grant number UL1RR024986 from the National Institutes of Health. JKS was supported by a postdoctoral award from Wayne State University.
Footnotes
DECLARATION OF INTEREST
The authors report no conflicts of interest.
References
- 1.Lawn JE, Wilczynska-Ketende K, Cousens SN. Estimating the causes of 4 million neonatal deaths in the year 2000. Int J Epidemiol. 2006;35(3):706–18. doi: 10.1093/ije/dyl043. [DOI] [PubMed] [Google Scholar]
- 2.Behrman RE, Butler AS, editors. Preterm Birth: Causes, Consequences, and Prevention. Washington, D.C: The National Academies Press; 2007. p. 792. [PubMed] [Google Scholar]
- 3.Regnault TR, Galan HL, Parker TA, Anthony RV. Placental development in normal and compromised pregnancies-- a review. Placenta. 2002;23 (Suppl A):S119–29. doi: 10.1053/plac.2002.0792. [DOI] [PubMed] [Google Scholar]
- 4.Kingdom J, Huppertz B, Seaward G, Kaufmann P. Development of the placental villous tree and its consequences for fetal growth. Eur J Obstet Gynecol Reprod Biol. 2000;92(1):35–43. doi: 10.1016/s0301-2115(00)00423-1. [DOI] [PubMed] [Google Scholar]
- 5.Thorp JM. Placental Vascular Compromise: unifying the etiologic pathways of perinatal compromise. Curr Probl Obstet Gynecol Fertil. 2001;24:203–220. [Google Scholar]
- 6.Torry DS, Hinrichs M, Torry RJ. Determinants of placental vascularity. Am J Reprod Immunol. 2004;51(4):257–68. doi: 10.1111/j.1600-0897.2004.00154.x. [DOI] [PubMed] [Google Scholar]
- 7.Arias F, Rodriquez L, Rayne SC, Kraus FT. Maternal placental vasculopathy and infection: two distinct subgroups among patients with preterm labor and preterm ruptured membranes. Am J Obstet Gynecol. 1993;168(2):585–91. doi: 10.1016/0002-9378(93)90499-9. [DOI] [PubMed] [Google Scholar]
- 8.Arias F, Victoria A, Cho K, Kraus F. Placental histology and clinical characteristics of patients with preterm premature rupture of membranes. Obstet Gynecol. 1997;89(2):265–71. doi: 10.1016/S0029-7844(96)00451-6. [DOI] [PubMed] [Google Scholar]
- 9.Kim YM, Chaiworapongsa T, Gomez R, Bujold E, Yoon BH, Rotmensch S, Thaler HT, Romero R. Failure of physiologic transformation of the spiral arteries in the placental bed in preterm premature rupture of membranes. Am J Obstet Gynecol. 2002;187(5):1137–42. doi: 10.1067/mob.2002.127720. [DOI] [PubMed] [Google Scholar]
- 10.Kim YM, Bujold E, Chaiworapongsa T, Gomez R, Yoon BH, Thaler HT, Rotmensch S, Romero R. Failure of physiologic transformation of the spiral arteries in patients with preterm labor and intact membranes. Am J Obstet Gynecol. 2003;189(4):1063–9. doi: 10.1067/s0002-9378(03)00838-x. [DOI] [PubMed] [Google Scholar]
- 11.Faye-Petersen OM. The placenta in preterm birth. Journal of Clinical Pathology. 2008;61(12):1261–1275. doi: 10.1136/jcp.2008.055244. [DOI] [PubMed] [Google Scholar]
- 12.Salafia CM, Ernst LM, Pezzullo JC, Wolf EJ, Rosenkrantz TS, Vintzileos AM. The very low birthweight infant: maternal complications leading to preterm birth, placental lesions, and intrauterine growth. Am J Perinatol. 1995;12(2):106–10. doi: 10.1055/s-2007-994417. [DOI] [PubMed] [Google Scholar]
- 13.Misra VK, Hobel CJ, Sing CF. Placental blood flow and the risk of preterm delivery. Placenta. 2009;30(7):619–24. doi: 10.1016/j.placenta.2009.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang A, Rana S, Karumanchi SA. Preeclampsia: the role of angiogenic factors in its pathogenesis. Physiology (Bethesda) 2009;24:147–58. doi: 10.1152/physiol.00043.2008. [DOI] [PubMed] [Google Scholar]
- 15.Maynard S, Epstein FH, Karumanchi SA. Preeclampsia and Angiogenic Imbalance. Annual Review of Medicine. 2008;59(1):61–78. doi: 10.1146/annurev.med.59.110106.214058. [DOI] [PubMed] [Google Scholar]
- 16.Smith GC, Crossley JA, Aitken DA, Jenkins N, Lyall F, Cameron AD, Connor JM, Dobbie R. Circulating angiogenic factors in early pregnancy and the risk of preeclampsia, intrauterine growth restriction, spontaneous preterm birth, and stillbirth. Obstet Gynecol. 2007;109(6):1316–24. doi: 10.1097/01.AOG.0000265804.09161.0d. [DOI] [PubMed] [Google Scholar]
- 17.Davey DA, MacGillivray I. The classification and definition of the hypertensive disorders of pregnancy. Am J Obstet Gynecol. 1988;158(4):892–8. doi: 10.1016/0002-9378(88)90090-7. [DOI] [PubMed] [Google Scholar]
- 18.Diggle P, Liang K-Y, Zeger SL. Analysis of longitudinal data. xi. New York: Clarendon Press; 1995. p. 253. [Google Scholar]
- 19.Verlohren S, Herraiz I, Lapaire O, Schlembach D, Moertl M, Zeisler H, Calda P, Holzgreve W, Galindo A, Engels T, et al. The sFlt-1/PlGF ratio in different types of hypertensive pregnancy disorders and its prognostic potential in preeclamptic patients. Am J Obstet Gynecol. 2011 doi: 10.1016/j.ajog.2011.07.037. [DOI] [PubMed] [Google Scholar]