Abstract
Polyamines are ubiquitous organic cations implicated in many physiological processes. Because they are positively charged at physiological pH, carrier-mediated systems are necessary for effective membrane permeation, but the identity of specific polyamine transporter proteins in eukaryotic cells remains unclear. Polyspecific organic cation transporters (OCTs) interact with many natural and xenobiotic monovalent cations and have been reported to transport dicationic compounds, including the short polyamine putrescine. In this study, we used Xenopus oocytes expressing mammalian OCT1 (SLC22A1), OCT2 (SLC22A2) or OCT3 (SLC22A3) to assess binding and transport of longer-chain polyvalent polyamines. In OCT-expressing oocytes, [3H]MPP+ uptake rates were 15– to 35–fold higher than in non-injected oocytes, whereas those for [3H]spermidine increased more modestly above the background, up to 3–fold. This reflected up to 20–fold lower affinity for spermidine than for MPP +; thus, K0.5 for MPP+ was ~50 μM in OCT1, ~170 μM in OCT2, and ~60 μM in OCT3, whereas for spermidine, K0.5 was ~1 mM in OCT1, OCT2 and OCT3. Jmax values for MPP+ and spermidine were within the same range, suggesting that both compounds are transported at a similar turnover rate. To gain further insight into OCT substrate specificity, we screened a selection of structural polyamine analogs for effect on [3H]MPP+ uptake. In general, blocking potency increased with overall hydrophobic character, which indicates that, as for monovalent cations, hydrophobicity is a major requirement for recognition in polyvalent OCT substrates and inhibitors. Our results demonstrate that the natural polyamines are low affinity, but relatively high turnover, substrates for OCTs. The identification of OCTs as polyamine transport systems may contribute to further understanding of the mechanisms involved in polyamine homeostasis, and aid in the design of polyamine-like OCT-targeted drugs.
Keywords: Organic cation transporter, polyspecific drug transporter, OCT, SLC22, OCT1, SLC22A1, OCT2, SLC22A2, OCT3, SLC22A3, OCT substrates and drugs, OCT pharmacophore, polyamines, putrescine, spermidine, spermine, polyamine analogs, cationic drugs, xenobiotics, rational drug design
Introduction
Polyamines are ubiquitous organic cations that are implicated in a broad range of physiological processes, including cell-cell interactions, signaling, cell proliferation and differentiation, and ion channel modulation.1 For example, naturally occurring cytoplasmic polyamines, such as spermidine (a7, see Fig. 1) and spermine (a10, Fig. 1), are responsible for inward rectification in Kir (KCNJ) potassium channels,2,3 and thus play an important role in shaping membrane potential responses. Extracellular polyamines have multiple effects in the central nervous system, including complex effects on NMDAR-type of glutamate receptors, enhancing NMDAR currents by channel opening and reducing activity by open channel block.4 Polyamine levels are highly regulated; exchange mechanisms are key to this regulation, and because polyamines are positively charged at physiological pH, carrier-mediated transport systems are necessary for effective membrane permeation. Polyamine transporters have been characterized in prokaryotic cells and in yeast,5,6 and connexin hemi-gap junctions have been shown to translocate polyamines in amphibian oocytes,7,8 but the molecular identity of specific polyamine carriers in mammalian cells is still unclear.9
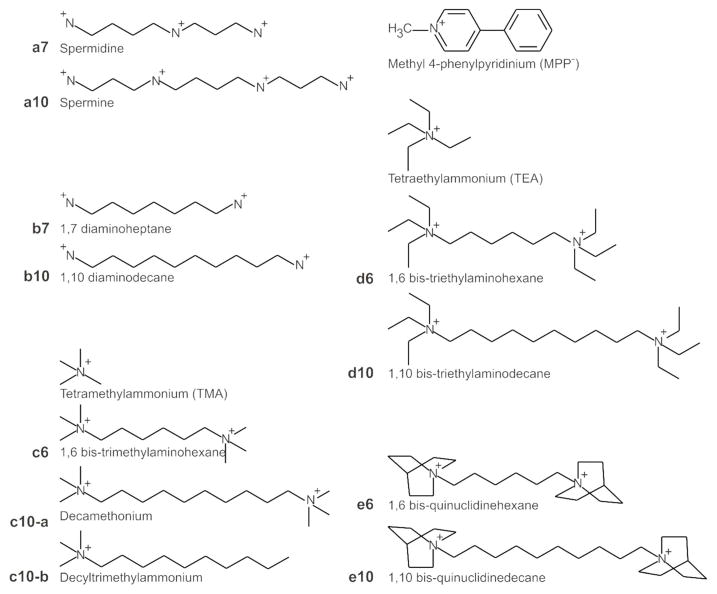
Figure 1. Compounds examined in this study.
Methyl 4-phenylpyridinium (MPP+) is a transported substrate for OCT 1, 2, and 3.13 Tetramethylammonium (TMA) and tetraethylammonium (TEA) are model cations. Spermidine (a7) and spermine (a10) are naturally occurring polyamines. Polyamine analogs (b–e) are classified according to the hydrophobicity of the end groups (letters, in ascending order); within each class, compounds are ordered according to the number of carbons between end groups. Note that decylmethylammonium only has one charged group, but has been designated c10-b to indicate its structural resemblance to decamethonium, c10-a.
The possibility that polyamines are substrates for organic cation transporters (OCTs) has received some attention,10,11 but the subject remains controversial.12 OCTs belong to the SLC22 family of polyspecific facilitative transporters, and are typically localized at the basolateral membrane of barrier epithelia, where they mediate the uptake, distribution, and efflux of cationic metabolites and drugs.13 In humans, OCT1 (SLC22A1) and OCT2 (SLC22A2) are predominantly expressed in hepatocytes and proximal tubular cells,14,15 whereas OCT3 (SLC22A3) exhibits a broader tissue distribution, and is found in placenta, bronchial and intestinal epithelium,16–18 astrocytes,19 as well as at the blood-cerebrospinal fluid barrier in choroid plexus epithelial cells.20 OCTs interact with many, structurally very diverse monovalent cations,21 including the model substrates tetraethylammonium (TEA; Fig. 1) and the neurotoxin methyl 4-phenylpyridinium (MPP+; Fig. 1). Recognized endogenous substrates include the metabolites choline and guanidine, and monoamine neurotransmitters such as the catecholamines.22 Additionally, OCTs have been shown to recognize a broad range of pharmacologically active compounds,13 and polymorphisms that modify OCT function have been found to alter the response of patients to certain drugs, such as metformin.23,24 Their broad substrate selectivity makes the OCTs good candidates for rational drug design, but it can also result in undesired side effects, such as accumulation and toxicity in non-target organs, as well as competitive drug interactions.25
Pharmacological and computational studies have established that hydrophobicity is a principal determinant for substrate recognition by OCTs.26–29 At least one positive charge is required for transport,30 but it has been reported that OCTs are also capable of transporting dicationic compounds, including the short polyamine putrescine.11 In this study, we used Xenopus oocytes expressing mammalian OCT1, OCT2 or OCT3 to assess whether the natural longer-chain spermidine (a7, Fig. 1) and spermine (a10, Fig. 1), which carry net physiological changes of +3 or +4, respectively, are substrates for OCTs. To gain further insights into the contribution of the number and spatial organization of charges to substrate hydrophobicity, recognition and binding, we investigated the interactions between the transporters and an extended array of polyamine analogs differing in hydrophobic character, charge number, and distribution of charged groups (Fig. 1). Our results may prove useful in the refinement of existing pharmacophore models, towards the design of OCT-targeted biopharmaceuticals and the development of strategies to prevent unwanted drug-transporter interactions.
Experimental Section
Chemicals
Unless otherwise noted, all chemicals were purchased from Sigma (St. Louis, MO). Restriction endonucleases were acquired from New England Biolabs (Ipswich, MA). Methyl [3H] 4-phenylpyridinium ([3H]MPP+) iodide (specific activity, 85 Ci/mmol) was from American Radiolabeled Chemicals (St. Louis, MO), and [3H]spermidine trihydrochloride (specific activity, 15 Ci/mmol) was from PerkinElmer (Boston, MA). The following test compounds were examined (Fig. 1): MPP+, tetramethylammonium (TMA) tetraethylammonium (TEA), spermidine (a7), spermine (a10), 1,7-diaminoheptane (b7), 1,10-diaminodecane (b10), 1,6 bis-trimethylaminohexane (c6), decamethonium (c10-a), decyltrimethylammonium (c10-b), 1,6 bis-triethylaminohexane (d6), 1,10 bis-triethylaminodecane (d10), bis-quinuclidinehexane (e6), and 1,10 bis-quinuclidinedecane (e10).
Functional Expression of OCTs in Oocytes
Mature female Xenopus laevis specimens were purchased from Xenopus Express (Brooksville, FL). All animal protocols followed guidelines approved by the Washington University School of Medicine and the National Institutes of Health. Frogs were anaesthetized with 0.1% tricaine buffered with 0.1% NaHCO3 prior to removal of a portion of the ovary, and were sacrificed by an overdose of tricaine. Stage V–VI oocytes were selected and maintained at 18 °C in modified Barth’s solution containing (mM) 88 NaCl, 2.4 NaHCO3, 1 KCl, 0.3 Ca(NO3)2, 0.4 CaCl2, 0.8 MgSO4 and 10 Hepes/Tris (pH 7.4), and supplemented with 50 mg/l gentamicin, 6 mg/l ciprofloxacin, and 100 mg/l streptomycin sulphate/100,000 units/l penicillin G sodium (Life Technologies, Carlsbad, CA). Mouse OCT1 and OCT2 (mOCT1 and mOCT2, in pBluescript II-SK) were linearized with XhoI and transcribed in vitro using the T3 mMessage mMachine kit (Applied Biosystems, Foster City, CA). Rat OCT3 (rOCT3, in pSPORT) was linearized with NotI and transcribed using the T7 mMessage mMachine kit (Applied Biosystems). Oocytes were injected one day after isolation with 25 ng mOCT1, mOCT2, or rOCT3 cRNA, and maintained at 18 °C for 4–7 days. Experiments were carried out at 20–22 °C. Non-injected oocytes served as controls.
Uptake Assays
For dose-response experiments, oocytes were incubated in the presence of 0.1 μM to 1 mM MPP+ (0.1 μM [3H]MPP+) or 0.1 μM to 10 mM spermidine (0.1 μM [3H]spermidine), in a buffer containing (mM) 100 NaCl, 3 KCl, 2 CaCl2, 1 MgCl2, and 10 Hepes/Tris (pH 7.4). For competition studies, oocytes were incubated in the presence of 0.1 μM [3H]MPP+ and 1 or 10 mM test compound (Fig. 1). After 30 min, oocytes were rinsed thoroughly with ice cold buffer, individually lysed with 2% sodium dodecyl sulfate, and assayed for radioactivity in commercial scintillation cocktail (Econo-Safe, Research Products International, Mount Prospect, IL). Unless otherwise indicated, data are shown as mean ± S.E. (standard error of the mean) of at least 3 experiments from different donor frogs, with 5–8 oocytes each.
Data Analysis
SigmaPlot 10.0 (Systat Software, San Jose, CA), Accelrys Draw 4.1 (Accelrys Inc., San Diego, CA) and CorelDRAW X3 13.0 (Corel Corporation, Mountain View, CA) were used for curve fitting, statistics, and figure preparation. The kinetic parameters of transport were calculated by nonlinear regression, using (SigmaPlot 10.0). In non-injected oocytes, concentration-dependent MPP+ and spermidine uptake values followed a linear relationship (see for example Fig. 2). Data from OCT-expressing oocytes were best fitted by a Michaelis-Menten relationship plus a non-saturable process (Equation 1), for which J is the uptake; S is the substrate concentration; Jmax and K0.5 are, respectively, the derived maximum rate and the apparent affinity constant of carrier-mediated transport; and Kd is the rate of non-saturable, diffusive transport:
| (1) |
Figure 2. Transport of MPP+ and spermidine into Xenopus laevis oocytes.
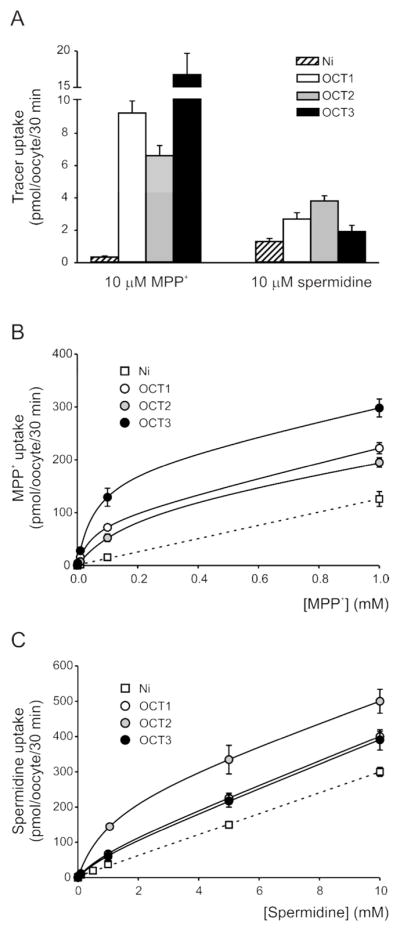
A, uptake of 10 μM MPP+ (0.1 μM [3H]MPP+) or 10 μM spermidine (0.1 μM [3H]spermidine) in non-injected oocytes (Ni, hatched bars) and in oocytes injected with mouse OCT1 (white bars), mouse OCT2 (gray bars) or rat OCT3 (black bars) cRNA. Results are means ± S.E. for 3 to 8 experiments from different oocyte preparations, each experiment with at least 6 oocytes per condition. B–C, concentration dependence of MPP+ (B) and spermidine (C) uptake into non-injected oocytes (squares) or into OCT-expressing oocytes (circles). Oocytes were incubated in presence of 0.1 μM [3H]MPP+ or 0.1 μM [3H]spermidine and increasing concentrations of unlabeled substrate. Results are shown as means ± S.E. for at least 6 oocytes. Dashed lines, MPP+ and spermidine uptake in non-injected oocytes was non-saturable, and followed a linear relationship (r2 = 0.9994 for both substrates) with estimated slopes of 125 ± 2 nl/oocyte/30 min (MPP+) and 30 ± 1 nl/oocyte/30 min (spermidine). Solid lines, uptake data from OCT-expressing oocytes were fitted with Equation 1 to estimate the kinetic parameters of OCT-mediated transport, plus a non-mediated component; results are shown in Table 1. All results are from one representative experiment, in which MPP+ and spermidine uptake were assayed in parallel, in non-injected oocytes and in oocytes from the same preparation that had been injected with OCT1, OCT2 or OCT3 cRNA, all on the same day. Each data set was confirmed at least once in a separate experiment, with oocytes from a different frog.
Where indicated, paired Student’s t test was applied to evaluate statistical differences between groups.
Results
OCT-mediated MPP+ and Spermidine Uptake
Figure 2A shows the uptake of 10 μM MPP+ and spermidine (0.1 μM [3H]MPP+ or [3H]spermidine) into non-injected oocytes, and into oocytes injected with mOCT1, mOCT2 or rOCT3 cRNA. In non-injected oocytes, MPP+ uptake was 0.5 ± 0.1 pmol/oocyte/30 min, and spermidine uptake was 2.0 ± 0.3 pmol/oocyte/30 min. In OCT-expressing oocytes, MPP+ transport rates were 15– to 35–fold higher than in non-injected oocytes, whereas those for spermidine increased more modestly above the background, up to 3–fold. To ascertain the kinetic properties of OCT-mediated and non-specific transport, we investigated the concentration dependence of MPP+ and spermidine uptake in the different experimental groups; results are presented in Figure 2B and 2C, respectively. In non-injected oocytes (squares), MPP+ (Fig. 2B) and spermidine (Fig. 2C) uptake was non-saturable, and the dose-response data followed a linear relationship (r2 > 0.999) with estimated slopes of 125 ± 2 nl/oocyte/30 min (MPP+) and 30 ± 1 nl/oocyte/30 min (spermidine). MPP+ and spermidine uptake data from OCT-injected oocytes (Fig. 2B and 2C, circles) were best fitted by Equation 1, that is, a Michaelis-Menten relationship with apparent affinity constants (K0.5) and maximum rates (Jmax) of carrier-mediated transport, plus a non-mediated component (Kd). As shown in Table 1, spermidine was transported by the OCTs with ~6– to 20–fold lower affinity than MPP+; thus, K0.5 for MPP+ was ~50 μM in OCT1, ~170 μM in OCT2, and ~60 μM in OCT3, whereas for spermidine, K0.5 was ~1 mM in OCT1, OCT2, and OCT3. On the other hand, Jmax values for MPP+ and spermidine were within the same range (Table 1), indicating that the turnover rate is similar for both compounds. Kd was estimated at ~100–140 nl/oocyte/30 min for MPP+ and ~30–34 nl/oocyte/30 min for spermidine (Table 1), consistnt with the linear rate of MPP+ and spermidine uptake in non-injected oocytes (Fig. 2B and 2C). This suggests that, in Xenopus oocytes, MPP+ and spermidine are taken up by endogenous mechanisms; because our experiments were conducted in presence of 2 mM extracellular Ca2+ (see Experimental Section), it is likely that these mechanisms are distinct from the Ca2+-sensitive connexin hemichannels described previously.7 Intriguingly, it has been observed that secondary and tertiary amines are transported across the oocyte membrane by a passive, Ca2+-insensitive carrier,31 but the molecular identity of this transport system remains elusive.32
Table 1. Kinetics of MPP+ and spermidine transport in OCT-expressing oocytes.
Data are from the experiments shown in Fig. 2B and 2C. Dose-response uptake values were fitted to Equation 1 to estimate the apparent affinity constants (K0.5) and highest rates (Jmax) of MPP+ and spermidine carrier-dependent transport, plus a non-mediated, diffusive component (Kd). The standard error of the fits is shown.
| K0.5 (μM) | Jmax (pmol/oocyte/30 min) | Kd (nl/oocyte/30 min) | |
|---|---|---|---|
| MPP+ | |||
| OCT1 | 50 ± 1 | 87 ± 1 | 139 ± 1 |
| OCT2 | 172 ± 19 | 117 ± 20 | 95 ± 14 |
| OCT3 | 57 ± 4 | 183 ± 6 | 125 ± 5 |
| Spermidine | |||
| OCT1 | 996 ± 21 | 67 ± 8 | 34 ± 1 |
| OCT2 | 1036 ± 19 | 225 ± 2 | 30 ± 1 |
| OCT3 | 983 ± 36 | 54 ± 11 | 34 ± 1 |
Inhibition of MPP+ Uptake by Polyamines and Polyamine Analogs
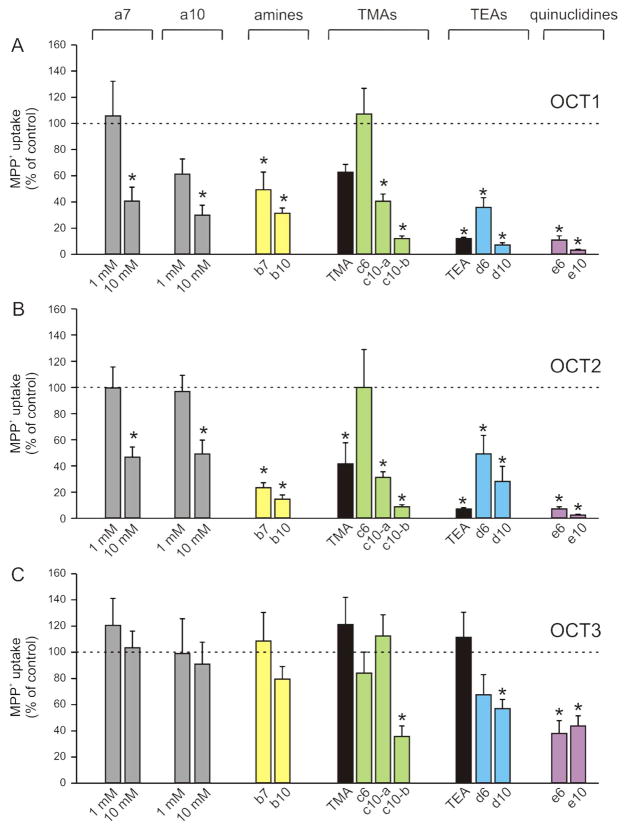
To gain further insights into OCT substrate specificity, we screened natural polyamines and a selection of structurally homologous chemicals (Fig. 1) for effect on the uptake of 0.1 μM [3H]MPP+ into oocytes expressing OCT1, OCT2 or OCT3. Spermidine (a7, Fig. 1) and spermine (a10) were used at 1 and 10 mM, and the other compounds were tested at 1 mM; results are shown in Figure 3. First, we compared spermidine (a7) and spermine (a10) with alkylamines that are equivalent in overall length but possessing only the two terminal charges, namely 1,7-diaminoheptane (b7) and 1,10-diaminodecane (b10). At 1 mM, spermidine had no effect on MPP+ uptake into OCT1 (Fig. 3A), or OCT2 (Fig. 3B) oocytes, but at 10 mM it blocked ~60 and ~55%. 1 mM spermine (a10) blocked only ~40% of the uptake in OCT1- (Fig. 3A), and failed to inhibit uptake in OCT2-expressing oocytes (Fig. 3B); at 10 mM, a10 blocked ~50% of the uptake in OCT1 and OCT2 oocytes. b7 and b10 inhibited ~50 and ~70% of MPP+ uptake in OCT1 (Fig. 3A), and about 80% in OCT2 oocytes (Fig. 3B). a7, a10, b7 and b10 did not have any significant blocking effect on MPP+ uptake into OCT3-expressing oocytes (Fig. 3C).
Figure 3. Effect of model cations, polyamines and polyamine analogs on MPP+ transport.
Uptake of 0.1 μM [3H]MPP+ in oocytes expressing OCT1 (A), OCT2 (B) or OCT3 (C) was measured in the absence or in the presence of TMA, TEA, natural polyamines (spermidine, a7; and spermine, a10), and selected polyamine analogs (b–e), as detailed in Figure 1. a7 and a10 were used at 1 and 10 mM, and the rest were tested at 1 mM. For TMA, TEA, b10, c10-a, and d10, data are means ± S.E. of 3 experiments with different donor frogs, and were normalized to the MPP+ uptake in the absence of external inhibitors (control), 180 ± 15 (OCT1), 231 ± 15 (OCT2) and 274 ± 20 (OCT3) fmol/oocyte/30 min. For a7, a10, b7, c6, c10-b, d6, e6 and e10, data are from individual experiments, where each compound was assayed in parallel in OCT1, OCT2 and OCT3-expressing oocytes from the same frog, and normalized to their own controls; each data set was confirmed in at least one additional trial with oocytes from a different preparation. Uptake in non-injected oocytes was fmol/oocyte/30 min (mean ± S.E. of 10 experiments), and was not affected by any of the test compounds. *p < 0.05 as compared to uptake in absence of external inhibitors (paired Student’s t test).
Next, we observed that increasing the hydrophobicity of the end charged group, and when more than one charge is present, the relative distance between them, improves recognition by the transporters. For example, in OCT1- and OCT2-expressing oocytes, MPP+ uptake dropped ~40% and ~60% in the presence of the monovalent cation TMA, but was unaffected in the presence of c6 (Fig. 3A and 3B), in which the two terminal trimethylamine groups are separated only by a hexyl chain (Fig. 1). In the presence of c10-a, in which the same terminal groups are separated by a decyl chain (Fig. 1), uptake decreased 60% in OCT1 (Fig. 3A) and 70% in OCT2 (Fig. 3B); c10-b, structurally analogous to c10-a but with only one charged group (Fig. 1), was a more potent blocker in both isoforms than its divalent counterpart (Fig. 3A and 3B). TEA, more hydrophobic than TMA, inhibited ~90% of the OCT1- and OCT2-mediated MPP+ uptake; blocking potency was reduced, but still significant, for d6, in which two end triethylamine groups are separated by a hexyl chain (Fig. 1), and restored in d10 (Fig. 3A and 3B), in which the two charges are more distant (Fig. 1). In OCT1 and OCT2 oocytes, MPP+ uptake was blocked over 90% in presence of quinuclidine analog c6, and almost abolished in presence of e10 (Fig. 3A and 3B). OCT3 appeared to be less tolerant than the other two isoforms: only monovalent c10-b, and highly hydrophobic divalents d6, d10, e6 and e10 inhibited the uptake of MPP+ into OCT3-expressing oocytes (Fig. 3C).
Discussion
Polyamines Are Substrates for the Organic Cation Transporters
The naturally occurring polyamines putrescine (1,4-diaminobutane), spermidine (a7, Fig. 1) and spermine (a10) are present in all cells, where they play prominent roles in cell growth, proliferation and differentiation.1 Control of the cytosolic polyamine pool is critical for normal physiology, and alterations in polyamine homeostasis have been linked to stroke, renal failure and cancer.33 Specific exchangers, transporters and multimeric uptake systems critical to the regulation of polyamine content have been described in bacteria and yeast.5,6 In higher organisms, connexin hemi-gap junctions mediate Ca2+-sensitive exchange of spermidine in Xenopus oocytes,7 and the diamine exporter complex SLC3-y+LAT has been shown to translocate putrescine and acetylpolyamine intermediaries in colon epithelial cells;34 otherwise, specific polyamine carrier proteins have yet to be identified.
Here, we have examined the hypothesis that polyamines are substrates for the organic cation transporters, namely OCT1 (SLC22A1), OCT2 (SLC22A2) and OCT3 (SLC22A3). Previously, it has been shown that putrescine and the polyamine precursor agmatine, both divalent at physiological pH, can be transported by human and rodent OCTs.11,12 We have extended this observation to demonstrate that spermidine, which is longer and carries an additional positive charge, is also a transported substrate of OCT1, OCT2 and OCT3 (Fig. 2 and Table 1). Modest inward currents were induced in presence of 1 mM spermine in Xenopus oocytes expressing rOCT1,35 suggesting that the longer, tetravalent polyamine is also transported, albeit at a low rate.
Though mouse or rat isoforms were used in our study, and interspecies differences in transport efficiency have been reported for certain compounds,28,36 it seems likely that spermidine will also be transported by human OCTs: residues that are essential for substrate specificity and binding in rodent OCTs are conserved in the respective human homologs.37–39
Physiological and Pharmacological Significance of OCT-Mediated Polyamine Transport
The identification of OCTs as relevant polyamine exchange systems may contribute to further our understanding of the physiological roles of polyamines, and the mechanisms involved in intracellular polyamine regulation. Circulating polyamine levels are low; for example, the concentration of spermidine in blood is ~300 nM.40 Here we have shown that the K0.5 for OCT-mediated spermidine uptake is ~1 mM (Table 1), and thus the OCTs may not play a significant role in cellular polyamine uptake under physiological conditions. However, intracellular polyamine concentrations in certain tissues, such as the glia, are in the mM range,41 and in these cells the OCTs could be implicated in polyamine efflux. Voltage-dependent block by cytosolic polyamines is the major process underlying inward rectification in Kir channels,3 which control membrane potential and potassium homeostasis in many tissues and organs, including the central nervous system. Human and rodent OCT2 and OCT3 are expressed in neurons of various areas of the brain42 and in glial cells,19,43 respectively, where they may participate in polyamine-mediated and Kir channel modulation and cell-to-cell communication.41,44 On the other hand, loss-of-function, overactive, or deregulated Kir channels result in disorders ranging from deafness, epilepsy and autism, to the systemic Andersen-Tawil, Barter and EAST/SeSAME syndromes;45–49 OCTs might be used as a vehicle to deliver high-affinity polyamine-like compounds to help alleviate some of these conditions.
Conversely, since OCTs are expressed in organs of drug absorption, disposition and excretion, such as the intestinal, liver, and kidney epithelia,13 they might be exploited to enhance the bioavailability of pharmaceuticals aimed to diagnose or treat diseases caused by polyamine imbalance. Carcinogenesis and tumor growth have been associated with increased intracellular polyamine levels,50 and thus OCTs might be targeted for the delivery of cytotoxic polyamine analogs or polyamine-conjugated imaging probes. This may aid particularly in the management of neuropathies and brain tumors: OCT1 and OCT2 have been shown to express in rodent and human brain microvascular epithelium,51 and have been hypothesized to mediate the blood-to-brain influx of agmatine,11 a neuromodulator which also has potent antiproliferative properties.52,53 OCT3 is found in the apical membrane of choroid plexus epithelial cells where it has been shown to participate in the cerebrospinal fluid-to-blood clearance of neurotoxic metabolites,20 and might thus be considered as a pathway for the elimination of polyamine drug byproducts.
Spermidine is a Low-Affinity Substrate for OCTs: Insights into OCT Substrate Specificity
Our results are consistent with, and complementary to, current understanding of OCT substrate selectivity. OCTs are known to interact with virtually all natural or xenobiotic primary, secondary, tertiary or quaternary amines, with K0.5, Ki or IC50 values in the μM (high affinity) to mM (low affinity) range.22 It has been established that the presence of one positive charge is essential for substrate translocation by OCTs,30 and one absolutely conserved residue within the substrate binding site (D475) is implicated in the ion-pair interaction that purportedly initiates substrate binding.37,39,54 Though the vast majority of known OCT substrates or blockers are monovalent cations, OCTs can also transport compounds that carry a net physiological charge of +2, as putrescine;11 +3, as seen here for spermidine; and perhaps even +4, as has been suggested for spermine.35
We have shown that spermidine is a substrate for OCT1, OCT2 and OCT3, but it is transported with low efficiency, i.e. at a similar turnover rate than the model substrate MPP+, but with up to 20–fold lower apparent affinity (Fig. 2 and Table 1). While the K0.5 for OCT-mediated spermidine transport was estimated at ~1 mM (Table 1), ~50% inhibition of MPP+ uptake was observed in OCT1 and OCT2-expressing oocytes only in presence of 10 mM spermidine (Fig. 3B), and neither 1 mM nor 10 mM spermidine had any blocking effect in OCT3-expressing oocytes (Fig. 3C). Based on mutagenesis, pharmacology and homology modeling data, it has been hypothesized that OCTs contain distinct, but partially overlapping, binding sites for different substrates.37,38,54 When administered together, cations bound to high-affinity sites can partially or totally compete away cations bound to low-affinity sites,55 and this may help explain the apparent discrepancy between our dose-response data and our competition assays. On the other hand, the low apparent affinity of OCTs for the polyamines may result from the relative hydrophilic nature of those charges, which in effect reduce the overall hydrophobicity of the compound, rather than the multiple charges carried by spermidine (a7; Fig. 1) or spermine (a10). Unlike spermidine or spermine, the diamines b7 and b10, which are significantly more hydrophobic than the polyamines,56 effectively inhibited MPP+ uptake in OCT1- and OCT2-expressing oocytes (Fig. 3A and 3B). Among the different dicationic analogs tested, the presence of more hydrophobic terminal groups generally resulted in a stronger block; within each class, elongating the aliphatic chain (and thus expanding the hydrophobic region) between charges seemed to improve recognition. For example, TMAs were overall weaker blockers than TEAs or quinuclidines, and among TMAs, c10-a and c10-b were stronger than c6 (Fig. 3).
Several previous studies have shown that there is a direct correlation between the hydrophobic character of a given compound and the strength of its interaction with the OCTs.26–29 In particular, it has been described that increasing the length of the alkyl chain in n-tetraalkylammonium compounds, which effectively increases their hydrophobicity, translates into a proportional increase in the IC50 values for inhibition of hOCT1-mediated radiolabelled TEA uptake in mammalian cells,27,57 and we observed that TEA was a more effective blocker of MPP+ uptake than TMA in OCT1- and OCT2-expressing oocytes (Fig. 3A and 3B). Though the relationship between hydrophobicity and binding affinity of OCT substrates and inhibitors with multiple charges has not been systematically addressed, our data suggest that, as with their monovalent counterparts, recognition and binding of divalent substrates depend largely on their overall hydrophobic character. Accordingly, whereas very hydrophobic, cyclic dicationic chemicals such as the broad-range herbicide paraquat,58 and the antiparasitic drugs pentamidine and furamidine,59 are high-affinity substrates or inhibitors of OCTs, the more hydrophilic diamines putrescine and agmatine are transported with very low efficiency.11
Most OCT substrates or blockers seem to interact less efficiently with OCT3 than with OCT1 or OCT2.22 Accordingly, neither the diamines, nor TMA or TEA, but only the most hydrophobic compounds used in our study, namely c10-b, d6, d10, e6 and e10 (Fig. 1), inhibited MPP+ uptake (Fig. 3C). This seems to indicate that OCT3 has more stringent binding requirements than its counterparts, in particular for hydrophobicity, which in turn may respond to differences in sequence and structural organization. No tridimensional structure of OCTs has yet been solved, but homology models of OCT1 and OCT2, based on extensive biochemical data and high-resolution crystal structures of E. coli LacY and GlpT,60,61 suggest that seven of the predicted twelve transmembrane helices (TM) of these proteins, namely TM 1, 2, 4, 5, 7, 10 and 11, fold in a large hydrophobic cleft capable of accommodating a wide variety of chemical species.39,62 OCT1 and OCT2 are 70% identical and have comparable substrate selectivity profiles, reflected in the relatively similar makeup of their predicted binding pockets.22,38,63 OCT3 orthologs share only 50% sequence identity with OCT1 or OCT2, which may translate into architectural differences substantial enough to impact transport or inhibition kinetics and may account for their apparently tighter binding constraints.
Acknowledgments
The authors wish to thank Simonne Francis and Valentina Ghisays for technical assistance. mOCT1 and mOCT2 were generously provided by Valentin Gorboulev (University of Wuerzburg, Germany), and rOCT3 was a gift from Vadivel Ganapathy (Georgia Health Sciences University, Augusta, GA). Major funding was provided by NIH grants HL54171 (to CGN) and NS065201 (to SNS). Synthesis of novel polyamine analogs 1,6 bis-trimethylaminohexane (c6), 1,6 bis-triethylaminohexane (d6), 1,10 bis-triethylaminodecane (d10), 1,6 bis-quinuclidinehexane (e6) and 1,10 bis-quinuclidinedecane (e10) was supported by National Science and Engineering Research Council of Canada (NSERC) Discovery Grants (to GRD and HTK).
References
- 1.Pegg AE. Mammalian polyamine metabolism and function. IUBMB Life. 2009;61:880–94. doi: 10.1002/iub.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Loussouarn G, Marton LJ, Nichols CG. Molecular basis of inward rectification: structural features of the blocker defined by extended polyamine analogs. Molecular pharmacology. 2005;68:298–304. doi: 10.1124/mol.105.012377. [DOI] [PubMed] [Google Scholar]
- 3.Nichols CG, Lopatin AN. Inward rectifier potassium channels. Annu Rev Physiol. 1997;59:171–91. doi: 10.1146/annurev.physiol.59.1.171. [DOI] [PubMed] [Google Scholar]
- 4.Rock DM, Macdonald RL. Polyamine regulation of N-methyl-D-aspartate receptor channels. Annual review of pharmacology and toxicology. 1995;35:463–82. doi: 10.1146/annurev.pa.35.040195.002335. [DOI] [PubMed] [Google Scholar]
- 5.Aouida M, Leduc A, Poulin R, Ramotar D. AGP2 encodes the major permease for high affinity polyamine import in Saccharomyces cerevisiae. The Journal of biological chemistry. 2005;280:24267–76. doi: 10.1074/jbc.M503071200. [DOI] [PubMed] [Google Scholar]
- 6.Igarashi K, Kashiwagi K. Modulation of cellular function by polyamines. Int J Biochem Cell Biol. 2010;42:39–51. doi: 10.1016/j.biocel.2009.07.009. [DOI] [PubMed] [Google Scholar]
- 7.Enkvetchakul D, Ebihara L, Nichols CG. Polyamine flux in Xenopus oocytes through hemi-gap junctional channels. J Physiol. 2003;553:95–100. doi: 10.1113/jphysiol.2003.047910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sha Q, Romano C, Lopatin AN, Nichols CG. Spermidine release from xenopus oocytes. Electrodiffusion through a membrane channel. The Journal of biological chemistry. 1996;271:3392–7. doi: 10.1074/jbc.271.7.3392. [DOI] [PubMed] [Google Scholar]
- 9.Poulin R, Casero RA, Soulet D. Recent advances in the molecular biology of metazoan polyamine transport. Amino acids. 2012;42:711–23. doi: 10.1007/s00726-011-0987-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Busch AE, Quester S, Ulzheimer JC, Waldegger S, Gorboulev V, Arndt P, Lang F, Koepsell H. Electrogenic properties and substrate specificity of the polyspecific rat cation transporter rOCT1. The Journal of biological chemistry. 1996;271:32599–604. doi: 10.1074/jbc.271.51.32599. [DOI] [PubMed] [Google Scholar]
- 11.Winter TN, Elmquist WF, Fairbanks CA. OCT2 and MATE1 provide bidirectional agmatine transport. Molecular pharmaceutics. 2011;8:133–42. doi: 10.1021/mp100180a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grundemann D, Hahne C, Berkels R, Schomig E. Agmatine is efficiently transported by non-neuronal monoamine transporters extraneuronal monoamine transporter (EMT) and organic cation transporter 2 (OCT2) The Journal of pharmacology and experimental therapeutics. 2003;304:810–7. doi: 10.1124/jpet.102.044404. [DOI] [PubMed] [Google Scholar]
- 13.Nies AT, Koepsell H, Damme K, Schwab M. Organic cation transporters (OCTs, MATEs), in vitro and in vivo evidence for the importance in drug therapy. Handb Exp Pharmacol. 2011:105–67. doi: 10.1007/978-3-642-14541-4_3. [DOI] [PubMed] [Google Scholar]
- 14.Gorboulev V, Ulzheimer JC, Akhoundova A, Ulzheimer-Teuber I, Karbach U, Quester S, Baumann C, Lang F, Busch AE, Koepsell H. Cloning and characterization of two human polyspecific organic cation transporters. DNA Cell Biol. 1997;16:871–81. doi: 10.1089/dna.1997.16.871. [DOI] [PubMed] [Google Scholar]
- 15.Nishimura M, Naito S. Tissue-specific mRNA expression profiles of human ATP-binding cassette and solute carrier transporter superfamilies. Drug metabolism and pharmacokinetics. 2005;20:452–77. doi: 10.2133/dmpk.20.452. [DOI] [PubMed] [Google Scholar]
- 16.Lips KS, Volk C, Schmitt BM, Pfeil U, Arndt P, Miska D, Ermert L, Kummer W, Koepsell H. Polyspecific cation transporters mediate luminal release of acetylcholine from bronchial epithelium. Am J Resp Cell Mol. 2005;33:79–88. doi: 10.1165/rcmb.2004-0363OC. [DOI] [PubMed] [Google Scholar]
- 17.Muller J, Lips KS, Metzner L, Neubert RHH, Koepsell H, Brandsch M. Drug specificity and intestinal membrane localization of human organic cation transporters (OCT) Biochem Pharmacol. 2005;70:1851–1860. doi: 10.1016/j.bcp.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 18.Sata R, Ohtani H, Tsujimoto M, Murakami H, Koyabu N, Nakamura T, Uchiumi T, Kuwano M, Nagata H, Tsukimori K, Nakano H, Sawada Y. Functional analysis of organic cation transporter 3 expressed in human placenta. The Journal of pharmacology and experimental therapeutics. 2005;315:888–95. doi: 10.1124/jpet.105.086827. [DOI] [PubMed] [Google Scholar]
- 19.Inazu M, Takeda H, Matsumiya T. Expression and functional characterization of the extraneuronal monoamine transporter in normal human astrocytes. Journal of neurochemistry. 2003;84:43–52. doi: 10.1046/j.1471-4159.2003.01566.x. [DOI] [PubMed] [Google Scholar]
- 20.Hosoya K, Tachikawa M. Roles of organic anion/cation transporters at the blood-brain and blood-cerebrospinal fluid barriers involving uremic toxins. Clin Exp Nephrol. 2011;15:478–85. doi: 10.1007/s10157-011-0460-y. [DOI] [PubMed] [Google Scholar]
- 21.Wright SH. Role of organic cation transporters in the renal handling of therapeutic agents and xenobiotics. Toxicology and applied pharmacology. 2005;204:309–19. doi: 10.1016/j.taap.2004.10.021. [DOI] [PubMed] [Google Scholar]
- 22.Koepsell H, Lips K, Volk C. Polyspecific organic cation transporters: structure, function, physiological roles, and biopharmaceutical implications. Pharmaceutical research. 2007;24:1227–51. doi: 10.1007/s11095-007-9254-z. [DOI] [PubMed] [Google Scholar]
- 23.Zolk O. Disposition of metformin: variability due to polymorphisms of organic cation transporters. Ann Med. 2012;44:119–29. doi: 10.3109/07853890.2010.549144. [DOI] [PubMed] [Google Scholar]
- 24.Choi MK, Song IS. Organic cation transporters and their pharmacokinetic and pharmacodynamic consequences. Drug metabolism and pharmacokinetics. 2008;23:243–53. doi: 10.2133/dmpk.23.243. [DOI] [PubMed] [Google Scholar]
- 25.Ciarimboli G. Role of organic cation transporters in drug-induced toxicity. Expert Opin Drug Metab Toxicol. 2011;7:159–74. doi: 10.1517/17425255.2011.547474. [DOI] [PubMed] [Google Scholar]
- 26.Ahlin G, Karlsson J, Pedersen JM, Gustavsson L, Larsson R, Matsson P, Norinder U, Bergstrom CA, Artursson P. Structural requirements for drug inhibition of the liver specific human organic cation transport protein 1. J Med Chem. 2008;51:5932–42. doi: 10.1021/jm8003152. [DOI] [PubMed] [Google Scholar]
- 27.Bednarczyk D, Ekins S, Wikel JH, Wright SH. Influence of molecular structure on substrate binding to the human organic cation transporter, hOCT1. Molecular pharmacology. 2003;63:489–98. doi: 10.1124/mol.63.3.489. [DOI] [PubMed] [Google Scholar]
- 28.Suhre WM, Ekins S, Chang C, Swaan PW, Wright SH. Molecular determinants of substrate/inhibitor binding to the human and rabbit renal organic cation transporters hOCT2 and rbOCT2. Molecular pharmacology. 2005;67:1067–77. doi: 10.1124/mol.104.004713. [DOI] [PubMed] [Google Scholar]
- 29.Zolk O, Solbach TF, Konig J, Fromm MF. Structural determinants of inhibitor interaction with the human organic cation transporter OCT2 (SLC22A2) Naunyn Schmiedebergs Arch Pharmacol. 2009;379:337–48. doi: 10.1007/s00210-008-0369-5. [DOI] [PubMed] [Google Scholar]
- 30.Harlfinger S, Fork C, Lazar A, Schomig E, Grundemann D. Are organic cation transporters capable of transporting prostaglandins? Naunyn Schmiedebergs Arch Pharmacol. 2005;372:125–30. doi: 10.1007/s00210-005-0011-8. [DOI] [PubMed] [Google Scholar]
- 31.Burckhardt BC, Thelen P. Effect of primary, secondary and tertiary amines on membrane potential and intracellular pH in Xenopus laevis oocytes. Pflugers Archiv: European journal of physiology. 1995;429:306–12. doi: 10.1007/BF00374144. [DOI] [PubMed] [Google Scholar]
- 32.Sobczak K, Bangel-Ruland N, Leier G, Weber WM. Endogenous transport systems in the Xenopus laevis oocyte plasma membrane. Methods. 2010;51:183–9. doi: 10.1016/j.ymeth.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 33.Casero RA, Pegg AE. Polyamine catabolism and disease. The Biochemical journal. 2009;421:323–38. doi: 10.1042/BJ20090598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Uemura T, Yerushalmi HF, Tsaprailis G, Stringer DE, Pastorian KE, Hawel L, 3rd, Byus CV, Gerner EW. Identification and characterization of a diamine exporter in colon epithelial cells. The Journal of biological chemistry. 2008;283:26428–35. doi: 10.1074/jbc.M804714200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Busch AE, Quester S, Ulzheimer JC, Gorboulev V, Akhoundova A, Waldegger S, Lang F, Koepsell H. Monoamine neurotransmitter transport mediated by the polyspecific cation transporter rOCT1. FEBS letters. 1996;395:153–6. doi: 10.1016/0014-5793(96)01030-7. [DOI] [PubMed] [Google Scholar]
- 36.Dresser MJ, Gray AT, Giacomini KM. Kinetic and selectivity differences between rodent, rabbit, and human organic cation transporters (OCT1) The Journal of pharmacology and experimental therapeutics. 2000;292:1146–52. [PubMed] [Google Scholar]
- 37.Gorboulev V, Shatskaya N, Volk C, Koepsell H. Subtype-specific affinity for corticosterone of rat organic cation transporters rOCT1 and rOCT2 depends on three amino acids within the substrate binding region. Molecular pharmacology. 2005;67:1612–9. doi: 10.1124/mol.104.008821. [DOI] [PubMed] [Google Scholar]
- 38.Koepsell H. Substrate recognition and translocation by polyspecific organic cation transporters. Biol Chem. 2011;392:95–101. doi: 10.1515/BC.2011.009. [DOI] [PubMed] [Google Scholar]
- 39.Popp C, Gorboulev V, Muller TD, Gorbunov D, Shatskaya N, Koepsell H. Amino acids critical for substrate affinity of rat organic cation transporter 1 line the substrate binding region in a model derived from the tertiary structure of lactose permease. Molecular pharmacology. 2005;67:1600–11. doi: 10.1124/mol.104.008839. [DOI] [PubMed] [Google Scholar]
- 40.Van Dobbenburgh OA, Houwen B, Jurjens H, Marrink J, Halie MR, Nieweg HO. Plasma spermidine concentrations as early indication of response to therapy in human myeloma. Journal of clinical pathology. 1983;36:804–7. doi: 10.1136/jcp.36.7.804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Benedikt J, Inyushin M, Kucheryavykh YV, Rivera Y, Kucheryavykh LY, Nichols CG, Eaton MJ, Skatchkov SN. Intracellular polyamines enhance astrocytic coupling. Neuroreport. 2012;23:1021–5. doi: 10.1097/WNR.0b013e32835aa04b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Busch AE, Karbach U, Miska D, Gorboulev V, Akhoundova A, Volk C, Arndt P, Ulzheimer JC, Sonders MS, Baumann C, Waldegger S, Lang F, Koepsell H. Human neurons express the polyspecific cation transporter hOCT2, which translocates monoamine neurotransmitters, amantadine, and memantine. Molecular pharmacology. 1998;54:342–52. doi: 10.1124/mol.54.2.342. [DOI] [PubMed] [Google Scholar]
- 43.Cui M, Aras R, Christian WV, Rappold PM, Hatwar M, Panza J, Jackson-Lewis V, Javitch JA, Ballatori N, Przedborski S, Tieu K. The organic cation transporter-3 is a pivotal modulator of neurodegeneration in the nigrostriatal dopaminergic pathway. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:8043–8. doi: 10.1073/pnas.0900358106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kucheryavykh YV, Shuba YM, Antonov SM, Inyushin MY, Cubano L, Pearson WL, Kurata H, Reichenbach A, Veh RW, Nichols CG, Eaton MJ, Skatchkov SN. Complex rectification of Muller cell Kir currents. Glia. 2008;56:775–90. doi: 10.1002/glia.20652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bockenhauer D, Feather S, Stanescu HC, Bandulik S, Zdebik AA, Reichold M, Tobin J, Lieberer E, Sterner C, Landoure G, Arora R, Sirimanna T, Thompson D, Cross JH, van’t Hoff W, Al Masri O, Tullus K, Yeung S, Anikster Y, Klootwijk E, Hubank M, Dillon MJ, Heitzmann D, Arcos-Burgos M, Knepper MA, Dobbie A, Gahl WA, Warth R, Sheridan E, Kleta R. Epilepsy, ataxia, sensorineural deafness, tubulopathy, and KCNJ10 mutations. The New England journal of medicine. 2009;360:1960–70. doi: 10.1056/NEJMoa0810276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hibino H, Inanobe A, Furutani K, Murakami S, Findlay I, Kurachi Y. Inwardly rectifying potassium channels: their structure, function, and physiological roles. Physiological reviews. 2010;90:291–366. doi: 10.1152/physrev.00021.2009. [DOI] [PubMed] [Google Scholar]
- 47.Inyushin M, Kucheryavykh LY, Kucheryavykh YV, Nichols CG, Buono RJ, Ferraro TN, Skatchkov SN, Eaton MJ. Potassium channel activity and glutamate uptake are impaired in astrocytes of seizure-susceptible DBA/2 mice. Epilepsia. 2010;51:1707–13. doi: 10.1111/j.1528-1167.2010.02592.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sala-Rabanal M, Kucheryavykh LY, Skatchkov SN, Eaton MJ, Nichols CG. Molecular mechanisms of EAST/SeSAME syndrome mutations in Kir4. 1 (KCNJ10) The Journal of biological chemistry. 2010;285:36040–8. doi: 10.1074/jbc.M110.163170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Scholl UI, Choi M, Liu T, Ramaekers VT, Hausler MG, Grimmer J, Tobe SW, Farhi A, Nelson-Williams C, Lifton RP. Seizures, sensorineural deafness, ataxia, mental retardation, and electrolyte imbalance (SeSAME syndrome) caused by mutations in KCNJ10. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:5842–7. doi: 10.1073/pnas.0901749106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Casero RA, Jr, Marton LJ. Targeting polyamine metabolism and function in cancer and other hyperproliferative diseases. Nature reviews Drug discovery. 2007;6:373–90. doi: 10.1038/nrd2243. [DOI] [PubMed] [Google Scholar]
- 51.Lin CJ, Tai Y, Huang MT, Tsai YF, Hsu HJ, Tzen KY, Liou HH. Cellular localization of the organic cation transporters, OCT1 and OCT2, in brain microvessel endothelial cells and its implication for MPTP transport across the blood-brain barrier and MPTP-induced dopaminergic toxicity in rodents. Journal of neurochemistry. 2010;114:717–27. doi: 10.1111/j.1471-4159.2010.06801.x. [DOI] [PubMed] [Google Scholar]
- 52.Isome M, Lortie MJ, Murakami Y, Parisi E, Matsufuji S, Satriano J. The antiproliferative effects of agmatine correlate with the rate of cellular proliferation. American journal of physiology Cell physiology. 2007;293:C705–11. doi: 10.1152/ajpcell.00084.2007. [DOI] [PubMed] [Google Scholar]
- 53.Wang JF, Su RB, Wu N, Xu B, Lu XQ, Liu Y, Li J. Inhibitory effect of agmatine on proliferation of tumor cells by modulation of polyamine metabolism. Acta pharmacologica Sinica. 2005;26:616–22. [PubMed] [Google Scholar]
- 54.Gorboulev V, Volk C, Arndt P, Akhoundova A, Koepsell H. Selectivity of the polyspecific cation transporter rOCT1 is changed by mutation of aspartate 475 to glutamate. Molecular pharmacology. 1999;56:1254–61. doi: 10.1124/mol.56.6.1254. [DOI] [PubMed] [Google Scholar]
- 55.Gorbunov D, Gorboulev V, Shatskaya N, Mueller T, Bamberg E, Friedrich T, Koepsell H. High-affinity cation binding to organic cation transporter 1 induces movement of helix 11 and blocks transport after mutations in a modeled interaction domain between two helices. Molecular pharmacology. 2008;73:50–61. doi: 10.1124/mol.107.040170. [DOI] [PubMed] [Google Scholar]
- 56.Weiger TM, Langer T, Hermann A. External action of di- and polyamines on maxi calcium-activated potassium channels: an electrophysiological and molecular modeling study. Biophysical journal. 1998;74:722–30. doi: 10.1016/S0006-3495(98)73997-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhang L, Gorset W, Dresser MJ, Giacomini KM. The interaction of n-tetraalkylammonium compounds with a human organic cation transporter, hOCT1. The Journal of pharmacology and experimental therapeutics. 1999;288:1192–8. [PubMed] [Google Scholar]
- 58.Chen Y, Zhang S, Sorani M, Giacomini KM. Transport of paraquat by human organic cation transporters and multidrug and toxic compound extrusion family. The Journal of pharmacology and experimental therapeutics. 2007;322:695–700. doi: 10.1124/jpet.107.123554. [DOI] [PubMed] [Google Scholar]
- 59.Ming X, Ju W, Wu H, Tidwell RR, Hall JE, Thakker DR. Transport of dicationic drugs pentamidine and furamidine by human organic cation transporters. Drug Metab Dispos. 2009;37:424–30. doi: 10.1124/dmd.108.024083. [DOI] [PubMed] [Google Scholar]
- 60.Abramson J, Smirnova I, Kasho V, Verner G, Kaback HR, Iwata S. Structure and mechanism of the lactose permease of Escherichia coli. Science. 2003;301:610–5. doi: 10.1126/science.1088196. [DOI] [PubMed] [Google Scholar]
- 61.Huang Y, Lemieux MJ, Song J, Auer M, Wang DN. Structure and mechanism of the glycerol-3-phosphate transporter from Escherichia coli. Science. 2003;301:616–20. doi: 10.1126/science.1087619. [DOI] [PubMed] [Google Scholar]
- 62.Zhang X, Shirahatti NV, Mahadevan D, Wright SH. A conserved glutamate residue in transmembrane helix 10 influences substrate specificity of rabbit OCT2 (SLC22A2) The Journal of biological chemistry. 2005;280:34813–22. doi: 10.1074/jbc.M506342200. [DOI] [PubMed] [Google Scholar]
- 63.Wright SH, Dantzler WH. Molecular and cellular physiology of renal organic cation and anion transport. Physiological reviews. 2004;84:987–1049. doi: 10.1152/physrev.00040.2003. [DOI] [PubMed] [Google Scholar]