Abstract
A fundamental question in cognitive neuroscience is how the human brain self-organizes to perform tasks. Multiple accounts of this self-organization are currently influential and in this article we survey one of these accounts. We begin by introducing a psychological model of task control and several neuroimaging signals it predicts. We then discuss where such signals are found across tasks with emphasis on brain regions where multiple control signals are present. We then present results derived from spontaneous task-free functional connectivity between control-related regions that dovetail with distinctions made by control signals present in these regions, leading to a proposal that there are at least two task control systems in the brain. This prompts consideration of whether and how such control systems distinguish themselves from other brain regions in a whole-brain context. We present evidence from whole-brain networks that such distinctions do occur and that control systems comprise some of the basic system-level organizational elements of the human brain. We close with observations from the whole-brain networks that may suggest parsimony between multiple accounts of cognitive control.
Introduction
Human beings can perform an endless variety of tasks with high accuracy despite little or no practice. To successfully perform a particular task, mental operations must be selected for precisely that task out of an infinite number of possible tasks and corresponding mental processes. How such processes are selected and implemented is the central question of cognitive control.
Prominent psychological models of task performance distinguish between ‘processors’ and ‘controllers’ [1]. Processors are specialized for particular operations. Controllers influence how processors operate. As information flows from sensory input to a decision or motor output in a task, processors operate on the information and controllers ensure that processors are arrayed in a manner that fulfills the task demands.
The anatomical basis for a system of ‘controllers’ was outlined shortly after the advent of noninvasive human neuroimaging in a review by Posner and Petersen [2]. The main arguments of the review were first, that attention is supported by a network of brain regions acting in concert, second, that these regions are physically distinct from regions that process specific inputs in a more automatic fashion, and third, that individual regions support particular cognitive operations involved in attention. In particular the review highlighted the importance of superior parietal cortex for orienting and anterior cingulate cortex for executive function.
These arguments remain operational more than two decades later. Different cognitive neuroscientific models of attention have been elaborated but the anatomical distinction between controllers and processors remains [3,4]. The anatomical basis for control systems has been elaborated, refined, and contested under various theoretical frameworks, but all frameworks rest upon discrete task control regions.
Extending beyond the Posner and Petersen account, Corbetta and Shulman outlined dorsal and ventral attention systems of parietal and frontal regions that had separate functions in top-down orienting and bottom-up disengagement of attention. Hierarchical accounts of cognitive control (based on temporal aspects of the task [5] or task complexity [6•]) focus on broad swathes of dorsolateral prefrontal cortex (DLPFC). Conflict monitoring accounts focus on the anterior cingulate cortex for conflict detection and DLPFC for executive control [7•]. Still other accounts have identified control regions across the brain, including frontal, parietal, cingulate, insular, subcortical, and even cerebellar regions [8•,9-11].
In this review we present one account of task control, summarizing a 20-year program of research in our laboratory. This account is not exclusive of all the other ideas about mechanisms and regions involved in attention networks, but it does organize a substantial fraction of the regions involved into two functionally distinct systems.
We begin by describing signals that would be expected from control regions under the psychological model outlined above. We then identify brain regions where such signals are found across many task fMRI experiments. We then describe the relationships found between these regions at rest (in the absence of task). These relationships indicate that control regions segregate into two systems and we relate these systems to different time scales of control during task performance. Finally, we assess the approximate network structure of the entire brain and find subnetworks in this whole-brain network that correspond to the two control systems. This last point illustrates that what we have identified as control systems are some of the basic building blocks of the brain’s functional organization.
Characteristics of control regions
Under the psychological model outlined above, at least three signals may be defined that control regions should display across a wide variety of tasks (Figure 1a). First, when a subject is given a cue to begin a particular task, control regions must send configuring signals to processors to establish the correct processing strategy needed for the task (the task set). A control region may therefore display start-cue activity as the task set is selected and instantiated. Second, for as long as a subject continues to perform the task, the task set must be maintained. A control region may therefore display sustained activity during task performance. Third, because successful control needs to recognize errors in performance and adjust task set accordingly, a control region may display error-specific activity. Figure 1a shows how each of these signals could manifest in a task fMRI experiment.
Figure 1. Characteristics of control regions.
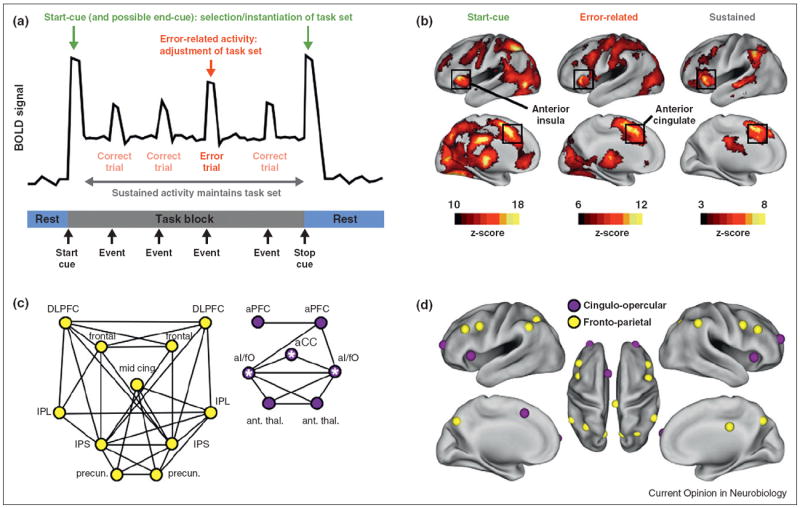
(a) A theoretical timecourse from a region that displays start-cue (green), sustained (gray), and error-related (red) activity. These three signals are characteristic of control regions. (b) The locations of each of the control signals across 10 task fMRI studies plotted as fixed-effect z-scores. Black squares indicate the anterior insula and anterior cingulate, the original ‘core’ of the task control system. Modified from [8•]. (c) Resting state correlations between select control regions (r > 0.175). At left in yellow, the fronto-parietal system. At right in purple, the cingulo-opercular system (‘core’ regions have white stars). Modified from [16]. (d) The regions in (c) on a PALS inflated brain surface.
We searched for such signals using a meta-analysis of 10 task fMRI datasets [8•]. These datasets used various sensory input (audio, visual) and motor responses (speech, button-pushing) and demanded a variety of intermediate operations (target detection, verb generation, semantic judgements, etc.). Start-cue, sustained, and error-related signals that were present across most studies could not be modality-specific or task-specific but instead must be task-general, consistent with control signals. Figure 1b shows where these signals were located. The distribution of each signal is unique and regions throughout the brain are identified — in frontal, parietal, temporal, insular, and cingulate cortex, as well as subcortical and cerebellar regions. Many brain regions display two of three signals, and some display all three. The boxed regions — in the dorsal anterior cingulate and dorsal anterior insula — display all three signals and were termed the ‘core’ of the task control system [8•].
The meta-analysis indicated that a surprisingly large number of brain regions may play roles in task control, and we began to search for organizing principles in these regions. By the mid-2000s it was clear that low-frequency BOLD signal obtained from subjects at rest — in the absence of a task — displayed remarkable properties (we abbreviate this signal RSFC for resting state functional connectivity MRI). Early experiments showed that over a 5–10 min period, the RSFC signal in a motor region would correlate selectively and highly with signal in other motor regions, the signal in visual regions would correlate selectively and highly with other visual regions, and likewise for auditory cortex [12-14]. Importantly, the default mode system, a ‘cognitive’ system, displayed similar selective RSFC correlations [15]. This suggested that if our control regions were in fact a basic functional system, they too should have high and selective RSFC correlations with one another.
We examined the RSFC signal in regions displaying control signals to see whether these regions also had selective patterns of RSFC correlations. We discovered that control regions appeared to group into at least two systems (Figure 1c,d) [16]. One system, termed the cingulo-opercular system, contained the ‘core’ regions with some additional regions such as anterior prefrontal cortex. The other system consisted mainly of frontal and parietal regions and was termed the fronto-parietal system. The cingulo-opercular system contains regions that display start-cue, error-related, and sustained activity, whereas the fronto-parietal system contains regions that displayed start-cue and error-related activity but not sustained activity during task set maintenance. RSFC correlations were high within each system but relatively low between systems, much as correlations are high within visual and auditory systems but are relatively low between such systems. We therefore refined our model of task control, suggesting that perhaps it is implemented by two relatively independent systems that operate on different time scales. The cingulo-opercular system could potentially operate over long (and short) timescales, playing a key role in task set maintenance, whereas the fronto-parietal system could preferentially operate over shorter time scales, playing a role in moment-to-moment task set adjustment [17].
Control systems in the context of whole-brain networks
The analyses described thus far were highly constrained: particular signal types defined control regions and the refinement into two control systems was based only on relationships between these select regions. It remained possible that the systems we described arose from a particular method of region selection and that our control systems were not really organizational elements of the human brain.
We therefore sought to define whole-brain networks and to assay RSFC relationships in these networks for specific subnetworks corresponding to our control systems. Whole-brain networks were formed in two complementary ways [18•]. The first way sought to define, incompletely but as best we could, the locations of areas and nuclei throughout the brain. We used a battery of task fMRI meta-analyses targeting button-pushing, reading, verb generation, task-induced deactivations, and other processes or signal types to define 165 areas and added a further 99 areas using fc-Mapping techniques [19,20]. This generated a collection of 264 regions that served as nodes in an ‘areal’ network. The second way of forming whole-brain networks was to consider every voxel in the brain as a node, leading to a ‘modified voxelwise’ network.
When the RSFC relationships between nodes in each of these whole-brain networks were examined, both control systems re-emerged as selective groupings of nodes in the larger network context [18•]. The fronto-parietal system is shown in yellow, and the cingulo-opercular system is shown in purple in Figure 2. These results indicated that what we identified as control systems are basic system-level elements of functional brain organization. It is important to recognize, too, that the subnetworks defined in the network analyses are more expansive than the originally proposed control systems. For example, the cingulo-opercular subnetwork contains not only the anterior insula, anterior cingulate, and anterior prefrontal cortex, but also representation in the supramarginal gyrus and other brain regions.
Figure 2. Control systems in the context of whole-brain networks.
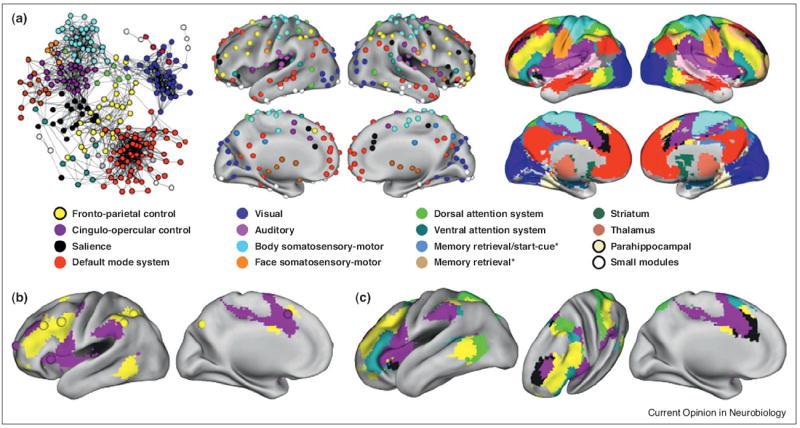
(a) A representative picture of the areal network is shown at left, where colors denote algorithmically determined subnetworks of the network (modified from [18•]). The middle and right pictures show consensus assignments of the areal and modified voxelwise networks. Nodes of the same color correlate highly and belong to the same subnetwork. (b) The control regions from Figure 1D are plotted over the modified voxelwise subnetworks to show the correspondence between subnetworks and control systems. (c) Five putative attention-related or control-related subnetworks are shown: the fronto-parietal control system, the cingulo-opercular control system, the salience system, the dorsal attention system, and the ventral attention system. Starred subnetwork names indicate tasks or signals that activate the subnetwork in task fMRI meta-analyses.
The modest difference between task-defined systems and RSFC subnetworks prompts two comments. First, because RSFC correlations are selectively high among functionally related regions (e.g., visual regions), we suspect that RSFC correlations reflect long-standing histories of co-activation among regions that perform similar operations. Accordingly, we consider it likely that the supramarginal gyrus routinely performs operations related to those performed in the regions identified as cingulo-opercular control regions. Second, the definition of RSFC subnetworks is not absolute but is instead somewhat flexible depending on methodological parameters [18•,21•]. That is to say that a subnetwork such as the cingulo-opercular system can be further broken into sub-subnetworks, which in this case act to separate the supramarginal gyrus from the ‘core’ regions of task control. Another way of viewing this is that the supramarginal gyrus is somewhat peripherally related to the cingulo-opercular ‘core’ regions.
Integrating whole-brain networks with other accounts of control
Our discussion so far neglects other sets of regions that have been posited to act as control systems. As noted above, Corbetta and Shulman have proposed the existence of dorsal and ventral attention systems that are respectively implicated in top-down visuospatial attention and bottom-up reorienting [22]. What we have presented so far is not meant to supercede or contradict the presence of these systems. In fact, in the whole brain networks, there are prominent subnetworks that appear to represent the dorsal and ventral attention systems (Figure 2). The implications of these multiple systems are discussed more fully in [23].
Another result of the whole-brain analysis is to suggest parsimony among some accounts of control. At the same time that our laboratory made fronto-parietal and cingulo-opercular distinctions between task control systems, Seeley et al. [10] reported two similar systems — an ‘executive’ system similar to the fronto-parietal system and a ‘salience’ system similar to the cingulo-opercular system. The ‘salience’ system offered a different explanation for the control-like signals in cingulo-opercular regions: that processes associated with autonomic arousal, rather than control per se, would also manifest as start-cue, sustained, and error-related activity. The whole-brain network provides a potential resolution to the two accounts of cingulo-opercular activity: in the modified voxelwise analysis there appear to be at least two cingulo-opercular systems (black and purple in Figure 2). The coordinates reported by Seeley and colleagues more closely reflect the black subnetwork, whereas the coordinates reported by Dosenbach and colleagues more closely reflect the purple subnetwork [18•]. Both subnetworks demonstrate sustained and error-related activity, but start-cue activity is more localized to the purple subnetwork.
To return to the psychological model of control, processors act in a relatively automatic fashion on specific types of inputs, whereas control regions act to influence the processors. One would therefore expect that processing regions might have relationships restricted mainly to related processors, whereas controllers would have a wider variety of relationships in the network reflecting their interactions with other control regions and a variety of processors. Network relationships are largely consistent with these expectations: in the areal network, processing regions such as visual regions are highly related to one another but are relatively segregated from the rest of the network, whereas regions of the fronto-parietal control system are centrally placed in the network and display relationships to a variety of systems [18•]. We note however that the extensive nature of the fronto-parietal system, and the relation of some of its regions to processing associated with specific cognitive activity during mathematical reasoning [24] or memory retrieval tasks [25] suggest that this large system may have interesting substructure in terms of both control and processing, or that for this particular system, the control/processing distinction may not be so pure.
Recent challenges to accounts of cognitive control
Not all accounts of cognitive control are supported by the network results, however. One perspective on control has been that DLPFC is organized hierarchically in rostro-caudal gradients, such that more complex (from a temporal [5] or rule abstraction [6•] standpoint) tasks recruit more anterior regions of frontal cortex, while simpler tasks recruit only more posterior regions of frontal cortex. In the functional connectivity networks, there is no obvious evidence of such hierarchy. Instead, it appears that frontal cortex is apportioned into several different systems, many of which have established task-related properties (e.g., the dorsal attention system). DLPFC in particular is part of a system of distributed brain regions including parietal, temporal, insula, and cingulate cortex.
Hierarchical accounts of DLPFC organization have been challenged by recent, targeted task fMRI experiments [26,27•]. The essence of hierarchical accounts is that only caudal regions of DLPFC are recruited for satisfying simple task structures, and that as task structures become more complex, more rostral regions are progressively recruited. Crittenden and Duncan [27•] performed a task fMRI experiment in which subjects were asked to discriminate vertical lines under several conditions, most of which are predicted to only elicit caudal DLPFC activity under hierarchical accounts. In conditions without extended temporal structure or multiple rules, robust activity was observed in rostral areas of DLPFC, a finding that clearly diverges from the predictions of hierarchical control. Relatedly, Reynolds and colleagues [26] found rostral DLPFC activity in conditions with low task complexity that are not expected to produce such activity under hierarchical accounts.
Conflict monitoring accounts [7•] of control have also been experimentally challenged. These accounts posit that dorsal medial prefrontal cortex (dmPFC) monitors response conflict and relays this information to DLPFC, which can adjust task set accordingly. In accord with such accounts, Grinband and colleagues [28•] performed a Stroop task and observed greater activity in dmPFC on incongruent trials than on congruent trials. However, they also made two critical observations. First, they observed that error likelihood was not linearly related to reaction time, which is a prediction of conflict monitoring accounts. Second, they observed that dmPFC activity, once reaction time was controlled, no longer distinguished between congruent and incongruent trials. Instead, a region in DLPFC selectively made such distinctions.
Conclusions
Despite considerable progress in the 25 years since neuroimaging became available to cognitive psychologists, the understanding of how tasks are implemented and controlled remains in its infancy. Several influential accounts of task control, such as hierarchical control or conflict monitoring, which focus on relatively few parts of the brain, have recently encountered strong experimental challenges [26,27•,28•]. Other accounts that consider a wider array of regions continue to gather supporting evidence. However, these distributed accounts suffer from their comprehensiveness — the roles of the many individual pieces comprising a control system are poorly understood and these accounts as yet have had difficulty making predictions that can be proved or disproved experimentally. A great deal of work remains to be done to characterize the specific contributions of particular regions to control and to build models of their interactions. Nevertheless, at this point it is clear that the human brain contains many distributed systems, and that among these systems, several of them are principally composed of regions that display signals consistent with task control.
Acknowledgments
This work was funded by a McDonnell Foundation Collaborative Action Award (S.E.P.) and NIH F30 MH940322 (J.D.P.).
Footnotes
Conflict of interest
The authors have no conflict of interest to declare.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
-
•
of special interest
- 1.Norman D, Shallice T. Consciousness and Self-Regulation: Advances in Research and Theory IV. Plenum Press; 1986. Attention to action: willed and automatic control of behavior. [Google Scholar]
- 2.Posner MI, Petersen SE. The attention system of the human brain. Annu Rev Neurosci. 1990;13:25–42. doi: 10.1146/annurev.ne.13.030190.000325. [DOI] [PubMed] [Google Scholar]
- 3.Desimone R, Duncan J. Neural mechanisms of selective visual attention. Annu Rev Neurosci. 1995;18:193–222. doi: 10.1146/annurev.ne.18.030195.001205. [DOI] [PubMed] [Google Scholar]
- 4.Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- 5.Koechlin E, Ody C, Kouneiher F. The architecture of cognitive control in the human prefrontal cortex. Science (New York, NY) 2003;302:1181–1185. doi: 10.1126/science.1088545. [DOI] [PubMed] [Google Scholar]
- 6•.Badre D, D’Esposito M. Functional magnetic resonance imaging evidence for a hierarchical organization of the prefrontal cortex. J Cogn Neurosci. 2007;19:2082–2099. doi: 10.1162/jocn.2007.19.12.2082. This study outlines the rule-abstraction hierarchical hypothesis of DLPFC organization. [DOI] [PubMed] [Google Scholar]
- 7•.Botvinick MM, Braver TS, Barch DM, Carter CS, Cohen JD. Conflict monitoring and cognitive control. Psychol Rev. 2001;108:624–652. doi: 10.1037/0033-295x.108.3.624. This report outlines the reasoning and predictions of the conflict monitoring account of cognitive control. [DOI] [PubMed] [Google Scholar]
- 8•.Dosenbach NUF, Visscher KM, Palmer ED, Miezin FM, Wenger KK, Kang HC, Burgund ED, Grimes AL, Schlaggar BL, Petersen SE. A core system for the implementation of task sets. Neuron. 2006;50:799–812. doi: 10.1016/j.neuron.2006.04.031. This study searched a large task fMRI dataset for regions that display signals consistent with psychological models of cognitive control, identifying regions of the dorsal anterior insula and the dorsal anterior cingulate as key regions for cognitive control. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cole MW, Schneider W. The cognitive control network: integrated cortical regions with dissociable functions. Neuroimage. 2007;37:343–360. doi: 10.1016/j.neuroimage.2007.03.071. [DOI] [PubMed] [Google Scholar]
- 10.Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, Reiss AL, Greicius MD. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci: Off J Soc Neurosci. 2007;27:2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Duncan J. The multiple-demand (MD) system of the primate brain: mental programs for intelligent behaviour. Trends Cogn Sci. 2010;14:172–179. doi: 10.1016/j.tics.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 12.Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magnet Reson Med: Off J Soc Magnet Reson Med/Soc Magnet Reson Med. 1995;34:537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- 13.Lowe MJ, Mock BJ, Sorenson JA. Functional connectivity in single and multislice echoplanar imaging using resting-state fluctuations. Neuroimage. 1998;7:119–132. doi: 10.1006/nimg.1997.0315. [DOI] [PubMed] [Google Scholar]
- 14.Cordes D, Haughton VM, Arfanakis K, Carew JD, Turski PA, Moritz CH, Quigley MA, Meyerand ME. Frequencies contributing to functional connectivity in the cerebral cortex in “resting-state” data. AJNR: Am J Neuroradiol. 2001;22:1326–1333. [PMC free article] [PubMed] [Google Scholar]
- 15.Greicius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc Natl Acad Sci U S A. 2003;100:253–258. doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dosenbach NUF, Fair DA, Miezin FM, Cohen AL, Wenger KK, Dosenbach RAT, Fox MD, Snyder AZ, Vincent JL, Raichle ME, et al. Distinct brain networks for adaptive and stable task control in humans. Proc Natl Acad Sci U S A. 2007;104:11073–11078. doi: 10.1073/pnas.0704320104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dosenbach NUF, Fair DA, Cohen AL, Schlaggar BL, Petersen SE. A dual-networks architecture of top-down control. Trends Cogn Sci. 2008;12:99–105. doi: 10.1016/j.tics.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18•.Power JD, Cohen AL, Nelson SM, Wig GS, Barnes KA, Church JA, Vogel AC, Laumann TO, Miezin FM, Schlaggar BL, et al. Functional network organization of the human brain. Neuron. 2011;72:665–678. doi: 10.1016/j.neuron.2011.09.006. This study proposed preliminary coordinates for brain areas and explored their network architecture in resting state fMRI data. One of the chief results was the emergence of fronto-parietal and cingulo-opercular subnetworks, indicating that control systems form some of the basic organizational elements of the brain. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dosenbach NUF, Nardos B, Cohen AL, Fair DA, Power JD, Church JA, Nelson SM, Wig GS, Vogel AC, Lessov-Schlaggar CN, et al. Prediction of individual brain maturity using fMRI. Science (New York, NY) 2010;329:1358–1361. doi: 10.1126/science.1194144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cohen AL, Fair DA, Dosenbach NUF, Miezin FM, Dierker D, Van Essen DC, Schlaggar BL, Petersen SE. Defining functional areas in individual human brains using resting functional connectivity MRI. Neuroimage. 2008;41:45–57. doi: 10.1016/j.neuroimage.2008.01.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21•.Yeo BTT, Krienen FM, Sepulcre J, Sabuncu MR, Lashkari D, Hollinshead M, Roffman JL, Smoller JW, Zollei L, Polimeni JR, et al. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J Neurophysiol. 2011;106:1125–1165. doi: 10.1152/jn.00338.2011. http://dx.doi.org/10.1152/jn.00338.2011. This comprehensive study of resting state fMRI data partitions the cortical surface into clusters, recovering select groupings of regions consistent with several control systems. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci. 2002;3:201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- 23.Petersen SE, Posner MI. The attention system of the human brain: 20 years after. Annu Rev Neurosci. 2012;35:73–89. doi: 10.1146/annurev-neuro-062111-150525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dehaene S, Spelke E, Pinel P, Stanescu R, Tsivkin S. Sources of mathematical thinking: behavioral and brain-imaging evidence. Science (New York, NY) 1999;284:970–974. doi: 10.1126/science.284.5416.970. [DOI] [PubMed] [Google Scholar]
- 25.Buckner RL, Wheeler ME. The cognitive neuroscience of remembering. Nat Rev Neurosci. 2001;2:624–634. doi: 10.1038/35090048. [DOI] [PubMed] [Google Scholar]
- 26.Reynolds JR, O’Reilly RC, Cohen JD, Braver TS. The function and organization of lateral prefrontal cortex: a test of competing hypotheses. PLoS ONE. 2012;7:e30284. doi: 10.1371/journal.pone.0030284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27•.Crittenden BM, Duncan J. Task difficulty manipulation reveals multiple demand activity but no frontal lobe hierarchy. Cerebral Cortex. 2012 Nov 6; doi: 10.1093/cercor/bhs333. [Epub ahead of print] This study poses substantial challenges to hierarchical accounts of cognitive control by demonstrating that much of prefrontal cortex is activated by simple discrimination tasks. This finding is counter to expectations under hierarchical models, which predict activity restricted to rostral prefrontal cortex under such simple task conditions. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28•.Grinband J, Savitskaya J, Wager TD, Teichert T, Ferrera VP, Hirsch J. The dorsal medial frontal cortex is sensitive to time on task, not response conflict or error likelihood. Neuroimage. 2011;57:303–311. doi: 10.1016/j.neuroimage.2010.12.027. This study poses substantial challenges to conflict monitoring accounts of cognitive control by demonstrating that activity in dorsal medial prefrontal cortex strongly reflects time on task and not response conflict. [DOI] [PMC free article] [PubMed] [Google Scholar]


