Abstract
Objectives:
To estimate the probability of response when intravesical BCG is given in combination with oral bropirimine for bladder carcinoma in-situ, and to evaluate toxicity when the two agents are combined.
Methods:
51 patients with histologic evidence of CIS and no prior treatment with BCG or bropirimine were enrolled in a cooperative group multi-center phase II trial. Initial treatment included Tice BCG 50 mg weekly for 6 weeks and oral bropirimine 3.0 gm per day for 3 consecutive days each week for 12 weeks. Response was assessed after 12 weeks by cystoscopy, biopsy, and barbotage cytology. Most patients received a second course followed by an identical assessment. Toxicity was recorded according to the SWOG toxicity criteria.
Results:
51 patients were enrolled and treated. 42 were eligible and evaluable for response and toxicity. There were 28 complete responders (67%, 50%-80% 95% confidence interval). 5-year progression-free survival estimate is 53%, and the 5-year survival estimate is 80%. There were no deaths, 2 with Grade 4 toxicity, 14 with Grade 3 toxicity, 17 with Grade 2 toxicity, 6 with Grade 1 toxicity, and only 3 with no toxicity reported as their worst toxicity grade.
Conclusions:
The combination failed to demonstrate an estimated response greater than 80%. It is not recommended that further evaluation of this combination be conducted.
Introduction
Despite improvements in the management of superficial bladder cancer, patients still fail to be cured of high-grade disease. Bacillus Calmette -Guerin has been shown to be the most effective intravesical therapy for the control of carcinoma in situ (CIS). However, up to 50% of patients may not respond to initial or even several courses of intravesical BCG therapy.1-3 Techniques and methods to improve upon this are needed.
Bropirimine is an oral interferon inducer that has been shown to have substantial immunomodulatory activity.4,5 Also, it has demonstrated clinical efficacy in transitional cell cancer.6-8 Response rates in single agent phase II trial have been noted in approximately 50% of patients. Even 32% of patients who have failed prior BCG therapy have demonstrated complete response to oral bropirimine, as have patients with upper tract positive urinary cytology.
Early laboratory studies of bropirimine demonstrated apparent synergy between BCG and bropirimine in cellular response to stimulation as well as in vivo efficacy in the MBT-2 tumor model.9 Based upon these and the early promising clinical results of bropirimine’s single agent efficacy, we performed a clinical trial to evaluate the combination of oral bropirimine with intravesical BCG in patients with CIS of the bladder.
Material and Methods
A multi-center cooperative group trial was designed to estimate the probability of response to the combination of oral bropirimine and intravesical BCG in patients with transitional cell CIS of the bladder. A second objective of the study was to assess the qualitative and quantitative toxicity of the combination of the two agents together.
Patients were required to have histologically-proven transitional cell CIS of the bladder on central review of surgical pathology specimens. Those found to have simultaneous papillary tumors were required to have had the tumor(s) completely resected, with no evidence of muscle invasive disease. No prior therapy with BCG, bropirimine, or systemic chemotherapy was allowed. At least one positive urinary cytology after each biopsy was required to assure the continued presence of CIS.
Treatment consisted of intravesical Tice BCG 50 mg. in 50 cc. normal saline weekly for six weeks. While not required, this was usually given on Monday or Tuesday of each treatment week. Forty-eight hours later, patients initiated self-administration of bropirimine for three consecutive days. Initially, daily dosage was 4.5 grams in three divided doses of 1.5 gram every two hours. A dose reduction to 3.0 gm. per day was made after recognition of possible cardiac toxicity in other studies that were ongoing simultaneously. Bropirimine was taken for 12 consecutive weeks, including the first six when BCG was given and the 6 weeks following that.
Response was assessed by obtaining repeat prostate and bladder mucosal biopsies and bladder wash for cytology. A second course of BCG and oral bropirimine was administered in patients who did not respond to the first or who elected to continue. No additional BCG was g iven after the second course, but bropirimine could be continued to complete one year of therapy. Subsequent treatments for patients who did not respond were not dictated by the protocol.
Patients were described by disease type as CIS alone vs. CIS with concurrent but completely resected papillary tumor, stage Ta vs. T1, and for prior intravesical therapy with either interferon vs. other agents vs. none.
All patients were required to have adequate bone marrow, renal, liver, and cardiac function status and a SWOG performance status of 0 to 2. Patients with clinically significant arrhythmia, unexplained syncope, recent myocardial infarction, angina, or congestive heart failure were excluded.
The accrual goal was an initial group of 25 patients. If 16 responses were seen in those 25, an additional 20 patients were to be accrued.
Results
A total of 51 patients were entered into the trial at a total of 16 institutions between December 1, 1992 and November 15, 1997. Nine patients were deemed to be ineligible due to insufficient documentation or failure to biopsy specified sites in the bladder, leaving 42 patients eligible for assessment of response. There were 38 males and 4 females with a median age of 67 years (range 44-84). Thirty-nine were White, one African American, one Hispanic, and one Asian or Pacific Islander.
Disease type included 16 with primary CIS only and 26 in whom CIS was present with a history of previously resected papillary tumor. Prior intravesical therapy had been used in o nly a single patient.
Of the 42 eligible patients, treatment was completed as planned in 14 patients and terminated for toxicity or side effects in 13. Four refused to complete the study for reasons unrelated to toxicity and 9 suffered progression or relapse of disease. Two additional patients were removed from the study for mon-protocol specified reasons not related to toxicity.
Of the 42 patients, 28 (67%) had a complete response. Disease was thought to be stable or to show no response in 5 (12%), increasing extent of disease was noted in 3 (7%) and post-treatment assessment was inadequate in 6 (14%). Those falling into this last group are considered non-responders.
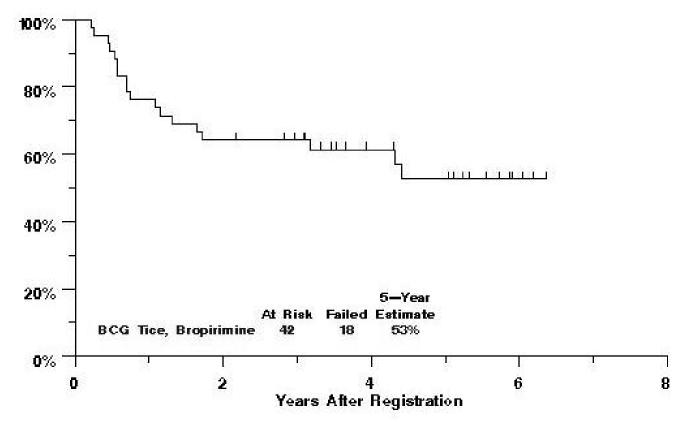
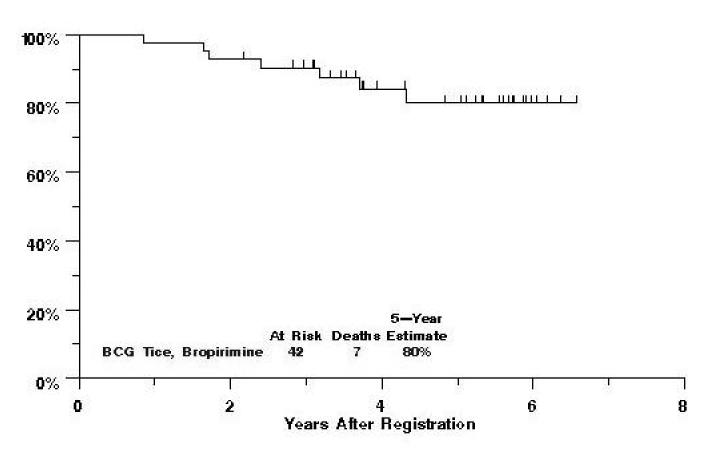
There have been 14 relapses documented on this study, and three of those patients have died. Additionally, 4 patients have died without documented relapse. Five-year progression-free survival is estimated at 53% (figure 1), while 5-year survival is estimated at 80% (figure 2).
Fig. 1.
Southwest Oncology Group Study 9140 Progression-Free Survival by Treatment Arm
Fig. 2.
Southwest Oncology Group Study 9140 Overall Survival by Treatment Arm
Toxicity was not substantially different than that seen with other BCG protocols involving more than a 6-week course of therapy.10 No patient died of treatment, while two did experience severe Grade 4 toxicity, including one with an allergic reaction to bropirimine classified as anaphylaxis. Fourteen patients experienced Grade 3 toxicity necessitating an interruption in therapy or the addition of medication to control side effects, generally of BCG-related bladder symptoms. Seventeen patients experienced Grade 2 toxicity, 6 reported Grade 1 or mild symptoms as their worst toxicity grade, while only 3 patients reported no toxicity.
The most common symptoms were urinary frequency and urgency, experienced in 50% of those with Grade 3, and fatigue, malaise, and lethargy, noted in 5 of the 14 at the Grade 3 le vel. The next most common side effect was hematuria where 4 patients experienced Grade 3 toxicity.
Possible cardiac-related side effects were noted in 5 patients. Included were 2 with Grade 1 arrhythmias (one of whom stopped due to that, while the other stopped for other reasons) and one with Grade 2 arrhythmia (who completed the treatment protocol). One patient with Grade 1 chest pain completed the protocol. One patient with Grade 4 dyspnea stopped the protocol treatment.
Discussion
In this multi-center, rigorously detailed trial, bropirimine failed to demonstrate an increase in the efficacy of intravesical BCG in controlling CIS of the bladder.
Bropirimine has been shown to have single agent activity in approximately 50% of patients with previously untreated CIS, 32% with BCG-failed CIS, and 50% with apparent CIS of the upper urinary tracts.6-8 Despite that, bropirimine is not currently available for research purposes. An application was made to obtain Food and Drug Administration clearance for bropirimine to be used in BCG-failed CIS. However, that approval was declined, and as of this writing, bropirimine remains unavailable for further research purposes.
Toxicity may have been increased over that seen with BCG alone, but this trial did not allow for a direct comparison. A detailed analysis of the toxicity of BCG when more than six weeks is given has not yet been provided from the large Southwest Oncology Group trial of maintenance BCG after a six-week induction course.10 It is known that toxicity was much higher in the group receiving maintenance, and less than 50% of patients in the maintenance arm received the intended 36 months of maintenance therapy.
Despite its single agent activity and the earlier laboratory evidence that bropirimine enhanced the activity of BCG, this study shows that if there is any enhancement of clinical BCG activity in CIS, it is not significant. Recombinant interferon alpha-2b has been shown to have single-agent activity in CIS.11,12 Efforts to promote the simultaneous use of intravesical interferon with BCG in CIS are being made, but we know of no such rigorous trial similar to ours. Other treatment methods are under investigation that might also provide alternatives to cystectomy for BCG failures.13-15 However, efforts to replace BCG with a more effective drug or enhance the initial efficacy of BCG should continue.
Footnotes
This investigation was supported in part by the following PHS Cooperative Agreement grant numbers awarded by the National Cancer Institute, DHHS: CA38926, CA32102, CA22433, CA96429, CA68183, CA42777, CA58416, CA37981, CA27057, CA20319, CA35431, CA46282, CA58882, CA13612, CA76447, CA76132, and supported in part by The Upjohn Company.
References
- 1.Lamm DL, Blumenstein BA, Crawford ED, et al. A randomized trial of intravesical doxorubicin and immunotherapy with bacillus Calmette-Guerin for transitional cell carcinoma of the bladder. N. Engl J Med. 1991;325:1205–1209. doi: 10.1056/NEJM199110243251703. [DOI] [PubMed] [Google Scholar]
- 2.Morales A, Nickel JC, Wilson JWL. Dose-response of bacillus Calmette-Guerin in the treatment of superficial bladder cancer. J Urol. 1992;147:1256–1258. doi: 10.1016/s0022-5347(17)37532-8. [DOI] [PubMed] [Google Scholar]
- 3.Nadler RB, Catalona WJ, Hudson MA, Ratliff TL. Durability of the tumor-free response for intravesical bacillus Calmette-Guerin therapy. J Urol. 1994;152:367–373. doi: 10.1016/s0022-5347(17)32741-6. [DOI] [PubMed] [Google Scholar]
- 4.Wierenga W. Antiviral and other bioactivities of pyrimidinones. Pharmacol. Ther. 1985;30:67. doi: 10.1016/0163-7258(85)90048-8. [DOI] [PubMed] [Google Scholar]
- 5.Lotzova E, Savary DS, Khan A, Stringfellow DS. Stimulation of natural killer cells in two random-bred strains of athymic mice by interferon-inducing pyrimidinones. J. Immunol., 132:2566–1984. [PubMed] [Google Scholar]
- 6.Sarosdy MF, Lamm DL, Williams RD, Moon TD, Flanigan RC, Crawford ED, Wilks NE, Earhart RH, Merritt JA. Phase I trial of oral bropirimine in superficial bladder cancer. J Urol. 1992;147:31–33. doi: 10.1016/s0022-5347(17)37126-4. [DOI] [PubMed] [Google Scholar]
- 7.arosdy MF, Lowe BA, Schellhammer PF, Lamm DL, Graham SD, Jr, Grossman HB, See WA, Peabody JO, Moon RD, Flanigan RC, Crawford ED, Morganroth J. Oral bropirimine immunotherapy of carcinoma in situ of the bladder: results of a phase II trial. Urology. 1996;48:21–27. doi: 10.1016/s0090-4295(96)90059-x. [DOI] [PubMed] [Google Scholar]
- 8.arosdy MF, Pisters LL, Carroll PR, Benson MC, Moon TD, Lamm DL, Hudson MA, Lerner SP, Koch MO, Schellhammer PF. Bropirimine immunotherapy of upper urinary tract carcinoma in situ. Urology. 1996;48:28–32. doi: 10.1016/s0090-4295(96)00080-5. [DOI] [PubMed] [Google Scholar]
- 9.Sarosdy MF, Kierum CA. Combination immunotherapy of murine transitional cell carcinoma using BCG and an interferon-inducing pyrimidinone. J. Urol. 1989;142(5):1376. doi: 10.1016/s0022-5347(17)39103-6. [DOI] [PubMed] [Google Scholar]
- 10.Lamm DL, Blumenstein BA, Crissman JD, Montie JE, Gottesman JE, Lowe BA, Sarosdy MF, Bohl RD, Grossman HB, Beck TM, Leimert JT, Crawford ED. Maintenance BCG Immunotherapy in Recurrent TA, T1 and Carcinoma in Situ Transitional Cell Carcinoma: A Randomized Southwest Oncology Group Study. J Urol. 2000;163(1124-1129) [PubMed] [Google Scholar]
- 11.Glashan R. A randomized controlled study of intravesical alpha-2b- interferon in carcinoma in situ of the bladder. J Urol. 1990;144:658–661. doi: 10.1016/s0022-5347(17)39547-2. [DOI] [PubMed] [Google Scholar]
- 12.Williams RD, Gleason DM, Smith AY, Zinner NR, Sagalowsky AI, Montie JE, Brosman SA, Marks LS, Brito G, Boxer RJ, Blank BH, Neri R, Rudeen J. Pilot study of intravesical alfa-2b interferon for treatment of bladder carcinoma in situ following BCG failure. J Urol. 1996;155:494A. [Google Scholar]
- 13.Bui TT, Schellhammer PF. Additional bacillus Calmette-Guerin therapy for recurrent transitional cell carcinoma after an initial complete response. Urology. 1997;49:687–690. doi: 10.1016/S0090-4295(97)00067-8. [DOI] [PubMed] [Google Scholar]
- 14.Manayak MJ. Photodynamic therapy for urologic malignancies. Urol Clin North Am. 1992;19:581–589. [Google Scholar]
- 15.Patterson AL, Greenberg RE, Weems L, et al. Pilot study of the tolerability and toxicity of intravesical valrubicin immediately after transurethral resection of superficial bladder cancer. Urology. Aug 1;200056(2):232–5. doi: 10.1016/s0090-4295(00)00654-3. [DOI] [PubMed] [Google Scholar]