Abstract
Twenty years of oncolytic virus (OV) development have created a field that is driven by the potential promise of lasting impact on our cancer treatment repertoire. With the field constantly expanding – over 20 viruses have been recognized as potential OVs – new virus candidates continue to emerge even as established viruses reach clinical trials. They all share the defining commonalities of selective replication in tumors, subsequent tumor cell lysis, and dispersion within the tumor. Members from diverse virus classes with distinctly different biologies and host species have been identified. Of these viruses, 15 have been tested on human glioblastoma multiforme (GBM). So far, 20 clinical trials have been conducted or initiated using attenuated strains of 7 different oncolytic viruses against GBM. In this review, we present an overview of viruses that have been developed or considered for GBM treatment. We outline the principles of tumor targeting and selective viral replication, which include mechanisms of tumor-selective binding, and molecular elements usurping cellular biosynthetic machinery in transformed cells. Results from clinical trials have clearly established the proof of concept and have confirmed the general safety of OV application in the brain. The moderate clinical efficacy has not yet matched the promising preclinical lab results; next-generation OVs that are either “armed” with therapeutic genes or that are embedded in a multimodality treatment regimen should enhance the clinical results.
Keywords: oncolytic virus, virotherapy, glioblastoma, glioma, clinical trial, herpes simplex, adenovirus, poliovirus, measles, Newcastle disease virus, reovirus, vesicular stomatitis virus, parvovirus, vaccinia virus, myxoma virus
INTRODUCTION AND GENERAL CONCEPTS
Introduction
Glioblastoma multiforme (GBM), the most common and most malignant primary brain tumor, is characterized by rapid and highly invasive growth, histological and genetic heterogeneity, resistance to most chemotherapeutic drugs, and local recurrence. This inauspicious mix of factors, and the tumor’s location in the body’s most complex and delicate organ, the brain, pose major challenges for curative therapy. Prognosis is dismal, with a median survival of 12–15 months 1. Notwithstanding recent progress using alkylating agents and tumor vasculature-targeting monoclonal antibodies that offered a survival benefit of several months in a subset of patients 2, overall survival numbers have only improved by a few months over the past 3 decades. Consequently, the development of novel alternative treatment options is crucial. Among these possibilities, the use of oncolytic viruses (OVs) holds substantial promise.
In oncology, the use of viruses as therapeutic agents has been extensively studied in two overlapping strategies: gene therapy and oncolytic virotherapy. In gene therapy, the main focus is on therapeutic genes and the virus plays an essential - but not defining - role as a gene delivery vehicle. Typically, replication-incompetent highly-attenuated viral vectors are used. In a fundamental departure from non-replicating viral vectors, oncolytic virotherapy employs viruses with a preserved potential for an active viral life cycle. The chief paradigm is tumor-selective conditional viral replication, resulting in lytic tumor cell destruction and release of thousands of viral progeny. Newly released viruses go on to infect neighboring tumor cells, theoretically causing and maintaining a wave of virus attack throughout the tumor. This multiplication of the input dose is a unique feature of oncolytic virotherapy that is not existent in any other form of treatment and leads to a local self-amplification of the therapeutic effect. Consequently, a much lower initial dose of virus would theoretically be required compared to non-replicating viral vector approaches. The success of oncolytic virotherapy depends on many critical factors, and a formulation of the optimal oncolytic virus would include the following characteristics: (I) distinct and well-defined mechanism of tumor selectivity, (II) strong cytolytic potential with low non-tumor toxicity, (III) potential for systemic application, (IV) rapid replication cycle with swift intratumoral spread, (V) accessibility to genetic engineering, (VI) easy manufacturing and high- titer-stock production, (VII) availability of antiviral agents to control unwanted viral spread, (VIII) genetic stability, (IX) no pre-existing immunity, and (X) stimulation of antitumor immunity.
The concept of virotherapy has a long history of nearly 100 years 3. In 1912, a woman with cervical cancer showed tumor regression after receiving an attenuated rabies virus vaccine. Additional anecdotal reports of cancer regression associated with concurrent virus infection appeared repeatedly over the intervening years. The modern era of virotherapy commenced in the early 1990s, when advances in technology allowing the generation of recombinant, laboratory engineered viruses met with an improved molecular-based understanding of virology and cancer genetics. The first laboratory-engineered virus used for oncolytic purposes was based on a Herpes simplex virus mutant and was reported in 1991 by Martuza and colleagues, 4 followed by an engineered oncolytic adenoviral mutant in 1996 5. Over the course of the last two decades, most cancer types have been experimentally targeted by virotherapy, and the list of potential oncolytic viral agents has extended to well over 20 viruses with countless variants and improved successor generations. From the beginning, glioblastoma multiforme has been a main focus of oncolytic virus development. This is in large part due to some features that make gliomas particular suitable for oncolytic virus therapy: (I) They are nearly exclusively confined to one organ compartment and distant metastases are not a characteristic of the disease, traits that may be complemented by the potential of OVs for local replication and intratumoral spread. (II) They grow surrounded by mostly postmitotic cells, making it particularly attractive for viruses that require active cell cycles for their replication.
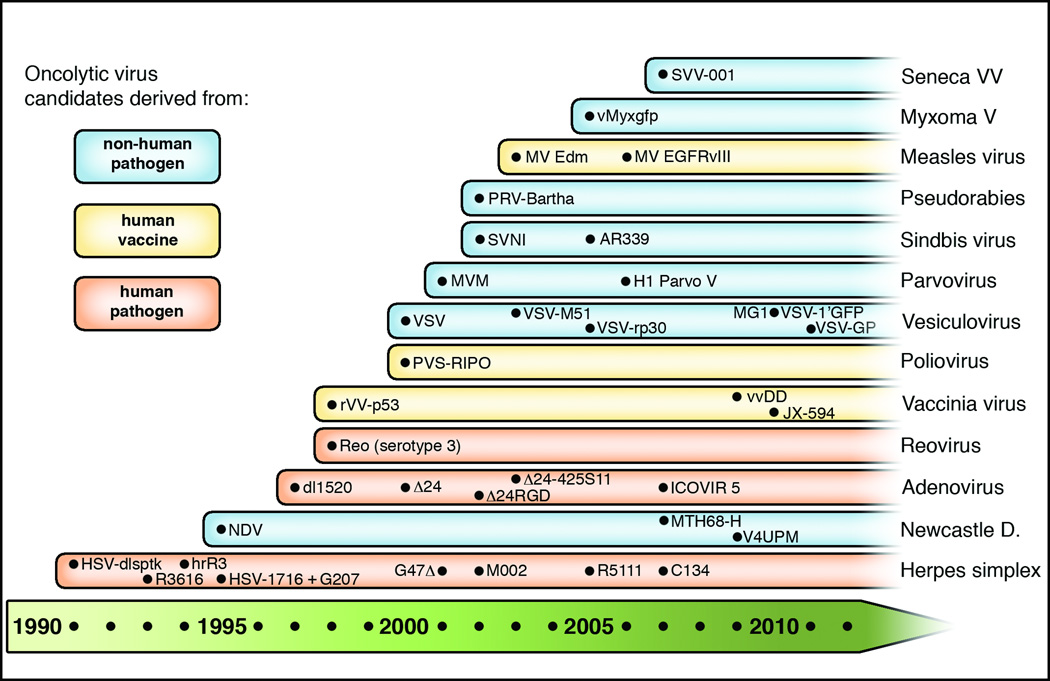
Since the Martuza group’s pioneering study in 1991, over 250 articles have been published on glioma oncolytic virotherapy, and about 15 viruses have been considered and examined for glioblastoma targeting (Table 1), of which 7 have advanced to clinical trials (herpes simplex virus [HSV], adenovirus [AdV], Newcastle disease virus [NDV], reovirus, H1 parvovirus, measles virus [MV], and poliovirus [PV]); several others are at advanced preclinical stages. The timeline of glioblastoma-directed oncolytic virus development is depicted in Figure 1. In the following, we outline general concepts of oncolytic virotherapy for glioblastoma, present a comprehensive overview of currently investigated oncolytic viruses for glioblastoma, and list the central findings of previous and ongoing clinical trials.
Table 1.
Oncolytic virus candidates for glioma therapy with summary of tumor selectivity factors
| Virus | Gemone and structure |
Host and virus family |
Determinants for tumor selective targeting or replication |
Phase of development relating to glioma |
|---|---|---|---|---|
| HSV1 | ds DNA Enveloped |
Human Herpesviridae |
1) HSV-TK and RR mutations compensated by activated cell cycle in tumors 2) PKR defects in tumors allow γ134.5 deleted HSV to replicate 3) ICP47 gene deletion acts immune -stimulatory |
Clinical phase I/II |
| NDV | ss (−) RNA Enveloped |
Avian Paramyxoviridae |
1) Mainly induction of antitumor cytokines and immune response 2) Possibly exploiting IFN defects |
Clinical phase I/II |
| Adeno | ds DNA Naked |
Human Adenoviridae |
1) Defects in p53 or RB pathway targeted by E1B and E1A mutants 2) RGD modification targets tumor integrins 3) E2F1 responsive elements control viral replication |
Clinical phase I |
| Reo | ds RNA Naked |
Mammalian Reoviridae |
1) Tropism for Ras – activated, transformed tumor cells | Clinical phase I |
| Vaccinia | ds DNA Enveloped |
Cow/Horse (?) Poxviridae |
1) TK deletion compensated by nucleotide abundance in transformed tumors 2) VGF deletions compensated by aberrant EGFR receptor activation 3) Large size requires leaky tumor vessels for viral extravasation |
Preclinical in vivo |
| Polio | ss (+) RNA Naked |
Human Picornaviridae |
1) Polio receptor CD46 expressed on glioma 2) Pathogenic polio IRES replaced with rhinovirus IRES |
Clinical phase I |
| VSV | ss (−) RNA Enveloped |
Livestock Mosquito Rhabdoviridae |
1) Selective replication depends on defective IFN pathway in tumor cells | Preclinical in vivo |
| MVM | ss DNA Naked |
Mouse Parvoviridae |
1) Actively dividing cells required for replication 2) Defects in PKR augment viral replication |
Preclinical in vitro |
| Sindbis | ss (+) RNA Enveloped |
Mammalian/ Mosquito Togaviridae |
1) Sindbis binds to 67kDa Laminin receptor, which is overexpressed on some tumors | Preclinical in vitro |
| PRV | ds DNA Enveloped |
Pig Herpesviridae |
1) HSV-TK and RR mutations compensated by activated cell cycle in tumors | Preclinical in vitro |
| Measles | ss (−) RNA Enveloped |
Human Paramyxoviridae |
1) Binding to CD-46 receptor, overexpressed on tumors | Clinical phase I |
| Myxoma | ds DNA Enveloped |
Rabbit Poxviridae |
1) Replication in IFN deficient tumor cells 2) High affinity to cells with activated Akt |
Preclinical in vivo |
| H1PV | ss DNA Naked |
Rat Parvoviridae |
1) Virus requires actively dividing cells in S-phase for replication 2) Defects in PKR augment viral replication |
Clinical phase I |
| SVV | ss (+) RNA Naked |
Pig Picornaviridae |
1) Strong tropism to neuroendocrine and solid pediatric tumors, possibly mediated through integrin α4 receptor binding | Preclinical in vitro |
Abbreviations: HSV1, herpes simplex virus 1; NDV, Newcastle disease virus; VSV, vesicular stomatitis virus; MVM, minute virus of mice; PRV, pseudorabies virus; H1PV, rat parvovirus H1; SVV, Seneca Valley virus; ds, double-stranded; ss, single-stranded; TK, thymidine kinase; RR, ribonucleotide reductase; PKR, protein kinase R; IFN, interferon; RB, retinoblastoma tumor suppressor; VGF, VGF nerve growth factor inducible; EGFR, epidermal growth factor receptor; IRES, internal ribosomal entry site.
Figure 1. Timeline of oncolytic virus development from glioblastoma therapy.
This schematic diagram lists viruses with oncolytic potential based on their first description in preclinical glioma studies starting in 1991. Subsequent major developments within one virus group are included. Viruses are color-coded based on their pathogenic background as human pathogen (orange), human vaccine (yellow), or non-human pathogen (blue).
General Concepts
Classification of viruses is based on viral families and characteristics of viral genomes and structures. In virotherapy, an additional categorization addresses pathogenicity of an oncolytic virus in relation to humans. With few exceptions, the viruses discussed herein fall into one of three groups: 1) human pathogen, 2) human attenuated vaccine, 3) non-human pathogen. In general terms, rendering a human pathogenic virus suitable for OV therapy requires targeted alterations of viral pathogenicity factors. These genetically engineered alterations result in a virus with selectively disabling mutations that lead to attenuated infection in normal cells, but not in tumor cells. The most widely known examples for OVs with a human pathogen background are HSV and AdV. Viruses based on human vaccine strains also require genetic engineering before application as OVs in order to enhance tumor targeting. However, given their pre-existing attenuated nature, the need to remove pathogenicity factors is less pronounced. Vaccinia, polio, and measles are prominent examples of vaccine viruses developed for glioma virotherapy. Finally, OV candidates with a non-human pathogen background do not necessarily require engineering under the premise that they are not associated with human disease, and infection in humans is either transient or non-productive. In tumor cells, on the other hand, various cellular aberrations result in enhanced permissiveness to replication of otherwise non-infectious viruses. NDV, myxoma virus (Myx), autonomous parvovirus, vesicular stomatitis virus (VSV), Sindbis virus (SIN), pseudorabies virus (PRV), and Seneca Valley virus (SVV) are included in the group of non-human pathogenic viruses investigated as therapeutic antiglioma agents.
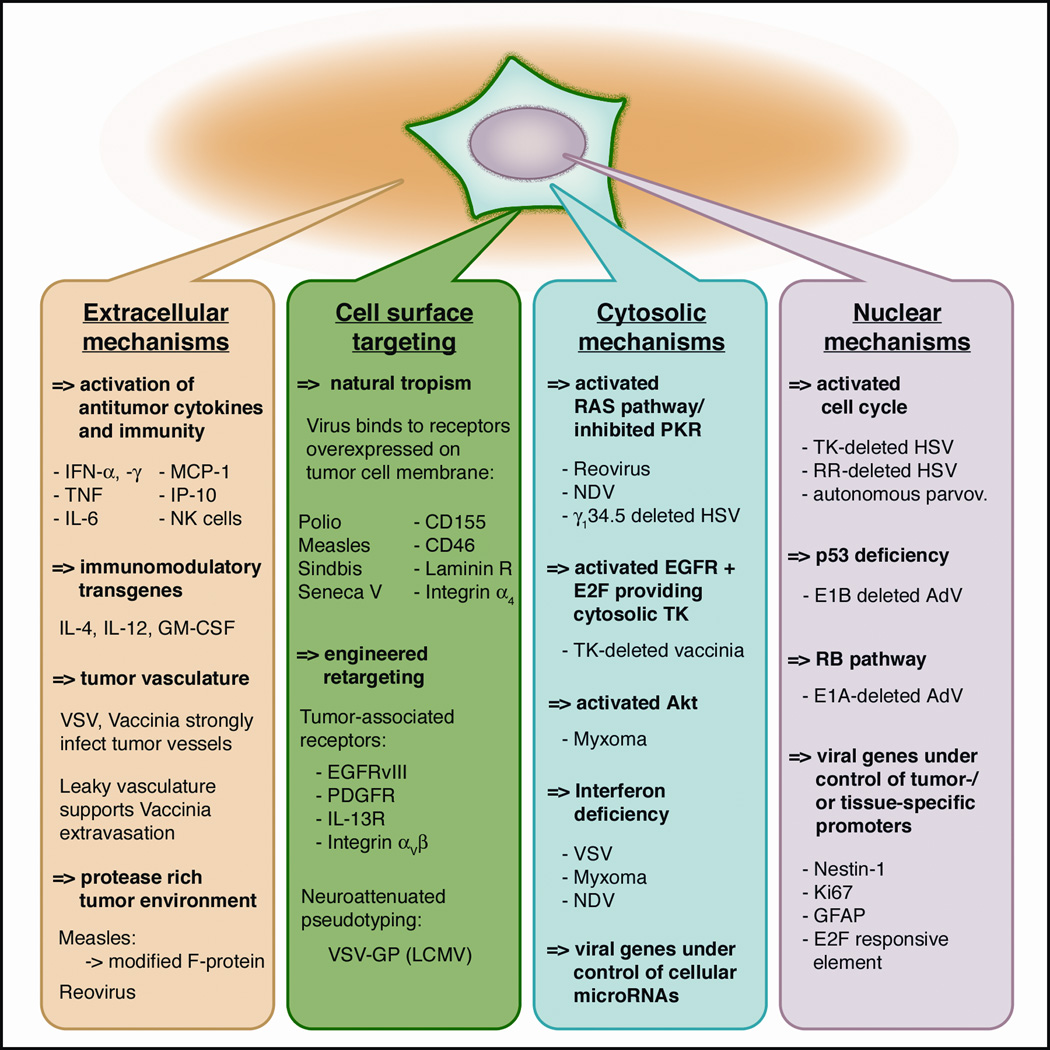
The defining concept of virotherapy is a conditional, tumor-restricted viral replication with subsequent lysis of tumor cells. This selective replication is based on inherent or engineered mechanisms that exploit tumor cell aberrations for viral propagation. As our understanding of glioblastoma genetics rapidly grows 6, 7, so do the number of potential molecular targets for virus interactions. The specific mechanisms underlying glioma-selective infection and replication will be discussed in detail for each virus individually in the respective sections below. A collection of the principles and locations of selective tumor-virus interaction in relation to different cellular compartments (membrane, cytosol, and nucleus) are shown in Fig. 2.
Figure 2. Mechanisms for selective oncotropism and oncolysis.
Molecular mechanisms determining tumor-selective viral infection and replication are grouped schematically based on the cellular compartment of tumor-virus interaction. (I) Extracellular compartment. Some oncolytic viruses (OVs) can exert selective tumor suppression through induction of cytokine production. OV’s can selectively attack vasculature in tumors and tumor-associated matrix proteases can augment virus attack. (II) Tumor cell membrane. Naturally occurring oncotropic viruses bind to tumor-associated surface receptors. OVs can be engineered to enhance tropism to aberrant tumor receptors. (III) Cytosol. Tumors with aberrant signaling cascades (RAS) and antiviral defense (PKR and IFN) can be targeted by a number of OVs. (IV) Nucleus. Activated cell cycle and nucleotide synthesis in transformed cells enable replication of gene-deleted OV mutants. Expression of viral genes can be engineered to be under control of tumor-specific promoters. Abbreviations: IL, interleukin; GM-CSF, Granulocyte-macrophage colony-stimulating factor; LCMV, lymphocytic choriomeningitis virus; PKR, protein kinase R; TK, thymidine kinase, GFAP, glial fibrillary acidic protein.
Cell surface mechanisms
Viral receptor-mediated attachment is the initial step of virus-cell interaction and a main target for tumor-selective mechanisms. For glioma, a number of associations have been proposed or established: (I) Measles virus receptor CD46 is overexpressed by numerous cancers 8. (II) Poliovirus co-receptor CD155 is overexpressed on glioma cells 9. (III) The receptor for sindbis virus, the 67-kDa high-affinity laminin receptor, has been shown to be overexpressed on numerous cancers 10. In addition to the inherent affinity of some viruses for receptors overexpressed on tumors, virus tropism can be retargeted towards tumor-associated receptors using genetic engineering. OVs have been designed that specifically target EGFRvIII, PDGFR, or IL-13R by viral expression of antibody domains or receptor ligands 11. In another modification, RGD peptide insertion into AdV fiber protein leads to high affinity binding to integrins αvβ3 and αvβ5, which are expressed on the surface of several tumors 12,13. Finally, narrowing the broad tissue tropism of VSV through glycoprotein pseudotyping has resulted in increased glioma tropism and reduction in neurotoxicity 14.
Cytosolic mechanisms
The cytoplasm contains potent antiviral effector elements. Double-stranded RNA formation, commonly seen with RNA viruses that have a cytosolic life cycle, causes activation of cellular antiviral defense mechanisms, most prominently activation of the protein kinase R (PKR) and interferon pathways. Activated PKR inhibits protein synthesis and promotes apoptosis, and interferon signals a downstream cascade leading to the activation of an orchestra of antiviral mediators. Tumor cells often show defects in antiviral defense, which is the basis for oncolysis by VSV, a virus otherwise extremely susceptible to antiviral cellular responses 15, 16. Defects in the interferon pathway are also responsible for the oncotropism of myxoma virus 17 and others. PKR is a strong inhibitor of viral protein synthesis, and tumors with an activated ras pathway display impaired PKR function. This forms the basis for the oncolytic action of Reovirus 18 and for γ134.5 gene-deleted HSV 19. An activated STAT-1 pathway has been shown to facilitate myxoma infection selectively 20. A new target for increased tumor selectivity is the altered expression of inhibitory microRNA elements in tumors. Gene expression and replication of VSV or measles virus containing complementary sequences to normal cell microRNA, such as let-7 or miR7, was strongly attenuated in normal cells but not in tumor cells lacking let-7 or miR7 21, 22.
Nuclear mechanisms
Autonomous parvoviruses require the S-phase of cell division of the host cell for successful replication 23, thereby reducing infection of postmitotic cells such as neurons. Thymidine kinase (TK) and ribonucleotide reductase (RR)-deleted HSV-mutants lack the ability to synthesize new nucleotides unless cellular DNA synthesis is active 24. Adenovirus E1A and E1B genes activate the cell cycle through Rb and p53 binding, respectively. In tumors with defective p53 or Rb function, AdV E1 genes become dispensable, allowing mutant AdVs lacking E1A or E1B genes to selectively replicate 25. Finally, for some oncolytic DNA viruses, expression of viral genes can be engineered to be under the control of tumor- or tissue-specific promoters, such as Nestin-1, GFAP, or Ki-67 26.
Extracellular mechanisms
The application of several viruses, such as HSV, NDV, VSV, reovirus and others can activate tumor-targeting effectors of the innate and adaptive immune system that act synergistically and contribute to tumor reduction 27, 28. In addition, VSV and vaccinia OVs have been shown to preferentially infect tumor vasculature 29, 30. Finally, tumor-associated proteases in the extracellular matrix augment the activity of measles and other OVs 31.
ONCOLYTIC VIRUS CANDIDATES FOR HIGH GRADE GLIOMA
In the second section, we present a comprehensive collection of all oncolytic viruses with proposed or established anti-glioma activity.
Herpes simplex virus
Herpes simplex virus 1 (HSV1) is the prototypic member of the herpesviridae, a family of large double-stranded DNA viruses and a common human pathogen that establishes lifelong infection through latency. Approximately 2/3 of the adult population are seropositive for HSV. In 1991, the first experimental application of a genetically modified oncolytic replication-competent virus for glioblastoma was reported by Martuza at al., using an HSV-thymidine kinase (TK)-deficient mutant, HSV-dlsptk, 32 that was shown to be highly attenuated in non-dividing cells such as neurons but effective in infecting, killing, and replicating in U87 human glioblastoma in vitro and in vivo 4. Since then, a multitude of oncolytic HSV mutants have been isolated or engineered, with R3616, HSV-1716, hrR3, G207, and G47Δ being the most-studied examples for targeting glioblastoma. All these mutants feature deleted or manipulated viral genes that reduce neurotoxicity but do not interfere with infection of actively dividing cells, particularly those with activated Ras pathway signaling and suppressed PKR function 19,33. R3616 and HSV-1716 have deletions in both copies of the viral γ134.5 genes, the gene product of which is essential for neurovirulence 34, 35. Another attenuation principle is employed by hrR3, a UL39 gene mutant with disrupted viral ribonucleotide reductase (RR) function, vital for viral nucleotide synthesis in post-mitotic but not dividing cells 36. With a significantly attenuated ability to infect neurons, R3616, HSV-1716, and hrR3 still effectively infect, kill, and replicate in several human glioma samples 37–40. Subsequently, second- and third-generation oncolytic HSV recombinants were introduced featuring multi-mutated genotypes. G207 combines the deletion of both copies of the γ134.5 gene with a gene-disrupting lacZ reporter gene insertion into the UL39 gene resulting in a highly attenuated HSV variant 41. Antiglioma activity in preclinical studies and an extensive safety assessment in rodents and non-human primates resulted in a clinical trial of an oncolytic HSV for glioblastoma that commenced in February 1998 42. In parallel, HSV-1716 was tested in a clinical study in Europe 43. G207 is also the foundation for the third-generation HSV G47Δ in which a third mutation was added 44. Deletion of the gene encoding for ICP47 results in enhanced MHC class 1 antigen presentation and enhanced lymphocytic tumor infiltration. In addition, this deletion causes a promoter shift for the US11 gene resulting in a faster viral life cycle and higher titers of oncolytic HSV progeny, ultimately increasing G47Δ’s oncolytic activity while preserving the G207 safety profile 45. “Arming” a γ134.5 gene-deleted HSV with immune-stimulatory IL-12 is the basis for oncolytic candidates designated M002 (murine IL-12) and M032 (human IL-12), and has been shown to enhance glioma cell killing in syngeneic and xenograft tumor models 46. Based on these findings, a clinical trial of M032 has been initiated (NSC 733972).
Additional efforts to “arm” attenuated HSV include adding alternative therapeutic transgenes and/or increasing tumor-selective targeting through receptor targeting and tumor-specific promoters. Transgenes that have been employed include CYP2B1, interleukins, and others 47–49. For receptor targeting, an IL-13Rα2 directed HSV was created 50. Additionally, a nestin promoter driving viral γ134.5 gene expression was shown to enhance glioma selectivity 51.
Adenovirus
A double-stranded non-enveloped DNA virus, adenovirus (AdV) is a very common human pathogen that causes mild and self-limiting upper respiratory symptoms. Genetically engineered recombinants of AdV serotype 5 (Ad5) that show conditional replication (CRAds) are some of the most studied oncolytic viruses. The 36 kb genome of the virus can be divided into two sets of genes, early (functional) and late (structural). Early gene products interact with cellular control and defense mechanisms and are indispensable for viral replication in normal cells. Of importance, proteins from the E1A gene region trigger cells to enter S-phase by interacting with cellular retinoblastoma tumor suppressor protein (pRb) and p300; E1B proteins suppress apoptosis by binding and inactivating p53, thereby inhibiting its pro-apoptotic response; E2 elements control viral DNA replication; E3 proteins counter various antiviral immune responses; E4 gene products inhibit cellular DNA repair mechanisms and apoptosis 52. This reliance on activated and uninhibited cell cycle mechanisms for viral replication renders adenoviruses with E1A or E1B deficiencies replication incompetent in normal cells, and therefore tumor-selective. A conditionally replicating E1B-55kDa gene deleted mutant dl1520 Adenovirus 5 (also known as ONYX-015) 5 was effective in killing p53 mutant U373 glioma cells but not p53 normal U87 cells 53. However, later studies in glioma tissue originating from primary tumor samples reported increased oncolytic activity of ONYX-015 in p53 wild-type glioma xenografts compared to p53 mutants 54. ONYX-015 was tested in a glioblastoma clinical trial 55. In addition to p53 targeting, Ad5 mutants have been developed in parallel that exploit defects in the Rb pathway through amino acid deletions in the Rb binding site of E1A 56, the resulting CRAd being designated Ad-delta-24. Alterations in expression levels of Rb and p16 were reported in nearly 50% of gliomas 57 and some reports suggest a correlation with advanced stages of glioma 58. In addition, receptor retargeting efforts have resulted in modifications of AdV tropism. An RGD peptide inserted into the Ad fiber protein - which normally binds the Coxsackie-Adenovirus receptor CAR - led instead to high affinity binding to integrins αvβ3 and αvβ5, which have been found on many tumor surfaces 12. A combined Ad-delta-24 with RGD modifications was shown to have increased oncolytic action against glioblastoma 13 and is currently undergoing testing in a clinical trial (NCT00805376). Additional retargeting strategies include a single chain antibody tagged to the AdV fiber knob that is directed against EGFR, which resulted in highly selective targeting of glioblastoma 59. The next generation of Ad-delta-24 includes the addition of an E2F1-responsive element as the promoter for E1A, making adenoviral E1A expression dependent on the presence of free E2F, commonly seen in tumors. This Ad-delta24-RGD-E2F1 construct (also known as ICOVIR) displayed enhanced efficacy in gliomas 60.
Newcastle Disease virus
This is an avian paramyxovirus with a negative stranded RNA genome, which is not associated with any serious human disease. Five strains PV701, 73-T, MTH-68/H, NDV-HUJ, and V4UPM have been tested for oncolytic potential 61, 62. The tumor-suppressive nature of NDV as an oncolytic virus and a tumor vaccine has been extensively studied in in vivo models and in clinical trials. However, the mechanisms underlying tumor selectivity are not well- defined. As with other oncolytic viruses, exploitation of defects in antiviral defense is likely to play a role 63. An activated Ras pathway might also contribute to NDV oncolysis 64. In addition, peripheral induction of TNF-α secretion by mononuclear cells and a boost in antitumor immune response have been proposed 65. In stark contrast to most other oncolytic virus candidates, NDV received relatively little preclinical investigation before being tested in clinical trials. We found only 4 experimental glioma studies, 66, 67,68,62 along with 4 clinical studies on NDV application against glioblastoma, with some promising reported outcomes 69–71. A 5th clinical trial is scheduled to start soon (NCT01174537).
Reovirus
Reoviruses are nonenveloped viruses that were initially described as Respiratory Enteric Orphan, “orphan” reflecting the belief that these viruses do not cause any apparent disease in humans. Reoviruses distinctively feature a double-stranded RNA genome, which together with double-stranded viral RNA transcripts cause a strong activation of cellular PKR pathways. PKR in turn blocks viral replication through the inhibition of protein synthesis and promotion of apoptosis. Wild-type reovirus displays an oncoselectivity for tumors with an activated Ras signaling pathway 72, which inhibits PKR function and is initially activated through receptor tyrosine kinases downstream of EGFR and PDGFR receptors. Reovirus showed promise as an anti-glioma agent in vitro 73 and in vivo 18. All 16 human glioma specimens and over 80% of established glioma cell lines tested were susceptible to reovirus oncolysis. Direct intratumoral injection resulted in effective oncolysis in subcutaneous and intracranial human glioblastoma xenografts in immunocompromised nude mice. Neurotoxicity initially observed in immunocompromised animals was absent in immune-competent rodent glioma models, and non-human primates injected intracranially with GMP-grade reovirus showed no neurotoxicity or measurable adverse effect 74, paving the way for two clinical trials 75. A recent study showed reovirus oncolytic effect against glioma generalized among several reovirus strains 76, opening the possibility for potentially repetitive application of serotype-switched reoviruses.
Vaccinia virus
Vaccinia virus (VV) is closely related to bovine poxvirus and is the vaccine agent used for the smallpox eradication program. It is a large double stranded enveloped DNA virus with a life cycle restricted to the non-nuclear cytoplasm.
A number of diverse strains of VV have been used as vaccines in humans 77. Of those, strongly attenuated strains show impaired replication and are mostly used as gene therapy vectors. In contrast, less attenuated VV strains Western Reserve, Lister, and Copenhagen have been used as a base for recombinant engineered oncolytic viruses. Although not regarded as tumor selective in a narrow sense by some authors 78, studies have shown a certain onco-preference by virtue of transformed cells providing a more favorable environment for VV replication 79. The first VVs studied for antiglioma activity were armed with a p53 transgene 80. To enhance oncoselectivity, recombinant VV strains were developed with targeted alterations to the VV genome, including tyrosine-kinase (TK) deletion and vaccinia growth factor deletion. Application of double-deleted VVs (vvDD) combined with immunosuppressive agents resulted in prolonged survival in rodent glioma models 81 and intravenous injection of vvDD in non-human primates was tolerated with no adverse effect 82. Recently, a VV (JX-594) armed with immune-modulatory human GM-CSF and LacZ inserted and disrupting TK gene was shown effective in prolonging survival in animal glioma models 83.
Poliovirus
Poliovirus is a positive stranded RNA virus belonging to the picornaviridae family and is the causative agent for human poliomyelitis. Neurotoxicity of poliovirus is attributed to two factors: 1) selective binding to the poliovirus receptor PVR (alternatively Necl-5 or CD155) expressed on motor neurons and 2) an internal ribosomal entry site (IRES) at the 5’ end of the viral RNA genome. Importantly, the receptor PVR/CD155 was found to be expressed in a majority of glioblastoma samples tested 9. In 1996, Gromeier et al. introduced an engineered intergenetic poliovirus with curtailed neurotoxicity by replacing the polio IRES sequence with a non-pathogenic version from human rhinovirus type 2 (HRV2) 84. The resulting poliovirus chimera PV1(RIPO) and PVS(RIPO) (with the attenuated Sabin polio vaccine strain as a backbone) showed good oncolytic potential against glioma in vitro and in vivo 85, while lacking poliomyelitis-like neurotoxicity in safety studies on CD155 transgenic mice and non-human primates 86, 87. PVS(RIPO) has received an IND for phase I clinical trials (#14735) and is scheduled to enter clinical trials in the upcoming months.
Measles virus
Measles virus (MV) is a negative stranded RNA virus belonging to the paramyxoviridae family, and is a well-known human pathogen causing common exanthemous disease and in rare circumstances severe encephalitis 88. Although early reports of MV growth on cultured glioma cells date back to 1975 89, it wasn’t until 2003 that the highly attenuated Edmonston strain (MV-Edm) used for widespread vaccination campaigns was shown to be of potential therapeutic value as an oncolytic agent for glioma 90. The two MV glycoproteins, the hemagglutinin protein H and fusion protein F, are crucial for oncolytic specificity and efficacy. Mutations in the viral H protein of the Edmonston strain cause the attenuated virus to display a high affinity to cellular CD46 receptors, which are overexpressed in a broad range of tumors 8, 91. The F protein is responsible for membrane fusion leading to syncytia formation and ultimately apoptosis 92. In 2002, a recombinant MV-Edm was engineered to express carcinoembryonic antigen (CEA) as a reporter gene to monitor viral activity in vivo 93. MV-CEA showed a favorable oncolytic and safety profile in a number of animal models including direct intracranial injection in CD46 transgenic mice and non-human primates 94 leading to the initiation of a phase 1 clinical trial for glioblastoma that is currently ongoing (NCT00390299). Recently, retargeted MVs have been engineered to express either a single chain antibody against glioblastoma-associated antigens EGFRvIII, or IL-13 as a ligand to glioblastoma-specific receptor IL-13Rα2 95, 96.
Vesicular Stomatitis Virus
VSV is an enveloped negative stranded RNA virus of the rhabdovirus family that is associated with mild and usually self-limiting disease in livestock. Infection in humans is rare and usually asymptomatic. As one of the most IFN-sensitive viruses, infections are rapidly controlled by normal cells that mount an IFN-mediated antiviral response 97. VSV was introduced as a potent oncolytic candidate in 2000 when it became apparent that VSV’s exquisite IFN sensitivity complemented the frequent aberrations in the IFN pathway in tumor cells 15, 98; a large number of various tumors were targeted and lysed by VSV, including glioblastoma. VSV has an extremely broad species and tissue tropism due to ubiquitous receptor binding, and it is not confined to specific transforming mutations like p53, ras, or myc 99. Numerous cancer types have been successfully targeted in preclinical studies 100. This broad tropism is particularly important in light of the heterogeneous mutation background of glioblastoma multiforme. In a recent study, we analyzed the susceptibility of a panel of human glioblastoma cell lines to VSV infection and found various levels of interferon impairment in these tumors, but not in non-tumor human brain samples 16. In orthotopic glioma models in rodent brain, VSV applied systemically was shown to selectively target and infect not only the tumor bulk, but also remote satellite glioma cell clusters 101, 102. A limitation to current efforts to develop VSV for potential therapeutic applications in the therapy of glioblastoma is the potential neurotoxicity observed in some rodent models 103. Consequently, one research focus has been on attenuating strategies, which have resulted in several mutants with reduced neurotoxicity. For example, VSV-M51 is a mutant that yields an enhanced interferon response 104, and VSV-p1-GFP displays a reduced replication speed 105. In addition, “armed” VSVs carrying therapeutic transgenes have been engineered 106, 107, however, none of these variants have been examined on glioblastomas yet. Alternatively, evolutionary pressure applied to VSV propagated in glioblastoma cultures resulted in the isolation of VSV-rp30, a mutated variant with enhanced tumor infectivity and lytic potency 108. Recently, a new VSV construct pseudotyped with a glycoprotein from lymphocytic choriomeningitis virus (LCMV) in place of VSV’s natural G protein was introduced with significantly reduced neurotoxicity in mice 14. VSV has recently been approved for a clinical trial as a vaccine vector for HIV (NCT01438606) and is in preclinical development as an oncolytic agent for a number of peripheral cancers 109. A close relative to VSV is Maraba virus, which recently excelled in a comparison study of the oncolytic potential of numerous rhabdovirus variants. Among the tumor lines targeted was human glioblastoma SNB19 (identical to U-373), which was particularly susceptible to Maraba virus infection 110.
Autonomous Parvovirus
Members of the family Parvoviridae are small non-enveloped single stranded DNA viruses and, with the exception of human parvovirus B19, are not pathogenic in humans. Parvoviruses can be divided into dependoviruses, such as adeno-associated virus AAV, which require coinfection with adeno-, herpesvirus or other helper viruses for replication, and autonomous parvoviruses that replicate independent of other viruses on permissive cells. A key requirement for virus replication is an inherent dependence on cell replication23. In addition, PKR disruption in transformed cells has been proposed to facilitate productive parvovirus infection 111. Rat H1 parvovirus has been extensively studied for glioblastoma oncolysis in vitro and in vivo 112 and is currently being tested in a phase 1 clinical study for glioblastoma in Germany 113 (NCT01301430). Another parvovirus, minute virus of mice (MVM) was subject to basic infectivity studies on glioblastoma cultures but has not yet advanced to in vivo studies 108, 114, 115.
Myxoma virus
Like vaccinia virus, myxoma virus (Myx) belongs to the poxviridae family and is a species-specific pathogen in European rabbits. As such, disease and pre-existing immunity in humans have not been reported116. Myx was found, however, to overcome the species barrier to selectively infect diverse human tumor cells. The oncotropism was shown to be based on tumor-associated aberrations like over-expression of Akt 20. Defects in the interferon pathway also contribute to permissiveness of myxoma infection in non-host species cells 17. Myxoma virus infected a large number of human glioma cell lines and human glioma surgery specimens; significantly prolonged survival was observed in Myx-injected immunocompromised intracranial glioma xenograft models117. In immunocompetent glioma models, intratumoral injection of Myx was effective in conjunction with the immune modulator rapamycin 118.
Seneca Valley virus
Seneca Valley virus (SSV-001) was initially discovered as a cytolytic cell culture contaminant. Subsequent isolation and sequencing revealed a strong relation to porcine picornaviruses, small single stranded RNA viruses. The virus is not known to cause any disease in humans or animals, but testing of a human tumor panel revealed a broad spectrum of cytolytic action, particularly on tumors with neuroendocrine features 119. Human glioblastoma cell lines, however, were mostly resistant (only 2 out of 9 lines were substantially infected). Nevertheless, discussing this virus in the context of glioblastoma virotherapy is merited for two reasons. First, it shows a strong affinity for pediatric tumors, and a 50% response rate (2 out of 4) in glioblastoma was found in a cell line panel of the Pediatric Preclinical Testing Program (PPTP) 120. Secondly, medulloblastoma, the most common pediatric brain tumor, was strongly targeted by SSV. The virus also targeted CD133+ cancer stem cells 121. In a remarkably fast development from virus isolation to clinical testing, 3 clinical trials were initiated for SSV under the trade name NTX-010 by Neotropix, Inc, including one trial for pediatric tumors (NCT01048892).
Sindbis virus
This alphavirus from the Togaviridae family is a positive stranded RNA virus with birds as the natural host and mosquitoes as the transfer vector. Infection in humans can occur after transmission through mosquito bites. Wild-type Sindbis virus (SIN) is neurotropic, causing encephalitis in mice, but also displays an inherent tropism for tumor cells 122, which is attributed to one of the surface receptors required for Sindbis virus infection, the 67-kDa high-affinity laminin receptor 123. This receptor has been shown to be overexpressed in numerous cancers, 10 and often correlates with more aggressive oncologic features 124. As a blood-borne pathogen it is stable in the blood stream and suitable for systemic application 125. The attenuated laboratory SIN strain AR339 has been studied as an oncolytic agent for numerous cancers 126. Our laboratory tested AR339 on glioblastoma and found productive infection of human U87 cells but no infection in a second line, MO59J 108.
Pseudorabies virus
Despite its name, pseudorabies virus (PRV) is not related to rabies virus but rather to HSV. PRV is a neurotropic pig alphaherpesvirus and the causative agent for Aujetzky’s disease in pigs. It is non-pathogenic in humans but displays a wide species tropism for infection of mammalian cells beyond its natural host, the swine 127,128. PRV is widely used in laboratories to study herpesvirus biology and is commonly used as a neuroanatomic circuit-tracing tool, owing to its well-defined transsynaptic spread 129. Its close relation to human herpesvirus HSV without any known human pathogenicity led to initial studies addressing PRV’s potential as an oncolytic virus. Using the laboratory strain PRV-Bartha as a backbone, several attenuated mutants have been engineered with TK and RR gene deletions analogue to attenuated HSV mutants. These PRV variants were shown to infect a number of cancer cell lines in vitro and in vivo, however affinity for human glioblastoma cells was low 130. Comparative studies in our laboratory found PRV only moderately suitable as an antiglioma virotherapy agent. Infection of human glioblastoma required a high viral titer and showed little selectivity over normal human control cells 108. In vivo, intratumoral viral spread was limited to the injection site, and analysis after systemic virus application found few signs of successful targeting of intracranial glioma xenografts 102.
CLINICAL TRIALS AND CASE STUDIES
Overview
As of the end of 2011, results from 8 clinical trials and 3 case reports have been published: five trials using HSV strains G207 and 1716, one trial using adenovirus ONYX-015, one trial using reovirus Reolysin, one trial using NDV strain HUJ, and three case series using NDV strain MTH-68. A total of nearly 120 patients were included in these trials. The important and encouraging outcome of all trials was the absence of any major virus-related complications and the assessment that no dose limiting toxicity (DLT), or maximum tolerated dose (MTD), was reached in any trial. Because these early studies were addressing safety and feasibility of this novel approach to treatment, dosing and application regimens were chosen conservatively in most cases, and the overall therapeutic effect was marginal, though some individual cases of treatment response were reported. Individual completed and ongoing trials are discussed in the following paragraphs. See TABLE 2 for a summary.
Table 2. Design and outcome of completed and ongoing clinical trials of oncolytic virotherapy in patients with malignant gliomas.
Standard font: Completed trials; Italic font: initiated or ongoing trials; (“x”) indicates the projected sample size for ongoing trials.
| Virus (strain) [Ref] |
Study phase |
# of Patients |
Application protocol | Efficacy | Toxicity |
|---|---|---|---|---|---|
| HSV G207 42 |
I | 21 | 1×106 –3×109pfu single IT injection | Decreased tumor volume on MRI in 8 patients | No toxicity, no serious adverse events |
| HSV G207 131 |
I | 6 | Two doses, totaling 1.5×109 pfu/intratumoral injections pre- and post-resection | Not efficient | transient fever, delirium, and hemiparesis in one patient that resolved with DXM treatment |
|
HSV G207 |
I | 9 | Two doses, totaling 1×109 pfu/intratumoral injections followed by radiotherapy on the next day | N/A | N/A |
| HSV 1716 43 |
II | 9 | 103–105 pfu single IT injection | 3 responders 5 stable disease | No toxicity, no serious adverse events |
| HSV 1716 132 |
I | 12 | 105 pfu single IT injection | Not assessed | No toxicity, no serious adverse events |
| HSV 1716 133 | I | 12 | 105 pfu single IT injection | 3 disease free for 15–22 months | No toxicity, no serious adverse events |
|
HSV 1716 |
II | N/A | N/A | N/A | N/A |
|
HSV G47Δ |
I | “21” | N/A | N/A | N/A |
|
HSV M032 |
I | N/A | N/A | N/A | N/A |
| AdV ONYX-015 55 |
I | 24 | 107– 1010pfu single injection to tumor resection cavity | Not efficient | No toxicity, no serious adverse events |
|
AdV Delta24-RGD |
I | N/A | N/A | N/A | N/A |
| ReoV 75 |
I | 12 | 107– 1010 pfu single intratumoral injection (to 3 dfferent foci) | 1 long term (>6years) survivor | No grade III or IV adverse events |
| ReoV | I | 18 | Convection enhanced delivery of 108–1010 TCID50 |
1 partial response 3 stable disease |
No toxicity, no serious adverse events |
| NDV HUJ 71 |
I/II | 14 | Part A: 108 – 1010 IU i.v. daily for 5 days each Part B: 3 cycles 5.5×109 IU i.v. |
1 complete response 3 longterm survivors |
No toxicity, no serious adverse events |
|
NDV HUJ |
I/II | “30” | 1010 IU i.v. daily | N/A | N/A |
| NDV MTH-68 69 |
CS | 1 | Daily intravenous injection of 2×107 to 2.5×108 pfu for years | Tumor shrinkage | No toxicity, no serious adverse events |
| NDV MTH-68 70 |
CS | 4 | Daily intravenous injection of 2×107 to 2.5×108 pfu for years | 4 long term survivors | No toxicity, no serious adverse events |
| NDV MTH-68 134 |
CS | 1 | Daily intravenous injection of 2×107 to 2.5×108 pfu for years | Tumor shrinkage | No toxicity, no serious adverse events |
|
Measles MV-CEA |
I | “40” | Intratumoral injection | N/A | N/A |
|
H1 H1PV |
I | “19” | Intratumoral injection prior to resection | N/A | N/A |
|
Polio PVS-RIPO |
I | N/A | Convection enhanced intratumoral injection prior to resection | N/A | N/A |
Abbreviations: HSV, herpes simplex virus; AdV, adenovirus; ReoV, reovirus, NDV, Newcastle disease virus; H1, rat H1 parvovirus, CS, case study/series; IT, intratumoral; i.v., intravenous; IU, infectious units; pfu, plaque forming units; TCID, tissue culture infective dose.
HSV
Two different oncolytic HSV-1 strains (G207 and 1716) have undergone phase I and II testing, and further phase III studies are on the way. Two other HSV therapeutics (G47delta and M032) are scheduled to enter clinical trials. Two phase I studies were conducted using G207 and a total of 27 patients were enrolled. In these studies published in 2000 and 2008, Markert et al. have reported no significant toxicity. In the first study, no serious adverse events were observed, and MTD was not reached after intratumoral injection of G207 in 21 patients. Eight of the 21 patients had clinical response, and one patient had long-term survival (>5.5 years) 42. In the follow-up phase 1b study, 1.15 × 109 plaque-forming units (pfU) of G207 were injected intratumorally or into the tumor bed before or after tumor resection in six patients with recurrent GBM 131. The authors reported that viral replication inside the tumor occurred in only 50% of the patients and no clinical response was observed in these 6 patients. A third study on intratumoral injection of 109 pfu of G207 into 5 different loci inside the tumor, followed by radiotherapy on the next day has been completed and publication of results is awaited (NCT00157703).
In parallel to G207 trials in the US, three phase I clinical trials employing HSV-1716 (SEPREHVIR®, Virttu biologics) have been conducted in Europe, and the first report was published simultaneously to the first G207 study in 2000 in the same issue of the journal “Gene Therapy” 43. A total of 33 patients were enrolled in these trials. In the first study, Rampling et al. administered a single injection of 103–105 pfu intratumorally in 9 patients with GBM with no resultant toxicities, and the MTD was not reached. Three patients had long-term survival longer than 3 years. Papanastassiou et al. performed a single intratumoral injection of 105 pfu of HSV-1716 in 12 GBM patients and subsequent tumor resection 4–9 days after injection 132. In the third study Harrow et al. injected 105 pfu into the peritumoral parenchyma in 12 patients with no resultant toxicity 133. Reportedly, a Phase II study is in preparation in Europe (www.virttu.com).
A clinical phase I trial applying a third generation oncolytic HSV strain G47delta to patients with progressive GBM was initiated at the University of Tokyo in 2009 49 and is currently open for recruitment with a target sample size of 21 patients (WHO trial number JPRN-UMIN000002661).
The latest representative of oncolytic HSV strains “armed” with a human IL-12 transgene, designated M032, is scheduled to enter clinical phase I for recurrent or progressive GBM, anaplastic astrocytoma or gliosarcoma at the University of Alabama in Birmingham (Gene Transfer Protocol Report 0801-899).
Adenovirus
A phase 1 trial conducted with intratumorally injected ONYX-015 was reported by Chiocca et al. in 2004 55. 24 patients with recurrent malignant glioma received up to 1010 pfu at 10 different sites at the tumor resection border. No significant toxicity was noted. ONYX-015 application was not associated with a therapeutic effect.
Currently, a phase I study using intra and peritumoral injection of 109–1011 pfu of double-modified Ad-Delta-24-RGD (DNATrix) is open for recruitment at MD Anderson Cancer Center in Houston, Texas (NCT00805376).
Reovirus
Reovirus (Reolysin- Oncolytics Ltd.) has been tested in two dose-escalation phase I studies conducted in Canada and the US. Forsyth et al. injected 107–109 pfu of reovirus to 3 intratumoral sites in 12 patients (9 grade IV and 3 grade III gliomas) with recurrent malignant glioma 75. No significant toxicity was reported and the MTD was not reached. There was one patient with a more than 6-year survival. A second multicenter trial in the USA with a dose escalating design and convection enhanced delivery of reovirus was done on 18 patients and no dose limiting toxicity was reported. Stable disease in three and partial response in one patient were noted, but no formal report has been published yet (NCT00528684).
NDV
One formal phase I study and several case reports/ series have been published on the use of NDV against glioblastoma. Freeman et al. reported a phase I study conducted in Israel on 14 patients with recurrent GBM (including one pediatric patient) using intravenously administered lentogenic (highly attenuated) NDV strain HUJ daily for 8 cycles 71. 11 patients completed the trial. 1 complete response and 3 long term survivals (> 3 years) were reported. No significant toxicity was reported and the MTD was not reached. A follow up phase I/II trial at the same institution has been initiated with a target enrollment of 30 patients, who are proposed to receive daily i.v. injection of NDV-HUJ (NCT01174537). Csatary et al. have published 3 case reports/series from Hungary using the mesogenic (medium virulent) MTH-68/H NDV strain. The first reported the case of a 14 year old boy with recurrent GBM who was treated more than 2 years with a combination of chemotherapy and MTH-68/H 69. The second report was that of one adult and 3 pediatric patients with GBM 70 with reported long term survival. The third case report was that of MTH-68/H and valproic acid in a pediatric patient with anaplastic astrocytomas 134. These case reports are encouraging; however, a better understanding of underlying mechanisms of action, and a randomized patient selection will benefit future studies of NDV. No formal clinical trials were reported with MTH-68/H so far.
H1 parvovirus
So far, H1 is the only parvovirus that has reached clinical studies. A phase I study involving intratumoral injection of H1-PV (ParvOryx- Oryx GmbH & Co. KG) before tumor resection has recently been started at the University of Heidelberg in Germany and is expected to enroll 19 patients with progressive primary or recurrent GBM (NCT01301430). The first patient reportedly received treatment in October 2011.
Measles virus
A phase I study using measles Edmonston vaccine strain derivate expressing human carcinoembrionic antigen (MV-CEA) in patients with recurrent GBM was initiated at Mayo Clinic in Rochester, MN and is currently recruiting patients to a planned enrollment number of 40 subjects. The virus is injected intratumorally or into the resection cavity (NCT00390299).
Poliovirus
PVS-RIPO, a recombinant chimeric polio construct based on Sabin’s live attenuated polio vaccine modified with a rhinovirus IRES element has been approved (IND# 14735) to enter clinical trial testing conducted at Duke University, Durham, USA 87. The proposed protocol includes single intratumoral convection-enhanced virus application before tumor resection in a dose escalation design.
Conclusion
Despite an impressive number of preclinical studies and valuable clinical trials confirming the general safety of the approach, the preclinical promise from laboratory experiments has not yet translated to significant therapeutic impact at the bedside. Given widespread initial hesitation at the use of replication competent lytic viruses in the treatment of the human brain, the decision to focus on the safest and most attenuated agents was paramount for the future of the field of oncolytic virus therapy. In this regard, the lack of virus-attributable adverse effects in over 100 patients should be considered a success and a foundation for next-generation studies. However, the field cannot survive on promise alone, particularly in light of the substantial costs involved with GMP grade virus production and testing. Without well-demonstrated efficacy that is required to reach treatment approval, the pharmaceutical industry and investment groups will remain cautious. So far, only one oncolytic virus has been approved by a governmental agency, AdV ONYX-015, registered in China for head and neck cancer therapy 135. Future trials will also incorporate multimodality treatment to investigate synergistic relationships between OVs and standard radiation or chemotherapeutic regimens, which have already been established in numerous preclinical models 136. An increasing number of studies have recently shown enhanced oncolytic activity when virus application is coupled with agents that temporarily suppress the antiviral immune response 137.
As nicely discussed in a recent commentary on the current state of oncolytic virotherapy 138, the development of oncolytic viruses preferably does not follow a unidirectional path but rather benefits from a reiterative feedback loop in which the findings from clinical trials influence and inform the design in future generations of viruses. This is particularly true for virotherapy in the brain, a field that faces unique challenges that can only incompletely be addressed in preclinical laboratory studies. Unlike small molecule therapeutics, biological effects of viruses are highly dependent on the species examined. Human viruses such as HSV, AdV, or poliovirus are highly attenuated in rodent tumor models but might be less so when applied in humans. Conversely, non-human pathogens such as VSV, SIN, and PRV can be pathogenic in mice, making preclinical survival studies particularly challenging. As these non-human pathogens move into clinical trials, a new dimension of safety considerations emerges as the consequences of environmental contamination and spread would need to be considered 139.
Despite the challenges and tremendous efforts involved in developing oncolytic viruses from “bench to bedside”, pursuing this path is justified given not only the potential efficacy of this treatment modality, but also given the accelerating rate of progress.
Acknowledgement
We thank John N Davis and Justin C Paglino for helpful suggestions on the manuscript.
Part of the work mentioned in this review article has been supported through grants NIH RO1 CA 124737 and NS48854.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
AN van den Pol has an equity interest in Azgardabio, which focuses on the development of viruses for therapeutic application.
REFERENCES
- 1.Wen PY, Kesari S. Malignant gliomas in adults. N Engl J Med. 2008;359:492–507. doi: 10.1056/NEJMra0708126. [DOI] [PubMed] [Google Scholar]
- 2.Van Meir EG, Hadjipanayis CG, Norden AD, Shu HK, Wen PY, Olson JJ. Exciting new advances in neuro-oncology: the avenue to a cure for malignant glioma. CA Cancer J Clin. 2010;60:166–193. doi: 10.3322/caac.20069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kelly E, Russell SJ. History of oncolytic viruses: genesis to genetic engineering. Mol Ther. 2007;15:651–659. doi: 10.1038/sj.mt.6300108. [DOI] [PubMed] [Google Scholar]
- 4.Martuza RL, Malick A, Markert JM, Ruffner KL, Coen DM. Experimental therapy of human glioma by means of a genetically engineered virus mutant. Science. 1991;252:854–856. doi: 10.1126/science.1851332. [DOI] [PubMed] [Google Scholar]
- 5.Bischoff JR, Kirn DH, Williams A, et al. An adenovirus mutant that replicates selectively in p53-deficient human tumor cells. Science. 1996;274:373–376. doi: 10.1126/science.274.5286.373. [DOI] [PubMed] [Google Scholar]
- 6.Parsons DW, Jones S, Zhang X, et al. An integrated genomic analysis of human glioblastoma multiforme. Science. 2008;321:1807–1812. doi: 10.1126/science.1164382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Verhaak RG, Hoadley KA, Purdom E, et al. Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell. 2010;17:98–110. doi: 10.1016/j.ccr.2009.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dorig RE, Marcil A, Chopra A, Richardson CD. The human CD46 molecule is a receptor for measles virus (Edmonston strain) Cell. 1993;75:295–305. doi: 10.1016/0092-8674(93)80071-l. [DOI] [PubMed] [Google Scholar]
- 9.Merrill MK, Bernhardt G, Sampson JH, Wikstrand CJ, Bigner DD, Gromeier M. Poliovirus receptor CD155-targeted oncolysis of glioma. Neuro Oncol. 2004;6:208–217. doi: 10.1215/S1152851703000577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van den Brule FA, Castronovo V, Menard S, et al. Expression of the 67 kD laminin receptor in human ovarian carcinomas as defined by a monoclonal antibody, MLuC5. Eur J Cancer. 1996;32A:1598–1602. doi: 10.1016/0959-8049(96)00119-0. [DOI] [PubMed] [Google Scholar]
- 11.Nakamura T, Peng KW, Harvey M, et al. Rescue and propagation of fully retargeted oncolytic measles viruses. Nat Biotechnol. 2005;23:209–214. doi: 10.1038/nbt1060. [DOI] [PubMed] [Google Scholar]
- 12.Pasqualini R, Koivunen E, Ruoslahti E. Alpha v integrins as receptors for tumor targeting by circulating ligands. Nat Biotechnol. 1997;15:542–546. doi: 10.1038/nbt0697-542. [DOI] [PubMed] [Google Scholar]
- 13.Fueyo J, Alemany R, Gomez-Manzano C, et al. Preclinical characterization of the antiglioma activity of a tropism-enhanced adenovirus targeted to the retinoblastoma pathway. J Natl Cancer Inst. 2003;95:652–660. doi: 10.1093/jnci/95.9.652. [DOI] [PubMed] [Google Scholar]
- 14.Muik A, Kneiske I, Werbizki M, et al. Pseudotyping vesicular stomatitis virus with lymphocytic choriomeningitis virus glycoproteins enhances infectivity for glioma cells and minimizes neurotropism. J Virol. 2011;85:5679–5684. doi: 10.1128/JVI.02511-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stojdl DF, Lichty B, Knowles S, et al. Exploiting tumor-specific defects in the interferon pathway with a previously unknown oncolytic virus. Nat Med. 2000;6:821–825. doi: 10.1038/77558. [DOI] [PubMed] [Google Scholar]
- 16.Wollmann G, Robek MD, van den Pol AN. Variable deficiencies in the interferon response enhance susceptibility to vesicular stomatitis virus oncolytic actions in glioblastoma cells but not in normal human glial cells. J Virol. 2007;81:1479–1491. doi: 10.1128/JVI.01861-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang F, Ma Y, Barrett JW, et al. Disruption of Erk-dependent type I interferon induction breaks the myxoma virus species barrier. Nat Immunol. 2004;5:1266–1274. doi: 10.1038/ni1132. [DOI] [PubMed] [Google Scholar]
- 18.Wilcox ME, Yang W, Senger D, et al. Reovirus as an oncolytic agent against experimental human malignant gliomas. J Natl Cancer Inst. 2001;93:903–912. doi: 10.1093/jnci/93.12.903. [DOI] [PubMed] [Google Scholar]
- 19.Smith KD, Mezhir JJ, Bickenbach K, et al. Activated MEK suppresses activation of PKR and enables efficient replication and in vivo oncolysis by Deltagamma(1)34.5 mutants of herpes simplex virus 1. J Virol. 2006;80:1110–1120. doi: 10.1128/JVI.80.3.1110-1120.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang G, Barrett JW, Stanford M, et al. Infection of human cancer cells with myxoma virus requires Akt activation via interaction with a viral ankyrin-repeat host range factor. Proc Natl Acad Sci U S A. 2006;103:4640–4645. doi: 10.1073/pnas.0509341103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leber MF, Bossow S, Leonard VH, et al. MicroRNA-sensitive oncolytic measles viruses for cancer-specific vector tropism. Mol Ther. 2011;19:1097–1106. doi: 10.1038/mt.2011.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Edge RE, Falls TJ, Brown CW, Lichty BD, Atkins H, Bell JC. A let-7 MicroRNA-sensitive vesicular stomatitis virus demonstrates tumor-specific replication. Mol Ther. 2008;16:1437–1443. doi: 10.1038/mt.2008.130. [DOI] [PubMed] [Google Scholar]
- 23.Rommelaere J, Geletneky K, Angelova AL, et al. Oncolytic parvoviruses as cancer therapeutics. Cytokine Growth Factor Rev. 2010;21:185–195. doi: 10.1016/j.cytogfr.2010.02.011. [DOI] [PubMed] [Google Scholar]
- 24.Parker JN, Bauer DF, Cody JJ, Markert JM. Oncolytic viral therapy of malignant glioma. Neurotherapeutics. 2009;6:558–569. doi: 10.1016/j.nurt.2009.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jiang H, Gomez-Manzano C, Lang FF, Alemany R, Fueyo J. Oncolytic adenovirus: preclinical and clinical studies in patients with human malignant gliomas. Curr Gene Ther. 2009;9:422–427. doi: 10.2174/156652309789753356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nandi S, Lesniak MS. Adenoviral virotherapy for malignant brain tumors. Expert Opin Biol Ther. 2009;9:737–747. doi: 10.1517/14712590902988451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.De Silva N, Atkins H, Kirn DH, Bell JC, Breitbach CJ. Double trouble for tumours: exploiting the tumour microenvironment to enhance anticancer effect of oncolytic viruses. Cytokine Growth Factor Rev. 2010;21:135–141. doi: 10.1016/j.cytogfr.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 28.Melcher A, Parato K, Rooney CM, Bell JC. Thunder and lightning: immunotherapy and oncolytic viruses collide. Mol Ther. 2011;19:1008–1016. doi: 10.1038/mt.2011.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Parato KA, Lichty BD, Bell JC. Diplomatic immunity: turning a foe into an ally. Curr Opin Mol Ther. 2009;11:13–21. [PubMed] [Google Scholar]
- 30.Breitbach CJ, De Silva NS, Falls TJ, et al. Targeting tumor vasculature with an oncolytic virus. Mol Ther. 2011;19:886–894. doi: 10.1038/mt.2011.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cattaneo R, Miest T, Shashkova EV, Barry MA. Reprogrammed viruses as cancer therapeutics: targeted, armed and shielded. Nat Rev Microbiol. 2008;6:529–540. doi: 10.1038/nrmicro1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Coen DM, Kosz-Vnenchak M, Jacobson JG, et al. Thymidine kinase-negative herpes simplex virus mutants establish latency in mouse trigeminal ganglia but do not reactivate. Proc Natl Acad Sci U S A. 1989;86:4736–4740. doi: 10.1073/pnas.86.12.4736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Farassati F, Yang AD, Lee PW. Oncogenes in Ras signalling pathway dictate host-cell permissiveness to herpes simplex virus 1. Nat Cell Biol. 2001;3:745–750. doi: 10.1038/35087061. [DOI] [PubMed] [Google Scholar]
- 34.Chou J, Kern ER, Whitley RJ, Roizman B. Mapping of herpes simplex virus-1 neurovirulence to gamma 134.5, a gene nonessential for growth in culture. Science. 1990;250:1262–1266. doi: 10.1126/science.2173860. [DOI] [PubMed] [Google Scholar]
- 35.MacLean AR, ul-Fareed M, Robertson L, Harland J, Brown SM. Herpes simplex virus type 1 deletion variants 1714 and 1716 pinpoint neurovirulence-related sequences in Glasgow strain 17+ between immediate early gene 1 and the 'a' sequence. J Gen Virol. 1991;72(Pt 3):631–639. doi: 10.1099/0022-1317-72-3-631. [DOI] [PubMed] [Google Scholar]
- 36.Yamada Y, Kimura H, Morishima T, Daikoku T, Maeno K, Nishiyama Y. The pathogenicity of ribonucleotide reductase-null mutants of herpes simplex virus type 1 in mice. J Infect Dis. 1991;164:1091–1097. doi: 10.1093/infdis/164.6.1091. [DOI] [PubMed] [Google Scholar]
- 37.Mineta T, Rabkin SD, Martuza RL. Treatment of malignant gliomas using ganciclovir-hypersensitive, ribonucleotide reductase-deficient herpes simplex viral mutant. Cancer Res. 1994;54:3963–3966. [PubMed] [Google Scholar]
- 38.Markert JM, Malick A, Coen DM, Martuza RL. Reduction and elimination of encephalitis in an experimental glioma therapy model with attenuated herpes simplex mutants that retain susceptibility to acyclovir. Neurosurgery. 1993;32:597–603. doi: 10.1227/00006123-199304000-00016. [DOI] [PubMed] [Google Scholar]
- 39.Kesari S, Randazzo BP, Valyi-Nagy T, et al. Therapy of experimental human brain tumors using a neuroattenuated herpes simplex virus mutant. Lab Invest. 1995;73:636–648. [PubMed] [Google Scholar]
- 40.McKie EA, MacLean AR, Lewis AD, et al. Selective in vitro replication of herpes simplex virus type 1 (HSV-1) ICP34.5 null mutants in primary human CNS tumours--evaluation of a potentially effective clinical therapy. Br J Cancer. 1996;74:745–752. doi: 10.1038/bjc.1996.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mineta T, Rabkin SD, Yazaki T, Hunter WD, Martuza RL. Attenuated multi-mutated herpes simplex virus-1 for the treatment of malignant gliomas. Nat Med. 1995;1:938–943. doi: 10.1038/nm0995-938. [DOI] [PubMed] [Google Scholar]
- 42.Markert JM, Medlock MD, Rabkin SD, et al. Conditionally replicating herpes simplex virus mutant, G207 for the treatment of malignant glioma: results of a phase I trial. Gene Ther. 2000;7:867–874. doi: 10.1038/sj.gt.3301205. [DOI] [PubMed] [Google Scholar]
- 43.Rampling R, Cruickshank G, Papanastassiou V, et al. Toxicity evaluation of replication-competent herpes simplex virus (ICP 34.5 null mutant 1716) in patients with recurrent malignant glioma. Gene Ther. 2000;7:859–866. doi: 10.1038/sj.gt.3301184. [DOI] [PubMed] [Google Scholar]
- 44.Todo T, Martuza RL, Rabkin SD, Johnson PA. Oncolytic herpes simplex virus vector with enhanced MHC class I presentation and tumor cell killing. Proc Natl Acad Sci U S A. 2001;98:6396–6401. doi: 10.1073/pnas.101136398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Todo T. Oncolytic virus therapy using genetically engineered herpes simplex viruses. Front Biosci. 2008;13:2060–2064. doi: 10.2741/2823. [DOI] [PubMed] [Google Scholar]
- 46.Parker JN, Gillespie GY, Love CE, Randall S, Whitley RJ, Markert JM. Engineered herpes simplex virus expressing IL-12 in the treatment of experimental murine brain tumors. Proc Natl Acad Sci U S A. 2000;97:2208–2213. doi: 10.1073/pnas.040557897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chase M, Chung RY, Chiocca EA. An oncolytic viral mutant that delivers the CYP2B1 transgene and augments cyclophosphamide chemotherapy. Nat Biotechnol. 1998;16:444–448. doi: 10.1038/nbt0598-444. [DOI] [PubMed] [Google Scholar]
- 48.Liu TC, Zhang T, Fukuhara H, et al. Oncolytic HSV armed with platelet factor 4, an antiangiogenic agent, shows enhanced efficacy. Mol Ther. 2006;14:789–797. doi: 10.1016/j.ymthe.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 49.Todo T. "Armed" oncolytic herpes simplex viruses for brain tumor therapy. Cell Adh Migr. 2008;2:208–213. doi: 10.4161/cam.2.3.6353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhou G, Ye GJ, Debinski W, Roizman B. Engineered herpes simplex virus 1 is dependent on IL13Ralpha 2 receptor for cell entry and independent of glycoprotein D receptor interaction. Proc Natl Acad Sci U S A. 2002;99:15124–15129. doi: 10.1073/pnas.232588699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kambara H, Okano H, Chiocca EA, Saeki Y. An oncolytic HSV-1 mutant expressing ICP34.5 under control of a nestin promoter increases survival of animals even when symptomatic from a brain tumor. Cancer Res. 2005;65:2832–2839. doi: 10.1158/0008-5472.CAN-04-3227. [DOI] [PubMed] [Google Scholar]
- 52.Toth K, Dhar D, Wold WS. Oncolytic (replication-competent) adenoviruses as anticancer agents. Expert Opin Biol Ther. 2010;10:353–368. doi: 10.1517/14712590903559822. [DOI] [PubMed] [Google Scholar]
- 53.Heise C, Sampson-Johannes A, Williams A, McCormick F, Von Hoff DD, Kirn DH. ONYX-015, an E1B gene-attenuated adenovirus, causes tumor-specific cytolysis and antitumoral efficacy that can be augmented by standard chemotherapeutic agents. Nat Med. 1997;3:639–645. doi: 10.1038/nm0697-639. [DOI] [PubMed] [Google Scholar]
- 54.Geoerger B, Grill J, Opolon P, et al. Oncolytic activity of the E1B-55 kDa-deleted adenovirus ONYX-015 is independent of cellular p53 status in human malignant glioma xenografts. Cancer Res. 2002;62:764–772. [PubMed] [Google Scholar]
- 55.Chiocca EA, Abbed KM, Tatter S, et al. A phase I open-label, dose-escalation, multi-institutional trial of injection with an E1B-Attenuated adenovirus, ONYX-015, into the peritumoral region of recurrent malignant gliomas, in the adjuvant setting. Mol Ther. 2004;10:958–966. doi: 10.1016/j.ymthe.2004.07.021. [DOI] [PubMed] [Google Scholar]
- 56.Fueyo J, Gomez-Manzano C, Alemany R, et al. A mutant oncolytic adenovirus targeting the Rb pathway produces anti-glioma effect in vivo. Oncogene. 2000;19:2–12. doi: 10.1038/sj.onc.1203251. [DOI] [PubMed] [Google Scholar]
- 57.Puduvalli VK, Kyritsis AP, Hess KR, et al. Patterns of expression of Rb and p16 in astrocytic gliomas, and correlation with survival. Int J Oncol. 2000;17:963–969. doi: 10.3892/ijo.17.5.963. [DOI] [PubMed] [Google Scholar]
- 58.Hamel W, Westphal M, Shepard HM. Loss in expression of the retinoblastoma gene product in human gliomas is associated with advanced disease. J Neurooncol. 1993;16:159–165. doi: 10.1007/BF01324703. [DOI] [PubMed] [Google Scholar]
- 59.van Beusechem VW, Mastenbroek DC, van den Doel PB, et al. Conditionally replicative adenovirus expressing a targeting adapter molecule exhibits enhanced oncolytic potency on CAR-deficient tumors. Gene Ther. 2003;10:1982–1991. doi: 10.1038/sj.gt.3302103. [DOI] [PubMed] [Google Scholar]
- 60.Alonso MM, Cascallo M, Gomez-Manzano C, et al. ICOVIR-5 shows E2F1 addiction and potent antiglioma effect in vivo. Cancer Res. 2007;67:8255–8263. doi: 10.1158/0008-5472.CAN-06-4675. [DOI] [PubMed] [Google Scholar]
- 61.Sinkovics JG, Horvath JC. Newcastle disease virus (NDV): brief history of its oncolytic strains. J Clin Virol. 2000;16:1–15. doi: 10.1016/s1386-6532(99)00072-4. [DOI] [PubMed] [Google Scholar]
- 62.Zulkifli MM, Ibrahim R, Ali AM, et al. Newcastle diseases virus strain V4UPM displayed oncolytic ability against experimental human malignant glioma. Neurol Res. 2009;31:3–10. doi: 10.1179/174313208X325218. [DOI] [PubMed] [Google Scholar]
- 63.Phuangsab A, Lorence RM, Reichard KW, Peeples ME, Walter RJ. Newcastle disease virus therapy of human tumor xenografts: antitumor effects of local or systemic administration. Cancer Lett. 2001;172:27–36. doi: 10.1016/s0304-3835(01)00617-6. [DOI] [PubMed] [Google Scholar]
- 64.Lorence RM, Katubig BB, Reichard KW, et al. Complete regression of human fibrosarcoma xenografts after local Newcastle disease virus therapy. Cancer Res. 1994;54:6017–6021. [PubMed] [Google Scholar]
- 65.Lorence RM, Rood PA, Kelley KW. Newcastle disease virus as an antineoplastic agent: induction of tumor necrosis factor-alpha and augmentation of its cytotoxicity. J Natl Cancer Inst. 1988;80:1305–1312. doi: 10.1093/jnci/80.16.1305. [DOI] [PubMed] [Google Scholar]
- 66.Naujocks G, Schmitz A, Schramm J, Wiestler O, Schirrmacher V. Peripheral immunization against malignant rat glioma can induce effective antitumor immunity in the brain. Int J Oncol. 1995;6:759–765. doi: 10.3892/ijo.6.4.759. [DOI] [PubMed] [Google Scholar]
- 67.Schneider T, Gerhards R, Kirches E, Firsching R. Preliminary results of active specific immunization with modified tumor cell vaccine in glioblastoma multiforme. J Neurooncol. 2001;53:39–46. doi: 10.1023/a:1011856406683. [DOI] [PubMed] [Google Scholar]
- 68.Fabian Z, Csatary CM, Szeberenyi J, Csatary LK. p53-independent endoplasmic reticulum stress-mediated cytotoxicity of a Newcastle disease virus strain in tumor cell lines. J Virol. 2007;81:2817–2830. doi: 10.1128/JVI.02490-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Csatary LK, Bakacs T. Use of Newcastle disease virus vaccine (MTH-68/H) in a patient with high-grade glioblastoma. Jama. 1999;281:1588–1589. doi: 10.1001/jama.281.17.1588-a. [DOI] [PubMed] [Google Scholar]
- 70.Csatary LK, Gosztonyi G, Szeberenyi J, et al. MTH-68/H oncolytic viral treatment in human high-grade gliomas. J Neurooncol. 2004;67:83–93. doi: 10.1023/b:neon.0000021735.85511.05. [DOI] [PubMed] [Google Scholar]
- 71.Freeman AI, Zakay-Rones Z, Gomori JM, et al. Phase I/II trial of intravenous NDV-HUJ oncolytic virus in recurrent glioblastoma multiforme. Mol Ther. 2006;13:221–228. doi: 10.1016/j.ymthe.2005.08.016. [DOI] [PubMed] [Google Scholar]
- 72.Strong JE, Coffey MC, Tang D, Sabinin P, Lee PW. The molecular basis of viral oncolysis: usurpation of the Ras signaling pathway by reovirus. EMBO J. 1998;17:3351–3362. doi: 10.1093/emboj/17.12.3351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Coffey MC, Strong JE, Forsyth PA, Lee PW. Reovirus therapy of tumors with activated Ras pathway. Science. 1998;282:1332–1334. doi: 10.1126/science.282.5392.1332. [DOI] [PubMed] [Google Scholar]
- 74.Yang WQ, Lun X, Palmer CA, et al. Efficacy and safety evaluation of human reovirus type 3 in immunocompetent animals: racine and nonhuman primates. Clin Cancer Res. 2004;10:8561–8576. doi: 10.1158/1078-0432.CCR-04-0940. [DOI] [PubMed] [Google Scholar]
- 75.Forsyth P, Roldan G, George D, et al. A phase I trial of intratumoral administration of reovirus in patients with histologically confirmed recurrent malignant gliomas. Mol Ther. 2008;16:627–632. doi: 10.1038/sj.mt.6300403. [DOI] [PubMed] [Google Scholar]
- 76.Alloussi SH, Alkassar M, Urbschat S, Graf N, Gartner B. All reovirus subtypes show oncolytic potential in primary cells of human high-grade glioma. Oncol Rep. 2011;26:645–649. doi: 10.3892/or.2011.1331. [DOI] [PubMed] [Google Scholar]
- 77.Guse K, Cerullo V, Hemminki A. Oncolytic vaccinia virus for the treatment of cancer. Expert Opin Biol Ther. 2011;11:595–608. doi: 10.1517/14712598.2011.558838. [DOI] [PubMed] [Google Scholar]
- 78.Eager RM, Nemunaitis J. Clinical development directions in oncolytic viral therapy. Cancer Gene Ther. 2011;18:305–317. doi: 10.1038/cgt.2011.7. [DOI] [PubMed] [Google Scholar]
- 79.Thorne SH, Hwang TH, O'Gorman WE, et al. Rational strain selection and engineering creates a broad-spectrum, systemically effective oncolytic poxvirus, JX-963. J Clin Invest. 2007;117:3350–3358. doi: 10.1172/JCI32727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gridley DS, Andres ML, Li J, Timiryasova T, Chen B, Fodor I. Evaluation of radiation effects against C6 glioma in combination with vaccinia virus-p53 gene therapy. Int J Oncol. 1998;13:1093–1098. doi: 10.3892/ijo.13.5.1093. [DOI] [PubMed] [Google Scholar]
- 81.Lun XQ, Jang JH, Tang N, et al. Efficacy of systemically administered oncolytic vaccinia virotherapy for malignant gliomas is enhanced by combination therapy with rapamycin or cyclophosphamide. Clin Cancer Res. 2009;15:2777–2788. doi: 10.1158/1078-0432.CCR-08-2342. [DOI] [PubMed] [Google Scholar]
- 82.Naik AM, Chalikonda S, McCart JA, et al. Intravenous and isolated limb perfusion delivery of wild type and a tumor-selective replicating mutant vaccinia virus in nonhuman primates. Hum Gene Ther. 2006;17:31–45. doi: 10.1089/hum.2006.17.31. [DOI] [PubMed] [Google Scholar]
- 83.Lun X, Chan J, Zhou H, et al. Efficacy and safety/toxicity study of recombinant vaccinia virus JX-594 in two immunocompetent animal models of glioma. Mol Ther. 2010;18:1927–1936. doi: 10.1038/mt.2010.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gromeier M, Alexander L, Wimmer E. Internal ribosomal entry site substitution eliminates neurovirulence in intergeneric poliovirus recombinants. Proc Natl Acad Sci U S A. 1996;93:2370–2375. doi: 10.1073/pnas.93.6.2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gromeier M, Lachmann S, Rosenfeld MR, Gutin PH, Wimmer E. Intergeneric poliovirus recombinants for the treatment of malignant glioma. Proc Natl Acad Sci U S A. 2000;97:6803–6808. doi: 10.1073/pnas.97.12.6803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Cello J, Toyoda H, Dejesus N, Dobrikova EY, Gromeier M, Wimmer E. Growth phenotypes and biosafety profiles in poliovirus-receptor transgenic mice of recombinant oncolytic polio/human rhinoviruses. J Med Virol. 2008;80:352–359. doi: 10.1002/jmv.21063. [DOI] [PubMed] [Google Scholar]
- 87.Goetz C, Gromeier M. Preparing an oncolytic poliovirus recombinant for clinical application against glioblastoma multiforme. Cytokine Growth Factor Rev. 2010;21:197–203. doi: 10.1016/j.cytogfr.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Reuter D, Schneider-Schaulies J. Measles virus infection of the CNS: human disease, animal models, and approaches to therapy. Med Microbiol Immunol. 2010;199:261–271. doi: 10.1007/s00430-010-0153-2. [DOI] [PubMed] [Google Scholar]
- 89.Nakamura K, Homma M, Ishida N. Growth of measles virus in cultures of rat glioma cells. Infect Immun. 1975;12:614–620. doi: 10.1128/iai.12.3.614-620.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Phuong LK, Allen C, Peng KW, et al. Use of a vaccine strain of measles virus genetically engineered to produce carcinoembryonic antigen as a novel therapeutic agent against glioblastoma multiforme. Cancer Res. 2003;63:2462–2469. [PubMed] [Google Scholar]
- 91.Jurianz K, Ziegler S, Garcia-Schuler H, et al. Complement resistance of tumor cells: basal and induced mechanisms. Mol Immunol. 1999;36:929–939. doi: 10.1016/s0161-5890(99)00115-7. [DOI] [PubMed] [Google Scholar]
- 92.Galanis E, Bateman A, Johnson K, et al. Use of viral fusogenic membrane glycoproteins as novel therapeutic transgenes in gliomas. Hum Gene Ther. 2001;12:811–821. doi: 10.1089/104303401750148766. [DOI] [PubMed] [Google Scholar]
- 93.Peng KW, Facteau S, Wegman T, O'Kane D, Russell SJ. Non-invasive in vivo monitoring of trackable viruses expressing soluble marker peptides. Nat Med. 2002;8:527–531. doi: 10.1038/nm0502-527. [DOI] [PubMed] [Google Scholar]
- 94.Allen C, Paraskevakou G, Liu C, et al. Oncolytic measles virus strains in the treatment of gliomas. Expert Opin Biol Ther. 2008;8:213–220. doi: 10.1517/14712598.8.2.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Allen C, Vongpunsawad S, Nakamura T, et al. Retargeted oncolytic measles strains entering via the EGFRvIII receptor maintain significant antitumor activity against gliomas with increased tumor specificity. Cancer Res. 2006;66:11840–11850. doi: 10.1158/0008-5472.CAN-06-1200. [DOI] [PubMed] [Google Scholar]
- 96.Allen C, Paraskevakou G, Iankov I, et al. Interleukin-13 displaying retargeted oncolytic measles virus strains have significant activity against gliomas with improved specificity. Mol Ther. 2008;16:1556–1564. doi: 10.1038/mt.2008.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Barber GN. Vesicular stomatitis virus as an oncolytic vector. Viral Immunol. 2004;17:516–527. doi: 10.1089/vim.2004.17.516. [DOI] [PubMed] [Google Scholar]
- 98.Balachandran S, Barber GN. Vesicular stomatitis virus (VSV) therapy of tumors. IUBMB Life. 2000;50:135–138. doi: 10.1080/713803696. [DOI] [PubMed] [Google Scholar]
- 99.Stanford MM, Bell JC, Vaha-Koskela MJ. Novel oncolytic viruses: riding high on the next wave? Cytokine Growth Factor Rev. 2010;21:177–183. doi: 10.1016/j.cytogfr.2010.02.012. [DOI] [PubMed] [Google Scholar]
- 100.Lichty BD, Power AT, Stojdl DF, Bell JC. Vesicular stomatitis virus: re-inventing the bullet. Trends Mol Med. 2004;10:210–216. doi: 10.1016/j.molmed.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 101.Lun X, Senger DL, Alain T, et al. Effects of intravenously administered recombinant vesicular stomatitis virus (VSV(deltaM51)) on multifocal and invasive gliomas. J Natl Cancer Inst. 2006;98:1546–1557. doi: 10.1093/jnci/djj413. [DOI] [PubMed] [Google Scholar]
- 102.Ozduman K, Wollmann G, Piepmeier JM, van den Pol AN. Systemic vesicular stomatitis virus selectively destroys multifocal glioma and metastatic carcinoma in brain. J Neurosci. 2008;28:1882–1893. doi: 10.1523/JNEUROSCI.4905-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.van den Pol AN, Dalton KP, Rose JK. Relative neurotropism of a recombinant rhabdovirus expressing a green fluorescent envelope glycoprotein. J Virol. 2002;76:1309–1327. doi: 10.1128/JVI.76.3.1309-1327.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Stojdl DF, Lichty BD, tenOever BR, et al. VSV strains with defects in their ability to shutdown innate immunity are potent systemic anti-cancer agents. Cancer Cell. 2003;4:263–275. doi: 10.1016/s1535-6108(03)00241-1. [DOI] [PubMed] [Google Scholar]
- 105.Wollmann G, Rogulin V, Simon I, Rose JK, van den Pol AN. Some attenuated variants of vesicular stomatitis virus show enhanced oncolytic activity against human glioblastoma cells relative to normal brain cells. J Virol. 2010;84:1563–1573. doi: 10.1128/JVI.02040-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Heiber JF, Barber GN. Vesicular stomatitis virus expressing tumor suppressor p53 is a highly attenuated, potent oncolytic agent. J Virol. 2011;85:10440–10450. doi: 10.1128/JVI.05408-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Fernandez M, Porosnicu M, Markovic D, Barber GN. Genetically engineered vesicular stomatitis virus in gene therapy: application for treatment of malignant disease. J Virol. 2002;76:895–904. doi: 10.1128/JVI.76.2.895-904.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wollmann G, Tattersall P, van den Pol AN. Targeting human glioblastoma cells: comparison of nine viruses with oncolytic potential. J Virol. 2005;79:6005–6022. doi: 10.1128/JVI.79.10.6005-6022.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ausubel LJ, Meseck M, Derecho I, et al. Current good manufacturing practice production of an oncolytic recombinant vesicular stomatitis viral vector for cancer treatment. Hum Gene Ther. 2011;22:489–497. doi: 10.1089/hum.2010.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Brun J, McManus D, Lefebvre C, et al. Identification of genetically modified Maraba virus as an oncolytic rhabdovirus. Mol Ther. 2010;18:1440–1449. doi: 10.1038/mt.2010.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ventoso I, Berlanga JJ, Almendral JM. Translation control by protein kinase R restricts minute virus of mice infection: role in parvovirus oncolysis. J Virol. 2010;84:5043–5051. doi: 10.1128/JVI.02188-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Herrero YCM, Cornelis JJ, Herold-Mende C, Rommelaere J, Schlehofer JR, Geletneky K. Parvovirus H-1 infection of human glioma cells leads to complete viral replication and efficient cell killing. Int J Cancer. 2004;109:76–84. doi: 10.1002/ijc.11626. [DOI] [PubMed] [Google Scholar]
- 113.Geletneky K, Hartkopf AD, Krempien R, Rommelaere J, Schlehofer JR. Therapeutic implications of the enhanced short and long-term cytotoxicity of radiation treatment followed by oncolytic parvovirus H-1 infection in high-grade glioma cells. Bioeng Bugs. 2010;1:429–433. doi: 10.4161/bbug.1.6.12943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Abschuetz A, Kehl T, Geibig R, Leuchs B, Rommelaere J, Regnier-Vigouroux A. Oncolytic murine autonomous parvovirus, a candidate vector for glioma gene therapy, is innocuous to normal and immunocompetent mouse glial cells. Cell Tissue Res. 2006;325:423–436. doi: 10.1007/s00441-006-0199-z. [DOI] [PubMed] [Google Scholar]
- 115.Rubio MP, Guerra S, Almendral JM. Genome replication and postencapsidation functions mapping to the nonstructural gene restrict the host range of a murine parvovirus in human cells. J Virol. 2001;75:11573–11582. doi: 10.1128/JVI.75.23.11573-11582.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kerr P, McFadden G. Immune responses to myxoma virus. Viral Immunol. 2002;15:229–246. doi: 10.1089/08828240260066198. [DOI] [PubMed] [Google Scholar]
- 117.Lun X, Yang W, Alain T, et al. Myxoma virus is a novel oncolytic virus with significant antitumor activity against experimental human gliomas. Cancer Res. 2005;65:9982–9990. doi: 10.1158/0008-5472.CAN-05-1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lun X, Alain T, Zemp FJ, et al. Myxoma virus virotherapy for glioma in immunocompetent animal models: optimizing administration routes and synergy with rapamycin. Cancer Res. 2010;70:598–608. doi: 10.1158/0008-5472.CAN-09-1510. [DOI] [PubMed] [Google Scholar]
- 119.Reddy PS, Burroughs KD, Hales LM, et al. Seneca Valley virus, a systemically deliverable oncolytic picornavirus, and the treatment of neuroendocrine cancers. J Natl Cancer Inst. 2007;99:1623–1633. doi: 10.1093/jnci/djm198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Morton CL, Houghton PJ, Kolb EA, et al. Initial testing of the replication competent Seneca Valley virus (NTX-010) by the pediatric preclinical testing program. Pediatr Blood Cancer. 2010;55:295–303. doi: 10.1002/pbc.22535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Yu L, Baxter PA, Zhao X, et al. A single intravenous injection of oncolytic picornavirus SVV-001 eliminates medulloblastomas in primary tumor-based orthotopic xenograft mouse models. Neuro Oncol. 2011;13:14–27. doi: 10.1093/neuonc/noq148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Tseng JC, Levin B, Hurtado A, et al. Systemic tumor targeting and killing by Sindbis viral vectors. Nat Biotechnol. 2004;22:70–77. doi: 10.1038/nbt917. [DOI] [PubMed] [Google Scholar]
- 123.Wang KS, Kuhn RJ, Strauss EG, Ou S, Strauss JH. High-affinity laminin receptor is a receptor for Sindbis virus in mammalian cells. J Virol. 1992;66:4992–5001. doi: 10.1128/jvi.66.8.4992-5001.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Menard S, Tagliabue E, Colnaghi MI. The 67 kDa laminin receptor as a prognostic factor in human cancer. Breast Cancer Res Treat. 1998;52:137–145. doi: 10.1023/a:1006171403765. [DOI] [PubMed] [Google Scholar]
- 125.Tseng JC, Granot T, DiGiacomo V, Levin B, Meruelo D. Enhanced specific delivery and targeting of oncolytic Sindbis viral vectors by modulating vascular leakiness in tumor. Cancer Gene Ther. 2010;17:244–255. doi: 10.1038/cgt.2009.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Unno Y, Shino Y, Kondo F, et al. Oncolytic viral therapy for cervical and ovarian cancer cells by Sindbis virus AR339 strain. Clin Cancer Res. 2005;11:4553–4560. doi: 10.1158/1078-0432.CCR-04-2610. [DOI] [PubMed] [Google Scholar]
- 127.Enquist LW, Husak PJ, Banfield BW, Smith GA. Infection and spread of alphaherpesviruses in the nervous system. Adv Virus Res. 1998;51:237–347. doi: 10.1016/s0065-3527(08)60787-3. [DOI] [PubMed] [Google Scholar]
- 128.Tischer BK, Osterrieder N. Herpesviruses--a zoonotic threat? Vet Microbiol. 2010;140:266–270. doi: 10.1016/j.vetmic.2009.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Card JP, Enquist LW. Transneuronal circuit analysis with pseudorabies viruses. Chapter 1. Curr Protoc Neurosci. 2001;(Unit1 5) doi: 10.1002/0471142301.ns0105s09. [DOI] [PubMed] [Google Scholar]
- 130.Boldogkoi Z, Bratincsak A, Fodor I. Evaluation of pseudorabies virus as a gene transfer vector and an oncolytic agent for human tumor cells. Anticancer Res. 2002;22:2153–2159. [PubMed] [Google Scholar]
- 131.Markert JM, Liechty PG, Wang W, et al. Phase Ib trial of mutant herpes simplex virus G207 inoculated pre-and post-tumor resection for recurrent GBM. Mol Ther. 2009;17:199–207. doi: 10.1038/mt.2008.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Papanastassiou V, Rampling R, Fraser M, et al. The potential for efficacy of the modified (ICP 34.5(-)) herpes simplex virus HSV1716 following intratumoural injection into human malignant glioma: a proof of principle study. Gene Ther. 2002;9:398–406. doi: 10.1038/sj.gt.3301664. [DOI] [PubMed] [Google Scholar]
- 133.Harrow S, Papanastassiou V, Harland J, et al. HSV1716 injection into the brain adjacent to tumour following surgical resection of high-grade glioma: safety data and long-term survival. Gene Ther. 2004;11:1648–1658. doi: 10.1038/sj.gt.3302289. [DOI] [PubMed] [Google Scholar]
- 134.Wagner S, Csatary CM, Gosztonyi G, et al. Combined treatment of pediatric high-grade glioma with the oncolytic viral strain MTH-68/H and oral valproic acid. Apmis. 2006;114:731–743. doi: 10.1111/j.1600-0463.2006.apm_516.x. [DOI] [PubMed] [Google Scholar]
- 135.Garber K. China approves world's first oncolytic virus therapy for cancer treatment. J Natl Cancer Inst. 2006;98:298–300. doi: 10.1093/jnci/djj111. [DOI] [PubMed] [Google Scholar]
- 136.Zemp FJ, Corredor JC, Lun X, Muruve DA, Forsyth PA. Oncolytic viruses as experimental treatments for malignant gliomas: using a scourge to treat a devil. Cytokine Growth Factor Rev. 2010;21:103–117. doi: 10.1016/j.cytogfr.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 137.Naik JD, Twelves CJ, Selby PJ, Vile RG, Chester JD. Immune recruitment and therapeutic synergy: keys to optimizing oncolytic viral therapy? Clin Cancer Res. 2011;17:4214–4224. doi: 10.1158/1078-0432.CCR-10-2848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Liu TC, Hwang TH, Bell JC, Kirn DH. Translation of targeted oncolytic virotherapeutics from the lab into the clinic, and back again: a high-value iterative loop. Mol Ther. 2008;16:1006–1008. doi: 10.1038/mt.2008.70. [DOI] [PubMed] [Google Scholar]
- 139.Koppers-Lalic D, Hoeben RC. Non-human viruses developed as therapeutic agent for use in humans. Rev Med Virol. 2011;21:227–239. doi: 10.1002/rmv.694. [DOI] [PMC free article] [PubMed] [Google Scholar]