Abstract
Purpose
Asthma interventions targeting urban adolescents are rare, despite a great need. Motivating adolescents to achieve better self-management of asthma is challenging, and the literature suggests that certain subgroups are more resistant than others. We conducted a school-based, randomized controlled trial to evaluate Puff City, a web-based, tailored asthma intervention, which included a referral coordinator, and incorporated theory-based strategies to target urban teens with characteristics previously found to be associated with lack of behavior change.
Methods
To identify eligible teens, questionnaires on asthma diagnoses and symptoms were administered to 9–12th graders of participating schools during a scheduled English class. Eligible, consenting students were randomized to Puff City (treatment) or generic asthma education (control).
Results
422 students were randomized (98% African-American, mean age=15.6 years). At 12 month follow-up, adjusted Odds Ratios (95% Confidence Intervals) indicated intervention benefit for treatment teens for symptom-days and restricted activity days (analyzed as categorical variables) aOR=0.49 (0.24–0.79), p=0.006 and 0.53 (0.32–0.86), p=0.010, respectively. Among teens meeting baseline criteria for rebelliousness, treatment teens reported fewer symptom-days, symptom-nights, school absences and restricted activity days, aOR=0.30 (0.11–0.80), 0.29 (0.14–0.64), 0.40 (0.20–0.78), and 0.23 (0.10–0.55); all p<0.05. Among teens reporting low perceived emotional support, treatment students reported only fewer symptom-days than controls, aOR=0.23 (0.06 – 0.88), p=0.031. Statistically significant differences in medical care use were not observed.
Conclusions
Results suggest a theory-based, tailored approach, with a referral coordinator, can improve asthma management in urban teens. Puff City represents a viable strategy for disseminating an effective intervention to high risk and hard-to-reach populations.
INTRODUCTION
The personal, social, and medical costs of asthma are largely borne by racial and ethnic groups disproportionately represented among the poor in the US.1;2 Racial disparities in asthma are also reflected in statistics describing asthma morbidity and mortality among US adolescents.1 Compared to White adolescents and children aged 0–14 years, African-American adolescents aged 15–19 years have lower rates of preventive care, such as primary care visits, but higher rates of acute care, such as hospitalizations and Emergency Department visits.1 Non-white teens also have higher asthma mortality rates.1 The literature suggests that these trends are most likely due to uncontrolled and under-managed disease, indicating the need for better clinical and patient self-management.3;4 Effective asthma interventions targeting this age group could help to improve patient self-management and encourage teens to partner with physicians toward the ultimate goal of better asthma control.
Few trials of asthma management programs have been conducted among high school students. In 2007, our group published on a web-based, computer-tailored intervention called Puff City.5 We developed a revised Puff City program that included new submodules designed to target teens with characteristics shown to be associated with lack of behavior change in the previous trial.6 The objective of this paper is to present the results of a randomized controlled trial (RCT) conducted to evaluate the new version of Puff City and to examine subgroups targeted by the added submodules. We hypothesized that urban, African-American high school students with asthma randomized to receive Puff City would report fewer symptom-days than teens randomized to a control group.
METHODS
Development of Puff City
Development and content of Puff City, with the exception of the submodules and booster, is also described in earlier publications.5;6 Briefly, Puff City focuses on three behaviors: controller medication adherence, keeping an inhaler nearby, and smoking reduction/cessation. Health messages and information based on theoretical models and approaches to behavior change relevant to asthma control (e.g., Health Belief Model, Attribution Theory, Motivational Interviewing) are presented for these three behaviors, allowing the delivery of information both central and peripheral to the behavior.7–10 The program also includes information on trigger avoidance, device usage (e.g., how to use a diskus, terbuhaler, spacer, etc.), and basic asthma physiology. A radio DJ delivers the scientifically sound advice that is tailored to each teen. All Puff City surveys are voiced-over to accommodate literacy limitations. A medication module with visual aids helps teens identify current asthma medications.
Puff City uses tailoring to apply behavioral theory. Tailoring is the “assessment and provision of feedback based on information that is known or hypothesized to be most relevant for each individual participant of a program”11;12 During sessions, computer algorithms use teen responses to questions on attitudes and beliefs to select the appropriate information from a message library and assemble pre-programmed tailored feedback, creating an extensive array of message permutations. The message library was created by medical experts and behavioral scientists.
Referral coordinator
As was done in the original Puff City, a risk assessment report, generated by the data management system, collated student responses to selected questions and was used by a referral coordinator to proactively contact students in the treatment group, referring them to community agencies and resources as needed. Flags for the referral coordinator included severe/persistent asthma symptoms, sharing asthma medication with a friend or relative, lack of a physician or health insurance, lack of any asthma medication, and a positive response to 5 of 7 questions about depressive symptoms from the Diagnosis Interview Schedule for Children Predictive Scales.13 Referral coordinators did not provide education.
Submodules
Based on previous analyses, we created submodules to address low perceived emotional support, low motivation, and potential resistance to change.
Low perceived emotional support
This characteristic was determined using questions adapted from the Multidimensional Scale of Perceived Social Support14 and was defined as < 2 on a scale of 1–5, where 5 = high support, in response to “How much support do you feel you have when it comes to controlling your asthma?” Students answered the question for family, friends, and others. Messages helped teens brainstorm on how they might capitalize on support within existing networks or how they might identify new sources of support.
Low motivation
Students with low motivation were defined as selecting a response of < 5 on a scale of 1–10, where 10 = high motivation in response to the question, “How motivated are you to change [core behavior]…?” Messages and exercises in this module borrowed from Motivational Interviewing concepts and used a values-based exercise to reveal dissonance between the behaviors and values reported by students.15;16
Resistant to change
This submodule targeted students who exhibited no change after one or more sessions. The submodule uses values-linkage to promote greater autonomous (or intrinsically-derived) self-regulation.17;18
Rebelliousness
The above submodules also addressed rebelliousness. Criteria for rebelliousness at baseline was a score >2.5 on a 5 point rebelliousness scale19, where 5 = high rebellion). These students also received messages from an edgier character using a tone designed to show empathy with the user.
During online sessions, the appropriate submodule was delivered to students meeting the above criteria. Afterward, students were returned to the original flow of the program.
A booster addressed resistance and attempted to correct early stages of relapse (defined as a return to negative behavior after showing positive behavior). The booster borrowed concepts from attribution, or relapse prevention, theories9;20 and Motivational Interviewing.15 During the 6 month follow-up survey, the computer retrieved information from previous sessions to detect relapse or resistance, and delivered booster messages accordingly.
Randomized trial
Study methods were approved by the Institutional Review Boards of Henry Ford Health System, Detroit Public Schools Office of Research, Evaluation, and Assessment, University of Michigan, and Georgia Health Sciences University. To identify students eligible for the RCT, caregivers of all 9th–12th graders of six public high schools were informed by mail of a screening questionnaire (Lung Health Survey) to be administered during a scheduled English class.5;21 Caregivers could opt out of having their student participate in the Lung Health Survey by signing and returning the letter to the school. The recruitment period was from fall of 2007 – fall of 2008.
Items on the Lung Health Survey requested information on asthma diagnosis, respiratory symptoms (including items from the International Survey of Asthma and Allergies in Childhood (ISAAC) questionnaire),22 and health care utilization. Students were eligible if they met study criteria for current asthma, defined as report of ever having a physician/health care provider diagnosis of asthma accompanied by day or nighttime symptoms, use of medication for asthma symptoms in the past 30 days, medical care use for asthma in the past year, and >1 refill(s) of beta agonists in the past year.5;21 Students were also eligible if they did not report a physician diagnosis, but answered positively to items on the ISAAC and reported symptom frequencies similar to those used in the EPR 2 and 3 for classification of mild, intermittent asthma.
Eligible students providing written assent and parental consent were invited to enroll in the RCT.5 Packets with study information and assent/consent/forms were mailed to the homes of eligible students by a District-affiliated contractor, in order to maintain student confidentiality.5;21
Using computers at school, participating students completed an online baseline survey followed by four online asthma management sessions (15–30 minutes in length) to be completed in ≤ 180 days, with ≥ 1 week between sessions. Follow-up surveys occurred at 6 and 12 months post-baseline. Caregivers completed phone surveys at baseline and at 12 month follow-up.
Control websites
Controls received 4 sessions of generic asthma education to match the experience of students in the treatment group. After log-in, control students were provided with a link to four generic asthma websites using a combination of Windows (tm) system policies and the school district’s proxy server. To regulate dosage, control teens received a “time expired” message after 30 minutes of browsing, which corresponded to the maximum time needed to complete a tailored session. Control websites were selected from recognized US and Canadian organizations with a history of providing evidence-based information on asthma management. 23
Asthma severity
Classification of asthma severity was adapted from the Expert Panel Report 3: Guidelines for Diagnosis and Treatment of Asthma, “Figure 14. Classification of Asthma Severity ≥ 12 months of age”, using nighttime symptoms.24 As done in previous studies, investigators interpreted and assigned numeric values when terms such as “frequent” and “continual” were used in the EP3 criteria. Classification of asthma control was adapted from “Figure 15. Classification of Asthma Control (≥ 12 years of age)”, from the EPR3, with the addition of school days missed and days had to change plans.24
Statistical analysis
A random number generator was used within each unique stratum (school, grade, gender, and asthma severity) to assign individuals to the treatment or control group. The balance between treatment and control groups was set to occur at random accrual points within each stratum. Because the intervention was delivered by the computer, research staff was blinded to group assignment, as were statisticians and investigators. All surveys were identical for treatment and control students.
Our original sample size approach was designed to apply globally across a variety of variables including ED visits, hospitalizations, days of restricted activity, and school absenteeism. With 80% power for a projected sample size of 150 per group, we could detect a relative risk (RR) between treatment and control groups for ED visits and hospitalizations of 0.47 and 0.32, respectively, or a difference in proportions of ≥ 15 percentage points. For continuous variables, with 80% power and 150/group, an effect size of 0.35 (e.g., ≥ 1.1 days for school days missed) could be detected.
Statistical significance was defined as a p value < 0.05 and we used an intention-to-treat approach. Comparisons by participation and by randomization arm were conducted using chi-square tests for categorical variables accompanied by pairwise comparisons when appropriate. Wilcoxon rank sum test was used for continuous and ordinal variables. Unbiased estimates of mean time to completion from session 1 to session 4 were obtained using the Kaplan-Meier method. For this analysis, if teen baseline had “don’t know” for physician diagnosis of asthma, caregiver’s baseline report for teen asthma diagnosis was used.
Treatment/control comparisons of outcomes at 12 month follow up were analyzed using negative binomial regression. Adjusted risk ratios (aRR) were calculated with corresponding 95% Confidence Intervals (95%CI). A base model consisted of school, sex and asthma severity. Baseline values for the outcome variable were included in the model when assessing treatment effect for specific outcomes. Potential confounding was assessed by including in the final model any variable found to change the risk estimate for the association of randomization group to study outcome by ≥ 20%. Potential confounders included age, physician diagnosis of asthma, Medicaid enrollment, caregiver education (SES), home environmental tobacco smoke (ETS), student smoking, and number of sessions completed. Comparisons of indicators of uncontrolled asthma (all dichotomous variables) were approached in similar fashion, with adjusted odds ratios (aOR) from logistic regression models calculated with 95% Confidence Intervals. Subgroup analyses were conducted by restricting the study sample to students meeting selected criteria (e.g., rebelliousness, moderate-severe asthma, etc.) and then applying the approach described above.
RESULTS
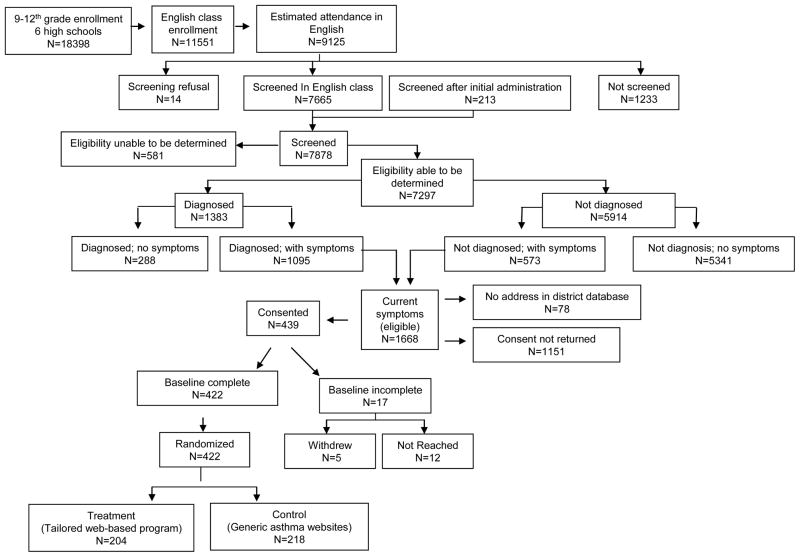
Across the six schools, 98% of students were African-American and 74% qualified for free/reduced price lunch.25 Of the 9125 students enrolled in an English class and present on the day of questionnaire administration, 7878 students (86.3%) completed the screening form, and 1668/7878 (21.2%) were eligible for the RCT. A total of 439 students (26.3% of eligible) provided assent and consent, of which 422 (96.1% of those consenting) completed a baseline and were randomized (Figure 1).
Figure 1.
Flow of study participants through each stage of a school-based randomized trial to evaluate Puff City
Using data from the screening Lung Health Survey for comparison, participating eligible students were significantly more likely than nonparticipating eligible students to have a physician diagnosis of asthma and report > 4 days of restricted activity in the past 30 days. Nonparticipants were similar to participants with respect to age, gender, exposure to environmental tobacco smoke, teen smoking, indicators of uncontrolled asthma (with exception of days of restricted activity noted above) and reported medical care use at screening (data not shown). After randomization, no significant differences were observed between treatment and control students (Table 1). Compared to controls, fewer treatment students had a rescue inhaler at baseline.
Table 1.
Study compliance and study sample characteristics at session 1, by randomization group,1 for students enrolled in a randomized trial of Puff City (n=422)
| Treatment N=204 | Controls N=218 | Overall p value | |||
|---|---|---|---|---|---|
| Study activity | |||||
| Failed to complete even 1 session, % (n) | 4.4 | (9) | 4.1 | (9) | 0.89 |
| Completed all four sessions, % (n) | 85.8 | (175) | 90.8 | (198) | 0.11 |
| Days from session 1 to last session completed 2, mean(SE) | 49.5 | (SE=2.7) | 54.2 | (SE=2.6) | 0.12 3 |
| Completed a 12-month follow up, % (n) | 89.7 | (183) | 90.4 | (197) | 0.82 |
| Completed baseline and 12-month follow up in same season, % (n) | 70.5 | (98) | 64.2 | (86) | 0.26 |
| Core behavior and report of medication at session 1 | |||||
| No controller medication reported, % (n) | 66.5 | (127) | 60.7 | (122) | 0.46 4 |
| Among students reporting a controller medication: | |||||
| Controller medication, adherent ≥ 5 of last 7 days | 8.4 | (16) | 11.4 | (23) | |
| Controller medication, not adherent < 5 of last 7 days | 25.1 | (48) | 27.9 | (56) | |
| Missing | (13) | (17) | |||
| No rescue inhaler reported, % (n) | 57.6 | (110) | 45.3 | (91) | 0.064 |
| Among students reporting a rescue medication: | |||||
| Rescue inhaler available ≥ 5 of last 7 days | 19.4 | (37) | 24.4 | (49) | |
| No rescue inhaler available < 5 of last 7 days | 23.0 | (44) | 30.3 | (61) | |
| Missing | (13) | (17) | |||
| Non-smoker, % (n) | 69.7 | (136) | 72.1 | (119) | 0.81 5 |
| Smoker, smoked ≥ 2 cigarette in last 30 days, % (n) | 7.7 | (15) | 7.9 | (13) | |
| Smoker, smoked < 2 cigarettes in last 30 days, % (n) | 22.6 | (44) | 20.0 | (33) | |
| Missing | (9) | (53) | |||
| Low perceived emotional support | 19.6 | (40) | 24.3 | (53) | 0.24 |
| Rebelliousness | 42.2 | (86) | 41.7 | (91) | 0.93 |
36 students returned consent forms but did not complete baseline surveys and were not randomized (see Figure 1);
Regardless of number of sessions completed.
Wilcoxon rank sum test;
Adjusted for school at enrollment and severity at baseline using multinomial logistic regression;
Chi-squared test
Overall, 88.4% of students completed all four computer sessions and 90% completed the 12 month follow up survey (Table 1). Students could access the program from school computers, but could also use non-school computers to access Puff City. Of the 240 sessions due over the summer of 2008 for enrolled students, 108 (45%) were completed during dates when schools were closed (data not shown). Also, 90.7% (88/97) of students without a home computer completed ≥ 3 of the 4 online sessions (data not shown).
For the outcome of symptom days, treatment students reported significantly fewer days than controls (Table 2). Among students meeting criteria for moderate-severe asthma, adjusted risk ratios were significant for symptom days, school days missed, school days missed due to asthma, and days of restricted activity (Table 2).
Table 2.
Self-report of functional status at 12 months for students enrolled in a randomized trial of Puff City, for all and for students meeting criteria for moderate-severe asthma
| Functional status | Treatment | Control | aRR1 | 95%CI | p |
|---|---|---|---|---|---|
|
| |||||
| (N=422) | Mean (sd) | Mean (sd) | |||
| Symptom days | 3.9 (5.9) | 5.2 (6.4) | 0.8 | (0.6, 1.0) | 0.019 |
| Symptom nights | 2.7 (5.6) | 2.8 (4.9) | 1.0 | (0.7, 1.6) | 0.82 |
| School days missed | 2.6 (4.3) | 3.1 (4.9) | 0.8 | (0.6, 1.0) | 0.08 |
| School days missed: asthma | 0.8 (2.1) | 1.4 (3.9) | 0.8 | (0.5, 1.2) | 0.25 |
| Days restricted activity | 3.2 (5.5) | 4.2 (6.0) | 0.8 | (0.6, 1.1) | 0.14 |
| Days had to change plans | 1.7 (4.5) | 1.8 (4.3) | 1.0 | (0.6, 1.6) | 0.96 |
| Medical Care Use2 | |||||
| ED visits | 0.9 (2.1) | 0.9 (2.4) | 1.0 | (0.7, 1.4)3 | 0.92 |
| Hospitalizations | 0.3 (1.2) | 0.3 (1.0) | 1.2 | (0.5, 2.6)4 | 0.66 |
| Moderate – Severe5 | Treatment | Control | aRR1 | 95%CI | p |
|
| |||||
| (n=104) | Mean (sd) | Mean (sd) | |||
|
| |||||
| Symptom days | 6.2 (7.7) | 9.2 (8.1) | 0.6 | (0.5,0.9) | 0.013 |
| Symptom nights | 5.1 (6.6) | 6.4 (7.9) | 0.7 | (0.4,1.2) | 0.210 |
| School days missed | 3.5 (5.6) | 5.1 (7.0) | 0.5 | (0.3–0.8) | 0.009 |
| School days missed: asthma | 1.3 (2.6) | 3.3 (6.6) | 0.4 | (0.2–0.8) | 0.007 |
| Days restricted activity | 5.3 (7.4) | 7.1 (7.6) | 0.6 | (0.4–0.9) | 0.025 |
| Days had to change plans | 3.0 (5.5) | 4.3 (6.8) | 0.6 | (0.3–1.0) | 0.064 |
| Medical Care Use 2 | |||||
| ED visits | 1.5 (3.4) | 1.7 (3.7) | 1.0 | (0.5, 2.0)3 | 0.95 |
| Hospitalizations | 0.3 (0.8) | 0.5 (1.1) | 0.6 | (0.2, 2.2)3 | 0.47 |
Adjusted risk ratio and corresponding 95% confidence interval
Self-report at 12 months
Adjusted risk ratio and corresponding 95% confidence interval from negative binomial regression, adjusting for baseline measurement, baseline asthma severity, gender, and school of enrollment
Adjusted risk ratio and corresponding 95% confidence interval from negative binomial regression, adjusting for baseline measurement, baseline asthma severity, gender, school of enrollment, age, and environmental tobacco smoke
Classification of asthma severity and cut-offs adapted from “Figure 14. Classification of Asthma Severity (≥ 12 years of age)”, using nighttime symptoms from Expert Panel 3: Guidelines for Diagnosis and Treatment of Asthma24
We evaluated self-report of emergency department (ED) visits and hospitalizations at 12 months (Table 2). One student reporting > 20 ED visits and one student reporting 22 hospitalizations were excluded from this analysis as outliers. No significant differences were observed at 12 months overall, or when restricting the analysis to students with moderate-severe asthma.
Presented in Table 3 are categorical variables corresponding to the cut offs used in the EPR 3 to represent indicators of uncontrolled asthma. Treatment/control comparisons were significant for the ooutcome of >8 symptom-days in the last 30 days (or ≥2 symptom days/week in the last 30 days), and >4 days of restricted activity in the last 30 days.
Table 3.
Indicators of uncontrolled asthma at 12 month follow-up for students enrolled in a randomized trial of Puff City
| Indicators of uncontrolled asthma1 | Treatment | Control | aOR2 | 95%CI | p | ||
|---|---|---|---|---|---|---|---|
|
| |||||||
| All (N=422) | |||||||
|
| |||||||
| n | (%)3 | n | (%) | ||||
| ≥ 2 days/wk/30 days | |||||||
| Yes | 23 | (11.3) | 46 | (21.1) | 0.5 | 0.2 – 0.8 | 0.006 |
| No | 181 | (43.6) | 172 | (78.9) | |||
| ≥ 3 Symptom nights | |||||||
| Yes | 50 | (24.5) | 68 | (31.3) | 0.6 | 0.4 – 1.0 | 0.074 |
| No | 154 | (75.5) | 149 | (68.7) | |||
| > 2 Sch days missed | |||||||
| Yes | 68 | (33.3) | 89 | (40.8) | 0.7 | 0.4 – 1.1 | 0.090 |
| No | 136 | (66.7) | 129 | (59.2) | |||
| > 2 Sch days missed (asthma) | |||||||
| Yes | 26 | (12.8) | 32 | (14.8) | 0.8 | 0.5 – 1.5 | 0.52 |
| No | 178 | (87.2) | 185 | (85.2) | |||
| > 4 Days of restricted activity | |||||||
| Yes | 39 | (19.1) | 68 | (31.2) | 0.5 | 0.3 – 0.9 | 0.010 |
| No | 165 | (80.9) | 150 | (68.8) | |||
| > 4 Days had to change plans | |||||||
| Yes | 20 | (9.8) | 24 | (11.1) | 0.8 | 0.4 – 1.7 | 0.58 |
| No | 184 | (90.2) | 193 | (88.9) | |||
Classification of asthma control adapted from “Figure 15. Classification of Asthma Control (≥ 12 years of age)”, with exception of days changed plans and school days missed from Expert Panel 3: Guidelines for Diagnosis and Treatment of Asthma24
Adjusted odds ratio and corresponding 95% confidence interval
n with risk factor in assignment group/total in assignment group
In analyses restricted to students meeting criteria for one or more submodules, compared to controls, treatment students meeting criteria for high rebellion reported fewer symptom-days, symptom-nights, school days missed, and days of restricted activity. These effects were not observed in data for low rebellion teens (Table 4). Among students meeting criteria for low perceived emotional support, fewer treatment students than controls had ≥ 2 symptom-days/week in a 30 day period. For students with mid – high perceived emotional support, treatment students were significantly less likely than controls to report > 2 school days missed/30 days and > 4 days of restricted activity/30 days (Table 4).
Table 4.
Results for analysis of indicators of uncontrolled asthma for students enrolled in a randomized controlled trial of Puff City and restricted to students receiving submodules, by randomization group.
| Indicators of uncontrolled asthma | High Rebellion2 (n=177) | Low Rebellion (N=245) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Treatment | Control | aOR3 | 95%CI | p | Treatment | Control | aOR | 95%CI | p | |||||
|
| ||||||||||||||
| n | (%) | n | (%) | n | (%) | n | (%) | |||||||
| ≥ 2 days/wk/30 days | 7 | (8.1) | 22 | (24.2) | 0.30 | 0.11 – 0.80 | 0.016 | 16 | (13.6) | 24 | (18.9) | 0.50 | 0.22 – 1.13 | 0.095 |
| ≥ 3 Symptom nights | 14 | (16.3) | 34 | (37.4) | 0.29 | 0.14 – 0.64 | 0.002 | 36 | (30.5) | 34 | (27.0) | 1.04 | 0.55 – 1.95 | 0.90 |
| > 2 Sch days missed | 25 | (29.1) | 44 | (48.4) | 0.40 | 0.20 – 0.78 | 0.007 | 43 | (36.4) | 45 | (35.4) | 1.18 | 0.65 – 2.12 | 0.59 |
| > 2 Sch days missed (asthma) | 11 | (12.8) | 21 | (23.1) | 0.51 | 0.21 – 1.21 | 0.12 | 15 | (12.7) | 11 | (8.7) | 1.40 | 0.57 – 3.43 | 0.46 |
| > 4 Days of restricted activity | 11 | (12.8) | 35 | (38.5) | 0.23 | 0.10 – 0.55 | 0.001 | 28 | (23.7) | 33 | (26.0) | 0.89 | 0.47 – 1.67 | 0.70 |
| > 4 Days had to change plans | 6 | (7.0) | 17 | (18.7) | 0.37 | 0.12 – 1.08 | 0.068 | 14 | (11.9) | 7 | (5.6) | 1.90 | 0.63 – 5.72 | 0.25 |
| High perceived emotional support4 (n=329) | Low perceived emotional support (n=93) | |||||||||||||
| Treatment | Control | aOR | 95%CI | p | Treatment | Control | aOR | 95%CI | p | |||||
|
| ||||||||||||||
| n | (%) | n | (%) | n | (%) | n | (%) | |||||||
|
| ||||||||||||||
| ≥ 2 days/wk/30 days | 18 | (11.0) | 30 | (18.2) | 0.52 | 0.26 – 1.04 | 0.065 | 5 | (12.5) | 16 | (30.2) | 0.23 | 0.06 – 0.88 | 0.031 |
| ≥ 3 Symptom nights | 41 | (25.0) | 50 | (35.0) | 0.68 | 0.40 – 1.16 | 0.16 | 9 | (22.5) | 18 | (34.0) | 0.42 | 0.14 – 1.32 | 0.14 |
| > 2 Sch days missed | 45 | (27.4) | 66 | (40.0) | 0.53 | 0.32 – 0.87 | 0.013 | 23 | (57.5) | 23 | (43.4) | 1.77 | 0.67 – 4.65 | 0.25 |
| > 2 Sch days missed (asthma) | 18 | (11.0) | 22 | (13.4) | 0.85 | 0.43 – 1.71 | 0.65 | 8 | (20.0) | 10 | (18.9) | 1.05 | 0.29 – 3.71 | 0.95 |
| > 4 Days of restricted activity | 28 | (17.1) | 46 | (27.9) | 0.54 | 0.31 – 0.96 | 0.035 | 11 | (27.5) | 22 | (41.5) | 0.48 | 0.17 – 1.39 | 0.18 |
| > 4 Days had to change plans | 15 | (9.2) | 15 | (9.2) | 0.94 | 0.41 – 2.13 | 0.87 | 5 | (12.5) | 9 | (17.0) | 0.73 | 0.19 – 2.82 | 0.65 |
Classification of asthma control adapted from “Figure 15. Classification of Asthma Control (≥ 12 years of age)”, with exception of days changed plans and school days missed from Guidelines for Diagnosis and Treatment of Asthma: Expert Panel Report 3.24
> 2.5 for responses to rebellion questions where 1=strongly disagree (less rebellious) and 5=strongly agree (more rebellious)
Adjusted odds ratio and corresponding 95% confidence interval
> 2 on average of three scales for emotional support, where 1=strongly disagree(less support) and 5=strongly agree (more support)
Finally, as an exploratory analysis (and with knowledge of inherent limitations) we restricted the analysis to students in the treatment group who did not have contact with the referral coordinator (n=33 out of 204) with all 218 students in the control group. In this restricted analysis, significant inverse odds ratios were observed for symptom-days, days of restricted activity, and school days missed, aOR=0.28, 0.46, and 0.57, respectively; all p ≤ 0.05. (Data not shown)
DISCUSSION
Evaluations of web-based asthma management interventions are few. In 2007, Bussey-Smith conducted a Cochrane Review of publications on the evaluation of interactive and web-based asthma interventions published since 1995.26 Of the 9 studies reviewed, four included youth 12 and older, of which two were in urban populations.27;28 Of the two urban studies, one study among 6–17 year old children showed significant reductions in hospitalizations (no difference in ED visits) with computer-assisted asthma management.27 One other qualitative assessment of an internet-based self-management tool in adolescents conducted in the Netherlands reported positive changes in asthma control as measured by the Asthma Control Questionnaire.29 We are not aware of other tailored, school- and web-based interventions, such as Puff City, that specifically target urban high school students.
The intervention had a positive effect on the most study outcomes, especially among teens meeting criteria for moderate-severe asthma. We might expect that students meeting criteria for moderate-severe asthma (more frequent symptoms) would be more likely to report benefit from the intervention. Alternatively, these teens may be the most difficult to help. Results were similar using indicators of uncontrolled asthma as the outcome, which may be more meaningful to patients and physicians than mean number of symptom-days.30
Results were also positive for students with high rebellion and emotional support levels. For the latter, aOR were generally < 1 (indicating intervention benefit), perhaps not reaching statistical significance due to sample size. The exceptions were school days missed overall and for asthma. At least one study has shown an association between emotional support and school attendance.31 The importance of emotional support for asthma management has been previously supported in the literature, and has been shown to be important for other chronic diseases and disease management behaviors, such as adherence.32 Online support, for example, through email or a chat room might be a reasonable format for Puff City, and an important addition to the program.
We did not observe a significant intervention effect for ED visits and results for students with moderate-severe asthma only suggested a trend in the hypothesized direction for hospitalizations. Krishna et al was able to show reductions in self-report of ED visits using an interactive multimedia asthma intervention among patients aged 0–17 years.33 In the Krishna study, only 8% of participants were African-American and the percentage of teens is not clear. Moreover, retention for the final visit in the Krishna study appears to be < 50% compared to almost 90% for our study. We were unable to find other comparable studies of multimedia interventions targeting urban teens with asthma. It is possible that our analyses may underestimate an intervention benefit as the control group received generic, web-based asthma education and not “usual care”. The intervention may have had a greater impact on emergency department and hospital use if it were initiated in a provider’s office or the, emergency department, or if referral coordinators had interacted more closely with the student’s primary care provider.
A limitation of our study is that the definitions of asthma severity and uncontrolled asthma used, although adapted from the EPR 3, are both based on symptom frequency and do not incorporate spirometry or clinician observation over a series of medical visits. Severity and control are distinct, but related, concepts that are not easily differentiated without objective measures and a medical history. Self-report, however, is routinely used by health care providers to determine level of control and severity, and is used in national surveys such as the National Health and Nutrition Examination Surveys (NHANES), Behavioral Risk Factor Surveillance System (BRFSS), and the National Asthma Survey, all of which use recall periods similar to those in our study.34–36 A second limitation is that our study design did not include randomization within the treatment group for receipt of submodules. As an integral part of the program, the submodules cannot be evaluated as a separate entity. Moreover, because the referral coordinator saw only treatment students, this aspect of the intervention cannot be evaluated separately from other aspects of the intervention. However, in a restricted analysis of treatment students who had not seen the referral coordinator, compared to controls, we did see similar effects, lending some support to the effectiveness of tailored content. Third, recruitment for this school-based study was relatively low (roughly 25% of those eligible), despite an ambitious recruitment campaign that included a variety of activities to encourage enrollment (e.g., mailings, contests, giveaways, presentations and school incentives). We note that in the present analysis (1) participants were similar to non-participants for most outcomes; and (2) baseline variables that differed significantly between treatment and control groups suggested slightly higher baseline morbidity for treatment students, which could bias our results toward the null. Finally, as Puff City was specifically designed for African American high school students, our results may be generalizable only to urban high school students with asthma symptoms and characteristics similar to that of our study participants.37 However, our research, and that of others, suggests there is value in the identification and engagement of high risk groups in the promotion of behavior change.11
The dissemination potential for web-based applications is great and tailoring allows delivery of personalized intervention content with high fidelity. The schools participating in Puff City were found to have ample computer resources to support Puff City prior to being approached about the study. Students also accessed the intervention from non-school computers. Mobile applications are another very feasible option yet to be explored. According to published reports, Non-Hispanic Black and Hispanic youth use web-based applications frequently for social networking.38
CONCLUSION
Ethnic minorities and underserved communities often are the last exposed to innovative interventions, despite having the greatest need.39;40 Puff City represents a viable strategy for improving disease self-management among urban adolescents with asthma. Our results highlight the unique needs of adolescents who struggle with self-identity. Even with a burgeoning sense of independence, teens still require support when managing a chronic disease. The means of integrating behavioral interventions such as Puff City into existing health care delivery systems as a clinical tool is an important next step for improving asthma control in hard-to-reach populations.
Acknowledgments
This research was funded by the National Institutes of Health, National Heart, Lung, and Blood Institute. Grant # R01 HL67462-01 ClinicalTrials.gov Id: NCT00201058
We thank the Detroit Public Schools Office of Research, Evaluation and Assessment and Anntinette McCain, Director of the Coordinated School Health Council. We also acknowledge the expert advice and consult of Sarah Lyon Callo, MA, MS and Ericka Garcia, MA, of the Michigan Department of Community Health.
Abbreviations
- aOR
Adjusted Odds Ratio
- aRR
Adjusted Relative Risk
- CI
Confidence Interval
- ED
Emergency Department
- EPR3
Expert Panel Report 3: Guidelines for Diagnosis and Treatment of Asthma
- ETS
Environmental Tobacco Smoke
- HFHS
Henry Ford Health System
- RTC
Randomized Controlled Trial
- SES
Socioeconomic Status
- QOL
Quality of Life
Footnotes
Financial Disclosure: The authors wish to disclose that one pharmaceutical company has expressed an interest in the use of Puff City. A formal agreement is pending.
Implications and Contribution
Asthma deaths are higher for African-American adolescents aged 15-19 years compared to younger children. Needed for adolescents are interventions encouraging patient self-management, in partnership with a physician. Results suggest Puff City, a theory-based, tailored intervention, is a viable strategy for improving asthma control in urban teens, including potentially unresponsive subgroups.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Moorman JE, Rudd RA, Johnson CA, King M, Minor P, Bailey C, et al. National surveillance for asthma--United States, 1980–2004. Morbidity & Mortality Weekly Report Surveillance Summaries. 2007;56(8):1–54. [PubMed] [Google Scholar]
- 2.Akinbami LJ, Moorman JE, Liu X. National Health Statistics Reports; No 32. Hyattsville, MD: National Center for Health Statistics; 2011. Asthma prevalence, health care use, and mortality: United States, 2005–2009. [PubMed] [Google Scholar]
- 3.Riera A, Walker DM. The impact of race and ethnicity on care in the pediatric emergency department. Current Opinion in Pediatrics. 2010;22(3):284–9. doi: 10.1097/MOP.0b013e32833973a5. [Review] [50 refs] [DOI] [PubMed] [Google Scholar]
- 4.Boudreaux ED, Emond SD, Clark S, Camargo CA., Jr Race/ethnicity and asthma among children presenting to the emergency department: differences in disease severity and management. Pediatr. 2003;111:e615–e621. doi: 10.1542/peds.111.5.e615. [DOI] [PubMed] [Google Scholar]
- 5.Joseph CLM, Peterson EL, Havstad SL, Johnson CC, Hoerauf S, Stringer S, et al. A web-based, tailored asthma management program for urban African-American high school students. Am J Respir Crit Care Med. 2007;175:888–895. doi: 10.1164/rccm.200608-1244OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Joseph CLM, Havstad SL, Johnson D, Saltzgaber J, Peterson EL, Resnicow K, et al. Factors Associated With Nonresponse to a Computer-Tailored Asthma Management Program for Urban Adolescents With Asthma. J Asthma. 2010;47:667–673. doi: 10.3109/02770900903518827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Janz NK, Becker MH. The Health Belief Model: a decade later. Health Educ Q. 1984;11:1–47. doi: 10.1177/109019818401100101. [DOI] [PubMed] [Google Scholar]
- 8.Rosenstock I. Historical Origins of the Health Belief Model. Health Education Monographs. 1974:2. doi: 10.1177/109019817800600406. [DOI] [PubMed] [Google Scholar]
- 9.Larimer ME, Palmer RS, Marlatt GA. Relapse prevention. An overview of Marlatt’s cognitive-behavioral model. Alcohol Res Health. 1999;23:151–160. [PMC free article] [PubMed] [Google Scholar]
- 10.Emmons KM, Rollnick S. Motivational interviewing in health care settings. Opportunities and limitations. Am J Prev Med. 2001;20:68–74. doi: 10.1016/s0749-3797(00)00254-3. [DOI] [PubMed] [Google Scholar]
- 11.Kreuter MW, Strecher VJ, Glassman B. One size does not fit all: the case for tailoring print materials. Ann Behav Med. 1999;21:276–283. doi: 10.1007/BF02895958. [DOI] [PubMed] [Google Scholar]
- 12.Kreuter MW, Skinner CS. Tailoring: what’s in a name? Health Educ Res. 2000;15:1–4. doi: 10.1093/her/15.1.1. [DOI] [PubMed] [Google Scholar]
- 13.Lucas CP, Zhang H, Fisher PW, Shaffer D, Regier DA, Narrow WE, et al. The DISC Predictive Scales (DPS): efficiently screening for diagnoses. J Am Acad Child Adolesc Psychiatry. 2001;40:443–449. doi: 10.1097/00004583-200104000-00013. [DOI] [PubMed] [Google Scholar]
- 14.Dahlem NW, Zimet GD, Walker RR. The Multidimensional Scale of Perceived Social Support: a confirmation study. J Clin Psychol. 1991;47:756–761. doi: 10.1002/1097-4679(199111)47:6<756::aid-jclp2270470605>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 15.Resnicow K, Baskin M, Rahotep S, Periasamy S, Rollnick S. Motivational Interviewing in Health Promotion and Behavioral Medicine. In: Cox W, Klinger E, editors. Handbook of Motivational Counseling. John Wiley & Sons, Ltd; 2004. pp. 457–76. [Google Scholar]
- 16.Borrelli B, Riekert KA, Weinstein A, Rathier L. Brief motivational interviewing as a clinical strategy to promote asthma medication adherence. J Allergy Clin Immunol. 2007;120:1023–1030. doi: 10.1016/j.jaci.2007.08.017. [DOI] [PubMed] [Google Scholar]
- 17.Dijkstra A, De VH. Clusters of precontemplating smokers defined by the perception of the pros, cons, and self-efficacy. Addict Behav. 2000;25:373–385. doi: 10.1016/s0306-4603(99)00073-8. [DOI] [PubMed] [Google Scholar]
- 18.Ryan RM, Deci EL. Intrinsic and Extrinsic Motivations: Classic Definitions and New Directions. Contemp Educ Psychol. 2000;25:54–67. doi: 10.1006/ceps.1999.1020. [DOI] [PubMed] [Google Scholar]
- 19.Tyc VL, Lensing S, Klosky J, Rai SN, Robinson L. A comparison of tobacco-related risk factors between adolescents with and without cancer 1. J Pediatr Psychol. 2005;30:359–370. doi: 10.1093/jpepsy/jsi030. [DOI] [PubMed] [Google Scholar]
- 20.Marlatt GA. Taxonomy of high-risk situations for alcohol relapse: evolution and development of a cognitive-behavioral model. Addiction. 1996;91 (Suppl):S37–S49. [PubMed] [Google Scholar]
- 21.Joseph CLM, Baptist AP, Stringer S, Havstad SL, Ownby DR, Johnson CC, et al. Identifying students with self-report of asthma and respiratory symptoms in an urban, high school setting. J Urban Health. 2007;84:60–69. doi: 10.1007/s11524-006-9121-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weiland SK, Bjorksten B, Brunekreef B, Cookson WO, von Mutius E, Strachan DP. Phase II of the International Study of Asthma and Allergies in Childhood (ISAAC II): rationale and methods. Eur Respir J. 2004;24:406–412. doi: 10.1183/09031936.04.00090303. [DOI] [PubMed] [Google Scholar]
- 23.Croft DR, Peterson MW. An evaluation of the quality and contents of asthma education on the World Wide Web. Chest. 2002;121:1301–1307. doi: 10.1378/chest.121.4.1301. [DOI] [PubMed] [Google Scholar]
- 24.NIH publication no 07-4051. Bethesda, MD: U.S. Department of Health and Human Services; National Institutes of Health; National Heart, Lung, and Blood Institute; National Asthma Education and Prevention Program; 2007. Expert Panel Report 3: Guidelines for the diagnosis and management of asthma. [Google Scholar]
- 25.U.S. Department of Education, Institute of Education Sciences (IES) - National Center for Education Statistics. 1990 K Street NW, Washington, DC 20006, USA, Phone: (202) 502–7300. 2007.
- 26.Bussey-Smith KL, Rossen RD. A systematic review of randomized control trials evaluating the effectiveness of interactive computerized asthma patient education programs. [Review] [28 refs] Annals of Allergy, Asthma, & Immunology. 2007;98:507–516. doi: 10.1016/S1081-1206(10)60727-2. [DOI] [PubMed] [Google Scholar]
- 27.Bartholomew LK, Gold RS, Parcel GS, Czyzewski DI, Sockrider MM, Fernandez M, et al. Watch, Discover, Think, and Act: evaluation of computer-assisted instruction to improve asthma self-management in inner-city children. Patient Education & Counseling. 2000;39(2–3):269–80. doi: 10.1016/s0738-3991(99)00046-4. [DOI] [PubMed] [Google Scholar]
- 28.Guendelman S, Meade K, Benson M, Chen YQ, Samuels S. Improving asthma outcomes and self-management behaviors of inner-city children: a randomized trial of the Health Buddy interactive device and an asthma diary. Arch Pediatr Adolesc Med. 2002;156:114–120. doi: 10.1001/archpedi.156.2.114. [DOI] [PubMed] [Google Scholar]
- 29.van dMV, van Stel HF, Detmar SB, Otten W, Sterk PJ, Sont JK. Internet-based self-management offers an opportunity to achieve better asthma control in adolescents. Chest. 2007;132(1):112–9. doi: 10.1378/chest.06-2787. [DOI] [PubMed] [Google Scholar]
- 30.Sonnad S, Goldsack MChem J, Mohr P, Mullins D, Whicher D. Pragmatic Phase 3 Pharmaceutical Trials: Recommendations for the design of clinical trials that are more informative for patients, clinicians, and payers. Center for Medical Technology Policy; 2010. Effectiveness Guidance Document. [Google Scholar]
- 31.Richman JM, Rosenfeld LB, Bowen GL. Social support for adolescents at risk of school failure. Social Work. 1998;43(4):309–23. doi: 10.1093/sw/43.4.309. [DOI] [PubMed] [Google Scholar]
- 32.Rhee H, Belyea MJ, Brasch J. Family support and asthma outcomes in adolescents: barriers to adherence as a mediator. Journal of Adolescent Health. 2010;47(5):472–8. doi: 10.1016/j.jadohealth.2010.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Krishna S, Francisco BD, Balas EA, Konig P, Graff GR, Madsen RW. Internet-enabled interactive multimedia asthma education program: a randomized trial. Pediatr. 2003;111:503–510. doi: 10.1542/peds.111.3.503. [DOI] [PubMed] [Google Scholar]
- 34.National Health and Nutrition Examination Survey (NHANES) CDC/National Center for Health Statistics. 2011. [Google Scholar]
- 35.Nelson DE, Holtzman D, Bolen J, Stanwyck CA, Mack KA. Reliability and validity of measures from the Behavioral Risk Factor Surveillance System (BRFSS) Preventive Med. 2001;46 (Suppl 1):S3–42. [PubMed] [Google Scholar]
- 36.O’Connor KS, Osborn L, Olson L, Blumberg SJ, Frankel MR, Srinath KP, et al. Design and operation of the National Asthma Survey. Vital & Health Statistics - Series 1: Programs & Collection Procedures. 2008;(46):1–122. [PubMed] [Google Scholar]
- 37.Joseph C, Saltzgaber J, Havstad S, Johnson C, Johnson D, Peterson E, et al. Comparison of early-, late-, and non-participants in a school-based asthma management program for urban high school students. Trials. 2011;12:141. doi: 10.1186/1745-6215-12-141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lenhart A, Madden M, Smith A, Purcell K, Zickuhr K, Rainie L. The Pew Research Center’s Internet & American Life Teen-Parent survey, April 19-July 14, 2011. [Accessed June 17, 2012. 2011];Pew Research Center. Available at: http://www.pewinternet.org/Reports/2011/Teens-and-social-media/Part-1/Social-media-demographics.aspx. Pew Research Center. Teens and Social Media: Part I.
- 39.Geller SE, Koch A, Pellettieri B, Carnes M. Inclusion, analysis, and reporting of sex and race/ethnicity in clinical trials: have we made progress? J Womens Health (Larchmt) 2011;20:315–320. doi: 10.1089/jwh.2010.2469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Institute of Medicine. Addressing Racial and Ethnic Health Care Disparities. Institute of Medicine; 2005. [Google Scholar]