Abstract
Background and Purpose
Atrial fibrillation (AF) has been associated with cognitive decline independant of stroke, suggesting additional effects of AF on the brain. We aimed to assess the association between AF and brain function and structure in a general elderly population.
Methods
This is a cross-sectional analysis on 4251 non-demented participants (mean age 76 ± 5 years) in the population-based AGES-Reykjavik Study. Medical record data were collected on the presence, subtype and time from first diagnosis of AF; 330 participants had AF. Brain volume measurements, adjusted for intracranial volume, and presence of cerebral infarcts were determined with MRI. Memory, speed of processing and executive function composites were calculated from a cognitive test battery. In a multivariable linear regression model, adjustments were made for demographic, cardiovascular risk factors and cerebral infarcts.
Results
Participants with AF had lower total brain volume compared to those without AF (p<0.001). The association was stronger with persistent/permanent than paroxysmal AF and with increased time from the first diagnosis of the disease. Of the brain tissue volumes, AF was associated with lower volume of gray and white matter (p<0.001 and p=0.008 respectively) but not of white matter hyperintesities (p=0.49). Participants with AF scored lower on tests on memory.
Conclusions
AF is associated with smaller brain volume and the association is stronger with increasing burden of the arrhythmia. These findings suggest that AF has a cumulative negative effect on the brain independent of cerebral infarcts.
Keywords: atrial fibrillation, brain imaging, cognition, cerebral infarct
Introduction
The number of individuals with AF is projected to triple in the next four decades (1). Data have emerged showing that AF may be a risk factor for cognitive impairment and dementia independent of stroke, indicating that the arrhythmia has additional adverse effects on the brain (2-5). Brain atrophy, both of the gray and white matter, as well as increased white matter hyperintesities have been associated with detriment of brain function but currently there is limited data on AF and brain volume (6).
Three previous studies did not find a significant association between AF and total brain atrophy (7-9). These studies did not report whether there was a difference between patients with paroxysmal and permanent AF or whether the time from the first diagnosis of AF was of any significance. They also did not report differences in specific brain tissue volumes. Gaining a better understanding of the relationship between AF and brain structure might prove useful for developing interventions to potentially delay the progression to dementia in these patients.
The aim of this study was to examine the association between AF and brain measures on MRI as well as cognitive function using data from the large population- based Age, Gene/Environment Susceptibility-Reykjavik (AGES-Reykjavik) study. Furthermore the purpose was to assess the significance of the duration and type of AF (paroxysmal versus persistent/permanent) on these outcome measures.
Materials and methods
The AGES-Reykjavik Study was designed to investigate the genetic and environmental factors contributing to clinical and subclinical disease at older age. The study is a continuation of the Reykjavik Study, which was a total population study of men and women born between 1907 and 1935 who were residents of the Greater Reykjavik area in 1967. The study was a longitudinal study carried out from 1967 to 1994 that collected mid-life data on cardiovascular traits (10-11). The AGES-Reykjavik cohort is a random recruitment of survivors from the Reykjavik study. It included 5,764 subjects (2,438 men and 3,326 women), aged 67 to 93 years. From September 2002 to February 2006, new data were collected at the research center: a questionnaire was administered, a clinical examination was performed, and images were acquired of the brain, musculoskeletal system, body composition, vasculature and heart. The study design and initial assessments of the cohort have been described previously in more detail (12).
The AGES-Reykjavik Study has been approved by the Icelandic National Bioethics Committee, which acts as the Institutional Review Board for the Icelandic Heart Association, and by the Institutional Review Board for the Intramural Research Program of the National Institute on Aging, National Institutes of Health, USA. Informed consent was obtained from all participants
Ascertainment of Atrial Fibrillation
AF was identified by: reviewing hospital records and private physician's records for all participants with the hospital discharge diagnosis codes for AF (ICD-9 code 427.9 or ICD-10 code I48) from any hospital in Reykjavik from January 1st 1987 till the day of the study examination, and by reviewing a twelve lead electrocardiogram (ECG) performed during the AGES-Reykjavik study comprehensive examination. Prolonged monitoring was not performed. Using information from the participants aforementioned records and from the study examination AF was classified as either paroxysmal or persistent/permanent according to recently published guidelines (13). Those who only had AF that occurred less than 4 weeks from open heart surgery were excluded. Duration of AF was calculated from the date of first diagnosis of AF till the date of the study examination.
Potential Confounders
Age, sex, education level (primary/secondary/college or university), smoking status (never smoker/ever smoker) and alcohol consumption (none or low (0-7 drinks/week)/moderate or high (>7 drinks/week)) were assessed by a questionnaire. High depressive symptomatology was classified as a score of 6 or greater on the 15-item Geriatric Depression Scale (14). Body mass index (BMI) was calculated from measured height and weight. Hypercholesterolemia was defined as total cholesterol level >6.0 mmol/L or current use of lipid lowering drugs. Hypertension was defined as self-reported doctor's diagnosis, use of hypertensive medications, systolic blood pressure >140 mmHg or diastolic blood pressure ≥90 mmHg. Myocardial infarction was defined as self-reported history of myocardial infarction or evidence on ECG of possible or probable myocardial infarction. Diabetes mellitus was defined as a self-reported doctor's diagnosis, use of diabetes-related medications or fasting blood glucose >7 mmol/L. The diagnosis of heart failure was based on hospital discharge diagnosis codes from all hospitals in Reykjavik. Presence of cerebral infarcts was determined with brain MRI (see below).
MRI Image Acquisition and Image Processing
All participants without contraindications were eligible for a brain MRI performed on a study-dedicated 1.5-T Signa Twinspeed system (General Electric Medical Systems). The AGES-Reykjavik MRI image protocol has previously been described in detail (15). In brief, the protocol included a T1-weighted 3-dimensional spoiled gradient echo sequence, a proton density/T2-weighted fast-spin echo sequence, a T2*-weighted gradient echo-type echo planar imaging sequence, and a T2-weighted fluid-attenuated inversion recovery (FLAIR) sequence. All images were acquired to give full brain coverage in the oblique-axial plane. The T1- and proton density/T2-weighted images were acquired to include the entire skull, inferiorly form the level of the foramen magnum. Brain tissue volumes including cerebrospinal fluid gray matter, white matter and white matter hyperintensities (WMH) were computed with an automatic image analysis pipeline (The AGES/MNI pipeline), which is based on the MNI pipeline and optimized for use in the AGES-Reykjavik Study (16). Total brain volume was computed as the sum of gray matter, white matter and WMH volumes. The intra-cranial volume was computed as the sum of total brain and cerebrospinal fluid volumes. The AGES/MNI pipeline has been validated and described in detail elsewhere (17). All brain volumes are presented as percentages of intracranial volume to correct for cranial size. All references to brain volume in Results and Discussion relate to brain volume relative to intracranial volume. Absolute brain volumes are presented in supplemental data (please see http://stroke.ahajournals.org).
The scoring of cerebral infarcts is described in detail elsewhere (18). Infarcts were scored by trained radiographers and defined as defects of the brain parenchyma with a signal intensity that was isotense to that of the cerebrospinal fluid on all pulse sequences and had associated hyperintensity on FLAIR images with a minimal diameter of 4 mm, except for infarcts in the cerebellum and brain stem which had no size criteria and did not require associated hyperintensity. Infarcts with cortical involvement had also no size criteria. The average inter-rater reliability (weighted kappa) was 0.7 for cerebral infarcts and the intra-rater reliability score was 0.9, in 5% of all scans re-read without knowledge of the previous reading.
Assessment of Cognitive Function
The battery of cognitive tests included multiple tests of 3 cognitive domains. Similar to other population-based studies, composites scores for memory (MEM), processing speed (SP) and executive function (EF) were constructed based on a theoretical grouping of tests. The MEM composite included the immediate and delayed recall of a modified version of the California Verbal Learning Test (19). The SP composite included the Digit Symbol Substitution Test, Figure Comparison Test, and the Stroop Test part 1 and 2 (20-22). The EF composite included the Digits Backward test, a shortened version of the Spatial Working Memory subtest of the Cambridge Neuropsychological Test Automated Battery, and the Stroop Test part 3 (21-23). Gender-specific composite measures were computed by converting raw scores on each test to standardized Z-scores separately by gender and averaging the Z-scores across the tests in each composite (18). Inter-rater reliability for all tests was excellent (Spearman correlations range, 0.96–0.99). Ascertainment of dementia was performed in a 3-step process that has been described elsewhere (12). Briefly, a consensus diagnosis of dementia based on Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, guidelines was made by a panel that included a geriatrician, a neurologist, a neuropsychologist, and a neuroradiologist taking into account neuropsychological data, MRI of the brain, a neurological exam and information from interviews of proxies about medical history and social, cognitive, and daily functioning relevant to the diagnosis.
Statistical Analyses
General characteristics and cardiovascular risk factors were compared among participants with and without AF with analysis of covariance for continuous outcomes and logistic regression for categorical outcomes. Analysis of covariance was used to assess the association between AF and each brain MRI measure and the three cognitive composite scores. Adjustment for covariates was done in two steps: basic model (age, sex and education) and multivariable model (basic model + the previously described demographic and cardiovascular risk factors and presence of cerebral infarcts). To assess if the association between AF and each cognitive composite score was mediated by brain volume we adjusted the multivariable model for each brain MRI measure. The association of duration of AF (divided into tertiles) and type of AF (paroxysmal versus persistent/permanent) was then assessed. WMH volume was highly skewed so the measure was log-transformed. The means of WMH volumes are reported as anti-logs.
In secondary analyses we assessed for effect modification of cerebral infarcts by including interaction terms in the multivariable model. We also checked for effect modification of anticoagulation use by adding current use of warfarin as a covariate to the multivariable model and including interaction terms. Significance was set at p<0.05 for all models. All analyses were performed using SAS version 9.2 (SAS Institute Inc, Cary, NC).
Analytical Sample
Of the 5764 participants of the AGES-Reykjavik study, 5706 gave informed consent to match the study data to the hospital and the private physician's records. Of these 4569 had brain MRI scans from which brain volumes could be computed. Demented participants and those where information on cognitive status was missing were then excluded, leaving 4251 subjects. A subgroup of 3960 had all three cognitive domain test composite scores.
Results
Characteristics of the cohort
Of the 4251 participants, 330 had a diagnosis of AF. Compared to those without AF, participants with AF were older, more often male, consumed more alcohol, were more often on warfarin and had a higher prevalence of hypertension, previous myocardial infarction, heart failure, diabetes mellitus and cerebral infarcts on MRI (Table 1). AF was paroxysmal in 41.8% (n=138) of cases and persistent/permanent in 58.2% (n=192). The date of first diagnosis of AF could be confirmed for 315 of 330 cases. The mean duration of AF was 7.6 ± 7.0 years (median value 6.0 years, range 0 to 41 years).
Table 1.
Characteristics of the study cohort by presence of atrial fibrillation (AF) Data are shown as mean (standard deviation) for continuous variables and percentages for categorical variables.
| No AF (n= 3921) | AF (n= 330) | |
|---|---|---|
| Age, years | 75.9 (5.3) | 78.0 (5.4) ‡ |
| Gender, % men | 39.8 | 62.4† |
| Education, % primary | 22.9 | 18.2 |
| MMSE | 27.1 (2.4) | 26.6 (2.5) |
| Body mass index | 27.0 (4.3) | 27.1 (4.4) |
| Height, centimeters | 166.8 (9.2) | 170.9 (10.1) ‡ |
| Ever smoker | 56.7 | 63.9 |
| Alcohol consumption, moderate/high | 1.3 | 4.3† |
| Depressive symptoms | 6.5 | 6.4 |
| Hypertension | 79.3 | 91.5‡ |
| Myocardial infarction | 13.6 | 23.9† |
| Heart failure | 1.6 | 14.9‡ |
| Diabetes mellitus | 10.6 | 15.2* |
| Hypercholesterolemia | 57.6 | 48.8 |
| Warfarin use | 1.9 | 46.4‡ |
| Cerebral infarct on MRI | 28.6 | 48.8‡ |
P < 0.05;
P < 0.01,
P < 0.001 for age- and sex-adjusted comparison
MMSE = mini mental state examination
MRI = magnetic resonance imaging
Brain volumes and AF
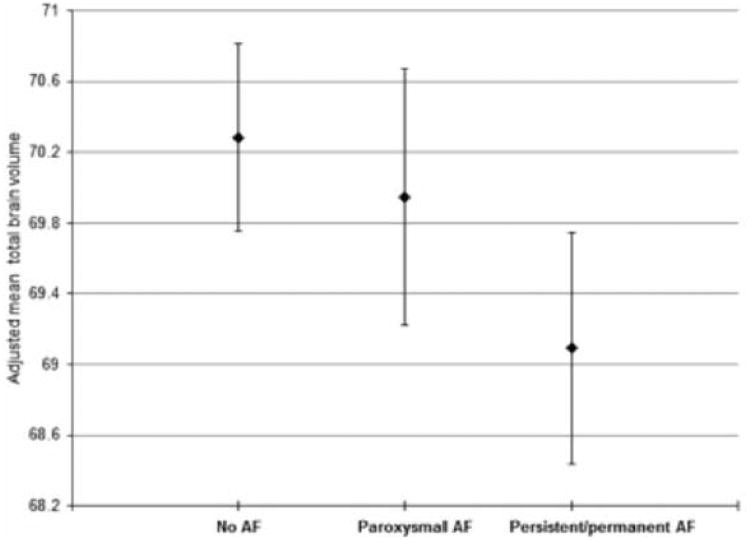
In the basic model AF was associated with lower volume of total brain, gray matter and white matter and higher volume of WMH (expressed as a percentage of total intracranial volume) (Table 2). After adjusting for further potential confounders, these associations remained significant except for WMH volume (absolute brain volumes are presented in supplemental table 1, please see http://stroke.ahajournals.org). There was a significant difference in total brain and gray matter volume depending on the type of AF, as those with persistent/permanent AF had lower volumes than those with paroxysmal AF (Figure 1 and table 3). There was a linear trend between increased time since first diagnosis of AF and lower total brain and gray matter volume (Table 4).
Table 2. Association between atrial fibrillation (AF) and MRI measured brain volumes: the AGES-Reykjavik Study.
| No AF | AF | |
|---|---|---|
| Total brain volume | ||
| Unadjusted | 72.4 (72.3-72.5) | 70.2 (69.8-70.6) ‡ |
| Model 1a | 72.1 (72.0-72.2) | 71.0 (70.6-71.4) ‡ |
| Model 2 b | 70.2 (69.7-70.8) | 69.4 (68.8-70.0) ‡ |
| Gray matter volume | ||
| Unadjusted | 45.3 (45.2-45.4) | 43.5 (43.2-43.9)‡ |
| Model 1a | 45.1 (45.0-45.2) | 44.2 (43.9-44.5) ‡ |
| Model 2 b | 43.7 (43.2-44.1) | 43.0 (42.5-43.5) ‡ |
| White matter volume | ||
| Unadjusted | 25.8 (25.7-25.8) | 25.0 (24.8-25.2) ‡ |
| Model 1a | 25.7 (25.7-25.8) | 25.3 (25.1-25.5) ‡ |
| Model 2 b | 25.2 (25.0-25.5) | 25.0 (24.7-25.3) † |
| WMH volume | ||
| Unadjusted | 0.88 (0.86-0.91) | 1.09 (0.99-1.20) ‡ |
| Model 1a | 0.89 (0.87-0.99) | 0.99(0.90-1.09) * |
| Model 2 b | 0.96 (0.84-1.10) | 1.00 (0.86-1.16) |
Data are adjusted mean percentage of total intracranial volume (95% confidence interval). Comparison to no AF:
P<0.05,
P<0.01,
P<0.001
Model 1: adjustedforage,sexandeducationlevel
Model 2: adjusted for age, sex, education level, body mass index, height, smoking, alcohol comsumption, hypercholesterolemia, hypertension, diabetes mellitus, myocardial infarction and heart failure and cerebral infarcts on MRI
WMH = white matter hyperintesities
MRI = magnetic resonance imaging
Figure 1.
Mean brain volume expressed as percentage of total intracranial volume (with standard error bars) in individuals without atrial fibrillation (AF), with paroxysmal AF and with persistent/permanent AF. Adjusted for age, sex, education level, hypertension, myocardial infarction, diabetes mellitus, heart failure, smoking, alcohol consumption, hypercholesterolemia, body mass index, height and cerebral infarcts on magnetic resonance imaging
Table 3. Association between types of atrial fibrillation (AF) and MRI measured brain volumes: The AGES-Reykjavik Study.
| Paroxysmal AF | Persistent/permanent AF | |
|---|---|---|
| Gray matter volume | -0.31 (0.24) | -0.96 (0.21) * |
| White matter volume | -0.11 (0.15) | -0.38 (0.13) |
| WMH volume | 0.03 (0.06) | 0.05 (0.08) |
| Total brain volume | -0.34 (0.29) | -1.20 (0.25) * |
Data are differences in brain volume expressed as percentage of total intracranial volume (standard error) in comparison to no AF
Paroxysmal compared to persistent/permanent AF:
P<0.05,
P<0.01,
P<0.001
Model is adjusted for age, sex, education, hypertension, myocardial infarction, diabetes mellitus, heart failure, smoking, alcohol use, hypercholesterolemia, body mass index, height and cerebral infarcts on MRI.
WMH = white matter hyperintesities
MRI = magnetic resonance imaging
Table 4. Association between duration of atrial fibrillation (AF) by tertile and MRI measured brain volumes: The AGES-Reykjavik Study.
| Duration of AF | Total brain volume | Gray matter volume | White matter volume |
|---|---|---|---|
| No AF (ref) | 70.2 | 43.7 | 25.2 |
| 1 | 69.5* | 43.1* | 25.0 |
| 2 | 69.4* | 42.9† | 24.9* |
| 3 | 69.3† | 43.0† | 25.0 |
| P for linear trend | 0.012 | 0.011 | 0.16 |
Data are adjusted mean percentage of total intracranial volume, adjusted for age, sex, education level, hypertension, myocardial infarction, diabetes mellitus, heart failure, smoking, alcohol use, hypercholesterolemia, body mass index, height and cerebral infarcts on MRI. For tertiles of AF duration the values are < 3.7 years for tertile 1 (n= 105), 3.7 – 8.6 years for tertile 2 (n=105) and > 8.6 years for tertile 3 (n=105). Comparison to no AF:
P<0.05,
P<0.01,
P<0.001
MRI = magnetic resonance imaging
In secondary analysis neither cerebral infarcts nor current warfarin therapy modified the association between AF and the brain volumes (data not shown)
Tests of Cognitive Function and AF
Participants with AF scored lower on tests of all three cognitive domains: MEM, SP and EF. After adjusting for cardiovascular risk factors and cerebral infarcts, AF was no longer significantly associated with SP or EF but the association between AF and MEM remained largely unchanged (Table 5). Finally the association between AF and MEM was no longer significant when we added to the models total brain and separately gray matter volume corrected for total intracranial volume (data not shown). There was no significant difference in any of the cognitive scores depending on the type of AF. Increased time since the first diagnosis of AF was associated with lower scores on test of MEM (ptrend = 0.045 for tertiles of AF duration). There was no significant interaction between cerebral infarcts and AF in their relation to MEM, SP or EF. Similarly current warfarin therapy was not a significant interaction factor (data not shown).
Table 5. Association between atrial fibrillation (AF) and cognitive domains (z scores): The AGES-Reykjavik Study.
| Model 1a | Model 2 b | |
|---|---|---|
| Memory | -0.10 (0.05)* | -0.10 (0.05)* |
| SP | -0.06 (0.04) | -0.03 (0.04) |
| EF | -0.07 (0.04)* | -0.05 (0.04) |
Data are differences in adjusted means of composite scores for cognitive domains (standard error) in comparison to no AF
Comparison to no AF:
P < 0.05,
P < 0.01,
P < 0.001.
Model 1: adjusted for age, sex and education level
Model 2: adjusted for age, sex, education level, depressive symptoms, hypertension, myocardial infarction, diabetes mellitus, heart failure, smoking, body mass index, alcohol consumption, hypercholesterolemia and cerebral infarcts on MRI
MRI = magnetic resonance imaging
Discussion
In this large cross sectional study of non-demented elderly individuals in the general population we found a significant association between AF and lower total brain, gray and white matter volume. For both total brain and gray matter volume the association was stronger with persistent/permanent compared to paroxysmal AF, and with increased time from first diagnosis of AF, suggesting a cumulative effect. In tests of cognitive function participants with AF scored significantly lower on tests of memory. As with brain volume, longer duration of AF was associated with worse outcome. These findings remained significant even after adjusting for the higher prevalence of cardiovascular risk factors and cerebral infarcts in AF population. The difference in total brain volume between individuals with and without AF equals a year and a half of normal loss of brain volume (17).
Atrial fibrillation and brain volume
Only a few previous studies have examined AF and brain volume. In a study published from the Cardiovascular Health Study on 303 adults 65-95 years of age, there was not a significant relation between AF and markers of total brain atrophy (7). However, as the authors point out, the relatively small sized study may have been underpowered to detect any association. A study from the Framingham Offspring Study on 1841 individuals did not find a significant association between AF and total brain volume (8). Similarly, in a recent case-control study, Knecht et al found no significant difference in total brain volume between those with and without AF (9). In both of these studies the average age of the cohort was around ten years younger than in the current study. As mentioned before we found a linear trend between increased duration of AF and lower total brain volume, i.e. the effect may be cumulative with increased AF burden. This may explain the lack of association between AF and total brain volume in younger cohorts.
Atrial fibrillation and cognitive function
After controlling for vascular factors, AF was associated with MEM but not with EP or SP. The latter two cognitive domains are closely associated with sub-cortical vascular disease that manifests primarily as infarcts and WMH (24). Those having AF for the longest time scored the lowest on tests of MEM. There was a trend towards lower scores in those with persistent/permanent compared to paroxysmal AF but it did not reach statistical significance. In the Framingham Offspring study on men free of symptoms of stroke, AF was associated with lower scores on a number of cognitive tests, mainly on SP and EF rather than memory (5). No adjustment was made for silent cerebral infarcts and, as previously mentioned, this pattern of cognitive performance is more consistent with subcortical vascular disease. In the previously mentioned study by Knecht et al, that included 87 patients with AF, all participants were free of cerebral infarcts on MRI and, similar to the results of the current study, AF had the strongest association with memory (9).
Potential mechanisms for the associations observed
The association between AF and brain atrophy and lower performance on tests of memory could only partially be explained by increased comorbidity and cerebral infarcts in the AF population. One possible explanation is that AF causes multiple microembolisms to the brain causing microinfarcts and subsequent atrophy. Additionally, altered cerebral blood perfusion, due to beat to beat variation in stroke volume, may also play a part. Cerebral hypoperfusion is associated with a reduction in both the gray and white matter volume of the brain. It however appears to have a greater negative effect on the gray matter, which could reflect the higher metabolic demand of the gray matter (25-26). We did find a stronger association between AF and lower gray matter than white matter and this finding may support the cerebral hypoperfusion hypothesis. There is some limited data on AF and cerebral blood flow. A small study on patients with no clinical symptoms of heart failure showed that those with AF had reduced regional cerebral blood flow compared to controls (27). In addition cerebral blood flow seems to increase after electrical cardioversion of AF (28). These observations might suggest that patients with persistent/permanent AF have on average less cerebral perfusion than those with only paroxysmal bursts of AF. We found that those with persistent/permanent AF had lower total brain and gray matter volume compared to with paroxysmal AF. It is, however, speculative that maintaining sinus rhythm could have an impact on the progress of brain atrophy and sequential cognitive decline in patients with AF. There are data, albeit limited, supporting this speculation. An observational study demonstrated that those who underwent radiofrequency ablation therapy for AF, which currently offers the most superior available rhythm control therapy for AF, had significantly lower risk for dementia than patients who did not have an ablation (29). The incidence of dementia in those undergoing ablation was similar to those without AF. Cognitive decline and brain atrophy need to be considered as endpoints in future prospective studies on treatment outcomes for AF.
Strength and limitations of the study
The main strength of this study is the large number of well described community dwelling subjects. This study also has some limitations. As this was a cross-sectional analysis any inference on direct cause and effect cannot be made. We did not have sufficient information on ejection fraction or stroke volume to include it as a covariate since echocardiography was performed on a proportion of the AGES-Reykjavik study cohort (30). Recently subclinical reductions in cardiac output have been associated with less total brain volume (31). However controlling for previous clinical diagnosis of heart failure did not affect the main conclusions of this study. Whether participants had paroxysmal or persistent/permanent AF was determined at the time of the study examination. We however did not have sufficient information on frequency or length of previous episodes of AF. This is a limitation in our ability to fully assess the actual burden of AF. In general individuals with AF are older, have increased co-morbidities and may more often have implanted cardiac electronic devices. This might have led to a selection bias in the study, if a higher percentage of persons with AF declined to participate in the study or could not undergo the brain MRI. As such, the results may actually underestimate the association between AF and brain volumes and cognition.
Conclusions
In the general elderly population, AF is associated with lower total brain volume independent of cerebral infarcts. The association is stronger for persistent/permanent AF than paroxysmal and with increased duration of the disease, suggesting a cumulative effect. The difference is evident both in the gray and white matter of the brain. Future prospective studies are needed to determine if maintenance of sinus rhythm is of benefit to attenuate brain atrophy and impaired memory performance.
Supplementary Material
Data are mean volume in ml (95% confidence interval). Comparison to no AF: * P<0.05, †P<0.01, ‡P<0.001
a Model 1: adjusted for age, sex and education level
b Model 2: adjusted for age, sex, education level, body mass index, height, smoking, alcohol comsumption, hypercholesterolemia, hypertension, diabetes, myocardial infarction and heart failure and cerebral infarcts on MRI
WMH = white matter hyperintesities
Acknowledgments
The authors thank the participants of the AGES-Reykjavik study and the Icelandic Heart Association staff.
Sources of funding: This work was supported by the Landspitali - The National University Hospital of Iceland Science Fund and the Helga Jonsdottir and Sigvaldi Kristjansson Memorial Fund. The AGES-Reykjavik study has been funded by NIH contract N01-AG-1-2100, the NIA Intramural Research Program, the Icelandic Heart Association and Althingi (the Icelandic Parliament).
Footnotes
Disclosures: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Stefansdottir H, Aspelund T, Gudnason V, Arnar DO. Trends in incidence and prevalence of atrial fibrillation in Iceland and future projections. Europace. 2011;13:1110–1117. doi: 10.1093/europace/eur132. [DOI] [PubMed] [Google Scholar]
- 2.Ott A, Breteler M, de Bruyne M, van Harskamp F, Grobbee DE, Hofman A. Atrial fibrillation and dementia in a population-based study: The Rotterdam Study. Stroke. 1997;28:316–321. doi: 10.1161/01.str.28.2.316. [DOI] [PubMed] [Google Scholar]
- 3.Bunch TJ, Weiss JP, Crandall BG, May HT, Bair TL, Osborn JS, et al. Atrial fibrillation is independently associatied with senile, vascular and alzheimer's dementia. Heart Rhythm. 2010;7:433–437. doi: 10.1016/j.hrthm.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 4.O'Connell JE, Gray CS, French JM, Robertson IH. Atrial fibrillation and cognitive function: case-control study. J Neurol Neurosurg Psychiatry. 1998;65:386–389. doi: 10.1136/jnnp.65.3.386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Elias MF, Sullivan LM, Elias PK, Vasan RS, D'Agostino RB, Seshadri S, et al. Atrial fibrillation is associated with lower cognitive performance in the Framingham offspring men. J Stroke Cerebrovasc Dis. 2006;15:214–222. doi: 10.1016/j.jstrokecerebrovasdis.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 6.Ikram MA, Vrooman HA, Vernooij MW, den Heijer T, Hofman A, Niessen WJ, et al. Brain tissue volumes in relation to cognitive function and risk of dementia. Neurobiol Aging. 2010;31:378–386. doi: 10.1016/j.neurobiolaging.2008.04.008. [DOI] [PubMed] [Google Scholar]
- 7.Manolio TA, Kronmal RA, Burke GL, Poirier V, O'Leary DH, Gardin JM, et al. Magnetic resonance abnormalities and cardiovascular disease in older adults. The Cardiovascular Health Study. Stroke. 1994;25:318–327. doi: 10.1161/01.str.25.2.318. [DOI] [PubMed] [Google Scholar]
- 8.Seshadri S, Wolf PA, Beiser A, Elias MF, Au R, Kase CS, et al. Stroke risk profile, brain volume, and cognitive function: The Framingham Offspring Study. Neurology. 2004;63:1591–1599. doi: 10.1212/01.wnl.0000142968.22691.70. [DOI] [PubMed] [Google Scholar]
- 9.Knecht S, Oelschläger C, Duning T, Lohmann H, Albers J, Stehling C, et al. Atrial fibrillation in stroke-free patients is associated with memory impairment and hippocampal atrophy. Eur Heart J. 2008;29:2125–2132. doi: 10.1093/eurheartj/ehn341. [DOI] [PubMed] [Google Scholar]
- 10.Bjornsson OJ, Davidsson D, Olafsson H, Olafsson O, Sigfusson N, Thorsteinsson T. Stages I–III, 1967–1969 and 1974–1976 Participants, Invitation, Response etc. The Icelandic Heart Association; Reykjavik: 1979. Report ABC XVIII Health Survey in the Reykjavik Area – Men. [Google Scholar]
- 11.Bjornsson G, Bjornsson OJ, Davidsson D, Kristj nsson BT, Olafsson O, Sigfusson N, Thorsteinsson T. Stages I–III 1968–1969 and 1976–1978 Participants Invitation Response etc. The Icelandic Heart Association; Reykjavik: 1982. Report ABC XXIV Health Survey in the Reykjavik Area – Women. [Google Scholar]
- 12.Harris TB, Launer LJ, Eiriksdottir G, Kjartansson O, Jonsson PV, Sigurdsson G, et al. Age, Gene/Environment Susceptibility–Reykjavik Study: multidisciplinary applied phenomics. Am J Epidemiol. 2007;16:1076–1087. doi: 10.1093/aje/kwk115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.European Heart Rhythm Association; European Association for Cardio-Thoracic Surgery. Camm AJ, Kirchhof P, Lip GY, Schotten U, Savelieva I, Ernst S, et al. Guidelines for the management of atrial fibrillation: the Task Force for the Management of Atrial Fibrillation of the European Society of Cardiology (ESC) Eur Heart J. 2010;31:2369–2429. doi: 10.1093/eurheartj/ehq278. [DOI] [PubMed] [Google Scholar]
- 14.Yesavage JA, Brink TL, Rose TL, Lum O, Huang V, Adey M, et al. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res. 1982;17:37–49. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]
- 15.Sveinbjornsdottir S, Sigurdsson S, Aspelund T, Kjartansson O, Eiriksdottir G, Valtysdottir B, et al. Cerebral microbleed in the population based AGES-Reykjavik study: prevalence and location. J Neurol Neurosurg Psychiatry. 2008;79:1002–1006. doi: 10.1136/jnnp.2007.121913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zijdenbos AP, Forghani R, Evans AC. Automatic “pipeline” analysis of 3-D MRI data for clinical trials: application to multiple sclerosis. IEEE Trans Med Imaging. 2002;21:1280–1291. doi: 10.1109/TMI.2002.806283. [DOI] [PubMed] [Google Scholar]
- 17.Sigurdsson S, Aspelund T, Forsberg L, Fredriksson J, Kjartansson O, Oskarsdottir B, et al. Brain tissue volumes in the general population of the elderly: the AGES-Reykjavik study. Neuroimage. 2012;59:3862–3870. doi: 10.1016/j.neuroimage.2011.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Saczynski JS, Jonsdottir MK, Sigurdsson S, Eiriksdottir G, Jonsson PV, Garcia ME, et al. White matter lesions and cognitive performance: the role of cognitively complex leisure activity. J Gerontol A Biol Sci Med Sci. 2008;63:848–854. doi: 10.1093/gerona/63.8.848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Delis DC, Kramer JH, Kaplan E, Ober BA. California Verbal Learning Test: Adult Version Manual. San Antonio, TX: The Psychological Corporation; 1987. [Google Scholar]
- 20.Salthouse TA, Babcock RL. Decomposing adult age differences in working memory. Dev Psychol. 1991;27:763–776. [Google Scholar]
- 21.Wechsler D. Administration and scoring manual for the Wechsler Adult Intelligence Scale-III. London: Psychological Corporation; 2008. [Google Scholar]
- 22.Stroop JR. Studies of interference in serial verbal reactions. J Exp Psychol. 1935;18:643–662. [Google Scholar]
- 23.Robbins TW, James M, Owen AM, Sahakian BJ, McInnes L, Rabbitt P. Cambridge Neuropsychological Test Automated Battery (CANTAB): a factor analytic study of a large sample of normal elderly volunteers. Dementia. 1994;5:266–281. doi: 10.1159/000106735. [DOI] [PubMed] [Google Scholar]
- 24.Pantoni L, Garcia JH. Pathogenesis of leukoaraiosis: a review. Stroke. 1997;28:652–659. doi: 10.1161/01.str.28.3.652. [DOI] [PubMed] [Google Scholar]
- 25.Payabvash S, Souza LC, Wang Y, Schaefer PW, Furie KL, Halpern EF, et al. Regional ischemic vulnerability of the brain to hypoperfusion: the need for location specific computed tomography perfusion thresholds in acute stroke patients. Stroke. 2011;42:1255–1260. doi: 10.1161/STROKEAHA.110.600940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marcoux FW, Morawetz RB, Crowell RM, DeGirolami U, Halsey JH., Jr Differential regional vulnerability in transient focal cerebral ischemia. Stroke. 1982;13:339–346. doi: 10.1161/01.str.13.3.339. [DOI] [PubMed] [Google Scholar]
- 27.Lavy S, Stern S, Melamed E, Cooper G, Keren A, Levy P. Effect of chronic atrial fibrillation on regional cerebral blood flow. Stroke. 1980;11:35–38. doi: 10.1161/01.str.11.1.35. [DOI] [PubMed] [Google Scholar]
- 28.Petersen P, Kastrup J, Videbaek R, Boysen G. Cerebral blood flow before and after cardioversion of atrial fibrillation. J Cereb Blood Flow Metab. 1989;9:422–5. doi: 10.1038/jcbfm.1989.62. [DOI] [PubMed] [Google Scholar]
- 29.Bunch TJ, Crandall BG, Weiss JP, May HT, Bair TL, Osborn JS, et al. Patients Treated with Catheter Ablation for Atrial Fibrillation Have Long-Term Rates of Death, Stroke, and Dementia Similar to Patients Without Atrial Fibrillation. J Cardiovasc Electrophysiol. 2011;22:839–845. doi: 10.1111/j.1540-8167.2011.02035.x. [DOI] [PubMed] [Google Scholar]
- 30.McAreavey D, Vidal JS, Aspelund T, Owens DS, Hughes T, Garcia M, et al. Correlation of Echocardiographic Findings With Cerebral Infarction in Elderly Adults: The AGES-Reykjavik Study. Stroke. 2010;41:2223–2228. doi: 10.1161/STROKEAHA.110.590430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Seshadri S, Wolf PA, Beiser A, Elias MF, Au R, Kase CS, et al. Stroke risk profile, brain volume, and cognitive function: The Framingham Offspring Study. Neurology. 2004;63:1591–1599. doi: 10.1212/01.wnl.0000142968.22691.70. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data are mean volume in ml (95% confidence interval). Comparison to no AF: * P<0.05, †P<0.01, ‡P<0.001
a Model 1: adjusted for age, sex and education level
b Model 2: adjusted for age, sex, education level, body mass index, height, smoking, alcohol comsumption, hypercholesterolemia, hypertension, diabetes, myocardial infarction and heart failure and cerebral infarcts on MRI
WMH = white matter hyperintesities