Abstract
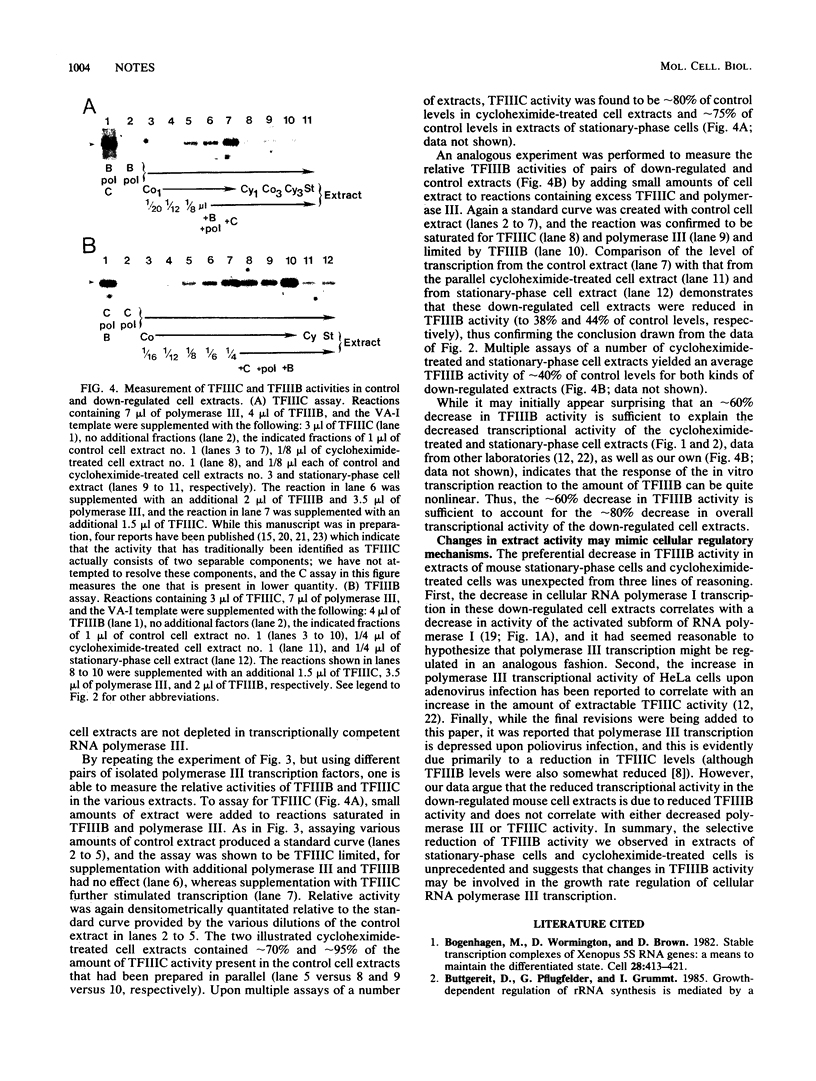
Extracts of cells that are down-regulated for transcription by RNA polymerase I and RNA polymerase III exhibit a reduced in vitro transcriptional capacity. We have recently demonstrated that the down-regulation of polymerase I transcription in extracts of cycloheximide-treated and stationary-phase cells results from a lack of an activated subform of RNA polymerase I which is essential for rDNA transcription. To examine whether polymerase III transcriptional down-regulation occurs by a similar mechanism, the polymerase III transcription factors were isolated and added singly and in pairs to control cell extracts and to extracts of cells that had reduced polymerase III transcriptional activity due to cycloheximide treatment or growth into stationary phase. These down-regulations result from a specific reduction in TFIIIB; TFIIIC and polymerase III activities remain relatively constant. Thus, although transcription by both polymerase III and polymerase I is substantially decreased in extracts of growth-arrested cells, this regulation is brought about by reduction of different kinds of activities: a component of the polymerase III stable transcription complex in the former case and the activated subform of RNA polymerase I in the latter.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bogenhagen D. F., Wormington W. M., Brown D. D. Stable transcription complexes of Xenopus 5S RNA genes: a means to maintain the differentiated state. Cell. 1982 Feb;28(2):413–421. doi: 10.1016/0092-8674(82)90359-2. [DOI] [PubMed] [Google Scholar]
- Buttgereit D., Pflugfelder G., Grummt I. Growth-dependent regulation of rRNA synthesis is mediated by a transcription initiation factor (TIF-IA). Nucleic Acids Res. 1985 Nov 25;13(22):8165–8180. doi: 10.1093/nar/13.22.8165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanaugh A. H., Gokal P. K., Lawther R. P., Thompson E. A., Jr Glucocorticoid inhibition of initiation of transcription of the DNA encoding rRNA (rDNA) in lymphosarcoma P1798 cells. Proc Natl Acad Sci U S A. 1984 Feb;81(3):718–721. doi: 10.1073/pnas.81.3.718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanaugh A. H., Thompson E. A., Jr Hormonal regulation of transcription of rDNA: glucocorticoid effects upon initiation and elongation in vitro. Nucleic Acids Res. 1985 May 10;13(9):3357–3369. doi: 10.1093/nar/13.9.3357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cizewski V., Sollner-Webb B. A stable transcription complex directs mouse ribosomal RNA synthesis by RNA polymerase I. Nucleic Acids Res. 1983 Oct 25;11(20):7043–7056. doi: 10.1093/nar/11.20.7043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culotta V. C., Wides R. J., Sollner-Webb B. Eucaryotic transcription complexes are specifically associated in large sedimentable structures: rapid isolation of polymerase I, II, and III transcription factors. Mol Cell Biol. 1985 Jul;5(7):1582–1590. doi: 10.1128/mcb.5.7.1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelke D. R., Ng S. Y., Shastry B. S., Roeder R. G. Specific interaction of a purified transcription factor with an internal control region of 5S RNA genes. Cell. 1980 Mar;19(3):717–728. doi: 10.1016/s0092-8674(80)80048-1. [DOI] [PubMed] [Google Scholar]
- Fradkin L. G., Yoshinaga S. K., Berk A. J., Dasgupta A. Inhibition of host cell RNA polymerase III-mediated transcription by poliovirus: inactivation of specific transcription factors. Mol Cell Biol. 1987 Nov;7(11):3880–3887. doi: 10.1128/mcb.7.11.3880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gokal P. K., Cavanaugh A. H., Thompson E. A., Jr The effects of cycloheximide upon transcription of rRNA, 5 S RNA, and tRNA genes. J Biol Chem. 1986 Feb 25;261(6):2536–2541. [PubMed] [Google Scholar]
- Grummt I., Smith V. A., Grummt F. Amino acid starvation affects the initiation frequency of nucleolar RNA polymerase. Cell. 1976 Mar;7(3):439–445. doi: 10.1016/0092-8674(76)90174-4. [DOI] [PubMed] [Google Scholar]
- Grummt I. Specific transcription of mouse ribosomal DNA in a cell-free system that mimics control in vivo. Proc Natl Acad Sci U S A. 1981 Feb;78(2):727–731. doi: 10.1073/pnas.78.2.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeffler W. K., Roeder R. G. Enhancement of RNA polymerase III transcription by the E1A gene product of adenovirus. Cell. 1985 Jul;41(3):955–963. doi: 10.1016/s0092-8674(85)80076-3. [DOI] [PubMed] [Google Scholar]
- Mann C., Buhler J. M., Treich I., Sentenac A. RPC40, a unique gene for a subunit shared between yeast RNA polymerases A and C. Cell. 1987 Feb 27;48(4):627–637. doi: 10.1016/0092-8674(87)90241-8. [DOI] [PubMed] [Google Scholar]
- Ottonello S., Rivier D. H., Doolittle G. M., Young L. S., Sprague K. U. The properties of a new polymerase III transcription factor reveal that transcription complexes can assemble by more than one pathway. EMBO J. 1987 Jul;6(7):1921–1927. doi: 10.1002/j.1460-2075.1987.tb02452.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segall J., Matsui T., Roeder R. G. Multiple factors are required for the accurate transcription of purified genes by RNA polymerase III. J Biol Chem. 1980 Dec 25;255(24):11986–11991. [PubMed] [Google Scholar]
- Sollner-Webb B., Tower J. Transcription of cloned eukaryotic ribosomal RNA genes. Annu Rev Biochem. 1986;55:801–830. doi: 10.1146/annurev.bi.55.070186.004101. [DOI] [PubMed] [Google Scholar]
- Tower J., Sollner-Webb B. Transcription of mouse rDNA is regulated by an activated subform of RNA polymerase I. Cell. 1987 Sep 11;50(6):873–883. doi: 10.1016/0092-8674(87)90514-9. [DOI] [PubMed] [Google Scholar]
- Van Dyke M. W., Roeder R. G. Multiple proteins bind to VA RNA genes of adenovirus type 2. Mol Cell Biol. 1987 Mar;7(3):1021–1031. doi: 10.1128/mcb.7.3.1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dyke M. W., Roeder R. G. Two forms of transcription factor TFIIIC in extracts from HeLa cells. Nucleic Acids Res. 1987 Jul 10;15(13):5031–5039. doi: 10.1093/nar/15.13.5031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshinaga S. K., Boulanger P. A., Berk A. J. Resolution of human transcription factor TFIIIC into two functional components. Proc Natl Acad Sci U S A. 1987 Jun;84(11):3585–3589. doi: 10.1073/pnas.84.11.3585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshinaga S., Dean N., Han M., Berk A. J. Adenovirus stimulation of transcription by RNA polymerase III: evidence for an E1A-dependent increase in transcription factor IIIC concentration. EMBO J. 1986 Feb;5(2):343–354. doi: 10.1002/j.1460-2075.1986.tb04218.x. [DOI] [PMC free article] [PubMed] [Google Scholar]