Introduction
Infections caused by human papillomavirus (HPV) result in considerable morbidity and mortality throughout the world.1–4 Conditions such as anogenital warts, caused by low-risk HPV genotypes and cervical dysplasia, more commonly caused by oncogenic HPV types, can affect females in all age groups.5–9 The prevalence of low and high risk HPV infection in females in the United States in 2003–2004 is 26.8%, estimated from the National Health and Nutrition Examination Survey.10 When analyzed by age group, the prevalence of HPV was 35% in females ages 14–19, 29% in ages 20–29, 13% in ages 30–39, 11% in ages 40–49, and 6.3% in ages 50–65.10,11
HPV is a nonencapsulated, double stranded DNA virus which can infect either cutaneous or mucosal surfaces.3,12 Over 200 types of HPV have been identified.13–15 Forty of those types are known to infect the anogenital tract14–17 and they are classified as low or high risk by their capacity to induce oncogenic changes.
HPV types that are unlikely to cause cancer include HPV 6, 11, 40, 42, 43, 44, 54, 61, 70, and 72.1,18 High risk types HR-HPV are more commonly associated with cancers and include types 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 68, 73, and 82.2–4,7 In North America, HPV 16 and 18 account for 70% of cervical cancer.4,18
HPV infections can cause anogenital and oral lesions in adults as well as the very young. This paper will review the prevalence, transmission, persistence, and clinical manifestations of HR-HPV in very young children.
Mechanism of Action
Microabrasions in the surface epithelium permit the entrance of HPV into the cell where it targets the basal layer of the stratified squamous epithelium. The metaplastic cells of the squamocolumnar junction of the cervix and oropharynx are also targets.1–3
There are five phases in the HPV life cycle, they include (1) infection and uncoating, (2) proliferation, (3) genomic phase, (4) viral synthesis, and (5) shedding.19 In the first phase, infection and uncoating, the basal cells are infected by HPV.19 The second phase is genomic maintenance. During this phase, early viral proteins (E1 andE2) are expressed. The virus maintains its genomic material with a low number of copies (10–200 copies per cell).19 The proliferative phase follows and early proteins E6 and E7 are expressed. These proteins stimulate cell cycle progression and control regulation in the parabasal layer. Genomic amplification follows in the suprabasal layer and early proteins are expressed (E1, E2, E4, and E5). Viral synthesis then occurs and late proteins (L1 and L2) are expressed. In the epithelial layer, these structural proteins enhance viral packaging. At the stratified layer of the epithelium, the virus is released when dead cells are shed and the virus is then free to infect other cells. This infectious cell cycle is thought to occur over a period of time of two to three weeks.4 The incubation period can range from 1–20 months.6
Another possibility in the HPV life cycle is latency. After initial infection, the immune system can induce regression of the viral life cycle and the virus can remain in a latent stage in the basal epithelium (Fig. 1).19
Fig. 1.
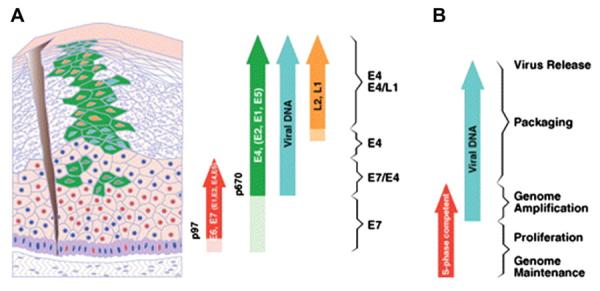
Life cycle of the Human Papillomavirus (reprinted with written permission of J. Doorbar).
Manifestation
Most infections with HPV are subclinical. In very young children (less than four years old), visible manifestations of HPV infection may include condyloma acuminatum.8 Cervical and anal infections in young children are the result of sexual abuse.9 Oral lesions include verrucae vulgaris, papillomas, condylomas, and focal epithelial hyperplasia.20–22 The majority of these lesions (75%) are the result of HPV 6 and 11. Juvenile onset recurrent respiratory papillomatosis (JORRP) is rare, more serious condition that can result is.21–24 Estimated to occur in 4.3/100,000 births, JORRP is caused by infection with HPV types 6 or 11 and is most likely to occur in first born, vaginally delivered children of women less than 20 years old.21,22,24,25
Modes of Transmission
Human Papillomavirus has been shown to be transmitted in several modes.1,2,5–7,9,12,14,20–22,26 The modes of transmission include: sexual contact, non-sexual contact (either directly or indirectly), and maternal contact either directly or indirectly.14,21,26,27 Sexual contact is the primary mode of transmission in both adults and children.7,14,21,28 Transmission can occur through penetrative sexual contact or intimate genital contact.4,26,29
Non-sexual acquisition can occur when HPV on skin surfaces or contaminated fomites comes in contact with microscopic injuries in the skin surface.21,22 This has been demonstrated in school aged children who acquire HPV from the sharing of school supplies.30 Auto inoculation occurs from the transmission of HPV from one site to another scratching or bathing.21,22,31
The possibility of maternal transmission was suggested by Hayek in 1956.32 He first described a condition in which “…multiple laryngeal papillomata are found in small children and adolescents. They are not hereditary, but in 20% of cases can be found at birth.”32 This condition is known as juvenile onset recurrent laryngeal papillomatosis. Thirty years later, maternal HPV infection was linked to these lesions.12,22,33,34 The possible mechanisms of vertical transmission are not well understood. HPV DNA has been isolated from the vas deferens, seminal fluid, and spermatozoa.22 Prenatal transmission of HPV has also been suggested. This is supported by the presence of HPV lesions on the infant at the time of birth.5,22 HPV has been detected in amniotic fluid that was obtained through amniocentesis prior to rupture of membranes35 and from amniotic fluid in a primary cesarean section in which artificial rupture of membranes was performed immediately prior to delivery.36 These examples suggest mechanisms of ascending infection rather than transplacental infection of HPV, since HPV infection does not result in viremia.5,22
Most neonatal HPV infection is vertical transmission at birth.22 There is also a possibility that the neonate is indirectly exposed to HPV on contaminated surfaces in the delivery room.5 HPV DNA can also be transmitted post-natally by caregivers during bathing or diapering.9,21,22
Transmission Rate
Vertical transmission of HR-HPV has been demonstrated when the maternal HPV type is concordant with the HPV type isolated from the newborn. In a large study, Smith found a 71% concordance rate of HPV genotypes in mother-baby pairs. They also found a high concordance (93%) and a low discordance rate when maternal and neonatal antibodies to HPV virus-like particles were analyzed.37 Rombaldi and Gajewska estimate that the prevalence of HPV in pregnant women is 25%.38,39
Several studies have evaluated the rate at which HPV is transmitted from mother to child, but the reported rates of transmission vary. Reported rates of transmission of HPV in women without clinical evidence of HPV to the neonate range from 1–18%.38,40,41 Watts reported a 1% transmission rate of HPV in infants who were born to women without clinical evidence of HPV or the presence of HPV DNA.40 Smith et al found similar rates of transmission in their study population of 203 infants born from 198 women. Of the 203 infants, two had detectable HPV in oral or genital swabs.41,42 At the other end of the spectrum, Fredericks detected HPV DNA in 1/19 (a transmission rate of 5%) infants born from 19 women who had HPV negative cervical swabs.43 Pararkain detected HPV in either buccal or genital swabs in 9% (1/11) of infants born to women without evidence of HPV in cervical swabs using PCR. This number increased to 18% when the infant were examined six weeks later.44
The rate of transmission was also examined in infants whose mothers had clinically evidence of HPV or cervical samples that were positive for HPV DNA at delivery. In women who had detectable HPV during pregnancy, reported HPV transmission rates range from 5–72%.38,42,43
Alberico et al evaluated cervical swabs from pregnant women each trimester; although they found no difference in HPV detection based on gestational age, they suggested that viral load was associated with neonatal transmission.45 Kaye et al studied 15 pregnant women known to be positive for HPV 16, eight women had infants that had HPV DNA at birth. The eight women had higher viral loads (35 to 5 ×106 copies per PCR sample) compared with the seven women who did not (17–195 copies per PCR sample).46
More recently, Rombaldi et al used multiple PCR and nested multiple PCR on maternal cervical swabs, neonatal nasopharyngeal specimens, to determine the rate of maternal HPV transmission.38 Sixty-three mother/ baby pairs were studied. Forty-nine (54.9%) of these women had detectable HPV DNA, 54.9% of which were high risk genotype. Eleven of the forty-nine (22.4%) infants had detectable HPV at delivery (six infants had HPV detected in buccal scrapings and body scrapings, five infants had HPV detected nasopharyngeal aspirate, and three infants had HPV detected in arterial cord blood). One infant had a new infection HPV between the first and sixth month of life (2%). They reported a transmission rate of 24.5%.
In order to clarify the risk of transmission, Medeiros performed a meta-analysis of prospective cohort studies. These studies included pregnant women of all races.47 Nine studies (conducted from 1994–2004) totaling 2111 women and 2113 offspring were included in the analysis. The estimated prevalence of HPV was 24.3%. The transmission rates in these studies varied from 1.5% to 46.6%. The rate of transmission for the pooled data was 6.5%. The pooled relative risk (RR) was 4.8. Analysis was also conducted on the seven studies that compared vaginal delivery versus cesarean delivery. The RR for HPV transmission was 18% in vaginal delivery compared with 8% in cesarean delivery.
Persistence
Few long-term studies have followed HPV in newborns from delivery through childhood. Puranen et al in 1995 followed 98 children born of 66 women.48 Buccal swabs were obtained from children and clinical oral exams were performed. Maternal genital swabs were also analyzed for HPV DNA. These children ranged in age from 4 months to 11.6 years old. Buccal swabs were positive in 31 of 98 (31.6%) samples. Hyperplastic oral changes were seen in 22.4% of the children's oral exams and one child had papilloma which was positive for HPV 16. Fifty-one percent of the children had HPV DNA that was concordant with the HPV DNA detected in their mothers. HPV 16 and 18 accounted for 80.6% of infections. This demonstrated that perinatally transmitted HPV can persist for a period of time.
Pakarian et al prospectively followed 31 women with HR-HPV and their 31 offspring for six weeks after delivery.44 Cervical swabs were positive for HR-HPV in 20 of the 31 (65%) women at the time of delivery. At 24 hours, HR-HPV DNA was isolated in 12/32(38%) of the infants in either buccal or genital samples. At six weeks, 8/30 (26%) infants were HR-HPV positive.
Sixty-two infants and 61 women were followed over a six-month period of time by Cason et al.49 Samples were collected from maternal cervix and vagina and the infants' mouth, penis, mons, and labia were swabbed at 24 hours of life, six weeks post delivery, and six months. HPV 16 was detected in 42/61 women (68.8%) and 13/61 (21.3%) of the women were HPV 18 positive. When DNA was taken from oral and genital swabs (at 24 hours) 33/62 infants were positive HPV 16, 10/62 of the infants were HPV 18 positive, and 9.6% were positive for both HPV 16 and 18. The transmission rate was 69% for HPV 16 and 76.9% for HPV 18. When specimens of the infants were evaluated 6 weeks later, 26/39 (66.6%) were HPV 16 positive (21 of these infants were positive at birth), 7/39 (17.9%) were HPV 18 positive, and 3/39 (7.6%) had both HPV 16 and 18. At six weeks the rate of persistence was 84% for HPV 16 and 75% for HPV 18. At the 6-month interval, 17 infants were available for follow-up. Eleven of seventeen were HPV 16 positive or a 83% rate of persistence. One of the five infants (20%) was positive for HPV 18 at 6-month follow-up.41
Although these earlier studies provided information about neonatal HPV, they are limited by their small sample size.
The Finnish Family Study followed 324 infants prospectively for 36 months.50 Oral scrapings and genital swabs were obtained at birth, day 3 of life, and at the end of the first, second, sixth, twelfth, and thirty-sixth month. At delivery, 14% of oral scrapings and 15% genital swabs were positive for HR-HPV. At 26 months, 10% of the infants had persistent oral carriage, 11% had cleared their HPV, 42% had acquired HR-HPV, and 37% were negative.
When the genital swabs were evaluated at 26 months, 1.5% of the infants had persistent HR-HPV, 14% had cleared their HPV, 36% had acquired HR-HPV, and 47% were negative. They concluded that persistent oral carriage in the infant was associated with persistent oral HR-HPV in the parents and the presence of hand warts on the mother.
Another component of the Finnish HPV Family Study, 76 families were followed over two years.51 They found the highest rate of HPV at 6 months time (18% in genital samples and 22% in oral). By 12 months, the oral HPV rate decreased by 7%. A change in the infants' immunity at 6 months was accredited.
Multivariant analysis was used to evaluate the dynamics of familial HPV. Persistent cervical HR-HPV in the mother (after 24 months) was a strong risk factor for the infants in the acquisition of oral HPV DNA. There was also a high odds ratio for the presence of HPV in the infants' genital if the mother's oral swabs were positive for HR-HPV six months postpartum.
The studies that summarize HPV persistence are listed in Table 1.
Table 1.
Results of Studies Evaluating HPV Transmission and Clearance in the Infant
| Study | Maternal Subjects | Newborn Subjects | Mothers with HPV DNA | Fathers with HPV DNA | Newborns with HPV DNA at Birth | Newborns with HPV DNA at Completion | Follow-up |
|---|---|---|---|---|---|---|---|
| Pakarian et al44 | 31 | 32 | 65% | NR | 38% | 26% | 6 weeks |
| Cason et al49 | 61 | 62 | 68.8% HPV 16 | NR | 54.1% with HPV 16 | 83% with HPV 16 | 6 months |
| 21.3% HPV 18 | 16.1% with HPV 18 | 20% with HPV 18 (17 infants were available for follow- up | |||||
| Rintala et al51 | 76 | 77 | 16% | 16% | 10% oral | 15% oral | 24 months |
| 10% genital | 10% genital | ||||||
| Rintala et al50 | 307 | 324 | NR | NR | 14% oral | 10% oral | 26.2 months |
| 15% genital | 1.5% genital |
NR, not reported
Conclusion
Given the prevalence of HR-HPV in the population, it is important to review the manner in which the virus can be transmitted to the newborn along with the clinical relevance. While the exact rate of transmission has not been determined, it is clear that HR-HPV can be transmitted during the delivery process. There is also a possibility that HR-HPV can be transmitted to the newborn or infant through by sexual or non-sexual contact. Several studies have demonstrated that HPV can be acquired in the neonatal period and in some infants HPV can persist for up to 26 months. More often, HPV can persist in the oral cavity and induce clinical changes characteristic of HPV long after delivery. It is not know why HPV persist in oral cavity, but Rintala et al suggested that the reservoir for HPV in the oral mucosa may be gingival pockets in emerging teeth.51
It is also not clear if there is a role for the current HPV vaccines in the children less than 9 years old. The current Advisory Committee on Immunization Practices (ACIP) recommendation is that vaccination of females with bivalent or quadrivalent HPV vaccine beginning at 11 or 12 years of age (the series can begin at age 9). The quadrivalent vaccine can also be given to males ages 9–26 years old, but it has not yet been added to the male vaccination schedule. The CDC also states that, “Ideally, vaccine should be administered before potential exposure to HPV through sexual contact.”52
The administration of children, youth, and families estimated 772,000 cases of child maltreatment were reported in 2008 and approximately 9% of these cases involved sexual abuse.53 Given these numbers, there is a real possibility that some children may be exposed to HPV before they are in the recommended age range for vaccination.
References
- 1.Ault KA. Epidemiology and natural history of human papillomavirus infections in the female genital tract. Infect Dis Obstet Gynecol. 2006;2006(Suppl):40470. doi: 10.1155/IDOG/2006/40470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schiffman M, Castle PE, Jeronimo J, et al. Human papillomavirus and cervical cancer. Lancet. 2007;370(9590):890. doi: 10.1016/S0140-6736(07)61416-0. [DOI] [PubMed] [Google Scholar]
- 3.Wiley D, Masongsong E. Human papillomavirus: the burden of infection. Obstet Gynecol Surv. 2006;61(6 Suppl 1):S3. doi: 10.1097/01.ogx.0000221010.82943.8c. [DOI] [PubMed] [Google Scholar]
- 4.Stanley M. Pathology and epidemiology of HPV infection in females. Gynecol Oncol. 2010;117:S5. doi: 10.1016/j.ygyno.2010.01.024. [DOI] [PubMed] [Google Scholar]
- 5.Jayasinghe Y, Garland SM. Genital warts in children: what do they mean? Arch Dis Child. 2006;91:696. doi: 10.1136/adc.2005.092080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Allen AL, Siegfried EC. The natural history of condyloma in children. J Am Acad Dermatol. 1998;39:951. doi: 10.1016/s0190-9622(98)70268-3. [DOI] [PubMed] [Google Scholar]
- 7.Stevens-Simon C, Nelligan D, Breese P, et al. The prevalence of genital human papillomavirus infections in abused and nonabused preadolescent girls. Pediatrics. 2000;106:645. doi: 10.1542/peds.106.4.645. [DOI] [PubMed] [Google Scholar]
- 8.Moscicki AB. Genital HPV infections in children and adolescents. Obstet Gynecol Clin North Am. 1996;23:675. [PubMed] [Google Scholar]
- 9.Gutman LT, St Claire KK, Everett VD, et al. Cervical-vaginal and intraanal human papillomavirus infection of young girls with external genital warts. J Infect Dis. 1994;170:339. doi: 10.1093/infdis/170.2.339. [DOI] [PubMed] [Google Scholar]
- 10.Datta SD, Koutsky LA, Ratelle S, et al. Human papillomavirus infection and cervical cytology in women screened for cervical cancer in the United States, 2003–2005. Ann Intern Med. 2008;148:493. doi: 10.7326/0003-4819-148-7-200804010-00004. [DOI] [PubMed] [Google Scholar]
- 11.Dunne EF, Unger ER, Sternberg M, et al. Prevalence of HPV infection among females in the United States. JAMA. 2007;297:813. doi: 10.1001/jama.297.8.813. [DOI] [PubMed] [Google Scholar]
- 12.Cason J, Mant CA. High-risk mucosal human papillomavirus infections during infancy & childhood. J Clin Virol. 2005;32(Suppl 1):S52. doi: 10.1016/j.jcv.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 13.Howley PM. Papillomavirus and their replication. In: Knipe DM, Howley PM, editors. Fields Virology. 4th ed Lippincott-Raven; Philadelphia: 2002. pp. 2197–2229. [Google Scholar]
- 14.American College of Obstetricians and Gynecologists ACOG Practice Bulletin: Clinical Management Guidelines for Obstetrician-Gynecologists. Number 61, April 2005. Human papillomavirus. Obstet Gynecol. 2005;105:905. doi: 10.1097/00006250-200504000-00056. [DOI] [PubMed] [Google Scholar]
- 15.Hager WD. Human papilloma virus infection and prevention in the adolescent population. J Pediatr Adolesc Gynecol. 2009;22:197. doi: 10.1016/j.jpag.2008.01.079. [DOI] [PubMed] [Google Scholar]
- 16.Schiffman M, Castle PE. Human papillomavirus: epidemiology and public health. Arch Pathol Lab Med. 2003;127:930. doi: 10.5858/2003-127-930-HPEAPH. [DOI] [PubMed] [Google Scholar]
- 17.Wiley DJ, Douglas J, Beutner K, et al. External genital warts: diagnosis, treatment, and prevention. Clin Infect Dis. 2002;35(Suppl 2):S210. doi: 10.1086/342109. [DOI] [PubMed] [Google Scholar]
- 18.Muñoz N, Bosch FX, de Sanjose S, et al. Epidemiologic classification of human papillomavirus types associated with cervical cancer. N Engl J Med. 2003;348:518. doi: 10.1056/NEJMoa021641. [DOI] [PubMed] [Google Scholar]
- 19.Doorbar J. The papillomavirus life cycle. J Clin Virol. 2005;32(Suppl 1):S7. doi: 10.1016/j.jcv.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 20.Summersgill KF, Smith EM, Levy BT, et al. Human papillomavirus in the oral cavities of children and adolescents. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2001;91:62. doi: 10.1067/moe.2001.108797. [DOI] [PubMed] [Google Scholar]
- 21.Syrjänen S, Puranen M. Human papillomavirus infections in children: the potential role of maternal transmission. Crit Rev Oral Biol Med. 2002;11:259. doi: 10.1177/10454411000110020801. [DOI] [PubMed] [Google Scholar]
- 22.Syrjänen S. Current concepts on human papillomavirus infections in children. APMIS. 2010;118:494. doi: 10.1111/j.1600-0463.2010.02620.x. [DOI] [PubMed] [Google Scholar]
- 23.Derkay CS, Wiatrak B. Recurrent respiratory papillomatosis: a review. Laryngoscope. 2008;118:1236. doi: 10.1097/MLG.0b013e31816a7135. [DOI] [PubMed] [Google Scholar]
- 24.Sun JD, Weatherly RA, Koopmann CF, Jr, et al. Mucosal swabs detect HPV in laryngeal papillomatosis patients but not family members. Int J Pediatr Otorhinolaryngol. 2000;53:95. doi: 10.1016/s0165-5876(00)00304-9. [DOI] [PubMed] [Google Scholar]
- 25.Sinclair KA, Woods CR, Kirse DJ, et al. Anogenital and respiratory tract human papillomavirus infections among children: age, gender, and potential transmission through sexual abuse. Pediatrics. 2005;116:815. doi: 10.1542/peds.2005-0652. [DOI] [PubMed] [Google Scholar]
- 26.Doerfler D, Bernhaus A, Kottmel A, et al. Human papilloma virus infection prior to coitarche. Am J Obstet Gynecol. 2009;200:487.e1. doi: 10.1016/j.ajog.2008.12.028. [DOI] [PubMed] [Google Scholar]
- 27.Kjaer SK, Chackerian B, van den Brule AJ, et al. High-risk human papillomavirus is sexually transmitted: evidence from a follow-up study of virgins starting sexual activity (intercourse) Cancer Epidemiol Biomarkers Prev. 2001;10:101. [PubMed] [Google Scholar]
- 28.Koch A, Hansen SV, Nielsen NM, et al. HPV detection in children prior to sexual debut. Int J Cancer. 1997;73:621. doi: 10.1002/(sici)1097-0215(19971127)73:5<621::aid-ijc1>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 29.Winer RL, Lee SK, Hughes JP, et al. Genital human papillomavirus infection: incidence and risk factors in a cohort of female university students. Am J Epidemiol. 2003;157:218. doi: 10.1093/aje/kwf180. [DOI] [PubMed] [Google Scholar]
- 30.Bunney MH, Barr BB, McLaren K, et al. Human papillomavirus type 5 and skin cancer in renal allograft recipients. Lancet. 1987;2(8551):151. doi: 10.1016/s0140-6736(87)92346-4. [DOI] [PubMed] [Google Scholar]
- 31.Myhre AK, Dalen A, Berntzen K, et al. Anogenital human papillomavirus in non-abused preschool children. Acta Paediatr. 2003;92:1445. [PubMed] [Google Scholar]
- 32.Hajek EF. Contribution to the etiology of laryngeal papilloma in children. J Laryngol Otol. 1956;70:166. doi: 10.1017/s0022215100052798. [DOI] [PubMed] [Google Scholar]
- 33.Cason J, Rice P, Best JM. Transmission of cervical cancer-associated human papilloma viruses from mother to child. Intervirology. 1998;41:213. doi: 10.1159/000024939. [DOI] [PubMed] [Google Scholar]
- 34.Sedlacek TV, Lindheim S, Eder C, et al. Mechanism for human papillomavirus transmission at birth. Am J Obstst Gynecol. 1989;161:55. doi: 10.1016/0002-9378(89)90232-9. [DOI] [PubMed] [Google Scholar]
- 35.Armbruster-Moraes E, Ioshimoto LM, Leão E, et al. Presence of human papillomavirus DNA in amniotic fluids of pregnant women with cervical lesions. Gynecol Oncol. 1994;54:152. doi: 10.1006/gyno.1994.1185. [DOI] [PubMed] [Google Scholar]
- 36.Rogo KO, Nyansera PN. Congenital condylomata acuminata with meconium staining of amniotic fluid and fetal hydrocephalus: case report. East Afr Med J. 1989;66:411. [PubMed] [Google Scholar]
- 37.Smith EM, Parker MA, Rubenstein LM, et al. Evidence for vertical transmission of HPV from mothers to infants. Infect Dis Obstet Gynecol. 2010;2010:326369. doi: 10.1155/2010/326369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rombaldi RL, Serafini EP, Mandelli J, et al. Perinatal transmission of human papilomavirus DNA. Virol J. 2009;6:83. doi: 10.1186/1743-422X-6-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gajewska M, Wielgos M, Kaminski P, et al. The occurrence of genital types of human papillomavirus in normal pregnancy and in pregnant renal transplant recipients. Neuro Endocrinol Lett. 2006;27:529. [PubMed] [Google Scholar]
- 40.Watts DH, Koutsky LA, Holmes KK, et al. Low risk of perinatal transmission of human papillomavirus: results from a prospective cohort study. Am J Obstet Gynecol. 1998;178:365. doi: 10.1016/s0002-9378(98)80027-6. [DOI] [PubMed] [Google Scholar]
- 41.Smith EM, Ritchie JM, Yankowitz J, et al. Human papillomavirus prevalence and types in newborns and parents: concordance and modes of transmission. Sex Transm Dis. 2004;31:57. doi: 10.1097/01.OLQ.0000105327.40288.DB. [DOI] [PubMed] [Google Scholar]
- 42.Smith EM, Johnson SR, Cripe T, et al. Perinatal transmission and maternal risks of human papillomavirus infection. Cancer Detect Prev. 1995;19:196. [PubMed] [Google Scholar]
- 43.Fredericks BD, Balkin A, Daniel HW, et al. Transmission of human papillomaviruses from mother to child. Aust N Z J Obstet Gynaecol. 1993;33:30. doi: 10.1111/j.1479-828x.1993.tb02047.x. [DOI] [PubMed] [Google Scholar]
- 44.Pakarian F, Kaye J, Cason J, et al. Cancer associated human papillomaviruses: perinatal transmission and persistence. Br J Obstet Gynaecol. 1994;101:514. doi: 10.1111/j.1471-0528.1994.tb13153.x. [DOI] [PubMed] [Google Scholar]
- 45.Alberico S, Pinzano R, Comar M, et al. [Maternal-fetal transmission of human papillomavirus] Minerva Ginecol. 1996;48:199. [PubMed] [Google Scholar]
- 46.Kaye JN, Cason J, Pakarian FB, et al. Viral load as a determinant for transmission of human papillomavirus type 16 from mother to child. J Med Virol. 1994;44:415. doi: 10.1002/jmv.1890440419. [DOI] [PubMed] [Google Scholar]
- 47.Medeiros LR, Ethur AB, Hilgert JB, et al. Vertical transmission of the human papillomavirus: a systematic quantitative review. Cad Saude Publica. 2005;21:1006. doi: 10.1590/s0102-311x2005000400003. [DOI] [PubMed] [Google Scholar]
- 48.Puranen M, Yliskoski M, Saarikoski S, et al. Vertical transmission of human papillomavirus from infected mothers to their newborn babies and persistence of the virus in childhood. Am J Obstet Gynecol. 1996;174:694. doi: 10.1016/s0002-9378(96)70452-0. [DOI] [PubMed] [Google Scholar]
- 49.Cason J, Kaye JN, Jewers RJ, et al. Perinatal infection and persistence of human papillomavirus types 16 and 18 in infants. J Med Virol. 1995;47:209. doi: 10.1002/jmv.1890470305. [DOI] [PubMed] [Google Scholar]
- 50.Rintala MA, Grénman SE, Järvenkylä ME, et al. High-risk types of human papillomavirus (HPV) DNA in oral and genital mucosa of infants during their first 3 years of life: experience from the Finnish HPV Family Study. Clin Infect Dis. 2005;41:1728. doi: 10.1086/498114. [DOI] [PubMed] [Google Scholar]
- 51.Rintala MA, Grenman SE, Puranen MH, et al. Transmission of high-risk human papillomavirus (HPV) between parents and infant: a prospective study of HPV in families in Finland. J Clin Microbiol. 2005;43:376. doi: 10.1128/JCM.43.1.376-381.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Centers for Disease Control and Prevention (CDC) FDA licensure of bivalent human papillomavirus vaccine (HPV2, Cervarix) for use in females and updated HPV vaccination recommendations from the Advisory Committee on Immunization Practices (ACIP) MMWR Morb Mortal Wkly Rep. 2010;59:626. [PubMed] [Google Scholar]
- 53.Department of Health and Human Services. Administration of Children, Youth, and Families . Child Maltreatment 2008. Government Printing Office; Washington, DC: 2008. [Google Scholar]


