Abstract
Vascular smooth muscle cells (VSM) dedifferentiate from the contractile to synthetic phenotype in response to acute vascular diseases such as restenosis and chronic vascular diseases such as atherosclerosis and contribute to growth of the neointima. We recently demonstrated that balloon catheter injury of rat carotid arteries resulted in increased expression of CaMKIIδ2 in the medial wall and the expanding neointima. These findings led us to hypothesize that increased expression of CaMKIIδ2 is a positive mediator of synthetic VSM. HDAC4 and HDAC5 function as transcriptional corepressers and are regulated in a CaMKII-dependent manner. In this study, we report that endogenous HDAC4 and HDAC5 in VSM are activated in a Ca2+- and CaMKIIδ2-dependent manner. We further show that AngII-and PDGF-dependent phosphorylation of HDAC4 and HDAC5 is reduced when CaMKIIδ2 expression is suppressed or CaMKIIδ2 activity is attenuated. The transcriptional activator MEF2 is an important determinant of VSM phenotype and is regulated in an HDAC-dependent manner. Herein, we report that stimulation of VSM cells with ionomycin or Ang II potentiates MEF2's ability to bind DNA and increases the expression of established MEF2 target genes Nur77 and MCP1. Suppression of CaMKIIδ2 attenuates increased MEF2 DNA binding activity and upregulation of Nur77 and MCP1. Finally, we show that HDAC5 is regulated by HDAC4 in VSM. Suppression of HDAC4 expression and activity prevents AngII- and PDGF- dependent phosphorylation of HDAC5. Taken together, these results illustrate a mechanism by which CaMKIIδ2 mediates MEF2-dependent gene transcription in VSM cells through regulation of HDAC4 and HDAC5.
Keywords: Ca2+/Calmodulin-dependent Protein Kinase II (CaMKII), Mef2, HDAC, vascular smooth muscle cells (VSM), gene transcription
Introduction
Vascular smooth muscle (VSM) cells are not terminally differentiated and can undergo dramatic changes in phenotype following acute and chronic vascular injuries [1]. This phenotype switch is characterized by loss in expression of transcription factors such as myocardin and contractile proteins including α-smooth muscle actin (α-SMA) and smooth muscle myosin heavy chain (SMMHC) that are required for differentiated smooth muscle contractile function. Concomitant with the reduced expression of these proteins is the upregulation of genes and proteins associated with the proliferative/synthetic state such as KLF4, versican, and PDGFR [1;2]. This phenotype switch also includes changes in a repertoire of Ca2+ signaling proteins including down-regulation of 1-type voltage-gated Ca2+ channels and ryanodine receptors [3;4], up-regulation of store-operated STIM1/Orai1 channels [5], and changes in relative expression of various TRP channels [6]and SERCA pump isoforms [4]. Changes in expression of Ca2+ signaling proteins can affect the kinetics and localization of Ca2+ signals and result in differential coupling of Ca2+ signals to transcriptional events, as summarized in recent reviews on excitation-transcription coupling in VSM [7] [8].
Consistent with this concept, our laboratory has documented that the multifunctional serine/threonine protein kinase, Ca2+/calmodulin-dependent protein kinase II (CaMKII) isoform expression pattern is altered in VSM after vascular injury [9]. Studies in intact rat carotid arteries revealed that CaMKIIγ, which has been associated with modulation of contractile function [10] predominates in differentiated medial smooth muscle, but its expression decreases rapidly after balloon catheter injury and is accompanied by a significant upregulation of the CaMKIIδ2 isoform in both injured medial wall and neointimal VSM[9]. Suppression of injury-induced CaMKIIδ2 upregulation with shRNA inhibited VSM proliferation and attenuated neointimal formation [9]. The function of CaMKIIδ in the response to vascular injury was recently confirmed using a carotid ligation injury model in a CaMKIIδ null mouse [11]. These results and previous in vitro studies are consistent with a function for CaMKIIδ isoforms in modulating VSM cell proliferation [12] and migration [13]. The rapid kinetics of the reciprocal CaMKII isoform switch in response to injury also raises the possibility that either the loss of CaMKIIγ and/or gain of CaMKIIδ may be involved in regulating the transition of differentiated VSM to the synthetic phenotype, potentially by differential coupling of these isoforms to specific transcriptional processes.
A potential transcriptional target for CaMKII is myocyte enhancing factor 2 (MEF2), a MADS-box family of DNA binding transcription factors with essential roles in a wide range of cell types including skeletal muscle, cardiomyocytes, neurons, and hematopoietic cells [14-16] [17;18]. Studies in transgenic mice demonstrate that MEF2 regulates smooth muscle expression of HRC and myocardin, which in turn positively regulates the transcription of most smooth muscle specific genes studied to date [19;20]. In contrast to these data indicating a role in the differentiated phenotype, two early studies demonstrated that MEF2 expression and activity are upregulated in the neointima of rat carotid arteries after balloon catheter injury and are required for synthetic-phenotype VSM cell proliferation in culture [21] [22]. Recently, a similar increase of MEF2 activity was reported in mouse neointima using a MEF2 reporter mouse [23].
MEF2 can act as either an activator or repressor depending on interaction with co-activators or co-repressors, respectively. HDAC4 and HDAC5 are class IIa HDACs that directly interact with MEF2 to promote its repressive activity [24]. Insight into the mechanisms coupling CaMKII activation to MEF2 activation are gained from studies in cardiomyocytes where MEF2 co-repressors HDAC4 and HDAC5 have been shown to be substrates for CaMKII phosphorylation leading to nuclear export and cytoplasmic sequestration through interaction with 14-3-3 proteins [25] [26] [27]. Studies in VSM evaluating CaMKII-dependent regulation of type IIa HDACs and MEF2 are limited and somewhat conflicting. An early study suggested that CaMKIIδ2 acts by phosphorylating 14-3-3 proteins in the cytosol, disrupting their ability to interact with and localize HDAC4 to the cytosol, thereby promoting interaction with nuclear MEF2 to repress transcription [28]. Conversely, treatment of VSM by the growth factor PDGFBB was shown to result in HDAC4 phosphorylation and cytoplasmic sequestration, with activation of MEF2 and subsequent c-jun expression in cultured VSM [23]. Similarly, CaMKII was implicated in angiotensin II (AII)-dependent VSM hypertrophy through phosphorylation of HDAC4 to de-repress the MEF2 complex [29]. A study by Pang et al indicated that CaMKIIδ2 forms a functional complex with the scaffolding protein GIT1 and HDAC5, mediating AII-dependent phosphorylation of HDAC5 and activation of MEF2 [30]. Conversely, Xu et al indicated that AII-dependent phosphorylation of HDAC5 and subsequent increases in MEF2 activity are PKC/PKD-dependent and do not involve CaMKII [31]. Thus, the conditions and mechanisms by which CaMKII regulates HDACs in VSM, and the relative contribution of this pathway in regulating MEF2 activity, remain unclear.
The goal of this study was to systematically evaluate the relative contribution of CaMKIIδ in the regulation of endogenous HDAC4, HDAC5, and MEF2 in VSM cells in response to two relevant stimuli, angiotensin II and PDGF, which activate Ca2+ signaling via distinct receptor coupled mechanisms. Using gain and loss of function molecular approaches we establish CaMKIIδ isoform-dependent regulation of endogenous HDAC4 and HDAC5 in response to both stimuli in VSM and demonstrate that HDAC4 is required for coupling of the Ca2+ signaling pathways to HDAC5. Downstream coupling of this regulatory mechanism to activation of MEF2 and transcription of the known MEF2 target genes Nur77 and MCP1 was stimulus-dependent. Angiotensin II-stimulated activation of MEF2 and induction of Nur77 and MCP1 was strongly dependent upon CaMKIIδ while PDGF stimulation of these transcription readouts was relatively independent of CaMKIIδ, suggesting additional redundant MEF2 activation pathways invoked by this growth factor.
Materials and methods
Cell Culture
Vascular smooth muscle cells (VSM) were obtained from the medial layer of the thoracic aorta of 200-300 gram Sprague-Dawley rats as described previously [32]. Briefly, the adventitial and endothelial layer of intact thoracic aortas was removed; the medial layer was then enzymatically dispersed and cultured in DMEM/F12 + 10% fetal bovine serum (Hyclone). The VSM cells were maintained at subconfluent densities by passaging twice a week using standard cell culture techniques and conditions (37°C with 5% CO2). All experiments were performed on cells passaged 3-10 times.
Cell Lysates and Immunoblotting
Cells were lysed (0.5 ml/60 mm dish or 1 ml/100 mm dish) in a modified RIPA buffer (10 mM Tris pH 7.4, 100 mM NaCL, 1 mM EDTA, 1 mM EGTA, 1 mM NaF, 20 mM Na4P2O7, 2 mM Na3VO4, 1% Triton X-100, 0.1 % SDS, 0.5 % deoxycholate, 10% glycerol, 1 mM DTT, 0.1 mM PMSF, and 0.2 U/ml aprotinin). Samples were resolved using standard SDS –PAGE procedures and transferred to nitropure nitrocellulose (GE Lifesciences) before immunoblotting. Standard immunoblotting using 5% nonfat dry milk or 3 % BSA was performed. Incubation with the primary antibody was either 1 hr at room temperature or overnight at 4°C as needed (indicated in the figure legends). Nitrocellulose membranes were incubated in Enhanced Chemiluminescent substrate (GE Lifesciences) and visualized using either standard X-Ray film (E.M. Parker) or a Fuji LAS 4000 (Fuji Inc.,Tokyo,Japan)).
Immunoprecipitations
Cell lysates were made using RIPA buffer as described above. The cell lysates were cleared by centrifugation at 14,000 × g for 10 min. After transferring to a clean tube, primary antibody was added and incubated overnight with rocking. Washed Protein A beads (Thermo Scientific, Rockford, Ill) were then added to the antibody/lysate mixture and incubated for an additional 90 min. The antibody/Protein A bead complex was then washed 3× with RIPA and resolved with SDS-PAGE and immunnoblotting as described above.
Immunoflourescence and Confocal Microscopy
VSM cells were seeded onto collagen (PureCol) coated glass coverslips previously coated with collagen. The cells were fixed in 4% paraformeldehyde and permeabolized with brief exposure to a triton-X100 containing buffer. After incubation with primary antibody, the cells were incubated with secondary antibody that fluoresces at specific wave lengths (Molecular Probes Inc.,) For indirect immunoflourescence, the cells were imaged with a Leica DM IRB inverted fluorescent microscope (Leica Microsystems, Bannockburn USA). For confocal microscopy, the cells were imaged with a Zeiss LSM 510 META confocal microscope (Carl Zeiss, Inc., Thornwood, NY).
Cell Transfections and adenoviral infections
For introduction of siRNA, VSM cells were transfected using the Amaxa Nucleofectin system (Lonza Group LTD., Switzerland) according to the prescribed specifications for VSM cells. For introduction of expression plasmids into the VSM cells, the Fugene HD (Roche Applied Science, Mannheim, Germany) transfection reagent was used according to manufacturer's specifications. For adenoviral infection, VSM cells at 70% confluence were infected at a multiplicity of infection (MOI) of 50 with purified adenovirus shDELTA2, shGFP, Kinase Negative CaMKIIδ2 in the presence of 10% FBS medium. All adenovirus stocks were propagated by adding small amounts of virus to HEK293 cells. When cells were approximately 50% lysed, cells and media were collected, subjected to 3X freeze/thaw cycles, aliquoted and stored at -80°C.
MEF2 DNA Binding Assay
MEF2 DNA binding was measured using a DNA binding assay (TransAm MEF2 Assay, Active Motif, Carlsbad,CA). Briefly, nuclear extracts were prepared from confluent 100 mm dishes of VSM cells using Active Motif's nuclear extract kit according to manufacturer's specifications. The nuclear extracts were then incubated in 96-well plates coated with oligonucleotide DNA corresponding to the MEF2 consensus binding site. Bound (active) MEF2 is detected by incubation with MEF2 antibody, a HRP-conjugated secondary antibody, and spectrophotometric analyses.
Quantitative PCR
Total RNA was extracted from cells after 1 hr treatment with 0.5 μM ionomycin or 100 nm Ang II with Trizol (Invitrogen, Carlsbad, CA) according to manufacturer's specifications. The first-strand cDNA was synthesized using QuantiTect Reverse Transcription (Qiagen, Valencia, CA). The quantitative RT-PCR was performed on M×3000P QPCR System using Brilliant III Ultra-Fast SYBR (Agilient, Santa Clara, CA). The primers for rat targets genes and internal controls were as follows: GAPDH forward 5′-TCG TCT CAT AGA CAA GAT GGT-3′, and reverse, 5′-GTA GTT GAG GTC AAT GAA GGG-3′; Nur77 forward 5′-AGT CCG CCT TTC TGG AGC TCT TTA-3′, and reverse, 5′-CAG GCA AAG GCA GGA ACA TCA ACA-3′; MCP1 forward 5′-TGT TCA CAG TTG CTG CCT GT-3′, and reverse 5-ACC TGC TGC TGG TGA TTC TCT T-3′
Materials
Polyclonal antibodies to HDAC4 and HDAC7 were purchased from Santa Cruz, Inc. (Santa Cruz, CA). Polyclonal antibody to HDAC5 was purchased from Signalway Antibody Inc. Antibody to phosphorylated HDAC4 (Ser 632) was purchased from Abnova and antibody to phosphorylated HDAC5 (ser498) was purchased from Abcam. Antibodies for CaMKIIδ2 and pan-CaMKII were produced as described previously [33] [34]. shDELTA2 was produced as described previously [12]. Smart pools for HDAC4 (SiHDAC4) and CaMKIIδ2 (SiCaMKIIδ2) were purchased from Dharmacon Inc. Adenoviral constructs expressing kinase negative CaMKIIδ2 and GFP were described previously [35]. Expression plasmids encoding GFP (pMAXGFP) was purchased fom Lonza. Expression plasmid encoding mutant HDAC4 (Ser278,478 and 632 to Ala) was purchased from Addgene Inc. All tissue culture media was purchased from Gibco-BRL (Life Technologies) unless specifically stated. Tissue culture supplies (dishes, pipettes, etc) were purchased from Fisher Scientific. SDS-PAGE and Western blotting supplies were purchased from Bio-Rad Inc. unless otherwise stated. All other chemicals were purchased from Sigma Inc. (St. Louis, MO).
Statistics
All data are expressed as means +/− SE. Mean values of groups were compared by ANOVA with post hoc comparisons using the Newman-Keuls test. For all comparisons, P<0.05 was considered statistically significant.
Results
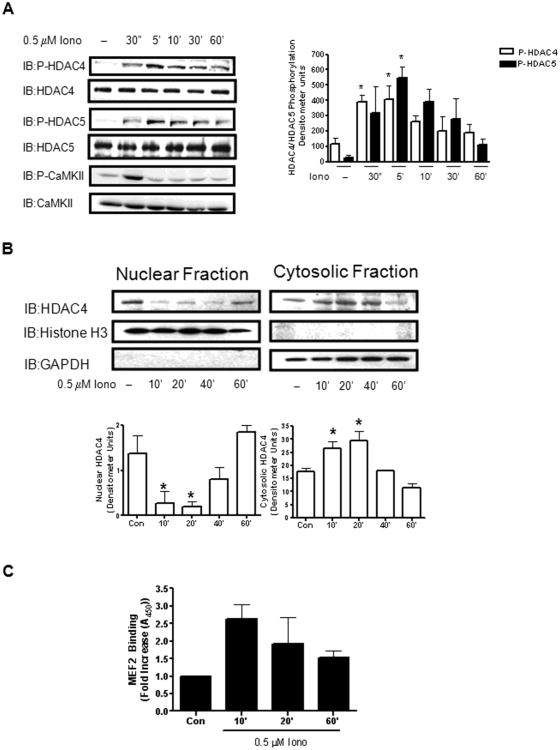
To determine the extent that an isolated Ca2+-dependent pathway mediates HDAC4 and HDAC5 regulation in VSM cells, we stimulated cultured VSM cells with the Ca2+ ionophore, ionomycin (Iono), over a time course of 60 min. Iono-dependent phosphorylation of HDAC4 and HDAC5 on serines 632 and 498, respectively, could be detected as early as 30 sec after stimulation, peaked after 5 min stimulation, and decreased to basal levels by 60 min (Figure 1A). Cell fractionation of VSM cells after a similar timecourse of Iono stimulation revealed that the levels of HDAC4 present in the nucleus decreased as compared to unstimulated cells as early as 10 min after Iono stimulation and remained low through 40 min Iono stimulation. After 60 min Iono stimulation, the levels of HDAC4 found in the nuclear fraction returned to levels found in unstimulated cells (Figure 1B, left panel). Conversely, stimulation of VSM cells with Iono resulted in an increase of HDAC4 levels found in the cytosol as compared to unstimulated cells as early as 10 min after stimulation. After 60 min Iono stimulation, HDAC4 levels in the cytosol were similar to those found in unstimulated cells (Figure 1B, right panel). These results are consistent with a previous finding [29] that phosphorylation of class IIa HDACs such as HDAC4 confines their intracellular location to VSM cell's cytosolic compartment.
Figure 1. Ca2+-dependent regulation of HDAC4 and HDAC5 in VSM.
(A) Whole cell lysates from cultured VSM cells stimulated with 0.5 μM ionomycin for the indicated times were immunoblotted for phosphorylated HDAC4 (IB:P-HDAC4ser632) or HDAC5 (IB:P-HDAC5ser 498). The lysates were further immunoblotted for active CaMKII (IB: P-CaMKII). Total HDAC4, HDAC5, and CaMKII was immunoblotted to insure equal protein loading. The histogram represents quantification of four immunoblots of phosphorylated HDAC4 or HDAC5. (B) Nuclear and cytosolic fractions were isolated from cultured VSM cells treated with 0.5 μM ionomycin (Iono) as indicated. These lysates were resolved by SDS-PAGE and immunoblotted for total HDAC4 (IB:HDAC4). Histograms represent quantification of three separate experiments. Fractions were immunoblotted for Histone H3 and GAPDH respectively to distinguish the nuclear fraction from the cytosolic fraction. (C) VSM cells were stimulated with Iono as indicated and MEF2 DNA binding was determined as described in the “Methods”.* P< 0.05.
Regulation of HDAC4 and HDAC5 has been closely linked to regulation of MEF2 activity [24]. To determine the extent to which MEF2's ability to bind DNA is regulated by Ca2+-dependent stimuli in VSM, cultured cells were stimulated with Iono over a 60 minute time course and MEF2's ability to bind its DNA consensus sequence was measured. MEF2 DNA binding increased nearly 3-fold after 10 min Iono stimulation as compared to unstimulated cells (Figure 1C). These results illustrate a tight relationship between HDAC4 and HDAC5's phosphorylation, their intracellular location, and their putative ability to mediate MEF2 DNA binding.
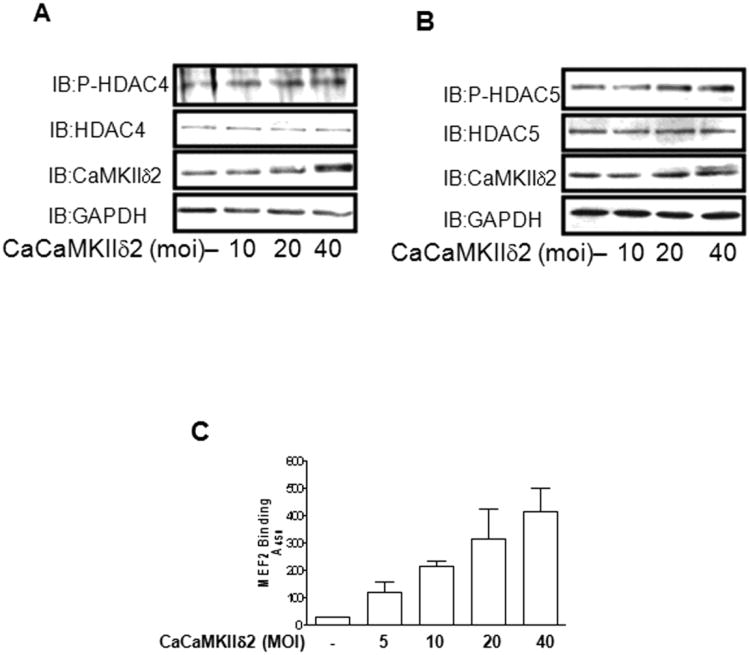
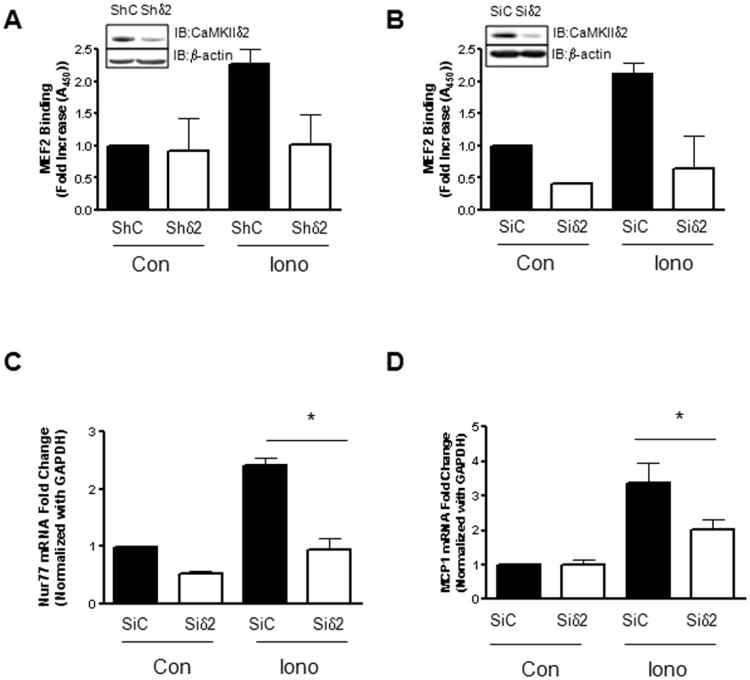
Along with the previously described ability to induce HDAC4 and HDAC5 phosphorylation in Figure 1, Iono stimulation is well known to result in a robust activation of CaMKII in VSM [36;37]. Peak activation of CaMKII was detected within 30 sec of ionomycin stimulation in the present study, as reflected by autophosphorylation on phospho-serine287 (Figure 1A). To investigate the potential link between CaMKIIδ2 activation and HDAC4/HDAC5 phosphorylation, we adenovirally over-expressed a constitutively active form of CaMKIIδ2 (T287D) [38] in VSM cells. Relatively low expression levels of constitutively active CaMKIIδ2 resulted in HDAC4 and HDAC5 phosphorylation (Figure 2A, B). Importantly, no apparent increase of PKD activity was detected (data not shown) as PKD has been shown to mediate HDAC4 and HDAC5 phosphorylation in VSM cells [31]. Similarly, over-expression of constitutively active CaMKIIδ2 increased MEF2 DNA binding consistent with the increasing levels of HDAC4 and HDAC5 phosphorylation detected (Figure 2C). To further assess the contribution of CaMKIIδ-dependent processes in Ca2+-dependent MEF2 DNA binding and gene transcription, CaMKIIδ2 was suppressed with a CaMKIIδ2 specific shRNA sequence introduced by adenoviral infection [39] (Figure 3A) or siRNA duplexes introduced by electroporation (Figure 3B). In both cases, suppression of CaMKIIδ2 attenuated ionomycin (Ca2+) - dependent increases in MEF2 DNA binding in VSM cells.
Figure 2. CaMKIIδ2 increases HDAC phosphorylation and MEF2 DNA binding.
VSM cells were infected with adenovirus encoding constitutively active CaMKIIδ2 (CaCaMKIIδ2) for 16 hours. Levels of phosphorylated HDAC4 (IB:P-HDAC4ser632) (A) or HDAC5 (IB:P-HDAC5ser498) (B) were detected by immunoblotting as described previously. Immunoblotting for CaMKIIδ2 (IB:CaMKIIδ2) was performed to determine the extent of CaMKII overexpression. GAPDH immunoblotting (IB: GAPDH) was performed to insure equal protein loading. (C) MEF2 DNA binding was determined in VSM cells infected with increasing amounts of adenovirus encoding constitutively active CaMKIIδ2 (CaCaMKIIδ2) for 16 hours.
Figure 3. CaMKIIδ2 mediates Ca2+-dependent gene transcription.
(A) MEF2 DNA binding was determined in VSM cells stimulated with 0.5 μM Iono for 10 min after adenoviral infection with either control shRNA (ShLuc) or shRNA targeting CaMKIIδ2 (Shδ2). (B) MEF2 DNA binding activity was determined in VSM cells stimulated with 0.5 μM Iono for 10 min after electroporation with either control (SiC) SiRNA or SiRNA targeting CaMKIIδ2 (Siδ). (C) Nur77 mRNA levels were determined by quantitative PCR in VSM cells stimulated with 0.25μM Iono for 1 hour after electroporation with either control (SiC) SiRNA or SiRNA targeting CaMKIIδ2 (Siδ). (D) MCP1 mRNA levels were determined by quantitative PCR in VSM cells stimulated with 0.25μM Iono for 1 hour after electroporation with either control (SiC) SiRNA or SiRNA targeting CaMKIIδ2 (Siδ). Each of these experiments was performed three separate times. * P<0.05.
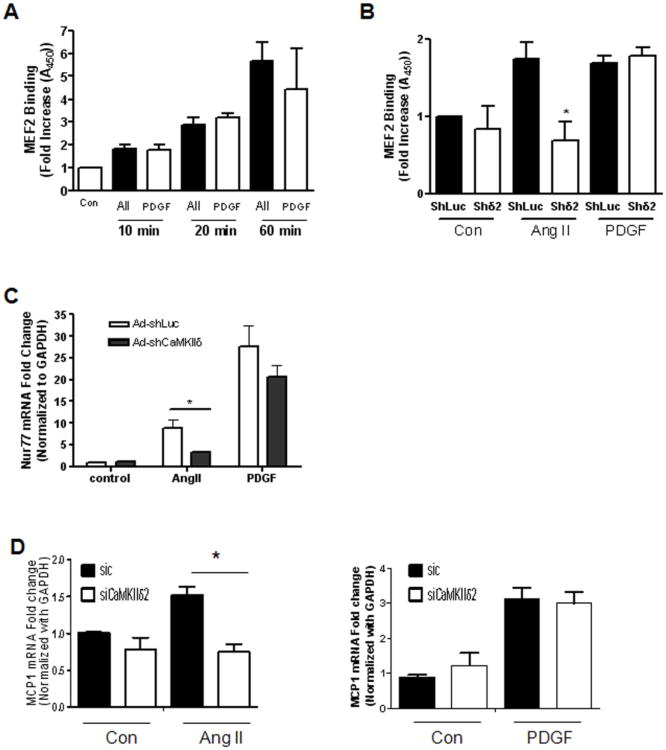
Transcriptional targets for MEF2 include the immediate response gene Nur77 [40] [41] and the chemokine MCP1 [42]. To determine if CaMKIIδ2 –dependent MEF2 regulation extends to a transcriptional readout in VSM cells, we measured Nur77 and MCP1 mRNA induction in response to Iono (Figure 3C, D). Suppression of CaMKIIδ2 protein levels significantly attenuated Iono- dependent increases in Nur77 and MCP1 mRNA levels in VSM cells. Taken together, these results describe an Iono (Ca2+)-dependent activation of CaMKIIδ2 in VSM cells that results in phosphorylation-dependent nucleocytoplasmic shuttling of HDAC4 and HDAC5 and mediates the expression of the MEF2-dependent transcription factors Nur77 and MCP1.
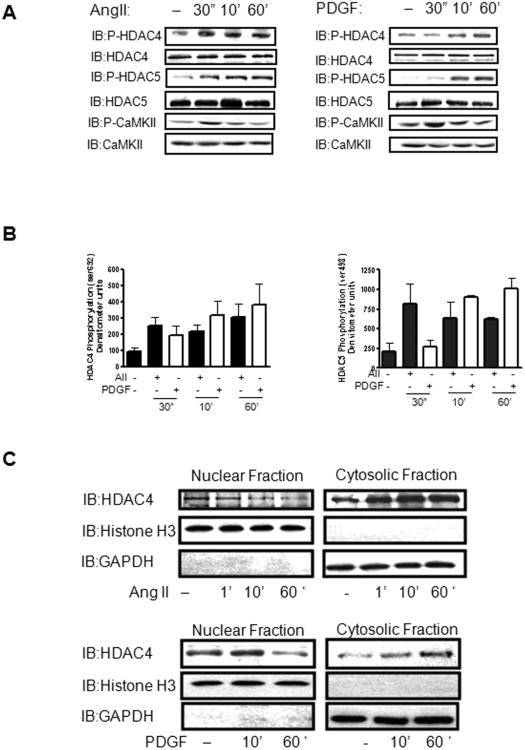
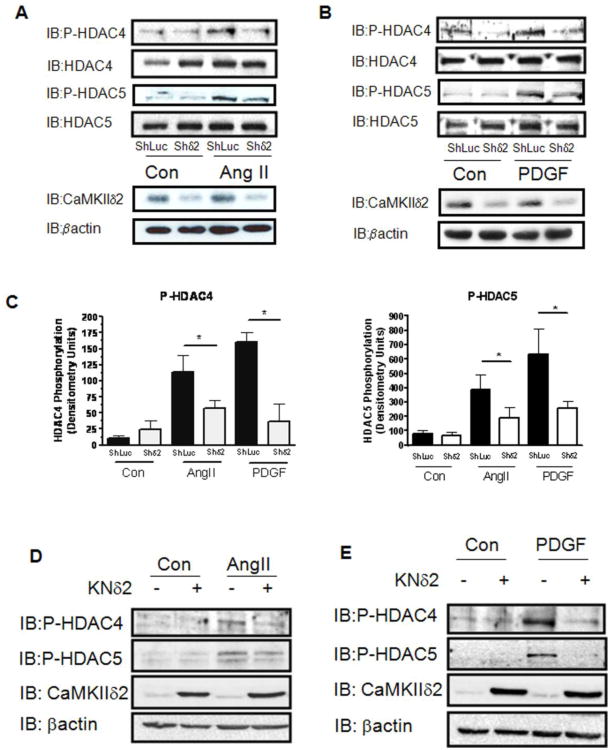
To investigate CaMKIIδ2's potential role in mediating relevant transcriptional pathways in VSM cells, we repeated the above experiments after stimulation with AngII and PDGF. Stimulation with AngII and PDGF has been reported to increase HDAC4 and HDAC5 phosphorylation in multiple cell types [26] [23] [31]. In cultured VSM, AngII and PDGF stimulated increases in HDAC4 and HDAC5 phosphorylation were observed after 30 sec and this phosphorylation was sustained for up to 3 hours (Figure 4A,B, data not shown). Cell fractionation studies indicated that AngII and PDGF stimulation induced a rapid loss of HDAC4 in the nuclear fraction as compared to unstimulated cells and a concomitant gain of HDAC4 in the cytoplasmic fraction (Figure 4C). Although the extent of HDAC4 phosphorylation was similar with both AngII and PDGF (Figure 4B, left panel) it appeared that AngII— dependent increases of HDAC5 phosphorylation at earlier time points were more robust than PDGF-dependent responses (Figure 4B, right panel). The implications of these results are not clear but may indicate AngII's differential ability to activate kinases that are specific to HDAC5 alone.
Figure 4. Agonist-dependent regulation of HDAC4 and HDAC5 in VSM.
(A) Whole cell lysates from cultured VSM cells stimulated with 100 nm Ang II (Left Panel) or 10 ng/ml PDGF (Right Panel) for the indicated times were immunoblotted for phosphorylated HDAC4 (IB:P-HDAC4ser632) or HDAC5 (IB:P-HDAC5ser 498). The lysates were also immunoblotted for active CaMKII (IB: P-CaMKII). Total HDAC4, HDAC5, and CaMKII were immunoblotted to insure equal protein loading. (B) Histograms represent quantification of four immunoblots of phosphorylated HDAC4 or HDAC5 as represented in panel A. (C) Nuclear and cytosolic fractions were isolated from cultured VSM cells treated with 100 nm Angiotensin II (Top Panel) or 10 ng/ml PDGF (Bottom Panel) as indicated. These lysates were resolved by SDS-PAGE and immunoblotted for total HDAC4 (IB:HDAC4). Fractions were immunoblotted for Histone H3 and GAPDH respectively to distinguish the nuclear fraction from the cytosolic fraction.
To determine CaMKIIδ2's ability to mediate AngII- and PDGF-dependent phosphorylation of HDAC4 and HDAC5, we suppressed CaMKIIδ2 protein levels using the shRNA approach described earlier. Silencing of CaMKIIδ2 expression attenuated AngII- and PDGF-dependent phosphorylation of HDAC4 and HDAC5 (Figure 5A, B). Similarly, overexpression of kinase negative CaMKIIδ2 [34] reduced AngII- and PDGF-dependent phosphorylation of HDAC4 and HDAC5 (Figure 5C, D). The results of this set of experiments are consistent with the results obtained using Iono (Figures 1 and 2) and further support a role for CaMKIIδ2 in regulating HDAC4 and HDAC5 in VSM cells.
Figure 5. CaMKIIδ2 mediates HDAC4 and HDAC5 phosphorylation.
(A) Levels of phosphorylated HDAC4 (IB:P-HDAC4ser632)or HDAC5 (IB:P-HDAC5ser498) were detected by immunoblotting in VSM cells stimulated with 100 nm Ang II for 10 min after adenoviral infection with either control shRNA (ShLuc) or shRNA targeting CaMKIIδ2 (Shδ2). Immunoblotting for CaMKIIδ2 (IB:CaMKIIδ2) was performed to determine the level of CaMKII knockdown. β-actin immunoblotting (IB:Pactin) was performed to insure equal protein loading. (B) Levels of phosphorylated HDAC4 (IB:P-HDAC4ser632)or HDAC5 (IB:P-HDAC5ser498) were detected by immunoblotting in VSM cells stimulated with 10 ng/ml PDGF for 10 min after adenoviral infection with either control shRNA (ShLuc) or shRNA targeting CaMKIIδ2 (Shδ2). (C) Histograms represent quantification of four experiments represented by panels A and B above. * indicates P<0.05. VSM cells were infected with 50 MOI kinase negative CaMKIIδ2 (KNδ2) and stimulated with 100 AngII for 10 min (D) or 10 ng/ml PDGF for 10 min (E) as indicated. The lysates were immunoblotted for phosphorylated HDAC4 or HDAC5. The lysates were further immunoblotted for CaMKIIδ2 and βactin to determine extent of CaMKIIδ2 overexpression and overall protein levels.
In contrast to the transient MEF2 DNA binding induced by Iono (Figure 1C), AngII and PDGF stimulation of VSM cells resulted in a sustained increase of MEF2 DNA binding over a 60 min time period (Figure 6A). Suppression of CaMKIIδ2 blocked the increase of MEF2 DNA binding after 10 min AngII stimulation (Figure 6B). Interestingly, loss of CaMKIIδ2 did not attenuate MEF2 DNA binding induced by 2 hour AngII treatment (data not shown) or PDGF-dependent increases of MEF2 DNA binding under any condition (Figure 6B). Consistent with results obtained with Iono stimulation (Figure 3), suppression of CaMKIIδ2 by shRNA significantly attenuated AngII-dependent increases in Nur77 and MCP1 mRNA levels in VSM cells (Figure 6C, D). Loss of CaMKIIδ2 was ineffective in suppressing PDGF-dependent increases of Nur77 or MCP1 (Figure 6C, data not shown). Taken together, these results indicate that a CaMKII/MEF2-dependent pathway selectively mediates Ca2+-and AngII-dependent increases in Nur77 and MCP1 induction in VSM cells.
Figure 6. CaMKIIδ2 mediates agonist-dependent MEF2 activity.
A) MEF2 DNA binding was determined in VSM cells stimulated with 100 nm Ang II or 10 ng/ml PDGF as indicated. This experiment was performed three separate times. B) MEF2 DNA binding was determined in VSM cells stimulated with 100 nm Ang II or 10 ng/ml PDGF for 10 min after adenoviral infection with either control shRNA (ShLuc) or shRNA targeting CaMKIIδ2 (Shδ2). This experiment was performed three separate times. (C) Nur77 mRNA levels were determined by quantitative PCR in VSM cells stimulated with 100 nm Ang II or 10 ng/ml PDGF for 1 hour after adenoviral infection with either control shRNA (ShLuc) or shRNA targeting CaMKIIδ2 (Shδ2). This experiment was performed three separate times. (D) MCP1 mRNA levels were determined by quantitative PCR in VSM cells stimulated with 100 nm Ang II or 10 ng/ml PDGF for 1 hour after electroporation with either control (SiC) SiRNA or SiRNA targeting CaMKIIδ2 (Siδ). This experiment was performed three separate times. * P< 0.05.
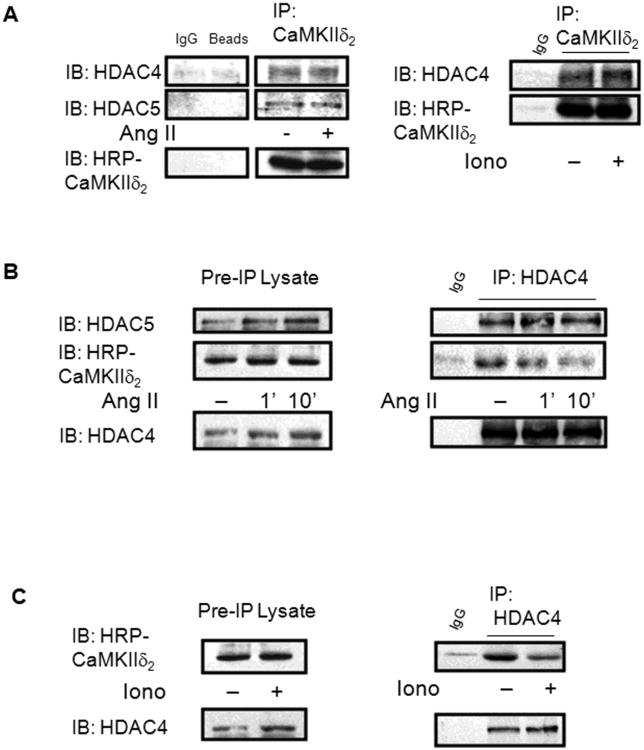
We next wanted to gain insight into the mechanism by which CaMKII regulates this HDAC/MEF2 signaling pathway. A recent study in cardiomyocytes using exogenously expressed proteins revealed that CaMKII physically interacts with HDAC4 at a site on HDAC4 identified as AA585-608 [26]. To determine if a similar interaction occurs in VSM cells with endogenous proteins, CaMKIIδ2 was immunoprecipitated from cell lysates of unstimulated and AngII-stimulated cells. Immunoblot analysis of these CaMKIIδ2 immunoprecipitates revealed the presence of both HDAC4 and HDAC5 (Figure 7A). Similarly, CaMKIIδ2 and HDAC5 were detected in HDAC4 immunoprecipitates of cell lysates from unstimulated VSM cells (Figure 7B). Interestingly, stimulation with AngII or ionomycin resulted in a reduction of CaMKIIδ2 that co-immunoprecipitates with HDAC4 (Figure 7B, C). These results indicate that CaMKIIδ2 directly interacts with HDAC4 and HDAC5 in VSM cells through a protein-protein interaction and suggest that this interaction is regulated in a stimulation-dependent manner.
Figure 7. CaMKIIδ2 physically interacts with HDAC4 and HDAC5.
A) CaMKIIδ2 was immunoprecipitated (IP: CaMKIIδ2) from untreated cultured VSM cells or cells stimulated with 100 nm AngII (Left Panel) or 0.5 μM Iono (Right Panel) for 10 min. The immunoprecipitates were resolved by SDS-PAGE and immunoblotted for HDAC4 (IB: HDAC4) or HDAC5 (IB: HDAC5). Because CaMKII runs at approximately 50 kd, the immunoprecipitates were immunoblotted with a CAMKII antibody conjugated with horseradish peroxidase (HRP) to directly detect CaMKII B) HDAC4 was immunoprecipitated (IP:HDAC4) from VSM cells treated as described above. The immunoprecipitates were immunoblotted for HDAC5 (IB:HDAC5) or HRP conjugated CaMKIIδ2 (IB:HRP-CaMKIIδ2). The immunoprecipitates were immunoblotted for HDAC4 to monitor the efficiency of the HDAC4 immunoprecipitation. C) HDAC4 was immunoprecipitated from cells stimulated with 0.5 μm Iono for 10 min and immunoblotted as indicated.
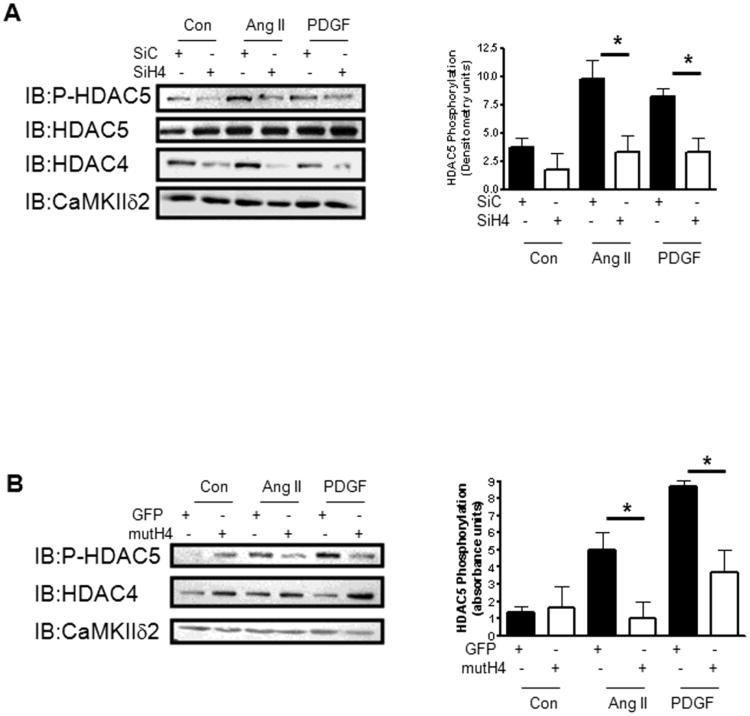
It was demonstrated that the CaMKII interaction site on HDAC4 is not conserved on HDAC5 and that CaMKII-dependent regulation of HDAC5 is dependent upon complex formation and heterodimerization with HDAC4 [27]. To evaluate the role of HDAC4 in HDAC5 regulation in VSM, we suppressed HDAC4 expression in VSM cells using siRNA (Figure 8A). Attenuation of HDAC4 levels in VSM resulted in a significant loss of both AngII- and PDGF-dependent phosphorylation of HDAC5 (Figure 8A, right panel). Previous work showed that a mutant of HDAC4 (S246A; S267A; S632) acts as a dominant repressor due to its inability to be phosphorylated and shuttled from the nucleus [43]. Over-expression of dominant repressive HDAC4 attenuated AngII- and PDGF-dependent increases in HDAC5 phosphorylation (Figure 8B). These results confirm a key role for HDAC4 in mediating HDAC5 phosphorylation in response to stimuli relevant to VSM function.
Figure 8. HDAC4 mediates agonist-dependent phosphorylation of HDAC5.
A) Levels of phosphorylated HDAC5 were determined in VSM cells stimulated with 100 nm Ang II or 10 ng/ml PDGF for 10 min after electroporation with either control (SiC) SiRNA or SiRNA targeting HDAC4 (SiH4). The lysates were immunoblotted for total HDAC4 (IB:HDAC4) to determine the extent of HDAC4 protein suppression and CaMKIIδ2 (IB:CaMKIIδ2) as a loading control. The histogram (right panel) represents quantification of immunoblots from three separate experiments. B) Levels of phosphorylated HDAC5 were determined in VSM cells stimulated with 100 nm Ang II or 10 ng/ml PDGF for 10 min after transfection with either a GFP expressing plasmid as control (GFP) or a plasmid encoding mutant HDAC4 (3S>A) (mutH4). The lysates were also immunoblotted for total HDAC4 (IB:HDAC4) to determine the extent of HDAC4 overexpression and CaMKIIδ2 (IB:CaMKIIδ2) as a loading control. The histograms represent quantification of immunoblots from three separate experiments. * indicates P< 0.05.
Discussion
Given the importance of MEF2 in VSM development [18] and regulation in response to injury [42], understanding the factors that mediate its activity are critical. Recent studies in VSM cells have implicated CaMKII and class IIa HDACs, HDAC4 and HDAC5 in particular, as being important regulators of MEF2 activity [29],[30],[23]. Examination of these studies reveals that there are several important questions remaining as to how CaMKII and HDACs coordinately regulate MEF2 function. Some of these questions include: how are HDACs regulated in response to different agonists?; which HDAC (s) are targeted by CaMKII in vivo?; and what MEF2 target genes are regulated in a CaMKII- and HDAC- dependent manner? In this study, we unambiguously established that HDAC4 and HDAC5 are regulated in response to increases in intracellular [Ca2+] and stimulation with Ang II and PDGF in VSM. We also provided evidence that CaMKIIδ2 is capable of mediating Ang II- and PDGF-dependent increases of HDAC4 and HDAC5 phosphorylation and Ca2+- and Ang II- dependent increases in MEF2 DNA binding activity. We further showed that these CaMKII-dependent increases in MEF2 DNA binding activity can be linked to CaMKII-dependent increases in expression of the MEF2 target genes Nur77 and MCP1. Finally, we demonstrated that HDAC5 phosphorylation is mediated by HDAC4 in VSM suggesting that CaMKIIδ2-dependent regulation of HDAC5 involves HDAC4.
Previous studies have shown that stimulation with Ang II [30] [29] and PDGF [23] results in HDAC4 and HDAC5 phosphorylation in VSM cells. In this study, we not only confirm these results but also demonstrate that stimulation with Iono, which causes rapid increases of intracellular [Ca2+], results in transient HDAC4 and HDAC5 phosphorylation and rapid and transient nucleocytoplasmic shuttling of HDAC4. These findings reveal the dynamic nature of HDAC regulation in vivo and provide a means of understanding the differential regulation of HDAC4 and HDAC5 by CaMKII verses other HDAC kinases such as PKD [44]. It is well established that CaMKIIδ2 is activated within 30 sec after Iono or agonist stimulation of VSM cells. Coupling this with Iono –dependent increases of MEF2 DNA binding activity and gene expression (Nur77 and MCP1) provides further evidence that transient activation of CaMKII is capable of mediating important downstream VSM functions.
The rapidity of agonist-stimulated CaMKII-dependent HDAC4/5 phosphorylation in VSM cells suggests that CaMKII is physically proximal to the HDACs, either in the nucleus or in the cytosol. In a previous study, we reported that CaMKIIδ2 is capable of being transported into the nucleus [45]. Perhaps there is some CaMKIIδ2 or even a small amount of nuclear targeting CaMKIIδ3 [34] in VSM cells which is responsible for the initial phosphorylation events necessary for HDAC4 and HDAC5 nucleocytosolic shuttling. The alternative is that CaMKII phosphorylates cytosolic HDACs. There is one report that suggests that CaMKII interacts with HDACs through 14-3-3 proteins in the cytosol and prevents HDAC reentry into the nucleus [28]. HDAC regulation also involves reciprocal regulation by phosphatases such as PP2A [46] and MYPT1 [47] in the cytosol. Given the evidence that CaMKII is capable of regulating phosphatase activity [48], it is possible that CaMKII may be regulating HDAC function in the cytosol in multiple ways.
Previous studies in cardiomyocytes using over-expressed HDAC4/5 reporters and mutants suggested that a physical complex of CaMKIIδ with HDAC4 and between HDAC4 and HDAC5 was required to couple CaMKII to HDAC5 regulation [27]. We were interested in testing this mechanism in VSM by evaluating endogenous proteins, in part, because the major CaMKIIδ isoform expressed in these cells is the δ2 isoform, which is largely cytosolic [49]. The physiological implications of this coupling are not clear but provide a selective means that increases of intracellular [Ca2+] and consequent CaMKII activation may directly mediate transcriptional events. For example, agonists like AngII that result in rapid and robust increases in intracellular calcium may be more likely to utilize the CaMKII/HDAC4 signaling axis and co-opt HDAC5 to enhance the transcriptional response, whereas stimuli that induce relatively low levels of intracellular calcium may bypass HDAC4 and signal primarily through HDAC5. This level of signaling specificity in regards to HDAC4 and HDAC5 has not been carefully studied in any system and may provide new insights into transcriptional regulation of immediate early response genes in VSM cells.
MEF2's role in VSM is poorly understood but is probably multifactorial, reflecting its known functions in regulating cell-type specific genes and as an integrator of cellular signals [18]. Based on its regulation of myocardin expression is cardiac and smooth muscle, and its control of contractile and metabolic genes in striated muscle, the prediction would be for MEF2 to promote the differentiated phenotype of VSM [15;18]. While compelling, this hypothesis remains to be tested in vivo through targeted MEF2 deletions. There is evidence, however, that MEF2 also has a role in regulating the synthetic VSM phenotype: MEF2 protein and activity are strongly upregulated in the neointima of rodent models of vascular injury, promote proliferation, and indirectly inhibit myocardin activity [21;23]. Indeed, a dominant negative MEF2 will reduce the size of the neointima following vascular injury [42]. Our data is consistent with this model in that CaMKIIδ2 is upregulated following balloon injury and its subsequent phosphorylation of HDAC4/5 could contribute to increased MEF2 activity in this system. Still, the mechanism by which MEF2 regulates the synthetic phenotype is poorly understood and the current data suggests multiple possible effectors. As stated above, MCP-1 is regulated by MEF2 and promotes macrophage infiltration and growth of a vascular lesion [42]. MEF2 may play a role in VSM migration from the media to the neointima because, in other cell types, MEF2 has been linked to migration and tissue invasion by its regulation of matrix metalloproteases, and chemokine ligands and receptors [50;51].
MEF2 is a well studied activator of Nur77 induction in many cell types including VSM (Figure 6) [40;52]. In the current studies the primary purpose of assaying Nur77 was as a “physiological” readout of MEF2 transcriptional activity. Previous studies have shown that, in contrast to MEF2 [21], Nur77 inhibits VSM proliferation in vitro and neointimal formation in vivo [53;54]. Reconciling into a cohesive model these apparently opposite functions of MEF2 and its transcriptional target, Nur77, in neointimal formation as well as understanding the functional significance of CaMKII-dependent regulation of Nur77 will require additional studies. Our model proposes a regulatory axis from CaMKII to MEF2 through regulation of HDAC4/5 nuclear localization. However, many transcription factors are targets of the class II HDACs, including serum response factor (SRF), which has been linked to CaMKII and HDAC4 [55] in other cell types. Thus, MEF2 is unlikely to be the only transcription factor targeted by CaMKII through HDAC4/5 in VSM.
These studies provide a mechanism by which CaMKIIδ2 is capable of mediating VSM cell transcriptional activity through regulation of class IIa HDACs, including a specific HDAC4/HDAC5/MEF2 pathway. Based on the current results it is apparent that a CaMKII/HDAC4,5/MEF2 signal axis will function in a stimulus and cell context-dependent manner. How ultimately such a pathway factors into functional responses including specific transcriptional events, VSM phenotype switching, or VSM function (proliferation, migration) will require additional studies.
Acknowledgments
We thank Virginia Foster and Scott Eichen for technical assistance in completing this project. We are also thankful to Wendy Vienneau for assistance in preparing the manuscript for submission.
Funding: This work was supported by National Heart, Lung, and Blood institute grants RO1-HL-092510 and RO1-HL- 49426 (to H.A. Singer).
Footnotes
The final version of record is available at http://www.biochemj.org.
Author Contribution: Roman Ginnan and Li Yan Sun conducted all experimental work, data analysis, and preparation of figures. The paper was written by Roman Ginnan with assistance from John J. Schwarz and Harold A. Singer. The overall study was conceived by Harold A. Singer with help from Roman Ginnan and John J. Schwarz.
Reference List
- 1.Owens GK, Kumar MS, Wamhoff BR. Molecular regulation of vascular smooth muscle cell differentiation in development and disease. Physiol Rev. 2004;84:767–801. doi: 10.1152/physrev.00041.2003. [DOI] [PubMed] [Google Scholar]
- 2.Lindqvist A, Nilsson BO, Ekblad E, Hellstrand P. Platelet-derived growth factor receptors expressed in response to injury of differentiated vascular smooth muscle in vitro: effects on Ca2+ and growth signals. Acta Physiol Scand. 2001;173:175–184. doi: 10.1046/j.1365-201X.2001.00873.x. [DOI] [PubMed] [Google Scholar]
- 3.Gollasch M, Haase H, Ried C, Lindschau C, Morano I, Luft FC, Haller H. L-type calcium channel expression depends on the differentiated state of vascular smooth muscle cells. FASEB J. 1998;12:593–601. doi: 10.1096/fasebj.12.7.593. [DOI] [PubMed] [Google Scholar]
- 4.Vallot O, Combettes L, Jourdon P, Inamo J, Marty I, Claret M, Lompre AM. Intracellular Ca(2+) handling in vascular smooth muscle cells is affected by proliferation. Arterioscler Thromb Vasc Biol. 2000;20:1225–1235. doi: 10.1161/01.atv.20.5.1225. [DOI] [PubMed] [Google Scholar]
- 5.Potier M, Gonzalez JC, Motiani RK, Abdullaev IF, Bisaillon JM, Singer HA, Trebak M. Evidence for S. FASEB J. 2009;23:2425–2437. doi: 10.1096/fj.09-131128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Inoue R, Jensen LJ, Shi J, Morita H, Nishida M, Honda A, Ito Y. Transient receptor potential channels in cardiovascular function and disease. Circ Res. 2006;99:119–131. doi: 10.1161/01.RES.0000233356.10630.8a. [DOI] [PubMed] [Google Scholar]
- 7.Wamhoff BR, Bowles DK, Owens GK. Excitation-transcription coupling in arterial smooth muscle. Circ Res. 2006;98:868–878. doi: 10.1161/01.RES.0000216596.73005.3c. [DOI] [PubMed] [Google Scholar]
- 8.Barlow CA, Rose P, Pulver-Kaste RA, Lounsbury KM. Excitation-transcription coupling in smooth muscle. J Physiol. 2006;570:59–64. doi: 10.1113/jphysiol.2005.098426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.House SJ, Singer HA. CaMKII-delta isoform regulation of neointima formation after vascular injury. Arterioscler Thromb Vasc Biol. 2008;28:441–447. doi: 10.1161/ATVBAHA.107.156810. [DOI] [PubMed] [Google Scholar]
- 10.Rokolya A, Singer HA. Inhibition of CaM kinase II activation and force maintenance by KN-93 in arterial smooth muscle. Am J Physiol Cell Physiol. 2000;278:C537–C545. doi: 10.1152/ajpcell.2000.278.3.C537. [DOI] [PubMed] [Google Scholar]
- 11.Li W, Li H, Sanders PN, Mohler PJ, Backs J, Olson EN, Anderson ME, Grumbach IM. The Multifunctional Ca2+/Calmodulin-dependent Kinase II {delta} (CaMKII{delta}) Controls Neointima Formation after Carotid Ligation and Vascular Smooth Muscle Cell Proliferation through Cell Cycle Regulation by p21. J Biol Chem. 2011;286:7990–7999. doi: 10.1074/jbc.M110.163006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.House SJ, Ginnan R, Armstrong S, Singer HA. Ca2+/Calmodulin-dependent protein kinase II-{delta} isoform regulation of vascular smooth muscle cell proliferation. Am J Physiol Cell Physiol. 2007;292:C2276–C2287. doi: 10.1152/ajpcell.00606.2006. [DOI] [PubMed] [Google Scholar]
- 13.Mercure MZ, Ginnan R, Singer HA. CaM kinase II delta2-dependent regulation of vascular smooth muscle cell polarization and migration. Am J Physiol Cell Physiol. 2008;294:C1465–C1475. doi: 10.1152/ajpcell.90638.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vong L, Bi W, O'Connor-Halligan KE, Li C, Cserjesi P, Schwarz JJ. MEF2C is required for the normal allocation of cells between the ventricular and sinoatrial precursors of the primary heart field. Dev Dyn. 2006;235:1809–1821. doi: 10.1002/dvdy.20828. [DOI] [PubMed] [Google Scholar]
- 15.Naya FJ, Black BL, Wu H, Bassel-Duby R, Richardson JA, Hill JA, Olson EN. Mitochondrial deficiency and cardiac sudden death in mice lacking the MEF2A transcription factor. Nat Med. 2002;8:1303–1309. doi: 10.1038/nm789. [DOI] [PubMed] [Google Scholar]
- 16.Li H, Radford JC, Ragusa MJ, Shea KL, McKercher SR, Zaremba JD, Soussou W, Nie Z, Kang YJ, Nakanishi N, Okamoto S, Roberts AJ, Schwarz JJ, Lipton SA. Transcription factor MEF2C influences neural stem/progenitor cell differentiation and maturation in vivo. Proc Natl Acad Sci U S A. 2008;105:9397–9402. doi: 10.1073/pnas.0802876105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.De VS, Anderson JP, Heidt AB, Khiem D, Xu SM, Black BL. Mef2c is activated directly by Ets transcription factors through an evolutionarily conserved endothelial cell-specific enhancer. Dev Biol. 2004;275:424–434. doi: 10.1016/j.ydbio.2004.08.016. [DOI] [PubMed] [Google Scholar]
- 18.Potthoff MJ, Olson EN. MEF2: a central regulator of diverse developmental programs. Development. 2007;134:4131–4140. doi: 10.1242/dev.008367. [DOI] [PubMed] [Google Scholar]
- 19.Anderson JP, Dodou E, Heidt AB, De Val SJ, Jaehnig EJ, Greene SB, Olson EN, Black BL. HRC is a direct transcriptional target of MEF2 during cardiac, skeletal, and arterial smooth muscle development in vivo. Mol Cell Biol. 2004;24:3757–3768. doi: 10.1128/MCB.24.9.3757-3768.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Creemers EE, Sutherland LB, McAnally J, Richardson JA, Olson EN. Myocardin is a direct transcriptional target of Mef2, Tead and Foxo proteins during cardiovascular development. Development. 2006;133:4245–4256. doi: 10.1242/dev.02610. [DOI] [PubMed] [Google Scholar]
- 21.Firulli AB, Miano JM, Bi W, Johnson AD, Casscells W, Olson EN, Schwarz JJ. Myocyte enhancer binding factor-2 expression and activity in vascular smooth muscle cells. Association with the activated phenotype. Circ Res. 1996;78:196–204. doi: 10.1161/01.res.78.2.196. [DOI] [PubMed] [Google Scholar]
- 22.Suzuki E, Guo K, Kolman M, Yu YT, Walsh K. Serum induction of MEF2/RSRF expression in vascular myocytes is mediated at the level of translation. Mol Cell Biol. 1995;15:3415–3423. doi: 10.1128/mcb.15.6.3415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gordon JW, Pagiatakis C, Salma J, Du M, Andreucci JJ, Zhao J, Hou G, Perry RL, Dan Q, Courtman D, Bendeck MP, McDermott JC. Protein kinase A-regulated assembly of a MEF2{middle dot}HDAC4 repressor complex controls c-Jun expression in vascular smooth muscle cells. J Biol Chem. 2009;284:19027–19042. doi: 10.1074/jbc.M109.000539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miska EA, Karlsson C, Langley E, Nielsen SJ, Pines J, Kouzarides T. HDAC4 deacetylase associates with and represses the MEF2 transcription factor. EMBO J. 1999;18:5099–5107. doi: 10.1093/emboj/18.18.5099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang T, Kohlhaas M, Backs J, Mishra S, Phillips W, Dybkova N, Chang S, Ling H, Bers DM, Maier LS, Olson EN, Brown JH. CaMKIIdelta isoforms differentially affect calcium handling but similarly regulate HDAC/MEF2 transcriptional responses. J Biol Chem. 2007 doi: 10.1074/jbc.M707083200. [DOI] [PubMed] [Google Scholar]
- 26.Backs J, Song K, Bezprozvannaya S, Chang S, Olson EN. CaM kinase II selectively signals to histone deacetylase 4 during cardiomyocyte hypertrophy. J Clin Invest. 2006;116:1853–1864. doi: 10.1172/JCI27438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Backs J, Backs T, Bezprozvannaya S, McKinsey TA, Olson EN. Histone deacetylase 5 acquires calcium/calmodulin-dependent kinase II responsiveness by oligomerization with histone deacetylase 4. Mol Cell Biol. 2008;28:3437–3445. doi: 10.1128/MCB.01611-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ellis JJ, Valencia TG, Zeng H, Roberts LD, Deaton RA, Grant SR. CaM kinase IIdeltaC phosphorylation of 14-3-3beta in vascular smooth muscle cells: activation of class II HDAC repression. Mol Cell Biochem. 2003;242:153–161. [PubMed] [Google Scholar]
- 29.Li H, Li W, Gupta AK, Mohler PJ, Anderson ME, Grumbach IM. Calmodulin kinase II is required for angiotensin II-mediated vascular smooth muscle hypertrophy. Am J Physiol Heart Circ Physiol. 2010;298:H688–H698. doi: 10.1152/ajpheart.01014.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pang J, Yan C, Natarajan K, Cavet ME, Massett MP, Yin G, Berk BC. GIT1 Mediates HDAC5 Activation by Angiotensin II in Vascular Smooth Muscle Cells. Arterioscler Thromb Vasc Biol. 2008 doi: 10.1161/ATVBAHA.107.161349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xu X, Ha CH, Wong C, Wang W, Hausser A, Pfizenmaier K, Olson EN, McKinsey TA, Jin ZG. Angiotensin II stimulates protein kinase D-dependent histone deacetylase 5 phosphorylation and nuclear export leading to vascular smooth muscle cell hypertrophy. Arterioscler Thromb Vasc Biol. 2007;27:2355–2362. doi: 10.1161/ATVBAHA.107.151704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Geisterfer AA, Peach MJ, Owens GK. Angiotensin II induces hypertrophy, not hyperplasia, of cultured rat aortic smooth muscle cells. Circ Res. 1988;62:749–756. doi: 10.1161/01.res.62.4.749. [DOI] [PubMed] [Google Scholar]
- 33.Singer HA, Benscoter HA, Schworer CM. Novel Ca2+/calmodulin-dependent protein kinase II gamma-subunit variants expressed in vascular smooth muscle, brain, and cardiomyocytes. J Biol Chem. 1997;272:9393–9400. doi: 10.1074/jbc.272.14.9393. [DOI] [PubMed] [Google Scholar]
- 34.Schworer CM, Rothblum LI, Thekkumkara TJ, Singer HA. Identification of novel isoforms of the delta subunit of Ca2+/calmodulin-dependent protein kinase II. Differential expression in rat brain and aorta. J Biol Chem. 1993;268:14443–14449. [PubMed] [Google Scholar]
- 35.Ginnan R, Pfleiderer PJ, Pumiglia KM, Singer HA. PKC {delta} and CaMKII{delta}2 Mediate ATP-Dependent Activation of ERK1/2 in Vascular Smooth Muscle. Am J Physiol Cell Physiol. 2004 doi: 10.1152/ajpcell.00202.2003. [DOI] [PubMed] [Google Scholar]
- 36.Abraham ST, Benscoter HA, Schworer CM, Singer HA. A role for Ca2+/calmodulin-dependent protein kinase II in the mitogen-activated protein kinase signaling cascade of cultured rat aortic vascular smooth muscle cells. Circ Res. 1997;81:575–584. doi: 10.1161/01.res.81.4.575. [DOI] [PubMed] [Google Scholar]
- 37.Abraham ST, Benscoter H, Schworer CM, Singer HA. In situ Ca2+ dependence for activation of Ca2+/calmodulin-dependent protein kinase II in vascular smooth muscle cells. J Biol Chem. 1996;271:2506–2513. doi: 10.1074/jbc.271.5.2506. [DOI] [PubMed] [Google Scholar]
- 38.Pfleiderer PJ, Lu KK, Crow MT, Keller RS, Singer HA. Modulation of Vascular Smooth muscle cell migration by CaMKII. Am J Physiol Cell Physiol. 2004 doi: 10.1152/ajpcell.00536.2003. [DOI] [PubMed] [Google Scholar]
- 39.Lu KK, Armstrong SE, Ginnan R, Singer HA. Adhesion-dependent activation of CaMKII and regulation of ERK activation in vascular smooth muscle. Am J Physiol Cell Physiol. 2005;289:C1343–C1350. doi: 10.1152/ajpcell.00064.2005. [DOI] [PubMed] [Google Scholar]
- 40.Dequiedt F, Kasler H, Fischle W, Kiermer V, Weinstein M, Herndier BG, Verdin E. HDAC7, a thymus-specific class II histone deacetylase, regulates Nur77 transcription and TCR-mediated apoptosis. Immunity. 2003;18:687–698. doi: 10.1016/s1074-7613(03)00109-2. [DOI] [PubMed] [Google Scholar]
- 41.Lam BY, Zhang W, Ng DC, Maruthappu M, Roderick HL, Chawla S. CREB-dependent Nur77 induction following depolarization in PC12 cells and neurons is modulated by MEF2 transcription factors. J Neurochem. 2010;112:1065–1073. doi: 10.1111/j.1471-4159.2009.06521.x. [DOI] [PubMed] [Google Scholar]
- 42.Suzuki E, Satonaka H, Nishimatsu H, Oba S, Takeda R, Omata M, Fujita T, Nagai R, Hirata Y. Myocyte enhancer factor 2 mediates vascular inflammation via the p38-dependent pathway. Circ Res. 2004;95:42–49. doi: 10.1161/01.RES.0000134631.75684.4A. [DOI] [PubMed] [Google Scholar]
- 43.Parra M, Verdin E. Regulatory signal transduction pathways for class IIa histone deacetylases. Curr Opin Pharmacol. 2010;10:454–460. doi: 10.1016/j.coph.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 44.Ha CH, Wang W, Jhun BS, Wong C, Hausser A, Pfizenmaier K, McKinsey TA, Olson EN, Jin ZG. Protein kinase D-dependent phosphorylation and nuclear export of histone deacetylase 5 mediates vascular endothelial growth factor-induced gene expression and angiogenesis. J Biol Chem. 2008;283:14590–14599. doi: 10.1074/jbc.M800264200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jones RJ, Jourd'heuil D, Salerno JC, Smith SM, Singer HA. iNOS Regulation by Calcium/Calmodulin-Dependent Protein Kinase II in Vascular Smooth Muscle. Am J Physiol Heart Circ Physiol. 2007 doi: 10.1152/ajpheart.01247.2006. [DOI] [PubMed] [Google Scholar]
- 46.Paroni G, Cernotta N, Dello RC, Gallinari P, Pallaoro M, Foti C, Talamo F, Orsatti L, Steinkuhler C, Brancolini C. PP2A regulates HDAC4 nuclear import. Mol Biol Cell. 2008;19:655–667. doi: 10.1091/mbc.E07-06-0623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Parra M, Mahmoudi T, Verdin E. Myosin phosphatase dephosphorylates HDAC7, controls its nucleocytoplasmic shuttling, and inhibits apoptosis in thymocytes. Genes Dev. 2007;21:638–643. doi: 10.1101/gad.1513107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Strack S, Barban MA, Wadzinski BE, Colbran RJ. Differential inactivation of postsynaptic density-associated and soluble Ca2+/calmodulin-dependent protein kinase II by protein phosphatases 1 and 2A. J Neurochem. 1997;68:2119–2128. doi: 10.1046/j.1471-4159.1997.68052119.x. [DOI] [PubMed] [Google Scholar]
- 49.Van Riper DA, Schworer CM, Singer HA. Ca2+-induced redistribution of Ca2+/calmodulin-dependent protein kinase II associated with an endoplasmic reticulum stress response in vascular smooth muscle. Mol Cell Biochem. 2000;213:83–92. doi: 10.1023/a:1007116231678. [DOI] [PubMed] [Google Scholar]
- 50.Chang S, Young BD, Li S, Qi X, Richardson JA, Olson EN. Histone deacetylase 7 maintains vascular integrity by repressing matrix metalloproteinase 10. Cell. 2006;126:321–334. doi: 10.1016/j.cell.2006.05.040. [DOI] [PubMed] [Google Scholar]
- 51.Fritzer A, Noiges B, Schweiger D, Rek A, Kungl AJ, von GA, Nagy E, Meinke AL. Chemokine degradation by the Group A streptococcal serine proteinase ScpC can be reconstituted in vitro and requires two separate domains. Biochem J. 2009;422:533–542. doi: 10.1042/BJ20090278. [DOI] [PubMed] [Google Scholar]
- 52.Youn HD, Liu JO. Cabin1 represses MEF2-dependent Nur77 expression and T cell apoptosis by controlling association of histone deacetylases and acetylases with MEF2. Immunity. 2000;13:85–94. doi: 10.1016/s1074-7613(00)00010-8. [DOI] [PubMed] [Google Scholar]
- 53.Pires NM, Pols TW, de Vries MR, van Tiel CM, Bonta PI, Vos M, Arkenbout EK, Pannekoek H, Jukema JW, Quax PH, de Vries CJ. Activation of nuclear receptor Nur77 by 6-mercaptopurine protects against neointima formation. Circulation. 2007;115:493–500. doi: 10.1161/CIRCULATIONAHA.106.626838. [DOI] [PubMed] [Google Scholar]
- 54.Arkenbout EK, de W V, van B M, van Achterberg TA, Grimbergen JM, Pichon B, Pannekoek H, de Vries CJ. Protective function of transcription factor TR3 orphan receptor in atherogenesis: decreased lesion formation in carotid artery ligation model in TR3 transgenic mice. Circulation. 2002;106:1530–1535. doi: 10.1161/01.cir.0000028811.03056.bf. [DOI] [PubMed] [Google Scholar]
- 55.Davis FJ, Gupta M, Camoretti-Mercado B, Schwartz RJ, Gupta MP. Calcium/calmodulin-dependent protein kinase activates serum response factor transcription activity by its dissociation from histone deacetylase, HDAC4. Implications in cardiac muscle gene regulation during hypertrophy. J Biol Chem. 2003;278:20047–20058. doi: 10.1074/jbc.M209998200. [DOI] [PubMed] [Google Scholar]