Abstract
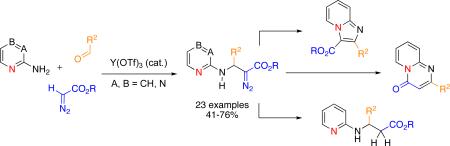
A novel three-component (3-CC) coupling reaction of 2-aminoazines, aromatic aldehydes and diazo-compounds producing polyfunctional β-amino-α-diazo-compounds has been developed. The reaction features an unprecedented heterocycle-assisted addition of a diazo-compound to an imine. The obtained diazoesters were efficiently converted into valuable heterocycles, as well as to β-amino acid derivatives.
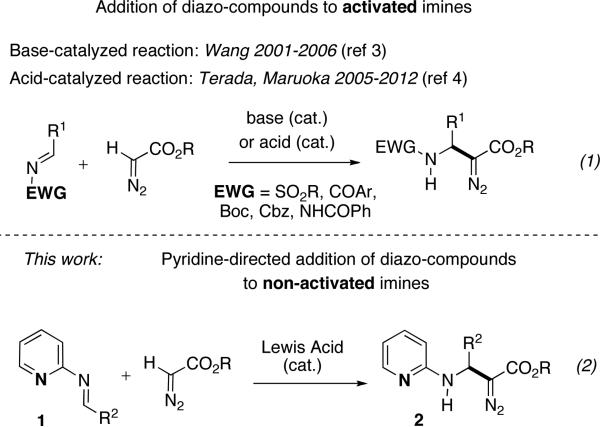
Nucleophilic addition of diazo-compounds to activated imines bearing strong electron-withdrawing groups at the nitrogen atom represents an important method of C–C bond formation, employed in the synthesis of β-amino acid derivatives, as well as other valuable products (Scheme 1, eq 1).1,2 Thus, Wang and co-workers reported a base-promoted reaction of N-SO2R imines with diazoesters producing β-amino-α-diazocarbonyl compounds.3 On the other hand, Terada, Maruoka, and others reported Brønsted acid-catalyzed addition of diazo-compounds to N-COAr and N-Boc imines (eq 1).4 However, these efficient methods are limited to activated imines only.
Scheme 1.
Pyridine-directed addition of diazo-compounds to imines
Herein we report an efficient Lewis acid-catalyzed addition of diazoesters to pyridine-containing imines 1 producing β-amino-α-diazocarbonyl compounds 2 (eq 2).5 Moreover, we also developed a 3-CC reaction of 2-aminoazines, aldehydes and diazo-compounds to form 2. The obtained β-amino-α-diazoesters represent useful synthetic scaffolds, which can be efficiently converted into diversely substituted heterocycles, such as imidazo[1,2-a]pyridine, pyrido[1,2-a]pyrimidine-4-one, as well as to N-pyridyl substituted β-amino acids.
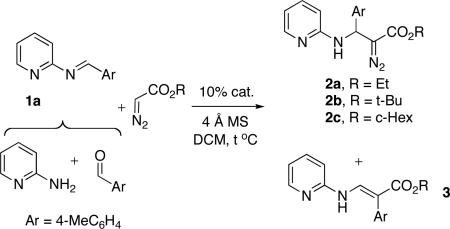
In continuation of our studies6 toward a multi-component synthesis of heterocycles,7 we explored a three-component coupling reaction of 2-aminopyridine, an aldehyde, and a diazoester. It was found that the reaction of imine of 2-aminopyridine 1a with ethyl diazoacetate in the presence of Py•TfOH (10 mol %) produced diazo-compound 2a8 along with some amounts of enamine 3a, a product of the 1,2-aryl shift (Table 1, entry 1).9 We considered this outcome quite interesting, as it represents the first efficient5 addition of diazo-Accordingly, optimization studies toward a more efficient formation of 2 were performed.
Table 1.
Optimization of the new 3-CC reaction conditionsa
| |||||
|---|---|---|---|---|---|
| entry | catalyst | R | t °C | 2 | 3 |
| 1b | Py•TfOH | Et | rt | 56% | 15% |
| 2b | TfOH | Et | rt | 45% | 15% |
| 3b | Tf2NH | Et | rt | 55% | 15% |
| 4b | CF3CO2H | Et | rt | - | - |
| 5b,c | PhP(OH)2 | Et | rt | 50% | 2% |
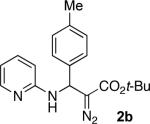
| 6b | Py•TfOH | t-Bu | rt | 59% | 14% |
| 7b | Py•TfOH | c-Hex | rt | 63% | 13% |
| 8b | Sc(OTf)3 | c-Hex | rt | 42% | 14% |
| 9b | La(OTf)3 | c-Hex | rt | 63% | 10% |
| 10b | Y(OTf)3 | c-Hex | rt | 52% | 1% |
| 11b | Y(OTf)3 | c-Hex | 10 °C | 15% | 3% |
| 12d | Y(OTf)3 | c-Hex | 10 °C | 14% | 4% |
NMR yields after 24 h.
2-CC reaction of imine 1a with diazocomponds.
Toluene was used as a solvent.
3-CC reaction from 2-aminopyridine, p-tolualdehyde, and c-Hex diazoacetate.
It was found that strong acids such as TfOH (entry 2), as well as Tf2NH (entry 3), can catalyze this reaction to produce 2a, together with a by-product enamine 3a. Employment of weaker acids, such as CF3CO2H (entry 4), did not give any product, whereas the use of phenylphosphinic acid catalyst produced the product 2a selectively, though in moderate yield only (entry 5). We found that the reaction of tert-Bu and c-Hex-diazoacetates afforded products 2b and 2c, respectively, in slightly better yield. However, formation of significant amounts of enamine 3 was observed (entries 6, 7). To our delight, the amount of enamine by-product 3 was significantly decreased when lanthanide triflates were used (entries 8-11). After this two-component coupling (2-CC) reaction was optimized, we aimed at the development of a more synthetically attractive 3-CC reaction. We found that this transformation, can indeed be performed in a three-component fashion starting from an aldehyde, a 2-aminopyridine, and a c-Hex diazoacetate which forms the product 2c in high yield (entry 12).
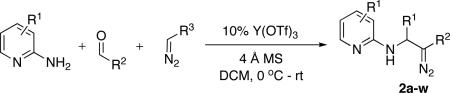
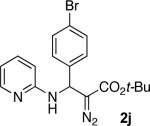
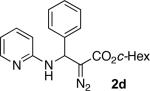
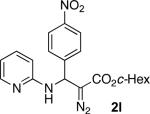
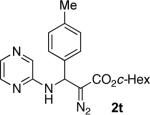
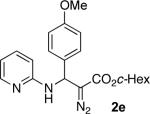
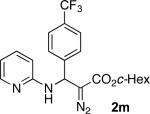
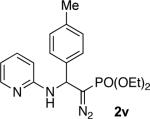
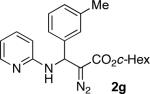
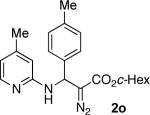
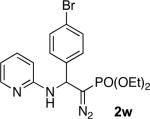
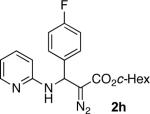
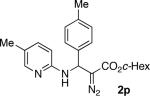
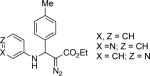
With optimized conditions in hand, we explored the scope of this novel 3-CC reaction. Thus, aromatic aldehydes bearing electron-donating and neutral groups (Table 2, entries 1-7), at the o-, m-, and p- positions reacted smoothly. Benzaldehydes having electron-withdrawing groups, such as fluoro (entry 8), bromo (entries 9,10), NO2 (entry 12), and CF3 (entry 13), produced the corresponding diazo esters in slightly lower yields (entries 8-13). In addition, an aldehyde bearing an unprotected hydroxy group (entry 11), as well as a heteroaromatic aldehyde, such as 2-thiophenecarboxaldehyde (entry 14), were tolerated under these reaction conditions. Substituted 2-aminopyridines were also competent partners for this 3-CC reaction (entries 15-18). However, the reaction of 2-aminopyridine, having an electron-withdrawing group, afforded the product in diminished yield (entry 18). The reaction could also be performed with other 2-aminoazines, namely 2-aminopyrimidine (entry 19), and 2-aminopyrazine (entry 20), as well as with 2-aminothiazole (entry 21), producing the corresponding products in reasonable yields. In addition to diazoesters, diethyl (diazomethyl)phosphonate can also be employed to form the corresponding β-amino-α-diazo-compounds 2v,w efficiently (entries 22 and 23). In general, the reaction shows high functional group tolerance with respect to all three components. Notably, aryl amines without a nitrogen atom at the α-position of the ring, such as aniline as well as 3- and 4-aminopyridines, do not produce detectable amounts of the corresponding diazo-products (entry 24).10
Table 2.
The scope of the new 3-CC reactiona
| ||||||||
|---|---|---|---|---|---|---|---|---|
| entry | product | yield (%) | entry | product | yield (%) | entry | product | yield (%) |
| 1 |
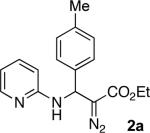
|
60 | 9 |
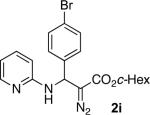
|
65 | 17 |
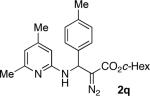
|
50 |
| 2 |
|
64 | 10 |
|
62 | 18b |
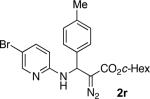
|
45 |
| 3 |
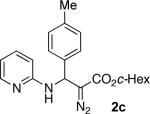
|
70 | 11 |
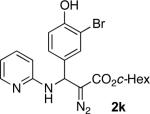
|
51 | 19 |
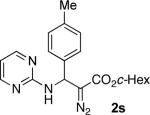
|
42 |
| 4 |
|
71 | 12b |
|
50 | 20 |
|
46 |
| 5 |
|
60 | 13 |
|
68 | 21 |
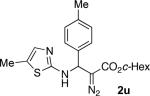
|
41 |
| 6 |
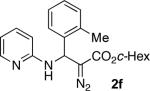
|
66 | 14 |
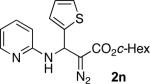
|
45 | 22 |
|
67 |
| 7 |
|
76 | 15 |
|
75 | 23 |
|
51 |
| 8 |
|
58 | 16 |
|
63 | 24 |
|
-c |
Unless otherwise noted: aldehyde (1 equiv), 2-aminoazine (1.1 equiv), diazo-compound (1.2 equiv), Y(OTf)3 (10%) and MS 4 Å (125 mg/mmol) in CH2Cl2 (0.3 M).
Pre-formed imine was used.
See ref 10.
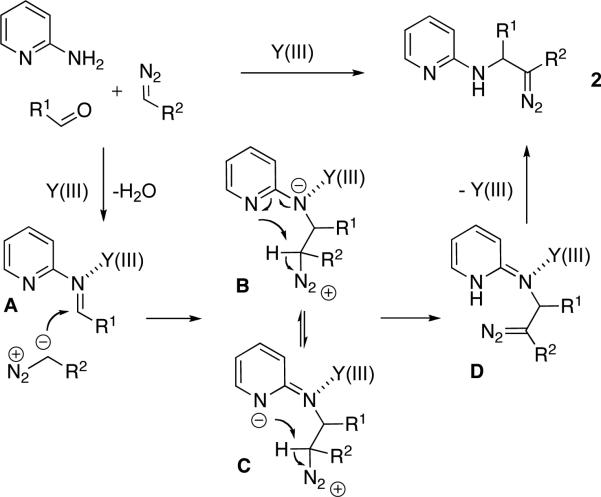
We rationalize these observations in the following way (Scheme 2). First, the formed Y(III)-activated imine A undergoes a nucleophilic attack by the diazo-compound to produce zwitter-ion B/C. It is likely that the nitrogen atom of pyridine ring serves as an intramolecular proton shuttle. Thus, deprotonation in B/C by the pyridine N-atom leads to diazo-intermediate D, producing diazo-compound 2 upon release of Y(III)-catalyst and tautomerization process. Therefore, the overall process can be considered as a pyridine group-assisted addition of diazo-compounds to imines. This mechanism is in good agreement with the fact that aniline, as well as 3- and 4-aminopyridines, which do not possess a properly situated N-atom, do not undergo this addition reaction (Table 2, entry 24).10
Scheme 2.
Proposed mechanism of new 3-CC reaction
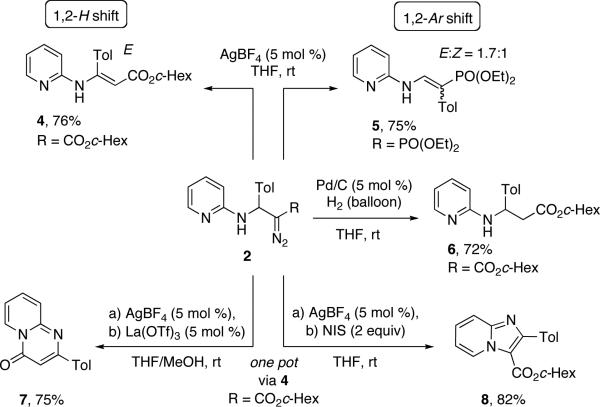
The obtained azine-containing β-amino-α-diazo-compounds 2 represent a versatile scaffold for various types of transformations. Thus, exploring the carbene reactivity of the obtained molecules, we found that diazoester 2c (R = CO2c-Hex) could undergo a selective 1,2-hydride shift11 in the presence of AgBF4 (5 mol %) to produce enamine 4.12 Interestingly, the corresponding α-diazoethylposphonate 2v (R = PO(OEt)2), under these reaction conditions, underwent an exclusive 1,2-aryl shift to form the enamine product 5.3c
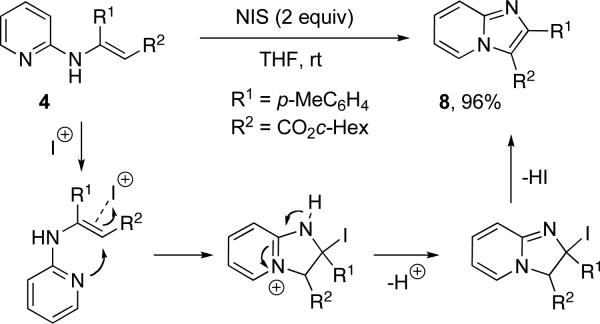
In addition, hydrogenation of the diazo-group of 2c efficiently converted it to β-amino acid derivative 6. The synthetic usefulness of the diazo-compounds 2 was further demonstrated in an efficient one-pot synthesis of N-fused heterocycles via cyclization of the in situ formed enamine 4. Thus, in the presence of La(OTf)3, it underwent lactamization into pyrido[1,2-a]pyrimidine-4-one 7. On the other hand, NIS-mediated cyclization converted 4 into imidazo[1,2-a]pyridine 8 (Scheme 3). Presumably, the cyclization of 4 into 8 proceeds via intramolecular attack of the pyridine nitrogen at the double bond of the enamine activated by an electrophilic agent, followed by a subsequent elimination and a tautomerization processes (Scheme 4).
Scheme 3.
Synthetic applications of diazocompounds 2
Scheme 4.
The proposed mechanism for formation of 8
In conclusion, we have developed a novel three-component coupling reaction of 2-aminoazines, aromatic aldehydes, and diazo compounds producing β-amino-α-diazoesters. This reaction features an unprecedented heterocycle-assisted addition of a diazocompound to an imine. The obtained β-amino-α-diazoesters represent an important polyfunctional synthetic scaffold suitable for useful transformations. Thus, the obtained diazo--compounds could be efficiently converted into valuable heterocyclic molecules such as imidazo[1,2-a]pyridines and pyrido[1,2-a]pyrimidine-4-ones, as well as β-(2-pyridyl)-amino acid derivatives.
Supplementary Material
Acknowledgment
We thank the National Institutes of Health (GM-64444 and 1P50 GM-086145) for financial support of this work. We thank Prof. V. H. Rawal and Prof. S. Kozmin (University of Chicago) for fruitful discussions.
Footnotes
Supporting Information Available Experimental procedures and spectroscopic data for all new compounds. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.For selected recent reviews on reactivity of diazo-compounds, see: Zhao X, Zhang Y, Wang J. Chem. Commun. 2012;48:10162. doi: 10.1039/c2cc34406h.Zhang Y, Wang J. Eur. J. Org. Chem. 2011:1015.Davies HML, Manning JR. Nature. 2008;451:417. doi: 10.1038/nature06485.Timmons DJ, Doyle MP. J. Organomet. Chem. 2001;617-618:98.
- 2.For recent review on reactions of diazo-compounds as nucleophiles, see: Zhang Y, Wang J. Chem. Commun. 2009:5350. doi: 10.1039/b908378b.
- 3.For base-promoted addition of diazo-compounds to activated imines, see: Jiang N, Qu Z, Wang J. Org. Lett. 2001;3:2989. doi: 10.1021/ol016324p.Jiang N, Wang J. Tetrahedron Lett. 2002;43:1285.Zhao Y, Jiang N, Wang J. Tetrahedron Lett. 2003;44:8339.Chen S, Zhao Y, Wang J. Synthesis. 2006:1705. For diastereoselective reaction, see: Zhao Y, Ma Z, Zhang X, Zou Y, Jin X, Wang J. Angew. Chem. Int. Ed. 2004;43:5977. doi: 10.1002/anie.200460730.
- 4.For enantioselective acid-catalyzed addition of diazo-compounds to activated imines, see: Uraguchi D, Sorimachi K, Terada M. J. Am. Chem. Soc. 2005;127:9360. doi: 10.1021/ja051922a.Hashimoto T, Maruoka K. J. Am. Chem. Soc. 2007;129:10054. doi: 10.1021/ja0713375.Maruoka K, Hashimoto T. Synthesis. 2008:3703.Hashimoto T, Kimura H, Nakatsu H, Maruoka K. J. Org. Chem. 2011;76:6030. doi: 10.1021/jo2005999.Hashimoto T, Kimura H, Kawamata Y, Maruoka K. Nat. Chem. 2011;3:642. doi: 10.1038/nchem.1096.Zhang H, Wen X, Gan L, Peng Y. Org. Lett. 2012;14:2126. doi: 10.1021/ol300664d. For heterogenous catalysis of this reaction, see: Kantam ML, Balasubrahmanyam V, Kumar KBS, Venkanna GT, Figueras F. Adv. Synth. Catal. 2007;349:1887.
- 5.A single example of a low-efficient (24% yield) Ag-mediated addition of ethyl diazoacetate to non-activated imine was reported: Wenkert E, McPherson CA. J. Am. Chem. Soc. 1972;94:8084.
- 6.a Chernyak N, Gevorgyan V. Angew. Chem. Int. Ed. 2010;49:2743. doi: 10.1002/anie.200907291. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Chernyak D, Chernyak N, Gevorgyan V. Adv. Synth. Catal. 2010;352:961. doi: 10.1002/adsc.201000015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.For selected reviews on multicomponent coupling reactions, see: Zhu J, Bienaymé H, editors. Multicomponent Reactions. Wiley-VCH; 2005. Ruijter E, Scheffelaar R, Orru RVA. Angew. Chem. Int. Ed. 2011;50:6234. doi: 10.1002/anie.201006515.Dömling A, Wang W, Wang K. Chem. Rev. 2012;112:3083. doi: 10.1021/cr100233r.Orru RVA, Ruijter E, editors. Synthesis of Heterocycles via Multicomponent Reactions I,II, in Topics in Heterocyclic Chemistry. Vol. 23. Springer; 2010.
- 8.The X-ray analysis of the product 2j confirmed the presence of the diazo-group in the obtained products (see Supportin Information for details). CCDC-916523 contains the supplementary crystallographic data for this compound. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
- 9.Notably, the likely aza-Darzens aziridination reaction of imine was not observed. For Py•TfOH-catalyzed aziridination of imines with diazocompounds, see: Bew SP, Carrington R, Hughes DL, Liddle J, Pesce P. Adv. Synth. Catal. 2009;351:2579. For Ln(OTf)3-catalyzed aziridination of imines with diazocompounds, see: Nagayama S, Kobayashi S. Chem. Lett. 1998:685.Xie W, Fang J, Li J, Wang PG. Tetrahedron. 1999;55:12929.
- 10.For attempts on 3-CC reaction employing aniline, 3- and 4-aminopyridines, see Supporting Information.
- 11.For 1,2-migrations in β-amino-α-diazoesters, see: Jiang N, Wang J. Synlett. 2002:149.Jiang N, Ma Z, Qu Z, Xing X, Xie L, Wang J. J. Org. Chem. 2003;68:893. doi: 10.1021/jo0259818.Shi W, Jiang N, Zhang S, Wu W, Du D, Wang J. Org. Lett. 2003;5:2243. doi: 10.1021/ol034550o.Shi W, Xiao F, Wang J. J. Org. Chem. 2005;70:4318. doi: 10.1021/jo050173c.Xu F, Zhang S, Wu X, Liu Y, Shi W, Wang J. Org. Lett. 2006;8:3207. doi: 10.1021/ol061047d.Xiao F, Wang J. J. Org. Chem. 2006;71:5789. doi: 10.1021/jo0605391. See also ref 3a, c, d.
- 12.See Supporting Information for full optimization results.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.