Abstract
This paper provides an assessment of the associations that weight loss patterns during the first year of an intensive lifestyle intervention have with four year maintenance and health outcomes. Two components described patterns of weight change during the first year of intervention: one reflected the typical pattern of weight loss over the 12 months, but distinguished those who lost larger amounts across the monthly intervals from those who lost less. The second component reflected the weight change trajectory, and distinguished a pattern of initial weight loss followed by regain versus a more sustained pattern of weight loss 2,438 individuals aged 45–76 years with type 2 diabetes mellitus, who enrolled in the weight loss intervention of a randomized clinical trial, were assigned scores according to how their weight losses reflected these patterns. Relationships these scores had with weight losses and health outcomes (glycosolated hemoglobin – HbA1c; systolic blood pressure, HDL-cholesterol, and triglycerides) over four years were described. Both individuals who had larger month-to-month weight losses in year 1 and whose weight loss was more sustained during the first year had better maintenance of weight loss over four years, independent of characteristics traditionally linked to weight loss success (p<0.001). While relationships with year 4 weight loss were stronger, the pattern of larger monthly weight loss during year 1 was also independently predictive of year 4 levels of HbA1c, HDL-cholesterol, and systolic blood pressure.
Keywords: weight loss, type 2 diabetes mellitus, principal components analysis
INTRODUCTION
There is great heterogeneity in how individuals’ weights change in response to lifestyle interventions designed to produce weight loss. It is possible that the initial patterns of changes predict longer term outcomes beyond what may be projected from an individual’s characteristics. It is also possible that early patterns in weight change may be related to longer term changes in markers of health, beyond what is captured by concurrent weight status.
This paper uses empirically-based descriptions of longitudinal patterns of weight change to describe short term responses to an intensive weight loss intervention. While this intervention was multifactorial and included individual tailoring and toolbox-approaches to facilitate weight loss, the goal for each individual was the same: to lose 10% of their bodyweight and maintain this loss (1,2). The intervention is viewed as an overall program and the intent is not to deconstruct its components. Instead, the monthly percent weight changes of individual responses to this program are decomposed. Based on previously published data from the Action for Health in Diabetes (Look AHEAD) study and others, longitudinal patterns of weight change can be reduced to a limited number of underlying components and an individual’s patterns of weight change can be described by combining these components according to how strongly they are expressed (3,4). For the first year of the Look AHEAD intervention program, two major components accounted for 95% of the total variability in weight loss patterns after random error was removed (3). These provide succinct empirically-generated descriptors for weight loss patterns. The major component reflects the typical negatively decelerating weight loss curve and distinguishes those individuals who lost relatively larger amount of weight during monthly intervals compared to those with smaller weight losses. The second, less pronounced, component reflected departures from the first component according to whether the weight loss followed a more curvilinear or linear trajectory. Each individual’s response to the intervention was ranked according to the extent to which these two components were empirically expressed. This description was used to examine whether the patterns of weight loss over the first year had longer term associations with better weight maintenance and other health outcomes.
Several questions are of interest. Is larger accumulation of month-to-month weight losses over the first year associated with better longer term profiles (i.e., weight loss and health outcomes such as cardiovascular disease risk factors)? Do the early components of weight loss patterns have associations with subsequent outcomes that are independent from later measures of weight? If these associations exist, do they reflect differences in characteristics of individuals with different early weight loss patterns?
If links can be identified between shorter term patterns of weight loss and longer-term weight maintenance and health outcomes, these may inform future intervention studies and help to identify individuals who may benefit from alternative strategies to reduce risks.
METHODS AND PROCEDURES
The Action for Health in Diabetes (Look AHEAD) study is a multi-center randomized clinical trial that has enrolled 5,145 overweight or obese volunteers with type 2 diabetes (4,5). It is assessing the long-term effects on cardiovascular outcomes of an intensive lifestyle intervention program designed to achieve and maintain weight loss by decreased caloric intake and increased physical activity. The comparison group receives diabetes support and education.
At enrollment, Look AHEAD participants had type 2 diabetes, were aged 45–76 years, and were overweight or obese (body mass index ≥25 kg/m2, or ≥27 kg/m2 if on insulin). Other inclusion requirements were a source of medical care, blood pressure <160/100 mmHg (treated or untreated), HbA1c <11%, plasma triglycerides < 600 mg/dl, and willingness to accept random assignment and participate in the study for up to 13.5 years.
Intensive Lifestyle Intervention
Approximately half (N=2,570) of Look AHEAD enrollees were assigned by randomization to participate in a weight loss intervention, which has been described previously (2). This intervention combined diet modification and increased physical activity and was designed to induce a weight loss of 10% of initial body weight during the first year and for this weight loss to be maintained thereafter. It was modeled on group behavioral programs developed for the treatment of obese patients with type 2 diabetes and included treatment components from the Diabetes Prevention Program (6). During the first 6 months, participants were seen weekly with 3 group meetings and 1 individual session per month. During months 7–12, participants were seen in the clinic at least twice per month: group sessions every other week and a monthly individual session. These sessions were led by interventionists trained in nutrition and exercise counseling. Each month, during months 13–48, participants had an individual, on-site session followed approximately 2 weeks later by a second individual contact by phone or email, with optional group sessions offered monthly. Participants were weighed at all intervention visits; principal components scores are based on these weights collected during the first year of the intervention (3).
Restriction of caloric intake was the primary method of achieving weight loss. To aim for a weight loss of 10% of initial weight, the calorie goals were 1200–1500 for individuals weighing 250 lbs (114 kg) or less at baseline and 1500–1800 for individuals weighing more than 250 lbs. The composition of the diet was structured to enhance glycemic control and to improve cardiovascular disease risk factors and included a maximum of 30% of total calories from fat, a maximum of 10% of total calories from saturated fat, and a minimum of 15% of total calories from protein.
The physical activity program of the weight loss intervention relied heavily on home-based exercise, with gradual progression toward a goal of 175 minutes of moderate intensity physical activity per week by the end of the first 6 months. Moderate-intensity walking was encouraged as the primary type of physical activity, however to enhance participation the intervention allowed for individual choices in types of moderate physical activities and the tailoring of exercise programs based on physical fitness test and safety issues.
The intervention plan called for 6 months of lifestyle strategies alone. After this, the “toolbox” algorithm included use of weight loss medication (orlistat) and/or advanced behavioral strategies for individuals having difficulty with weight loss. Advanced behavioral strategies included, for example, the provision of exercise equipment or enrolling participants in a cooking class. During years 2–4, the focus of intervention shifted to maintaining the weight losses and high levels of physical activity achieved during the first year (7). Those who had not achieved the recommended goals were encouraged to do so. Each month, participants had an individual, on-site meeting (20–30 minutes) with their interventionist, with a second individual contact by telephone or e-mail approximately 2 weeks later. Participants had individualized calorie goals, based on their desire to maintain their weight, lose more (if their BMI was > 23 kg/m2), or reverse weight gain. All participants were encouraged to replace one meal or snack per day with liquid shakes or meal bars. They also were instructed to continue to exercise at least 175 minutes/week.
Data Collection Protocols
Standardized interviewer-administered questionnaires were used to obtain self-reported data on markers of demography and medical history at baseline. History of cardiovascular disease was defined as self-report of prior myocardial infarction, stroke, coronary or lower extremity angioplasty, carotid endarterectomy, or coronary bypass surgery. Hypertension was defined as systolic blood pressure ≥140 mmHg or diastolic blood pressure ≥90 mmHg, or use of anti-hypertensive medications. Scores ≥10 on the Beck Depression Inventory were used to mark symptoms of depression (8). Weight was measured on all participants at baseline and at yearly assessment visits, using a digital scale (Tanitia, model BWB-800), by study staff who were masked to participants’ intervention status. These weights were used in analyses of annual weight changes from baseline.
Four longer term outcomes were assessed, for which the Look AHEAD intervention has been previously been shown to be beneficial over four years (9): fasting HbA1c levels from whole-blood samples, systolic blood pressure measured in duplicate with an automated device, and fasting HDL-cholesterol and triglyceride levels from serum. The Look AHEAD Central Biochemistry Laboratory was located at Northwest Lipid Research Laboratories, University of Washington, Seattle, WA.
Statistical Analysis
Analyses were restricted to the 2,438 (94.9%) of participants in the intensive lifestyle intervention on whom weight was measured at least once at an intervention visit during the first year of follow-up (so that component scores could be estimated) and at least one annual clinic visit through year 4 of follow-up (so that longer term weight and outcomes were available). The analyses conducted at year 1 on the longitudinal series of monthly percent changes in weight, called “principal components analyses” (10), yielded two underlying components that accounted for 95% of the intra-subject differences in patterns of change, which are summarized below; greater detail and the linear equations defining these components have been published earlier (11). The remaining 10 principal components collectively accounted for only 5% of the total variation and are not considered in this report.
For participants with incomplete data, component scores were estimated using least squares and subsequently, relative weights based on standard errors of these estimates were used in analyses to diminish the influence of individuals for whom there were only partial sequences of measurements. The rates of missing data for year 1 monthly visits recorded in the intervention tracking system were 13.8% (missing one month), 9.3% (missing 2–3 months), 4.3% (missing 4–6 months), and 3.2% (missing >6 months) (3). The two component scores were used to rank individuals according to percentiles. Differences in the mean percentile ranks were compared across subgroups based on individual’s characteristics at baseline and compared using weighted analyses of variance. To portray the patterns of weight changes defined by the two components, scores that corresponded to the mid-points of tertile groups for one component were added to the mean scores of the other component. Participants were grouped according to tertiles of these scores and weighted analyses of covariance were used to contrast four-year changes in weight, HbA1c, systolic blood pressure, HDL-cholesterol, and triglyceride concentrations with varying levels of covariate adjustment. General linear models (11) were fitted using weighted maximum likelihood to describe patterns in changes across time.
RESULTS
Description of Principal Components
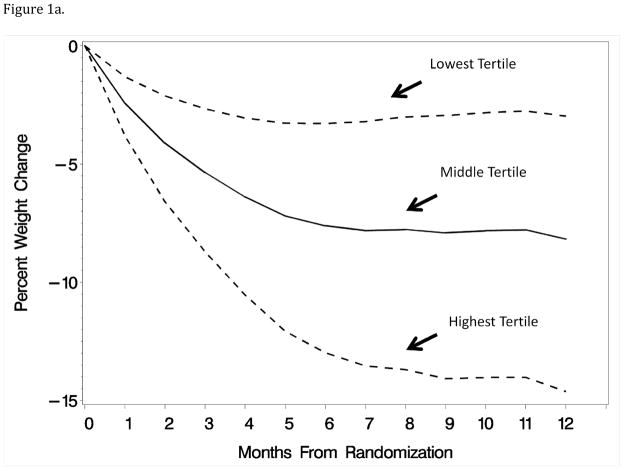
The first component reflected the typical trend of weight loss over the first year (88% of variation); scores on this component ranked participants according to the magnitude of their month-to-month weight losses. Individuals with higher scores on this component had relatively greater month-to-month weight losses, particularly if those were accumulated throughout the year. Thus, an individual who had little or no weight loss until the very end of year 1 would not score as highly on this component as one who reached the same weight loss through a series of incremental losses. In this sense, the component differs from a single measurement of weight loss at year 1 by capturing the pattern of weight losses over time, rather than at a single time point. Figure 1a portrays the fitted patterns at the midpoints of the tertiles of first component scores (i.e. at the 16.7%ile, 50.0%ile, and 83.3%ile) for individuals with the mean score of the second component.
Figure 1.
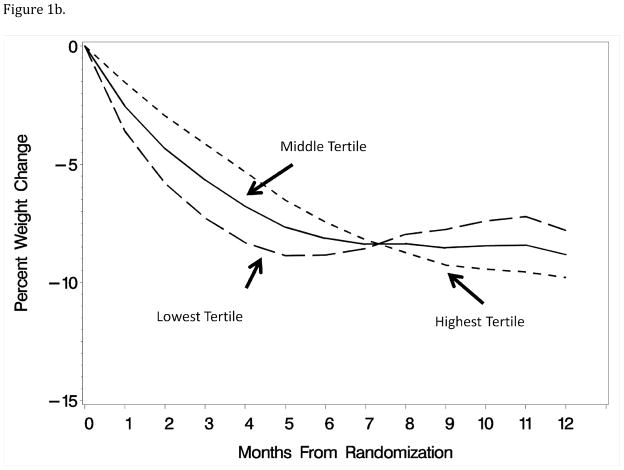
Figures 1a–1b: Patterns of weight loss during the first year of an intervention associated with the tertiles of principal component scores for month-to-month weight losses and linear versus curvilinear trajectory of weight loss. Curves were computed by adding to each principal component the mean levels of the other component.
The second component described how the patterns of weight losses varied from the first component, distinguishing individuals for whom weight losses occurred earlier followed by a leveling or slight regain (i.e. slightly U-shaped) or whether weight losses occurred more gradually and continued throughout the year (i.e. more linear). As can be seen in Figure 1b, adding increasing levels of this component to the first reduces the inflection point in the overall pattern of weight losses.
Relation of Principal Components with Baseline Characteristics
Table 1 describes participant’s characteristics at baseline and provides mean percentiles of component scores for individuals grouped according to these characteristics. Women tended to be in the lowest tertile of the first component, with smaller month-to-month weight losses over the year. They had higher scores on the second component, exhibiting a tendency for weight losses to occur more gradually and be sustained. Each of the characteristics in Table 1, except hypertension, had significant (p≤0.05) associations with one or both components of the weight change patterns.
Table 1.
Baseline characteristics of participants assigned to the Look AHEAD intensive lifestyle intervention and mean percentile rank of components describing patterns in percent weight changes over the first year of intervention: the magnitude of month-to-month weight losses and the linear versus curvilinear trajectory of weight losses. Higher percentiles mark individuals with greater month-to-month accumulations of weight loss and those whose weight losses occurred more gradually and were sustained.
| Baseline Characteristic | N (Percent) | Month-to- Month Weight Losses Mean (SD) Percentile |
Linear or Curvilinear Trajectory of Weight Losses Mean (SD) Percentile |
|---|---|---|---|
|
| |||
| Sex | |||
| Female | 1450 (59.5) | 47.1 (28.6) | 53.8 (27.8) |
| Male | 988 (40.5) | 54.3 (28.8) | 44.5 (29.5) |
| P<0.001 | P<0.001 | ||
|
| |||
| Age, yrs* | |||
| 45–54 | 604 (24.8) | 47.4 (29.2) | 49.7 (29.5) |
| 55–64 | 1357 (55.7) | 50.0 (29.2) | 50.0 (28.7) |
| 65–76 | 477 (19.6) | 53.3 (27.1) | 51.0 (28.5) |
| P=0.004 | P=0.62 | ||
|
| |||
| Race/Ethnicity* | |||
| African-American | 373 (15.3) | 40.1 (26.5) | 56.6 (27.4) |
| American Indian/Alaska Native | 128 (5.2) | 32.1 (23.6) | 61.1 (27.3) |
| Asian/Pacific Islander | 28 (1.2) | 46.2 (28.7) | 45.6 (25.7) |
| Hispanic/Latino | 321 (13.2) | 49.4 (27.8) | 54.3 (27.6) |
| Non-Hispanic White | 1539 (63.2) | 54.4 (28.8) | 46.8 (29.1) |
| Other/multiple | 48 (2.0) | 41.9 (29.5) | 45.7 (26.2) |
| P<0.001 | P<0.001 | ||
|
| |||
| BMI, kg/m2* | |||
| 25–29 | 384 (15.8) | 45.4 (26.5) | 50.3 (28.5) |
| 30–34 | 869 (35.6) | 51.4 (28.7) | 47.1 (28.2) |
| 35–39 | 642 (26.3) | 50.0 (29.6) | 50.8 (29.3) |
| ≥ 40 | 543 (22.3) | 51.0 (29.6) | 53.6 (29.3) |
| P=0.006 | P<0.001 | ||
|
| |||
| HbA1C, %* | |||
| < 7.0 | 1135 (46.6) | 54.3 (28.7) | 50.1 (29.5) |
| 7.0 – 8.9 | 738 (30.3) | 48.9 (28.5) | 49.8 (28.4) |
| ≥ 9.0 | 565 (23.2) | 42.9 (28.2) | 50.1 (28.3) |
| P<0.001 | P=0.96 | ||
|
| |||
| Insulin use | |||
| No | 2080 (85.3) | 50.9 (28.7) | 49.5 (29.0) |
| Yes | 358 (14.7) | 44.8 (29.6) | 52.9 (28.2) |
| P<0.001 | P=0.04 | ||
|
| |||
| Hypertension | |||
| No | 380 (15.6) | 48.0 (28.5) | 50.2 (28.2) |
| Yes | 2058 (84.4) | 50.4 (28.9) | 50.0 (29.0) |
| P=0.13 | P=0.88 | ||
|
| |||
| Prior CVD | |||
| No | 2090 (85.7) | 50.5 (28.9) | 50.3 (28.8) |
| Yes | 348 (14.3) | 47.1 (28.7) | 48.2 (29.0) |
| P=0.05 | P=0.20 | ||
|
| |||
| Beck Depression Inventory | |||
| <10 (no depression) | 1981 (81.5) | 50.9 (28.8) | 49.0 (29.0) |
| ≥ 10 | 451 (18.5) | 46.2 (28.8) | 54.7 (28.0) |
| P=0.002 | P<0.001 | ||
The following pairwise comparisons reach statistical significance based on a Scheffe test (p≤0.05):
For Monthly Weight Loss Accumulation:
- Age: 45–54 Years vs 65–76 Years
- Race/Ethnicity: African-American vs Non-Hispanic White; American Indian/Alaska Native vs Non-Hispanic White; African-American vs Hispanic/Latino; American Indian/Alaska Native vs Hispanic/Latino
- Body Mass Index: 25–29 vs 30–34 kg/m2; 25–29 vs ≥ 40 kg/m2
- HbA1c: All pairwise comparisons
For Linear Trajectory of Weight Losses:
- Race/Ethnicity: American Indian/Alaska Native vs Non-Hispanic White; Hispanic/Latino vs Non-Hispanic White; African-American vs Non-Hispanic White
- Body Mass Index: 30–34 vs ≥ 40 kg/m2
Relation of Principal Components with Weight Loss
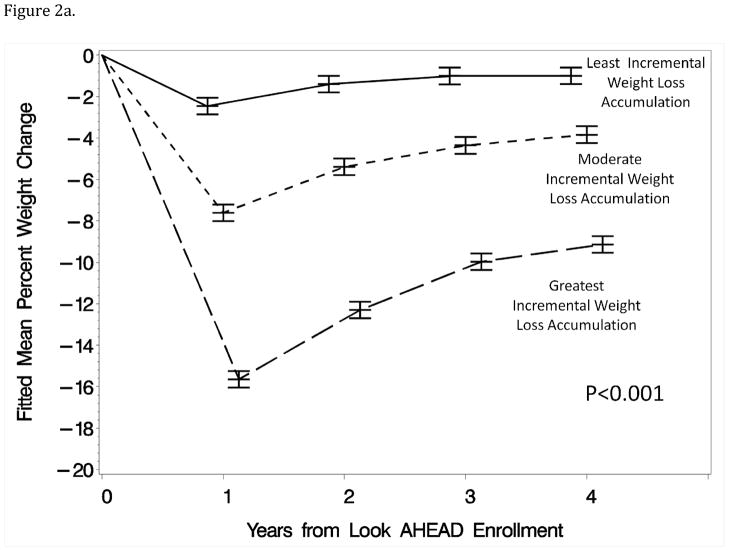
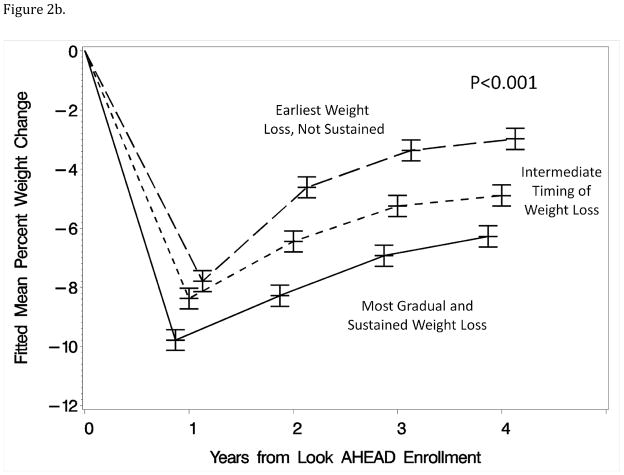
Of the 2,438 individuals included in these analyses, annual weight measurements were recorded on 98.6% (year 1), 95.5% (year 2), 94.3% (year 3), and 93.6% (year 4). Figure 2a portrays the mean weight changes from baseline at these visits for individuals grouped according to the first component, which were generated by fitting general linear models. Figure 2b is a similar presentation for individuals grouped according to the second component, with covariate adjustment for the first component. (Covariate adjustment for weight loss at year 1 produced similar patterns of mean differences at years 2–4.) In both cases, the mean trajectories across four years differed markedly among percentile groups (each p<0.001).
Figure 2.
Figure 2a–b: Mean percent changes in weight from baseline across four years for individuals grouped by tertile of component scores from the first year of the intervention. The time scales for the means have been perturbed slightly to reduce overlap in the figures.
Table 2 focuses on changes at year 4 and lists mean percent weight losses at this time for the percentile groups from analysis of these cross-sectional data, without and with full covariate adjustment. Differences were independent of adjustment and highly statistically significant. Participants who had, across year 1, relatively larger month-to-month weight losses and for which the timing of these weight losses was slower but more sustained maintained the largest weight losses at year 4. Extensive covariate-adjustment did not materially alter these relationships, i.e. the independent associations between initial weight loss patterns and year 4 weight losses could not be accounted for by baseline characteristics of participants.
Table 2.
Fitted mean year 4 percent changes in weight for individuals grouped by tertiles of principal component scores that express patterns of accumulated month-to-month weight losses and linear versus curvilinear trajectory of weight losses during the first year. Means are adjusted for the other principal component, without and with further adjustment for factors in Table 1.
| Principal Component Tertile | Covariate Adjustment Only for Other Component | Full Covariate Adjustment | ||
|---|---|---|---|---|
|
| ||||
| Mean (SE) | p-value | Mean (SE) | p-value | |
|
| ||||
| Month-to-Month Weight Losses | ||||
| 1st tertile (smallest monthly losses) | −0.54 (0.26) | <0.001 | −0.62 (0.26) | <0.001 |
| 2nd tertile | −4.01 (0.25) | −4.02 (0.25) | ||
| 3rd tertile (greatest monthly losses) | −9.37 (0.25) | −9.20 (0.26) | ||
|
| ||||
| Linear Versus Curvilinear Trajectory of Weight Losses | ||||
| 1st tertile (early but not sustained) | −3.30 (0.25) | <0.001 | −3.33 (0.25) | <0.001 |
| 2nd tertile | −4.64 (0.25) | −4.66 (0.25) | ||
| 3rd tertile (gradual and sustained) | −6.17 (0.25) | −6.11 (0.26) | ||
The following pairwise comparisons reach statistical significance based on a Scheffe test (p≤0.05):
For Monthly Weight Loss Accumulation:
- Limited Covariate Adjustment: All pairwise comparisons
- Covariate Adjustment: All pairwise comparisons
For Linear Versus Curvilinear Trajectory of Weight Losses:
- Limited Covariate Adjustment: 1st tertile vs 2nd tertile; 1st tertile vs 3rd tertile
- Covariate Adjustment: 1 st tertile vs 2nd tertile; 1st tertile vs 3rd tertile
Relation of Principal Components with Cardiovascular Disease Risk Factors
Table 3 portrays associations that weight loss patterns over the first year had with changes from baseline in HbAlc, systolic blood pressure, HDL-cholesterol, and triglycerides at year 4. For each outcome measure, four models were fitted to include: no covariates, Table 1 covariates, Table 1 covariates and year 4 percent weight change, or Table 1 covariates and change in relevant medications (i.e. oral diabetes medications and insulin use, antihypertensive medications, and lipid-lowering medications). Participants with greater month-to-month weight losses during year 1 had significantly better levels of HbA1c and HDL-cholesterol at year 4 (p<0.0001), with mean differences of approximately 0.4 % HbA1c and 3.5 mg/dl HDL-cholesterol between the lowest and highest percentile groups. Controlling for participant characteristics and for changes in use of diabetes or lipid lowering drugs use from baseline had little effect on the magnitude of these differences. At annual visits, individuals with greater year 4 weight changes had more favorable concurrent changes in HbA1c, systolic blood pressure, HDL-cholesterol, and triglycerides (each p<0.001). At year 4, controlling for corresponding weight loss attenuated the relationships that these measures had with magnitude of month-to-month weight losses during year 1, but these remained statistically significant, i.e. while relationships with current weight loss were much stronger, the pattern of weight loss accumulation during year 1 was independently predictive of these future outcomes.
Table 3.
Associations that patterns of weight loss during the first year of intervention (as expressed by tertile of principal component scores) have with changes in cardiovascular risk factors from baseline to year 4, with and without adjustment for covariates, percent weight change, and drug therapy.
| Cardiovascular Disease Risk Factor | Level of Covariate Adjustment | Tertile | Changes From Baseline At Year 4
|
|||
|---|---|---|---|---|---|---|
| Month-To-Month Weight Losses: Least to Greatest | Linear Versus Curvilinear Trajectory of Weight Losses: Early/Not Sustained vs Gradual and Sustained | |||||
|
| ||||||
| Mean (SE) | p-value | Mean (SE) | p-value | |||
|
| ||||||
| HbA1c, %* | None | 1st | −0.01 (0.05) | <0.001 | −0.21 (0.05) | 0.70 |
| 2nd | −0.17 (0.05) | −0.17 (0.05) | ||||
| 3rd | −0.42 (0.05) | −0.22 (0.05) | ||||
|
| ||||||
| Baseline factors1 | 1st | 0.03 (0.04) | <0.001 | −0.19 (0.04) | 0.47 | |
| 2nd | −0.17 (0.04) | −0.17 (0.04) | ||||
| 3rd | −0.47 (0.04) | −0.25 (0.04) | ||||
|
| ||||||
| Baseline factors and Year 4 weight change percent | 1st | −0.06 (0.05) | 0.01 | −0.21 (0.04) | 0.80 | |
| 2nd | −0.19 (0.04) | −0.18 (0.04) | ||||
| 3rd | −0.36 (0.05) | −0.22 (0.04) | ||||
|
| ||||||
| Baseline factors and change in diabetes drug use | 1st | 0.03 (0.05) | <0.001 | −0.20 (0.05) | 0.47 | |
| 2nd | −0.20 (0.04) | −0.19 (0.05) | ||||
| 3rd | −0.46 (0.05) | −0.26 (0.05) | ||||
|
| ||||||
| Systolic Blood Pressure (mmHg)* | None | 1st | −1.24 (0.69) | <0.001 | −5.27 (0.69) | 0.09 |
| 2nd | −4.83 (0.69) | −4.07 (0.70) | ||||
| 3rd | −6.38 (0.68) | −3.15 (0.69) | ||||
|
| ||||||
| Baseline factors1 | 1st | −1.34 (0.70) | <0.001 | −5.07 (0.69) | 0.24 | |
| 2nd | −5.02 (0.79) | −4.14 (0.70) | ||||
| 3rd | −6.21 (0.69) | −3.40 (0.70) | ||||
|
| ||||||
| Baseline factors and Year 4 weight change percent | 1st | −3.06 (0.72) | 0.05 | −5.27 (0.68) | 0.07 | |
| 2nd | −5.42 (0.68) | −4.31 (0.68) | ||||
| 3rd | −4.17 (0.73) | −3.04 (0.68) | ||||
|
| ||||||
| Baseline factors and change in antihypertensive medications | 1st | −1.28 (0.73) | <0.001 | −5.15 (0.71) | 0.13 | |
| 2nd | −4.82 (0.71) | −4.49 (0.72) | ||||
| 3rd | −6.59 (0.71) | −3.14 (0.72) | ||||
|
| ||||||
| HDL-C, mg/dl* | None | 1st | 2.32 (0.30) | <0.001 | 4.33 (0.30) | 0.13 |
| 2nd | 3.56 (0.3) | 3.46 (0.30) | ||||
| 3rd | 5.82 (0.29) | 3.95 (0.30) | ||||
|
| ||||||
| Baseline factors1 | 1st | 2.26 (0.30) | <0.001 | 4.30 (0.30) | 0.12 | |
| 2nd | 3.51 (0.30) | 3.44 (0.30) | ||||
| 3rd | 5.97 (0.30) | 4.05 (0.35) | ||||
|
| ||||||
| Baseline factors and Year 4 weight change percent | 1st | 2.85 (0.31) | <0.001 | 4.40 (0.30) | 0.11 | |
| 2nd | 3.65 (0.30) | 3.52 (0.30) | ||||
| 3rd | 5.25 (0.32) | 3.87 (0.30) | ||||
|
| ||||||
| Baseline factors and change in lipid medications | 1st | 2.29 (0.32) | <0.001 | 4.46 (0.32) | 0.10 | |
| 2nd | 3.60 (0.31) | 3.49 (0.32) | ||||
| 3rd | 5.97 (0.32) | 3.96 (0.32) | ||||
|
| ||||||
| Triglycerides, mg/dl | None | 1st | −17.62 (4.52) | 0.12 | −20.32 (4.47) | 0.71 |
| 2nd | −21.66 (4.54) | −24.81 (4.54) | ||||
| 3rd | −30.41 (4.46) | −24.86 (4.53) | ||||
|
| ||||||
| Baseline factors1 | 1st | −17.00 (4.62) | 0.11 | −19.11 (4.54) | 0.52 | |
| 2nd | −21.92 (4.56) | −25.21 (4.55) | ||||
| 3rd | −30.83 (4.57) | −25.76 (4.57) | ||||
|
| ||||||
| Baseline factors and Year 4 weight change percent | 1st | −22.54 (4.80) | 0.98 | −19.79 (4.52) | 0.62 | |
| 2nd | −23.29 (4.55) | −25.77 (4.53) | ||||
| 3rd | −24.16 (4.85) | −24.55 (4.56) | ||||
|
| ||||||
| Baseline factors and change in lipid medications | 1st | −15.72 (4.87) | 0.07 | −18.66 (4.72) | 0.48 | |
| 2nd | −22.08 (4.77) | −24.95 (4.78) | ||||
| 3rd | −31.63 (4.78) | −26.38 (4.81) | ||||
Baseline factors include sex, age, race/ethnicity, BMI, HbA1c, insulin use, hypertension, prior CVD, and depression.
The following pairwise comparisons reach statistical significance based on a Scheffe test (p≤0.05):
For Monthly Weight Loss Accumulation:
- HbA1c
- No Covariate Adjustment: All pairwise comparisons
- Baseline Covariate Adjustment: All pairwise comparisons
- Baseline and Year 4 Weight Covariate Adjustment: All pairwise comparisons
- Baseline and Diabetes Drug Use Covariate: All pairwise comparisons
- Systolic Blood Pressure
- No Covariate Adjustment: 1st vs 2nd tertile; 1st vs 3rd tertile
- Baseline Covariate Adjustment: 1st vs 2nd tertile; 1st vs 3rd tertile
- Baseline and Year 4 Weight Covariate Adjustment: 1st vs 2nd tertile; 1st vs 3rd tertile
- Baseline and Diabetes Drug Use Covariate: 1st vs 2nd tertile; 1st vs 3rd tertile
- HDL-cholesterol
- No Covariate Adjustment: All pairwise comparisons
- Baseline Covariate Adjustment: All pairwise comparisons
- Baseline and Year 4 Weight Covariate Adjustment: All pairwise comparisons
- Baseline and Diabetes Drug Use Covariate: All pairwise comparisons
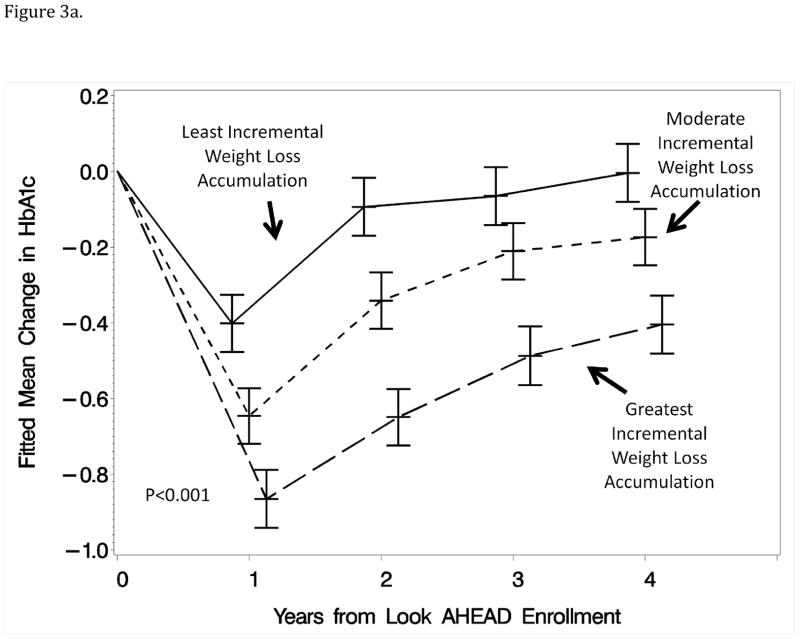
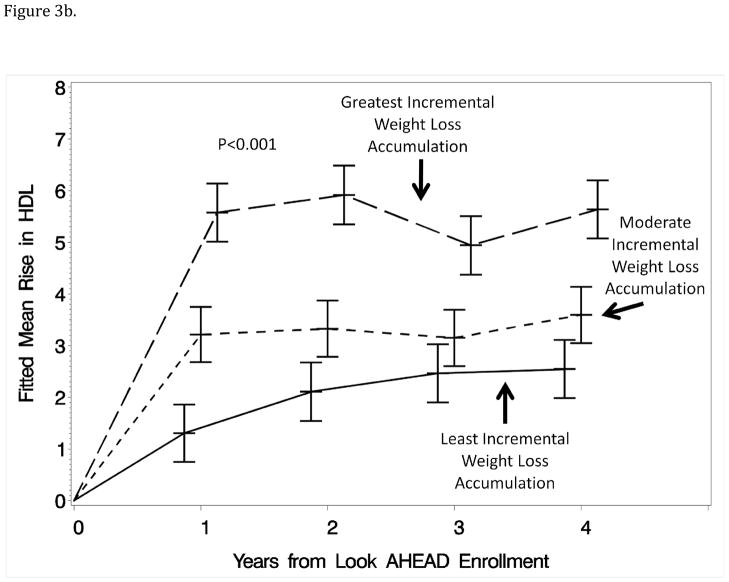
General linear models were used to examine how these differences unfolded over time to estimate mean differences at each of the annual exams with covariate adjustments for weight changes measured at those time points. Figure 3 portrays these means for HbA1c and HDL-cholesterol: it can be seen that differences were consistently maintained over time among tertile groups and that, even at year 1, the pattern of weight losses captured by the first component was predictive of change in HbA1c independently of the year 1 weight loss measurement.
Figure 3.
Figure 3a: Mean changes in HbA1c (%) for participants grouped (tertile) according to the relative magnitude of month-to-month weight losses during year 1 with covariate adjustment for all factors in Table 1 and weight loss from baseline measured at the same time as the HbA1c measures.
Figure 3b: Mean increases in HDL-cholesterol (mg/dl) for participants grouped (tertile) according to the relative magnitudes of month-to-month weight losses during year 1 with covariate adjustment for all factors in Table 1 and change in weight at the time of HCL-cholesterol measures.
Greater month-to-month weight losses over year 1 were associated with a greater reduction in year 4 systolic blood pressure, averaging about 5 mmHg between the first and third tertile groups. For this outcome, including year 4 weight change as a covariate attenuated mean differences so that they were of only marginal statistical significance (p=0.05) with mean differences of less than 2 mmHg.
None of the cardiovascular risk factors at year 4 were significantly related to the second component.
DISCUSSION
There was great diversity in the patterns of weight loss over the first year the Look AHEAD intensive multifactorial intervention, despite its common protocol and goals. Of the eight baseline characteristics considered, seven had significant relationships with the magnitudes of the month-to-month weight loss and/or the trajectory of weight loss. The principal component analysis allowed these associations to be succinctly described.
Both patterns of response to the first year of the intervention predicted successful maintenance of weight loss after four years. Greater month-to-month weight loss accumulation and more gradual and sustained weight loss during the first year were each associated with better longer term weight loss. These associations were independent of each other and of a panel of participant characteristics: markers of demography, health, and lifestyle, including baseline body mass index and diabetes control.
Many authors have stressed the importance that the initial magnitude of weight loss has for longer term success with behavioral interventions (12–15). A separate analysis of Look AHEAD data found that year 1 weight loss was the strongest determinant of year 4 weight loss, accounting for 22% of its overall variability (7). The Diabetes Prevention Program found that achieving an initial 7% weight loss strongly predicted longer term weight loss (16).
Several authors have found that losing weight more gradually is associated with greater long term maintenance of weight loss (17,18), however others have not (19). A complexity in this discussion is that the rate of initial weight loss is often not differentiated from the overall weight loss. Toubro, et al. attempted to examine this experimentally, using different intervention approaches to vary the rate of weight loss while achieving similar longer-term overall levels (20). They found that more rapid weight losses were associated with slightly better maintenance. This approach differs from our analyses in that we examine rates of response to a common intervention. The principal components analysis distinguished the timing of year 1 weight loss from its overall accumulation. Both independently were important predictors of longer term maintenance of weight loss. A weight loss that is achieved through gradual and sustained increments, rather than a more rapid loss weight loss that is not sustained, is associated with better long term maintenance, perhaps reflecting both the rate and the maintenance of adopting lifestyle changes during the first year.
Greater month-to-month weight losses during the first year of the weight loss intervention was associated with longer term benefits in markers of diabetes control, blood pressure control, and lipid control, independent of current weight loss. The factors that were most strongly associated with year 1 weight losses – HbA1c, HDL-cholesterol, and systolic blood pressure – have all been shown to be influenced by the overall Look AHEAD intervention relative to its control condition (9) and to be influenced by weight loss interventions of shorter duration (21). It may be that early success in losing weight serves to mark a group of individuals who are better able to maintain their health long term, however covariate adjustment for a number of personal characteristics did not materially alter these associations.
Importantly, the magnitude of month-to-month weight losses during the first year of the intervention was associated with improved HbA1c, systolic blood pressure, and HDL-cholesterol even after controlling for weight changes from baseline to the time that these outcomes were assessed. These associations could not be explained by changes in medications nor by a number of other factors that might be related to overall adherence and medical care. It is possible that this is an example of a “legacy” effect, i.e. an intervention effect that is carried forward that is not explained fully by current measures (22). Another possibility is that, due to short-term fluctuations in weight, a measurement at a single point does not fully capture associations between weight change and health outcomes that have emerged over time.
Whether or not weight losses during the first year were gradual and sustained or early with slight regain did not appear to have marked longer term influence on markers of health, with or without adjustment for year 4 weight status. It may be that the differences in longer term weight gain that are associated with the trajectory of initial weight loss are less meaningful and can be largely overcome with medical management and continued lifestyle intervention.
There are several qualifications to the findings. Patterns in weight loss with individual components have not been linked to targets of the intervention (behavioral strategies, physical activity, change in fitness, use of meal replacements, orlistat, etc.) and it is possible that these may be variously associated with weight loss patterns and with longer term outcomes. Findings are based on the Look AHEAD intervention and adults with type 2 diabetes who were eligible and volunteered for a clinical trial: it is possible that they will not generalize to other settings or groups.
The associations that are described between weight loss patterns over the initial year of a long-term intervention stress the importance of the initial success of the intervention. While current weight status is the strongest predictor of outcomes, individuals who were most responsive to the intervention (i.e. those who accumulated greater month-to-month weight losses during the first year) tended to have better longer term outcomes in addition to what could be predicted from their current weight. Individuals with more gradual and sustained weight losses during the first year had longer term better weight loss maintenance, but this pattern produced no additional benefits on the health outcomes we assessed.
Acknowledgments
This study is supported by the Department of Health and Human Services through the following cooperative agreements from the National Institutes of Health: DK57136, DK57149, DK56990, DK57177, DK57171, DK57151, DK57182, DK57131, DK57002, DK57078, DK57154, DK57178, DK57219, DK57008, DK57135, and DK56992. The following federal agencies have contributed support: National Institute of Diabetes and Digestive and Kidney Diseases; National Heart, Lung, and Blood Institute; National Institute of Nursing Research; National Center on Minority Health and Health Disparities; Office of Research on Women’s Health; the Centers for Disease Control and Prevention; and the Department of Veterans Affairs. This research was supported in part by the Intramural Research Program of the National Institute of Diabetes and Digestive and Kidney Diseases. The Indian Health Service (I.H.S.) provided personnel, medical oversight, and use of facilities. The opinions expressed in this paper are those of the authors and do not necessarily reflect the views of the I.H.S. or other funding sources.
Additional support was received from The Johns Hopkins Medical Institutions Bayview General Clinical Research Center (M01RR02719); the Massachusetts General Hospital Mallinckrodt General Clinical Research Center and the Massachusetts Institute of Technology General Clinical Research Center (M01RR01066); the University of Colorado Health Sciences Center General Clinical Research Center (M01RR00051) and Clinical Nutrition Research Unit (P30 DK48520); the University of Tennessee at Memphis General Clinical Research Center (M01RR0021140); the University of Pittsburgh General Clinical Research Center (GCRC) (M01RR000056), the Clinical Translational Research Center (CTRC) funded by the Clinical & Translational Science Award (UL1 RR 024153) and NIH grant (DK 046204); and the Frederic C. Bartter General Clinical Research Center (M01RR01346)
The following organizations have committed to make major contributions to Look AHEAD: FedEx Corporation; Health Management Resources; LifeScan, Inc., a Johnson & Johnson Company; OPTIFAST® of Nestle HealthCare Nutrition, Inc.; Hoffmann-La Roche Inc.; Abbott Nutrition; and Slim-Fast Brand of Unilever North America.
Appendix: Look AHEAD Research Group at Year 4
Clinical Sites
The Johns Hopkins Medical Institutions
Frederick L. Brancati, MD, MHS1; Lee Swartz2; Lawrence Cheskin, MD3; Jeanne M. Clark, MD, MPH3; Kerry Stewart, EdD3; Richard Rubin, PhD3; Jean Arceci, RN; Suzanne Ball; Jeanne Charleston, RN; Danielle Diggins; Mia Johnson; Joyce Lambert; Kathy Michalski, RD; Dawn Jiggetts; Chanchai Sapun
Pennington Biomedical Research Center
George A. Bray, MD1; Kristi Rau2; Allison Strate, RN2; Frank L. Greenway, MD3; Donna H. Ryan, MD3; Donald Williamson, PhD3; Brandi Armand, LPN; Jennifer Arceneaux; Amy Bachand, MA; Michelle Begnaud, LDN, RD, CDE; Betsy Berhard; Elizabeth Caderette; Barbara Cerniauskas, LDN, RD, CDE; David Creel, MA; Diane Crow; Crystal Duncan; Helen Guay, LDN, LPC, RD; Carolyn Johnson, Lisa Jones; Nancy Kora; Kelly LaFleur; Kim Landry; Missy Lingle; Jennifer Perault; Cindy Puckett; Mandy Shipp, RD; Marisa Smith; Elizabeth Tucker
The University of Alabama at Birmingham
Cora E. Lewis, MD, MSPH1; Sheikilya Thomas MPH2; Monika Safford, MD3; Vicki DiLillo, PhD; Charlotte Bragg, MS, RD, LD; Amy Dobelstein; Stacey Gilbert, MPH; Stephen Glasser, MD3; Sara Hannum, MA; Anne Hubbell, MS; Jennifer Jones, MA; DeLavallade Lee; Ruth Luketic, MA, MBA, MPH; L. Christie Oden; Janet Raines, MS; Cathy Roche, RN, BSN; Janet Truman; Nita Webb, MA; Casey Azuero, MPH; Jane King, MLT; Andre Morgan
Harvard Center
Massachusetts General Hospital
David M. Nathan, MD1; Enrico Cagliero, MD3; Kathryn Hayward, MD3; Heather Turgeon, RN, BS, CDE2; Linda Delahanty, MS, RD3; Ellen Anderson, MS, RD3; Laurie Bissett, MS, RD; Valerie Goldman, MS, RD; Virginia Harlan, MSW; Theresa Michel, DPT, DSc, CCS; Mary Larkin, RN; Christine Stevens, RN; Kylee Miller, BA; Jimmy Chen, BA; Karen Blumenthal, BA; Gail Winning, BA; Rita Tsay, RD; Helen Cyr, RD; Maria Pinto
Joslin Diabetes Center
Edward S. Horton, MD1; Sharon D. Jackson, MS, RD, CDE2; Osama Hamdy, MD, PhD3; A. Enrique Caballero, MD3; Sarah Bain, BS; Elizabeth Bovaird, BSN, RN; Barbara Fargnoli, MS, RD; Jeanne Spellman, BS, RD; Ann Goebel-Fabbri, PhD; Lori Lambert, MS, RD; Sarah Ledbury, MEd, RD; Maureen Malloy, BS; Kerry Ovalle, MS, RCEP, CDE
Beth Israel Deaconess Medical Center
George Blackburn, MD, PhD1; Christos Mantzoros, MD, DSc3; Ann McNamara, RN; Kristina Spellman, RD
University of Colorado Health Sciences Center
James O. Hill, PhD1; Marsha Miller, MS, RD2; Brent Van Dorsten, PhD3; Judith Regensteiner, PhD3; Ligia Coelho, BS; Paulette Cohrs, RN, BSN; Susan Green; April Hamilton, BS, CCRC; Jere Hamilton, BA; Eugene Leshchinskiy; Lindsey Munkwitz, BS; Loretta Rome, TRS; Terra Worley, BA; Kirstie Craul, RD, CDE; Sheila Smith, BS
Baylor College of Medicine
John P. Foreyt, PhD1; Rebecca S. Reeves, DrPH, RD2; Henry Pownall, PhD3; Ashok Balasubramanyam, MBBS3; Peter Jones, MD3; Michele Burrington, RD, RN; Chu-Huang Chen, MD, PhD; Allyson Clark Gardner, MS, RD; Molly Gee, MEd, RD; Sharon Griggs; Michelle Hamilton; Veronica Holley; Jayne Joseph, RD; Julieta Palencia, RN; Jennifer Schmidt; Carolyn White
The University of Tennessee Health Science Center
University of Tennessee East
Karen C. Johnson, MD, MPH1; Carolyn Gresham, RN2; Stephanie Connelly, MD, MPH3; Amy Brewer, RD, MS; Mace Coday, PhD; Lisa Jones, RN; Lynne Lichtermann, RN, BSN; Shirley Vosburg, RD, MPH; J. Lee Taylor, MEd, MBA
University of Tennessee Downtown
Abbas E. Kitabchi, PhD, MD1; Ebenezer Nyenwe, MD3; Helen Lambeth, RN, BSN2; Amy Brewer, MS, RD, LDN; Debra Clark, LPN; Andrea Crisler, MT; Debra Force, MS, RD, LDN; Donna Green, RN; Robert Kores, PhD
University of Minnesota
Robert W. Jeffery, PhD1; Carolyn Thorson, CCRP2; John P. Bantle, MD3; J. Bruce Redmon, MD3; Richard S. Crow, MD3; Scott Crow, MD3; Susan K Raatz, PhD, RD3; Kerrin Brelje, MPH, RD; Carolyne Campbell; Jeanne Carls, MEd; Tara Carmean-Mihm, BA; Julia Devonish, MS; Emily Finch, MA; Anna Fox, MA; Elizabeth Hoelscher, MPH, RD, CHES; La Donna James; Vicki A. Maddy, BS, RD; Therese Ockenden, RN; Birgitta I. Rice, MS, RPh, CHES; Tricia Skarphol, BS; Ann D. Tucker, BA; Mary Susan Voeller, BA; Cara Walcheck, BS, RD
St. Luke’s Roosevelt Hospital Center
Xavier Pi-Sunyer, MD1; Jennifer Patricio, MS2; Stanley Heshka, PhD3; Carmen Pal, MD3; Lynn Allen, MD; Lolline Chong, BS, RD; Marci Gluck, PhD; Diane Hirsch, RNC, MS, CDE; Mary Anne Holowaty, MS, CN; Michelle Horowitz, MS, RD; Nancy Rau, MS, RD, CDE; Dori Brill Steinberg, BS
University of Pennsylvania
Thomas A. Wadden, PhD1; Barbara J Maschak-Carey, MSN, CDE2; Robert I. Berkowitz, MD3; Seth Braunstein, MD, PhD 3 ; Gary Foster, PhD3; Henry Glick, PhD 3; Shiriki Kumanyika, PhD, RD, MPH 3; Stanley S. Schwartz, MD3 ; Michael Allen, RN; Yuliis Bell; Johanna Brock; Susan Brozena, MD; Ray Carvajal, MA; Helen Chomentowski; Canice Crerand, PhD; Renee Davenport; Andrea Diamond, MS, RD; Anthony Fabricatore, PhD; Lee Goldberg, MD; Louise Hesson, MSN, CRNP; Thomas Hudak, MS; Nayyar Iqbal, MD; LaShanda Jones-Corneille, PhD; Andrew Kao, MD; Robert Kuehnel, PhD; Patricia Lipschutz, MSN; Monica Mullen, RD, MPH
University of Pittsburgh
John M. Jakicic, PhD1, David E. Kelley, MD1; Jacqueline Wesche-Thobaben, RN, BSN, CDE2; Lewis H. Kuller, MD, DrPH3; Andrea Kriska, PhD3; Amy D. Otto, PhD, RD, LDN3, Lin Ewing, PhD, RN3, Mary Korytkowski, MD3, Daniel Edmundowicz, MD3; Monica E. Yamamoto, DrPH, RD, FADA 3; Rebecca Danchenko, BS; Barbara Elnyczky; David O. Garcia, MS; George A. Grove, MS; Patricia H. Harper, MS, RD, LDN; Susan Harrier, BS; Nicole L. Helbling, MS, RN; Diane Ives, MPH; Juliet Mancino, MS, RD, CDE, LDN; Anne Mathews, PhD, RD, LDN; Tracey Y. Murray, BS; Joan R. Ritchea; Susan Urda, BS, CTR; Donna L. Wolf, PhD
The Miriam Hospital/Brown Medical School
Rena R. Wing, PhD1; Renee Bright, MS2; Vincent Pera, MD3; John Jakicic, PhD3; Deborah Tate, PhD3; Amy Gorin, PhD3; Kara Gallagher, PhD3; Amy Bach, PhD; Barbara Bancroft, RN, MS; Anna Bertorelli, MBA, RD; Richard Carey, BS; Tatum Charron, BS; Heather Chenot, MS; Kimberley Chula-Maguire, MS; Pamela Coward, MS, RD; Lisa Cronkite, BS; Julie Currin, MD; Maureen Daly, RN; Caitlin Egan, MS; Erica Ferguson, BS, RD; Linda Foss, MPH; Jennifer Gauvin, BS; Don Kieffer, PhD; Lauren Lessard, BS; Deborah Maier, MS; JP Massaro, BS; Tammy Monk, MS; Rob Nicholson, PhD; Erin Patterson, BS; Suzanne Phelan, PhD; Hollie Raynor, PhD, RD; Douglas Raynor, PhD; Natalie Robinson, MS, RD; Deborah Robles; Jane Tavares, BS
The University of Texas Health Science Center at San Antonio
Steven M. Haffner, MD1; Helen P. Hazuda, Ph.D.1; Maria G. Montez, RN, MSHP, CDE2; Carlos Lorenzo, MD3; Charles F. Coleman, MS, RD; Domingo Granado, RN; Kathy Hathaway, MS, RD; Juan Carlos Isaac, RC, BSN; Nora Ramirez, RN, BSN; Ronda Saenz, MS, RD
VA Puget Sound Health Care System/University of Washington
Steven Kahn MB, ChB1; Brenda Montgomery, RN, MS, CDE2; Robert Knopp, MD3; Edward Lipkin, MD3; Dace Trence, MD3; Terry Barrett, BS; Joli Bartell, BA; Diane Greenberg, PhD; Anne Murillo, BS; Betty Ann Richmond, MEd; Jolanta Socha, BS; April Thomas, MPH, RD; Alan Wesley, BA
Southwestern American Indian Center, Phoenix, Arizona and Shiprock, New Mexico
William C. Knowler, MD, DrPH1; Paula Bolin, RN, MC2; Tina Killean, BS2; Cathy Manus, LPN3; Jonathan Krakoff, MD3; Jeffrey M. Curtis, MD, MPH3; Justin Glass, MD3; Sara Michaels, MD3; Peter H. Bennett, MB, FRCP3; Tina Morgan3; Shandiin Begay, MPH; Paul Bloomquist, MD; Teddy Costa, BS; Bernadita Fallis RN, RHIT, CCS; Jeanette Hermes, MS, RD; Diane F. Hollowbreast; Ruby Johnson; Maria Meacham, BSN, RN, CDE; Julie Nelson, RD; Carol Percy, RN; Patricia Poorthunder; Sandra Sangster; Nancy Scurlock, MSN, ANP-C, CDE; Leigh A. Shovestull, RD, CDE; Janelia Smiley; Katie Toledo, MS, LPC; Christina Tomchee, BA; Darryl Tonemah, PhD
University of Southern California
Anne Peters, MD1; Valerie Ruelas, MSW, LCSW2; Siran Ghazarian Sengardi, MD2; Kathryn (Mandy) Graves Hillstrom, EdD, RD, CDE; Kati Konersman, MA, RD, CDE; Sara Serafin-Dokhan
Coordinating Center
Wake Forest University
Mark A. Espeland, PhD1; Judy L. Bahnson, BA, CCRP3; Lynne E. Wagenknecht, DrPH3; David Reboussin, PhD3; W. Jack Rejeski, PhD3; Alain G. Bertoni, MD, MPH3; Wei Lang, PhD3; Michael S. Lawlor, PhD3; David Lefkowitz, MD3; Gary D. Miller, PhD3; Patrick S. Reynolds, MD3; Paul M. Ribisl, PhD3; Mara Vitolins, DrPH3; Haiying Chen, PhD3; Delia S. West, PhD3; Lawrence M. Friedman, MD3; Brenda L. Craven, MS, CCRP2; Kathy M. Dotson, BA2; Amelia Hodges, BS, CCRP2; Carrie C. Williams, BS, CCRP2; Andrea Anderson, MS; Jerry M. Barnes, MA, Mary Barr; Daniel P. Beavers, PhD; Tara Beckner; Cralen Davis, MS; Thania Del Valle-Fagan, MD; Esther J. Espeland; Patricia A. Feeney, MS; Candace Goode; Jason Griffin, BS; Lea Harvin, BS; Patricia Hogan, MS; Sarah A. Gaussoin, MS; Mark King, BS; Kathy Lane, BS; Rebecca H. Neiberg, MS; Michael P. Walkup, MS; Karen Wall, AAS; Terri Windham
Central Resources Centers
DXA Reading Center, University of California at San Francisco
Michael Nevitt, PhD1; Ann Schwartz, PhD2; John Shepherd, PhD3; Michaela Rahorst; Lisa Palermo, MS, MA; Susan Ewing, MS; Cynthia Hayashi; Jason Maeda, MPH
Central Laboratory, Northwest Lipid Metabolism and Diabetes Research Laboratories
Santica M. Marcovina, PhD, ScD1; Jessica Chmielewski2; Vinod Gaur, PhD4
ECG Reading Center, EPICARE, Wake Forest University School of Medicine
Elsayed Z. Soliman MD, MSc, MS1; Ronald J. Prineas, MD, PhD1; Charles Campbell2; Zhu-Ming Zhang, MD3; Teresa Alexander; Lisa Keasler; Susan Hensley; Yabing Li, MD
Diet Assessment Center, University of South Carolina, Arnold School of Public Health, Center for Research in Nutrition and Health Disparities
Robert Moran, PhD1
Hall-Foushee Communications, Inc
Richard Foushee, PhD; Nancy J. Hall, MA
Federal Sponsors
National Institute of Diabetes and Digestive and Kidney Diseases
Mary Evans, PhD; Barbara Harrison, MS; Van S. Hubbard, MD, PhD; Susan Z. Yanovski, MD; Robert Kuczmarski, PhD
National Heart, Lung, and Blood Institute
Lawton S. Cooper, MD, MPH; Peter Kaufman, PhD, FABMR
Centers for Disease Control and Prevention
Edward W. Gregg, PhD; David F. Williamson, PhD; Ping Zhang, PhD
Footnotes
Principal Investigator
Program Coordinator
Co-Investigator
All other Look AHEAD staffs are listed alphabetically by site.
References
- 1.The Look AHEAD Research Group. Look AHEAD: Action for Health in Diabetes. Design and methods for a clinical trial of weight loss for the prevention of cardiovascular disease in type 2 diabetes. Control Clin Trials. 2003;24:610–28. doi: 10.1016/s0197-2456(03)00064-3. [DOI] [PubMed] [Google Scholar]
- 2.The Look AHEAD Research Group. The Look AHEAD Study: A description of the lifestyle intervention and the evidence supporting it. Obesity. 2006a;14:737–52. doi: 10.1038/oby.2006.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Espeland MA, Bray GA, Neiberg R, et al. Describing patterns of weight changes using principal components analysis: results from the Action for Health in Diabetes (Look AHEAD) Research Group. Ann Epidemiol. 2009;19:701–710. doi: 10.1016/j.annepidem.2009.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Waring ME, Eaton CB, Lasater TM, Lapane KL. Correlates of weight patterns during middle age characterized by function principal components analysis. Ann Epidemiol. 2010;20:201–209. doi: 10.1016/j.annepidem.2009.11.013. [DOI] [PubMed] [Google Scholar]
- 5.The Look AHEAD Research Group. Baseline characteristics of the randomized cohort from the Look AHEAD (Action for Health in Diabetes) Research Study. Diab Vasc Dis Res. 2006b;3:202–215. doi: 10.3132/dvdr.2006.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.The Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. NEJM. 2003;346:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wadden TA, Neiberg RH, Wing RR, et al. Four-year weight losses in the Look AHEAD Study: factors associated with success. Obesity. 2011;19:1987–1998. doi: 10.1038/oby.2011.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beck AT, Steer RA, Garbin MG. Psychometric properties of the Beck Depression Inventory: twenty five years of evaluation. Clin Psychol Rev. 1988;8:77–100. [Google Scholar]
- 9.The Look AHEAD Research Group. Long term effects of lifestyle intervention on weight and cardiovascular risk factors in individuals with type 2 diabetes: four year results of the Look AHEAD trial. Arch Intern Med. 2010;170:1566–75. doi: 10.1001/archinternmed.2010.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Seber GAF. Multivariate Observations. New York: Wiley and Sons; 1984. [Google Scholar]
- 11.Littell RC, Milliken GA, Stroup WW, Wolfinger RD. SAS System for Mixed Models. Cary, NC: SAS Institute, Inc; 1996. [Google Scholar]
- 12.Jeffery RW, Wing RR, Mayer RR. Are smaller weight losses of more achievable weight loss goals better in the long term for obese patients? J Consult Clin Psychol. 1998;66:641–645. doi: 10.1037//0022-006x.66.4.641. [DOI] [PubMed] [Google Scholar]
- 13.Astrup A, Rossner S. Lessons from obesity management progammes: greater initial weight loss improves long-term maintenance. Obes Rev. 2000;1:17–19. doi: 10.1046/j.1467-789x.2000.00004.x. [DOI] [PubMed] [Google Scholar]
- 14.Elfhag K, Rossner S. Who succeeds in maintaining weight loss? A conceptual review of factors associated with weight loss maintenance and weight regain. Obes Rev. 2005;6:67–85. doi: 10.1111/j.1467-789X.2005.00170.x. [DOI] [PubMed] [Google Scholar]
- 15.Greenberg H, Stampfer MJ, Schwarzfuchs D, et al. Adherence and success in long-term weight loss diets: the Dietary Intervention Randomized Controlled Trial (DIRECT) J Am Coll Nutr. 2009;28:159–168. doi: 10.1080/07315724.2009.10719767. [DOI] [PubMed] [Google Scholar]
- 16.Wing RR, Haman RF, Bray GA, et al. Achieving weight and activity goals among Diabetes Prevention Program lifestyle participants. Obes Res. 2004;12:1426–1434. doi: 10.1038/oby.2004.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sbrocco T, Nedegaard RC, Stone JM, Lewis EL. Behavioral choice treatment promotes continuing weight loss: preliminary results of a cognitive-behavioral decision-based treatment for obesity. J Consult Clin Pyschol. 1999;67:260–266. doi: 10.1037//0022-006x.67.2.260. [DOI] [PubMed] [Google Scholar]
- 18.Lutes LD, Winett RA, Barger SE, et al. Small changes in nutrition and physical activity promote weight loss and maintenance: 3-month evidence from the ASPIRE randomized trial. Ann Behav Med. 2008;35:351–357. doi: 10.1007/s12160-008-9033-z. [DOI] [PubMed] [Google Scholar]
- 19.Nackers LM, Ross K, Perri MG. The association between rate of initial weight loss and long-term success in obesity treatment: does slow and steady win the race? Int J Behav Med. 2010;17:161–167. doi: 10.1007/s12529-010-9092-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Toubro S, Astrup A. Randomised comparison of diets for maintaining obese subjects’ weight after major weight loss: ad lib, low fat, high carbohydrate diet vs fixed energy intake. BMJ. 1997;314:29–34. doi: 10.1136/bmj.314.7073.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.The Look AHEAD Research Group. Reduction in weight and cardiovascular disease (CVD) risk factors in subjects with type 2 diabetes (T2DM): one-year results of Look AHEAD. Diab Care. 2007;30:1374–1383. doi: 10.2337/dc07-0048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Murray P, Chune GW, Raghaven VA. Legacy effects from DCCT and UKPDS: what they mean and implications for future diabetes trials. Curr Atheroscler Rep. 2010;12:432–9. doi: 10.1007/s11883-010-0128-1. [DOI] [PubMed] [Google Scholar]
- 23.Nackers LM, Ross K, Perri MG. The association between rate of initial weight loss and long-term success in obesity treatment: does slow and steady win the race? Int J Behav Med. 2010;17:161–167. doi: 10.1007/s12529-010-9092-y. [DOI] [PMC free article] [PubMed] [Google Scholar]