Abstract
The following on endoscopic treatments of Barrett's esophagus includes commentaries on animal experiments on cryotherapy; indications for cryotherapy, choice of dosimetry, number of sessions, and role in Barrett's esophagus and adenocarcinoma; recent technical developments of RFA technology and long-term effects; the comparative effects of diverse ablation procedures and the rate of recurrence following treatment; and the indications for treatment of dysplasia and the role of radiofrequency ablation.
Keywords: cryosurgery, esophageal neoplasms, Barrett's esophagus, dysplasia, adenocarcinoma, specialized intestinal metaplasia, subsquamous glandular mucosa, buried glands, radiofrequency ablation, argon plasma coagulation, multipolar electrocautery, PDT, Halo90, Halo180, Halo360, balloon sizing, carcinoma, subsquamous glands, proton pump inhibitor therapy, AspECT trial, multifocal disease, neosquamous epithelium
1. What are the results of cryotherapy in animal models?
Bruce D. Greenwald
bgreenwa@medicine. umaryland.edu
This discussion focuses on spray (noncontact) cryotherapy delivered to the esophagus via standard upper endoscope. Two cryotherapy devices are currently available in the United States—a low-pressure liquid nitrogen device (CryoSpray Ablation System, CSAMedical, Inc., Baltimore, MD, USA) and a high-pressure system using CO2 (Polar Wand, GI Supply, Camp Hill, PA, USA). This device generates cold by the Joule–Thomson effect, whereby rapid expansion of gas at the catheter tip produces a significant decrease in temperature. In both techniques, tissue is repeatedly frozen then allowed to thaw. These freeze-thaw cycles induce tissue necrosis by direct freezing (protein denaturation, extracellular, and intracellular ice formation, and cell membrane disruption), tissue necrosis (vascular stasis, platelet aggregation, and thrombosis), and cryotherapy-induced apoptosis. The procedures are performed using standard endoscopic sedation and techniques in the outpatient setting. To vent gas during the procedure, a modified orogastric tube (cryo-decompression tube) is placed prior to spraying and removed after treatment for the liquid nitrogen system, and a suction catheter is attached to the tip of the endoscope in the CO2 system. Endoscopic treatment sessions are repeated every four to six weeks as needed.
Animal models were developed to test whether these devices could successfully freeze esophageal tissue and to develop appropriate dosimetry. Initial studies using a prototype of the liquid nitrogen device involved 20 swine, with freeze time varying between 10 and 60 sec.1 Follow-up endoscopy with biopsy was performed on days 2, 7, 14, 21, and 28 to assess cryotherapy effects and healing. Animals were sacrificed after complete healing was documented, and full-thickness samples of the treated area were evaluated. Cryotherapy produced mucosal freezing in all animals within 30sec, and tissue thawed within one min after cessation of the spray. Follow-up endoscopy demonstrated varying degrees of injury from superficial erosions to severe mucosal blistering. Histologically, biopsies showed mild superficial inflammation extending to the lamina propria in mild cases but severe acute esophagitis extending to the submucosa with separation of the squamous epithelium in severe cases. Three swine developed esophageal strictures, and histology showed transmural acute inflammation from treatment in these cases.
In initial studies of a prototype CO2 system, mucosa was frozen in two dogs by spraying until a white frost was seen.2 Superficial necrosis and acute inflammation was seen on day 1 without submucosal involvement. Re-epithelialization began by day 4 with complete healing by week 3. A later study using the commercial CO2 device evaluated eight swine treated with varying duration of spray (15, 30, 45, 60, 120 sec) followed by necropsy two days later.3 Depth of necrosis and injury correlated with spray duration, with necrosis of the mucosa/submucosa at 15–30 sec, muscularis propria at 45–60 sec, and adventitia (transmural) at 120 sec.
A human depth of injury study is ongoing at the University of Miami, Miami, Florida.4 The liquid nitrogen device is being tested on normal esophageal tissue seven days prior to planned cancer-related esophagectomy, with assessment of inflammation, hemorrhage, and necrosis. Dosimetries used are 10 sec freeze followed by thaw for four cycles and 20 sec freeze then thaw for two cycles. Preliminary results in three patients indicate that inflammation extends to the submucosa/muscularis propria and necrosis to the mucosa/submucosa in the 10 sec group, with inflammation to the muscularis propria and necrosis to the submucosa in the patient treated for 20 sec.
In summary, the following has been learned through the study of cryotherapy in animal and human models:
spray cryotherapy can induce necrosis extending through the esophageal wall;
depth of injury is determined by duration of the cryotherapy spray and number of freeze-thaw cycles; and
in humans, spray cryotherapy induces necrosis extending into the submucosa and inflammation into the muscularis propria at doses currently being used clinically.
2. What is the difference between liquid nitrogen and carbon dioxide cryotherapy systems with respect to efficacy and to side effects?
Charles J. Lightdale, MD
cjl18@columbia.edu
Liquid nitrogen cryotherapy
This method uses a low-pressure spray of liquid nitrogen to freeze tissue at a very cold −196 ° C.5 The special plastic catheter carrying the liquid nitrogen gets very cold, as does the endoscope itself, and the catheter routinely becomes unmovable in the biopsy channel. There is a warm air pump to unfreeze the system more rapidly. As the liquid nitrogen spray freezes tissue, it warms to nitrogen gas, which expands and must be evacuated from the upper GI tract. For this purpose, a special oral-gastric suction tube with both active and passive suction is utilized. This tube can sometimes be a technical obstacle. Abdominal massage is also required during treatment to help evacuate gas through the tube and through the mouth. A clear plastic cap on the tip of the endoscope can help decrease the lens fogging associated with the freezing spray.
Carbon dioxide cryotherapy
This method uses compressed carbon dioxide gas spray through a catheter with a tiny opening at the tip (0.005 inch diameter). The rapid expansion of the room temperature carbon dioxide gas causes cooling (Joule–Thomson effect) to −78° C, freezing the targeted tissue.6 For gas evacuation, a cap-like suction device is fixed to the endoscope tip with a connecting flat suction tube along the side of the endoscope. The suction cap can make endoscope passage into the esophagus more difficult in some. The endoscope and catheter do not freeze, and abdominal massage is not required. The compressed carbon dioxide gas reservoir is more stable than liquid nitrogen, and the carbon dioxide system is less expensive. Lens fogging during the treatment is also a problem with this system.
Comparison of efficacy and side effects
Liquid nitrogen
In a series of 17 patients with HGD in BE, the complete response (CR) for HGD was 94%, for all dysplasia (D) 88%, and for intestinal metaplasia (IM) 53%.5 In a larger series of 60 patients with HGD, CR for HGD was 97%, D was 87%, and IM was 57%.7 In 49 patients who completed cryotherapy for stage T1 esophageal cancer, the CR was 75%.8 Reported side effects in these series include variable chest pain and dysphagia, stricture rate 3–13%, mostly related to prior therapies, and one gastric perforation in a patient with Marfan's syndrome.
Carbon dioxide
In 44 patients, the CR for HGD was 86%, D was 84%, and IM was 50%.9 Side effects were minimal chest discomfort, and no serious adverse events.
Conclusions
There are very limited data available to compare the two cryotherapy methods, but high efficacy in elimination of early neoplasia in BE and excellent safety profiles appear similar at this time. Further studies are also needed to compare cryotherapy to other ablative methods.
3. What is the recommended dosimetry for cryotherapy?
Julian A. Abrams
ja660@columbia.edu
Cryotherapy is a means of tissue destruction that can be used for the successful ablation of BE with or without dysplasia. Short-term data suggest that this treatment modality is both effective and safe. However, there are limited dosimetry data for cryotherapy in BE. Cryotherapy results in two processes, the freeze and the thaw, that both result in tissue damage and destruction. During the freeze, there is direct tissue injury via intra- and extra cellular ice crystal formation. This in turn can lead to both rapid cell death as well as delayed apoptosis several days after treatment. Both faster rates of cooling and longer duration of freeze increase the amount of ice crystal formation. During the thaw period, there is recrystallization and growth of ice crystals, which lead to shear injury. Additionally, thrombosis of small blood vessels occurs, leading to tissue hypoxia and cell death. It is unclear whether the freeze or thaw period is more important, or whether the number of freeze-thaw cycles is the most important factor in determining treatment effect.
Only a few dosimetry studies have been performed with cryotherapy in the esophagus.3,10,11 Johnston et al. used liquid nitrogen spray to treat the esophagus of 20 pigs, with freeze times ranging from 10 to 60 sec.10 Observed tissue injury ranged from superficial inflammation to coagulative necrosis on the submucosa. The degree of injury did not appear to correlate with freeze time. In a pig model study using cooled carbon dioxide, tissue damage was limited to the submucosa for freeze durations of 15 and 30 sec, but affected deeper layers with longer durations.3 The clinical relevance of tissue injury and necrosis to deeper layers of the esophagus is unclear, as cryotherapy can result in preservation of the extracellular matrix despite surrounding cell death.
Clinical experience with various doses of cryotherapy has demonstrated seemingly consistent efficacy with a good safety profile. The initial dosing regimen for liquid nitrogen spray in studies of BE with HGD or adenocarcinoma was 20 sec times three cycles. After a case of a patient with Marfan's syndrome who developed a gastric perforation due to overdistention, the dosing protocol was changed to 10 sec times four cycles. Both of these studies demonstrated that liquid nitrogen cryotherapy was efficacious and safe,7,8 although analyses comparing efficacy in the patients who received (20 sec × 3) versus (10 sec × 4) were not performed. There is an ongoing study of CO2 cryotherapy, although dosimetry, efficacy, and safety data are not yet available.
In summary, cryotherapy produces a variable depth of tissue injury based on duration of freeze, the number of freeze-thaw cycles, and the distance from the spray origin. The clinical experience to date seems to suggest that 10–15 sec freeze times may be adequate for short-term efficacy in ablation of BE. The optimum number of freeze-thaw cycles for tissue ablation is still unclear. Palliative treatment of esophageal tumors is less clear. Longer freeze times may be more effective in this population; this may be accompanied by a theoretical increased risk of perforation, although this has not been observed clinically. Studies that compare various freeze durations and numbers of freeze-thaw cycles with clinical outcomes are lacking.
4. What are the criteria that determine the mean number of cryotherapy sessions and total length of treatment—is PPI therapy concomitant to cryotherapy mandatory?
John D. Horwhat
john.david.horwhat@us.army.mil
As with any endoscopic technique, ideal application will be influenced by factors that relate to the patient, the procedure and the physician. Presently, cryotherapy remains a largely fledgling technology that is only slowly finding a niche with endoscopists that ablate BE. All (nonabstract) published data relating to cryotherapy and BE have been with the CSA™ Medical device, and there is no published consensus on dosimetry.7,8,12
With respect to patient-related factors, the major determinants are the length of the segment being treated and the goal of therapy. The aim of each treatment cycle is to keep an area of mucosa “painted” with a uniform white cryofrost for 20 sec in the treatment of dysplasia and 30 sec for cancer. Two cycles are typically given for a total treatment dose of 40 sec (dysplasia) and 60 sec (cancer) respectively. If one attempts to treat too large of a segment, there is the risk that the distal mucosa could begin to thaw as the scope is moved proximally, thus diminishing the uniformity of effect. In order to both maintain direct visualization and to ensure that the spray is applied for uniform freezing effect, a 3–4 cm length is the largest segment that should be targeted with each cycle. Whether one chooses to treat in a hemicircumferential or fully circumferential manner depends on the operators experience and ability to maneuver around the decompression tube.
Another patient factor is the goal of therapy. If the goal is regression from HGD to nondysplastic BE, one may not need as many sessions as when the goal is complete re-epithelialization to squamous mucosa. Just as small islands of columnar appearing tissue can remain after the application of RFA or PDT that require addition HALO90™ or spot treatment with APC, the same can occur with CSA™ and result in the need for an additional treatment session.
Procedural factors relate to dosimetry, the ability to maintain visualization and to ventilate the volume of gas that is instilled. During the earliest work with CSA™, dosimetry required either four 10-sec freeze-thaw cycles or two 20-sec cycles per segment treated. As experience developed and refinements in the dual-lumen decompression tube (CDT™) were made, most now use the 2 × 20 sec dosimetry as this is quicker, easier to perform and appears to give a more robust response than the 10-sec freeze-thaw cycles. Visualization and venting are interrelated in that one may encounter some fogging of the lumen after 12–15 sec of freezing. This can be ameliorated by temporarily stopping the flow of gas and allowing decompression to catch up. Recall that liquid nitrogen expands nearly 700 times in volume when changing from a liquid to gas with rewarming, and it is imperative that the decompression tube ventilates this volume to reduce the risk of barotrauma. Since the inception of the CDT™, overdistention, and resultant barotrauma have been all but eliminated.
Recent work performed by Ribiero et al. has contributed greatly to our knowledge of dosimetry.13 Patients scheduled for esophagectomy had a 2 × 2 cm2 area of mucosa targeted for cryospray with either 4 × 10 sec or 2 × 20 sec. Seven days later at esophagectomy the treated area was analyzed histologically. The areas treated with 2 × 20 sec sprays consistently achieved a depth of necrosis to the level of the superficial or deep submucosa. This evidence adds support to the basis for a dosimetry of 2 × 20 sec in patients with dysplasia and further work in this area is ongoing by these investigators.
Table 1 shows data from several centers as it relates to dosimetry and includes nondysplastic Barrett's, dysplasia, and cancer. Abstract data presented at OESO 2010 are included. The data from Dumot et al. from the Cleveland Clinic are not shown as the prototype devices and dosimetry changed multiple times over the course of his study as the technology evolved, and the data are too heterogeneous to display. Even allowing for the evolution from hemicircumferential spraying to circumferential spraying then from 4 ×10 sec sprays to 2×20 sec sprays, there is a striking consistency among the various centers for a number of treatment sessions that ranges from 3 to 4. Regarding the issue of whether acid control is necessary during the application of cryotherapy, the only data relating to CSA™ ablation come from the original pilot study with CSA™ from Johnston et al.12 All patients that entered into the trial that had reversal of their Barrett's to normal squamous had complete acid control demonstrated by 24 h-pH analysis upon entry to the study. Doses of medication were escalated for patients without acid control and repeat testing done at higher dose to ensure an anacid intraesophageal lumen was present during the treatment and healing phases of the study. Similarly, work with RFA from the Stanford group has shown a statistically significant (P < 0.05) relationship between acid control and treatment response, emphasizing the need for acid control in patients undergoing ablation.14
Table 1. Number of treatment sessions required for nondysplastic and dysplastic Barrett's esophagus and esophageal cancer.
| Cohort | N | Population | Segment length (cm) | Number of treatments/patient | Dosimetry used |
|---|---|---|---|---|---|
| NNMC cohort(includes Johnston GIE) | 335 | Nondysplastic and LGD/HGD | 3.9(1–11) | 3 | 10 @ 2 × 20 sec hemicircumferential, 25 @ 4 ×10 sec circumferential |
| WRAMC cohort(unpublished data) | 119 | Nondysplastic and LGD | 2.9(1–10) | 4 | 14 @ 4 × 10 sec, 5 @ 2 × 20 sec |
| Shaheen (GIE) | 998 | HGD | 5.3(1–13) | 3.4 | Mixture of 4 × 10 and 2 × 20 sec |
| Greenwald (GIE) | 779 | Cancer | 4(1–15) tumor length | 3 | 3 × 20 sec |
| Sreenarasimhaiah(OESO) | 223 | LGD, HGD, and ImCA | 5.84 | 2.25 | 4 × 10 and 2 × 20 |
NNMC, National Naval Medical Center; WRAMC, Walter Reed Army Medical Center; LGD, low-grade dysplasia; HGD, high-grade dysplasia; GIE, gastrointestinal endoscopy; OESO, 10th World Congress, World Organization for Specialized Studies on Diseases of the Esophagus; sec, seconds.
Summary
The current literature demonstrates the ability to accomplish eradication of HGD and intramucosal esophageal cancer with three to four applications of CSA™ therapy. Recent work presented at the 2010 OESO World Congress suggests an ability to accomplish this with less than three treatments, on average, and this may reflect advantages gained by the shift toward a dosimetry of 2 × 20 sec yielding a more robust treatment response than 4 × 10 sec sprays. Certainly, we await the full results from Ribiero et al.13 as they study more patients undergoing esophagectomy in an effort to confirm whether 2 × 20 sec dosimetry will continue to consistently demonstrate full thickness mucosal necrosis. If proven in subsequent patients, this full thickness histology would confirm that which we see endoscopically and solidify our ability to embrace this as our standard noncancer dosimetry. And with regard to acid control, we should aim to control acid exposure in all patients undergoing any ablation procedure—whether radiofrequency, resectional procedures, or cryotherapy—to ensure the greatest opportunity for healing with a neosquamous epithelium.
5. Endoscopic spray cryotherapy for esophageal cancer: cure or palliation?
Bruce D. Greenwald
bgreenwa@medicine.umaryland.edu
This review discusses the use of low-pressure endoscopic spray cryotherapy with liquid nitrogen (CryoSpray Ablation System) for the treatment of esophageal cancer. With this technique, esophageal tissue is frozen with liquid nitrogen delivered in a noncontact method via a catheter passed through the working channel of a standard upper endoscope. In cancer, tissue is typically frozen for 20 sec, allowed to thaw completely (approximately one min) then refrozen repeatedly, typically in at least three freeze-thaw cycles. These cycles induce tissue necrosis by direct freezing (protein denaturation, extracellular and intracellular ice formation, cell membrane disruption), tissue necrosis (vascular stasis, platelet aggregation, and thrombosis), and cryotherapy-induced apoptosis. The procedure is performed using standard endoscopic sedation and techniques in the outpatient setting. To vent nitrogen gas formed by evaporation during the procedure, a modified orogastric tube (cryodecompression tube) is placed prior to spraying and removed after treatment. Endoscopic treatment sessions are repeated every four to six weeks as needed.
The first case of endoscopic spray cryotherapy in esophageal cancer was reported by Cash et al. in 2007.15 A 73-year-old man with previous T2N1 tonsillar cancer seven years prior and T4N0 esophageal squamous cell carcinoma three years prior, both treated with concurrent chemotherapy and external beam radiotherapy, presented with a T2N0 esophageal squamous cell carcinoma outside the field of the previous esophageal cancer. He was treated with two sessions of cryotherapy and remained disease free for over two years. He developed an esophageal stricture from treatment that required dilation and placement of a temporary esophageal stent.
The largest reported study on the use of endoscopic spray cryotherapy in esophageal cancer was published earlier this year.8 In this retrospective study, 79 patients from 10 sites around the U.S. were treated. These patients failed, refused, or were not candidates for standard therapy, including chemotherapy, radiation therapy, or esophagectomy. In this cohort, 74 (94%) had adenocarcinoma, 5 (6%) had squamous cell cancer, and 64 (81%) were male. Median age was 76 (range 51–93) years, and tumor stage at enrollment was T1: 60 (T1a: 33, T1b: 23, not specified: 4; T2: 16, T3/4: 3. Many participants were treated with previous therapy including endoscopic mucosal resection: 27 (42%), concurrent chemotherapy/external beam radiotherapy: 12 (19%), photodynamic therapy (PDT):11(17%), external beam radiotherapy alone: 7 (11%), concurrent chemotherapy and external beam radiotherapy then esophagectomy: 2 (3%), argon plasma coagulation (APC): 2 (3%), and one each for chemotherapy alone, esophageal stent, and RFA.
An efficacy cohort of 49 participants completed treatment, either through endoscopic and biopsyconfirmed tumor eradication (CR) or because of tumor progression, patient preference to stop therapy, or comorbid condition precluding further therapy (treatment failure). In this group, tumor stage was T1: 36 (T1a: 24, T1b: 10, not specified: 2); T2: 10, T3/4: 3. Overall CR was 61.2%, including 72.2% for T1 tumors (T1a: 75%, T1b: 60%). Mean follow-up was 10.6 months for all and 11.5 months in the T1a group. The median number of cryotherapy sessions needed to produce a CR was three. Concurrent treatments at the time of cryotherapy included endoscopic resection: 9, external beam radiotherapy: 2, argon plasma coagulation: 3, RFA:2, and esophagectomy: 4. In a number of patients, cryotherapy was not able to eradicate the tumor but was able to slow or halt tumor progression for extended periods of time, over one year in some cases. In a safety analysis including 332 treatments for the 79 participants, no serious adverse events were reported. Esophageal stricture developed in 10 (13%), with 9/10 noted to have esophageal narrowing due to previous treatment prior to initiation of cryotherapy.
An earlier study of spray cryotherapy included five patients with intramucosal carcinoma.16 Three of five responded to the treatment, downgrading histology to IM without HGD or carcinoma. Another study, primarily assessing safety and tolerability of spray cryotherapy in 77 patients, included treatment data on seven patients with intramucosal or mucosal carcinoma.5 Cancer was eliminated in all patients and IM was eliminated in 5/7 (although follow-up was limited).
Limited data are available on the use of spray cryotherapy for debulking of advanced esophageal neoplasms. Treatment in this setting is technically challenging due to luminal stenosis and the presence of the cryodecompression tube, which may make scope manipulation difficult.
In summary, liquid nitrogen spray cryotherapy:
eradicates T1a (mucosal) esophageal adenocarcinoma in 75% of cases;
can be used in T1b (submucosal) disease, but risk of lymph node metastasis must be considered;
is probably no better than other ablation modalities for palliation of bulky esophageal cancers, but experience is limited; and
appears to cause tumor regression for extended periods even if eradication is not possible.
Disclosures
The author has received research funding from and serves as a consultant and medical advisory board member for CSA Medical, Inc., the manufacturer of the CryoSpray Ablation system.
6. Compared to other thermal ablation modalities, what is the rate of development of subsquamous glandular mucosa following RFA?
Ram Chuttani
rchuttan@bidmc.harvard.edu
Key issues
Incomplete ablation by any method may result in the glandular mucosa getting “buried” under regenerating neosquamous epithelium.
This subsquamous glandular mucosa may not be visible endoscopically.
Inadequate endoscopic surveillance may lead to development of undetected malignancy.
Argon plasma coagulation
In several series, incidence of subsquamous glandular mucosa after APC ablation varies between 4–44%.17–21 The key factors predicting lower incidence with APC are higher power settings, higher PPI dose, and shorter Barrett's segment.
Photo dynamic therapy ablation and multipolar electro coagulation
Three series compared APC versus PDT; PDT had between 4% and 24% incidence of subsquamous glandular mucosa.18–20 In addition, the PHOBAR Trial compared PDT with porfimer sodium combined with acid suppression (PHOPDT) to acid suppression alone (OM) in patients with HGD. At baseline, 5.8% of patients in the PHOPDT group, and 2.9% of patients in the OM group had SSIM. After treatment, the percentage of patients with squamous overgrowth increased, but there was no significant difference between the two groups: PHOPDT (30%) and OM (33%) groups (P = 0.63).21
The data on the incidence of subsquamous glandular mucosa after MPEC therapy are limited. Sharma et al. reported SSIM in 27% of NDBE/LGD patients (3/11) who had undergone MPEC and achieved endoscopic and histologic cure (mean follow-up of 24 months after endoscopic reversal of Barrett's).22
Other key factors
Adequate biopsy specimen must be obtained— large capacity forceps are essential to sample lamina propria.
All of these thermal methods have an unacceptably high incidence of subsquamous “buried” glandular mucosa.
There are reports of undetected subsquamous adenocarcinoma developing after APC and PDT.19,23,24
Radiofrequency ablation
In multiple studies, RFA has resulted in no subsquamous glandular mucosa (Table 2). AIM Trial at 2.5 years showed no subsquamous glandular epithelium in nearly 4,000 total biopsy specimens.25 AIM-II 5-year durability trial had 1,473 biopsy specimens with no buried glands. A total of 85% of the specimens included lamina propria or deeper tissue.26 The highest published post-RFA subsquamous glandular mucosa rate to date is 5% of patients in the AIM Dysplasia Trial, a randomized control trial that evaluated RFA versus control (biopsy surveillance and high-dose PPI acid suppression) in LGD and HGD patients. At baseline, 25% of patients had subsquamous glandular mucosa. At the 12-month primary endpoint, this decreased to 5% in the RFA arm and increased to 40% in the control arm.34 Overall, in the published RFA literature, which includes over 900 patients, there have been six patients with post-RFA biopsy proven subsquamous glandular mucosa (one subsquamous glandular mucosa containing fragment per patient).27–29 This translates to less than 0.7% of patients having subsquamous glandular mucosa post-RFA.28–39
Table 2. In multiple studies, RFA has resulted in no subsquamous glandular mucosa.
| N | FU | CR-IM | CR-D C | CR-HGD | Buried glands | Stricture rate | |
|---|---|---|---|---|---|---|---|
| AIM-II trial25 | 61 | 30 months | 98.4% | None | 0% | ||
| 50 | 60 months | 92% | None | 0% | |||
| AIM-LGD96 | 10 | 24 months | 90% | 100% | None | 0% | |
| HGD Registry | 92 | 12 months | 54% | 80% | 90% | None | 0.4% |
| AMC-I30 | 11 | 14 months | 100% | 100% | None | 0% | |
| AMC-II31 | 12 | 14 months | 100% | 100% | None | 0% | |
| Comm Registry68 | 429 | 20 months | 77% | 100% | None | 1.1% | |
| EURO-I69 | 24 | 15 months | 90% | 100% | None | 4.0% | |
| EURO-II32 | 118 | 12+ months | 96% | 100% | |||
| Emory70 | 27 | <12 months | 100% | 100% | None | 0% | |
| Dartmouth71 | 25 | 20 months | 78 | ||||
| Henry Ford33 | 66 | Varied | 93% | None | 6.0% | ||
| Mayo34 | 63 | 24 months | 79% | 89% | None | 0% | |
| LGD72 | 39 | 24 months | 87% | 95% | None | 0% | |
| HGD36 | 24 | 23 months | 67% | 79% | None | 0% | |
| AIM RCT (primary)27 | 127 (RFA84) | 12 months | 77% (83%) | 86% (92%) | 5.1% | 6.0% | |
| Long-term FU73 | 106 | 24 months | 93% | 95% | 3.8% | 7.6% | |
| EMR vs. RFARCT38 | 22 | 24 months | 96% | 96% | None | 14% |
7. What is expected from future technical developments of the RFA technology (automated process) to optimally size the ablation balloon?
Charles J. Lightdale
cjl18@columbia.edu
Current sizing balloon and procedure
In choosing the Halo360 ablation catheter for circumferential RFA, a sizing balloon is used to select the appropriate catheter, which is made in five inner diameter (ID) sizes: 18 mm, 22 mm, 25 mm, 28 mm, and 31 mm.40 Sizing is performed at 1 cm intervals beginning at 12 cm above the esophagogastric junction measured as the top of the gastric folds. At every sizing location, the Halo generator provides a measure of the esophageal ID and recommends the ablation catheter size. Data are recorded on a sizing worksheet, and the smallest recommended ablation catheter is selected. Generally 5–7 sizing steps are required, which adds 5–10 min to the procedure.41 The sizing procedure is generally safe, although it can cause some intraprocedural discomfort and small amounts of bleeding associated with breaks in the mucosa.26,42,43 When sizing and ablation catheter selection are performed according to instructions, circumferential ablation is a safe, effective procedure with a < 0.02% perforation rate and no patient deaths.
Recent sizing balloon modification
A recent improvement in the sizing balloon involves a change in the balloon material from polyethylene terephthalate (PET) to the softer polyurethane (PU). The earlier PET balloon could measure esophageal ID to ≤ 33.7 mm, while the new PU sizing balloons can measure esophageal ID to ≤45 mm. This allows better differentiation between the esophagus and cardia.
Sizing balloon modifications under review
The current inflation pressure of the sizing balloon is 4 pounds/square inch (PSI). A new modification is being considered that will decrease the sizing balloon pressure to 3 PSI. The advantage of a lower-pressure sizing balloon (25% less force applied to esophageal wall) would be to make it less prone to migration within the esophagus and also to further decrease the risk of mucosal tears and bleeding. Using this method, the smallest size ablation catheter should theoretically be used with less stricture formation, although this risk is already low (0–6%). If this modification results in more residual islands of Barrett's mucosa, these are still easily treated using the Halo90 focal RFA device.
Circumferential use of larger focal ablation device
Another way to improve the sizing balloon step would be to avoid it altogether by using the Halo90 focal RFA device in a circumferential manner. The Halo90 has a 13 mm × 20 mm footprint compared to the new Halo Ultra90 that has a 13 mm × 40 mm footprint, allowing faster ablation of larger Barrett's areas.
Future concepts
The possibility of even larger Halo180 devices and “one size fits all” balloon devices that unfurl or unroll to accommodate individual esophageal sizes and shapes are also in the development pipeline, and these would obviate the need for balloon staging.
8. What are the comparative adverse effects of the diverse ablation procedures?
Srinadh Komanduri
koman1973@gmail.com
There has been an evolution in strategies for endoscopic ablation of dysplastic Barrett's epithelium and early intramucosal adenocarcinoma. The advances in endoscopic mucosal resection have limited ablative strategies to flat dysplastic disease. Prior ablative strategies including multipolar electrocoagulation (MPEC) and APC have fallen out of favor due to lack of predictability as to depth of ablation. PDT was the mainstay of ablative strategies for many years. PDT has been very effective for treatment of HGD, but it has carried a stricture rate up to 30%, side effects of photosensitivity, nausea, and dehydration.44 Furthermore, the risk of buried glandular disease, which was reported frequently with PDT, appears too extremely rare with newer technology such as RFA. RFA has the most thoroughly investigated safety profile and safety reporting via the ongoing registry.
Current overall complication rates are <0.25% with stricture rate <1%, nearly nonexistent buried glandular disease despite specifically sampling for this, and minimal bleeding or perforation risk (0.02%).26 Perforation really has not been a significant issue with any of the therapies but can occur and generally is operator and technique dependent. Finally, the newest therapy for ablation has been cryotherapy. While data have been limited, a recently published study evaluating safety and efficacy of cryotherapy7 in HGD demonstrated strictures in 3% of patients, buried glands in 3% and one minor case of bleeding. With both RFA and cryotherapy, the strictures that have been reported have for the most part been responsive to single balloon dilation. Overall, the safety profile with RFA and cryotherapy is significantly improved over PDT with RFA; currently with the most data in support of its safety profile with RFA, cryotherapy is significantly improved over PDT, and RFA currently with the most data in support of its safety profile.
9. Is the potential rate of recurrence of Barrett's epithelium following ablation currently known? What are the reasons for variability in published results?
Melissa P. Upton
mupton@u.washington.edu
“Recurrence” or persistence?
Some papers use the term “recurrence,” but there is currently no data to show if Barrett's mucosa or Barrett's-associated neoplasia detected after ablation represents recurrence or persistence. The more precise term, incomplete response (IR), is recommended, when findings such as Barrett's epithelium or neoplastic alterations are seen within a defined interval after ablation.
The results of radiofrequency ablation are promising, but long-term follow-up is not yet available. Table 3 summarizes some of the recent papers in this area.
Table 3. The results of radiofrequency ablation are promising, but long-term follow-up is not yet available.
| Results of radiofrequency ablation (RFA) | |||
|---|---|---|---|
|
| |||
| Follow-up duration | |||
|
|
|||
| Trial | 1 year | 2.5 years | 5 years |
| AIM trial RFA27N = 60 | CR-IM 70% | CR IM 98% | CR-IM 92% N = 50 |
| US Multicenter | CR-LGD 90.5% | ||
| RFA | CR-HGD 81% | ||
| CR-IM 77.4% | |||
| Velanovitch et al. N = 60 | CR-IM 93% | ||
| US Multicenter for HGD | CR-HGD 90.2% | ||
| for HGD | CR-D 80.4% | ||
| CR-IM 54.3% | |||
| Sharma et al.22 (eight centers) N = 70 | CR-IM 70% | ||
| Roorda et al.14 | CR-IM 46% | ||
| CR-D 71% | |||
(CR, complete response; IM, intestinal metaplasia; LDG, low-grade dysplasia; HGD, high-grade dysplasia; D, cysplasia).
A recent Cochrane review of a number of ablative techniques summarized the results of 16 studies, including 1,074 patients, with a mean number of 49 patients per study (range 8–108).45 Studies using PDT had a mean eradication rate of 52% for BE and eradication rates of dysplasia ranging between 56% and 100%. Factors that affected PDT efficacy included variations in drug, light source, and dose of light. Results reported for RFA included a mean 82% eradication rate of BE and a 94% eradication rate of dysplasia.45
Factors that may affect detection of Barrett's mucosa following ablation include limitations of endoscopic identification, variations in biopsy strategy and sampling, extent of ablation or incomplete ablation, and buried Barrett's glands that may remain below squamous re-epithelialization. There are limits to standard white light endoscopy, which can only identify luminal contour and luminal lining, visualizing columnar tongues, but lack the resolution to differentiate subtypes of columnar epithelium.
After squamous re-epithelialization, buried glands cannot be seen without specialized endoscopic techniques, such as optical coherence tomography (OCT). Follow-up biopsy strategy must employ careful surveillance such as the Seattle protocol, which includes four-quadrant large-jaw biopsies at each 2 cm of esophagus previously documented to have columnar metaplasia and at each 1 cm of esophagus in patients with history of neoplastic changes. Biopsies must be deep enough to sample subsquamous lamina propria to rule out buried Barrett's glands or neoplasia, and it appears that the rate of buried glands is similar comparing postablation biopsies with sham/PPI biopsies.46
Investigators in the AIM Dysplasia Trial reported that 80% of biopsies post-RFA were deep enough to evaluate for subsquamous glands; however, they considered biopsies adequate if they included lamina propria, muscularis mucosae, or submucosa. Squamous mucosa was most apt to provide limited or superficial biopsies. Subepithelial sampling was present in only 78.5% of squamous-only samples, compared to 98.8% of columnar mucosa samples (P < 0.001). The investigators stated that “a biopsy that included LP papillae was categorized as LP.” Their figure 1F shows a tangentially oriented biopsy of squamous mucosa that does not include subsquamous tissue.46 If this figure is representative of a portion of the samples that were deemed adequate, more studies and longer follow-up are needed to determine the rate and biologic potential of buried glands post-RFA. It is also worth emphasizing that these results are from experienced investigators using standardized procedures. We do not yet know whether the excellent ablation results reported so far can be achieved in other sites of practice and whether they will be lasting results.
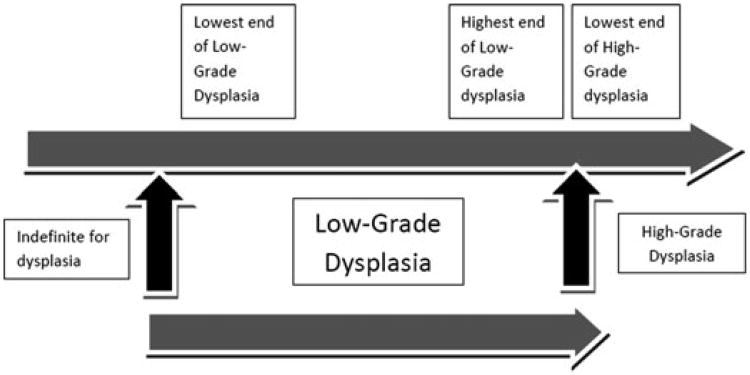
Figure 1.
The low-grade dysplasia spectrum: not all things given the diagnosis of low-grade dysplasia are the same.
What factors have been associated with “recurrence” (persistence)?
A total of 75% of “recurrences” after cryotherapy or RFA were in cardia.47 This raises the question of whether the GEJ may be harder to treat, or more difficult to biopsy and screen, than tubular esophagus. Molecular signature may also be associated with difficulty in eradicating disease with RFA. Investigators performed microdissection of mucosal biopsies and studied a panel of 16 allelic imbalances in 21 patients with LGD and BE. If more than 75% of cells carried mutations, the patients were more resistant to RFA treatment, and additional treatment sessions were needed to eradicate mucosa with mutations.48 Other reported associations with IR include longer lengths of the Barrett's segments, older age of the patients, multifocality of dysplasia, obesity, location of IM at the top of the gastric folds, and longer duration of dysplasia prior to treatment. In one study including pH control, adequate pH was achieved in fewer than 50% of patients undergoing RFA, and ablation efficacy was lower in patients with inadequate pH control.14
In conclusion, endoscopic ablation appears to achieve excellent rates of ablation of Barrett's epithelium and Barrett's-associated neoplasia, however, longer follow-up is needed to identify risk factors for persistence and recurrence, using regular and careful biopsy surveillance strategies. Additional investigation and clinical research is needed to optimize control of esophageal pH, and to identify patients at risk for IR or “recurrence,” including those with longer segments and increased mutation rates. Better strategies are also needed for surveillance of GEJ and cardia.
10. Given the low risk for cancer development, should LGD be treated?
Henry D. Appelman
appelman@umich.edu
Actually, a more appropriate question is, “given the low risk of cancer development, should LGD even require more stringent surveillance?” In order to answer this question, we need a clear definition of LGD. The textbooks offer various definitions that are filled with words like “slightly,” “reduced,” “larger,” “minimal,” “more atypical,” “mild,” “irregular,” and “inconspicuous” that are used to define LGD, yet these words have no clear meanings. Therefore, definitions of LGD currently in print are only marginally useful at best.
Regardless of these definitional problems, is it still possible that, in practice, the diagnosis of LGD by different pathologists is good enough for clinicians to use in determining patient management? Tobegin with, clinicians and pathologists have to recognize that LGD is not a single entity but a group of epithelial changes that look worse than normal, but they do not look as bad as HGD. We know that the histologic diagnosis of LGD has poor agreement even among experienced pathologists.49 Presumably, LGD has a lowest end and a highest end, and the highest end is next to the lowest end of HGD. (Fig. 1)
This leads to other questions. Is the cancer risk the same at both ends of the low-grade dysplastic spectrum? Is the cancer risk for highest LGD like that for the lowest HGD or is it more like the lowest LGD? Clearly we don't have answers to these questions, because no study has separated LGD into the two groups of highest and lowest ends and evaluated cancer risk for each groups separately. The reason why this has not been done is that no pathologist or groups of pathologists know the criteria for epithelia at both ends of the LGD spectrum. What is obvious is that the diagnosis of LGD is not very dependable, and so clinicians have to decide if they really want to make patient care decisions based upon such an undependable diagnosis.
Presumably, this has lead to the variability in recommendations for dealing with LGD. These recommendations vary from continuing endoscopic biopsy surveillance every six months for LGD for an indefinite period of time to antireflux therapy followed by endoscopic surveillance every one to three years. The Practice Parameters Committee of the American College of Gastroenterology suggests that LGD requires expert pathologist confirmation with follow-up endoscopy at six months and yearly endoscopy until there is no dysplasia on two consecutive yearly endoscopies.50 To emphasize the problems in histologic diagnosis of LGD, there is a recent study from the Netherlands of 147 patients who had a diagnosis of LGD from six community hospitals. The biopsies were reviewed by two “expert” pathologists, who downgraded the diagnosis to negative or indefinite for dysplasia in 85% of the cases.51 They only agreed with the diagnosis of LGD in 15% of these patients. In these 15%, there was an 85% cumulative risk of progression to HGD or carcinoma in 109 months compared to only a 5% risk at 107 months for those cases that were downgraded to negative or indefinite. In another study from four U.S. centers of 618 patients, 147 cases were diagnosed as LGD, but there was no central pathologist review.52 In this study, it was concluded that the progression to carcinoma from LGD is not significantly different from that of the entire group, most of whom did not have dysplasia. This suggested that LGD patients might have been managed similarly to nondysplastic Barrett's patients in terms of surveillance.
To conclude, based upon this information, more frequent follow-up is clearly indicated for cases of LGD that have been verified by the two expert Dutch pathologists, because there is a high risk of progression to HGD and carcinoma. However, regular follow-up might be recommended for cases of LGD from the four U.S. centers as long as they were not reviewed by the Dutch pathologists, because of their low-risk of progression.
11. What is the effect of continuous therapy with PPIs compared to intermittent prescription on the rate of progression of BE to HGD and adenocarcinoma?
Helen M. Shields
hshields@caregroup.harvard.edu
The data supporting a chemopreventative effect of acid suppression in BE leading to a reduction of the risk of HGD and esophageal cancer are conflicting. In 1996, using organ culture of endoscopic biopsies of BE, Fitzgerald et al. showed that continuous acid exposure resulted in increased villin expression, indicating differentiation because villin is one of the first cytoskeletal proteins to be localized to the apical membrane in development and is widely used as a marker of cell differentiation.53 They also showed reduced cell proliferation as indicated by proliferating cell nuclear antigen expression (PCNA), suggesting a differentiated phenotype.53
In contrast, short acid-pulses dramatically increased cell PCNA expression and proliferation. These authors concluded that variations in acid exposure may contribute to the heterogeneity seen both molecularly and structurally in Barrett's patients. They postulated that acid suppression would need to be effective enough to inhibit acid pulses and cell proliferation. However, Lao-Sirieix et al. found that long-term acid suppression reduces proliferation in BE biopsy samples, but it had no advantageous effect on c-myc, apoptosis, or COX-2.54 Lao-Sirieix noted that the AspECT trial is important because it will evaluate aspirin and esomeprazole for their effect on the risk of HGD and/or esophageal cancer in a prospective, randomized manner.54
Additional controversial results of the effect of acid on proliferation were published in 2007 by Feagins et al., who treated Barrett's cells with short exposures to acid.55 Acid exposure significantly decreased total cell numbers and resulted in cell cycle prolongation that was associated with greater expression of p53.55 The authors concluded that acid has p53-mediated, antiproliferative effects in nonneoplastic Barrett's epithelial cells.55 They speculated that antisecretory medication in dosages beyond that necessary to heal esophagitis may be detrimental rather than helpful. They called for prospective clinical trials to determine the optimal level of acid suppression in BE.55
In 2006, Cooper et al. reported that BE patients who were continuously treated with PPIs had a low incidence of adenocarcinoma (0.31%) compared to previously published reports.56 However, de Jonge recently published the largest reported nationwide cohort of unselected patients from the Netherlands with BE and found a 0.4% annual risk of cancer in histologically proven BE patients, which is lower than the frequently quoted 0.5% annual incidence of cancer in Barrett's patients.57 Male gender, older age (greater than 75 years), and the diagnosis of low-grade dysplasia are independent predictors of malignant progression.57
In summary, the role of acid in increasing or decreasing proliferation is still controversial and uncertain. Physicians must balance the safety of the long-term use of PPIs in patients with BE and their possible, but not proven, potential for decreasing the risk of neoplasia over the long term. The annual incidence of esophageal cancer in patients with BE is lower than previously thought making comparisons to older and higher estimates of the risk not useful for decision making. The results of the 10-year 2,513 patient prospective controlled trial AspECT to be reported in 2014 will be of great importance to making decisions concerning the use of PPIs on a continuous basis to specifically decrease the risk of HGD and adenocarcinoma in Barrett's patients.
12. Can the role of RFA in ablating dysplasia be evaluated? What are the late results?
Nicholas J. Shaheen
nshaheen@med.unc.edu
Endoscopic ablative therapy for BE is a rapidly evolving field featuring potentially landscapeshifting technology for the care of these patients. The prospect of improving care in BE is especially enticing given that our practices have been largely unchanged over the last 20 years, during which time the incidence of esophageal adenocarcinoma, the disease to which BE predisposes, has risen dramatically.59 Because the technology to perform ablation has evolved and become more accessible, and because the morbidity associated with these procedures is generally low, these therapies have gained in popularity. However, considerable questions persist about when in the course of BE these techniques should be used, and which of the several available techniques should be preferred.
It appears that ablation can change the natural history of BE, at least in the short and midterm. Multiple modalities have been demonstrated to effectively cause reversion of IM to neosquamous epithelium.59–62 Importantly, high-dose acid suppression with PPIs must be given in conjunction with the treatments to induce this change.
Two modalities have been subjected to comparison with intensive endoscopic surveillance in randomized controlled trials, PDT and RFA. Overholt et al.63 randomized 208 subjects with BE and HGD in a 2:1 ratio to either treatment with a PPI, or treatment with PDT plus PPI therapy. The primary outcome was eradication of all HGD at any time during follow-up. Secondary outcomes included complete eradication of IM, cancer incidence, and safety profile. Subjects were assessed with upper endoscopy with biopsies every six months. The mean follow-up in the PDT group was 24 months, and in the PPI group, 19 months. Seventy-seven percent of subjects in the PDT + PPI arm achieved the primary outcome, compared to 39% in the PPI group. Of treated subjects, 52% had complete eradication of IM at some time during follow-up. Cancer incidence was 13% in the PDT + PPI group, compared to 28% in the PPI group, with all differences between groups statistically significant. Esophageal strictures occurred in 12% of subjects undergoing one PDT treatment, and 38% after two treatments. The authors concluded that PDT was effective in eradication of HGD, and decreased cancer incidence.
A second study compared RFA to intensive endoscopic surveillance.42 One hundred and twenty-seven subjects with either low-grade (n = 64) or high-grade (n = 63) dysplasia were randomized in a 2:1 ratio to either RFA + PPI or PPI alone. The primary outcomes were complete eradication of dysplasia and IM at 12 months. Secondary outcomes included cancer incidence and safety profile. Subjects were assessed by upper endoscopy with biopsies every three months (HGD) or every six months (low-grade dysplasia). In the treatment groups, 81% (HGD) and 91% (LGD) achieved eradication of dysplasia, and 77% achieved eradication of IM. In contrast, 21% of controls achieved eradication of dysplasia, and 2% achieved eradication of IM. The cancer risk at one year was 9.3% in controls and 1.2% in treated patients (P < 0.05 for all).
A recent study assessing the ability of cryotherapy with liquid nitrogen also presented promising results. Of 98 patients with BE and HGD treated at 10 U.S. sites, 97% were able to be made free of HGD, 86% were free of all dysplasia, and 57% were free of all IM. The side effect profile of the treatment was favorable, with no perforations, three strictures, and two subjects with chest pain managed with narcotics. For ease of use, efficacy, and cost, current data suggest that RFA is a logical choice for ablative therapy in BE. It has a side effect profile superior to that of PDT, with fewer strictures. Efficacy data from rigorous studies demonstrate efficacy as good, or better, than other forms of ablation, and the balloon-based device allows treatment of even long segments of disease in a time-effective manner compared to other devices.
But which patients deserve consideration as candidates for ablation? Is the only good BE burned BE, even if it was nondysplastic before combusted? Both cost-effectiveness analysis data,64 as well as the data from trials reviewed above, suggest that subjects with HGD are reasonable candidates. While cancer risk is substantially lower in subjects with low-grade dysplasia, certain subgroups of LGD subjects may also deserve consideration. Multifocal disease, dysplasia confirmed by multiple pathologists, and longer segment disease have all been suggested as potential risk factors for progression in LGD. Interestingly, even among subjects with nondysplastic disease, cost-effectiveness data suggest that ablative therapy may confer a survival advantage at a reasonable cost.64 The treatment of nondysplastic patients faces an especially high bar, because the vast majority of these subjects will never develop cancer.65 Therefore, any treatment directed at this subgroup needs to be safe, reasonably priced, and effective. Although this appears to be a formidable task, ablation may in fact turn out to be preferable to our current strategy of endoscopic surveillance,66 because of the high costs associated with endoscopic surveillance, as well as the extremely high costs and mortality associated with the development of esophageal adenocarcinoma. This is especially true if endoscopic surveillance intervals can be altered after successful ablation. However, to date, vitally needed outcomes data regarding the efficacy of ablation in less severe forms of Barrett's make it impossible to draw firm conclusions about the role of these therapies in subjects with nondysplastic Barrett's and those with LGD.
Much remains unknown regarding the utility of ablative therapy in Barrett's. Although preliminary data suggest that in both dysplastic and nondysplastic Barrett's, the neosquamous epithelium is durable in mid-term results,26,67 further work will define the long-term durability of the neosquamous reversion. The cancer risk in treated subjects requires further definition. As noted above, further work will be necessary to optimize candidate selection. Until these issues are settled, the use of ablative technologies in BE will continue to be an evolving picture.
13. What are the current indications for cryo-ablative therapy in patients with Barrett's esophagus?
Stephen Sontag
sontagsjs@aol.com
In the past five years a number of institutions have utilized cryo-ablative therapies to destroy unwanted malignant tissues. In general, there are five reports that contain the bulk of the information that is so often referred to when discussing the use of cryoablation. These reports are as follows:
Greenwald et al.8 (10 centers, 79 pts with LGD, HGD, adenocarcinoma—curable but inoperable)
Endoscopic spray cryo-therapy for esophageal cancer – safety and efficacy. GIE 2010
Greenwald et al.5 (4 centers, 77 patients with adenocarcinoma/SCC T1, T2, T3—inoperable)
Safety, tolerability and efficacy of endoscopic low-pressure liquid nitrogen spray cryotherapy in the esophagus. Diseases of the Esophagus 2010
Shaheen et al.7 (9 centers, 98 pts with HGD— curable)
Safety & efficacy of endoscopic spray Cryotherapy for BE with HGD. GIE 2010
Dumot et al.16 (1 center, 30 pts with HGD, Adenocarcinoma—curable but inoperable)
CSA for Barrett's esophagus with HGD and early esophageal carcinoma in high risk patients. GIE 2009
Johnston et al.12 (1 center, 11 pts with BE with or without dysplasia—eradicatable)
Cryo-ablation of Barrett's esophagus: a pilot study. GIE 2005
All together, these five reports provide 230 patients with some type of “bad” esophageal disease. The company itself, C.S.A. Medical of Baltimore Maryland provides a “cryo-therapy ablation kit” pamphlet that contains the indications, contraindications and warnings as well as instructions on the use of cryo-therapy. The stated indications for use are contained in a simple generalizable sentence: “The CSA System is intended to be used as a cryosurgical tool for the destruction of unwanted tissue in the field of general surgery, specifically endoscopic applications.”
The stated contraindications are somewhat lengthier (Table 4). Of interest is the way a large insurance company looks at use of newer therapies such as cryo-ablation. Aetna Insurance Company in their Clinical Policy Bulletin for Barrett's esophagus surgery states the following:
- Aetna considers any of the following interventions medically necessary for the treatment of members with Barrett's esophagus who have HGD when medical therapy (e.g., PPI's, H-2 RA's, or prokinetic agents) has failed:
- esophagectomy
- fundoplication
- photodynamic therapy
- endoscopic mucosal resection
- radiofrequency ablation
- Aetna considers any of the following ablative interventions experimental and investigational for the treatment of members with Barrett's esophagus because their effectiveness for this indication has not been established:
- Argon plasma coagulation
- cryo-therapy
- Laser therapy
- Multi-polar electro-coagulation
- Ultrasonic therapy
Table 4.
| Category | Contraindication |
|---|---|
| General |
|
| Compromised Tissue |
|
| Anatomical Volume/Compliance (Gas Evacuation) |
|
The wisdom and basis of these decisions is beyond the scope of this paper. Indeed, a conference of endoscopic investigators charged with determining which therapies should and should not be approved would likely make for a very entertaining weekend.
Discussion
Large-scale studies on the benefits of cryo-ablation therapy (or any ablative therapies) are unlikely to be performed soon, and even more unlikely to be funded. In all likelihood, the role of ablative therapies, including cryo-ablation, will be defined by a large number of individual experiences, most important of which would be the safety of the method. Until that time, individual and group experience will be the source upon which we depend. With the knowledge that some therapies considered safe today may have unrecognized long-term deleterious effects, I offer some morsels of food for thought:
Our everyday beloved sun provides us with light and warmth.
But, our treasured sun also provides a high energy assault on our human tissues.
The modalities we use to destroy the evil Barrett's…
–MPEC, heat, APC, laser, PDT, radiofrequency, freezing–
Also provide a high energy assault on our human tissues.
And
Skin cancer is linked not so much to chronic exposure,
But rather to a single severe sun burn from years earlier…
perhaps to that one really bad burn—when you were a teen.
Conclusion
Without evidence for or against the long-term benefit of ablation of Barrett's dysplasia, I offer the following, which is important to recognize is based only on feelings and opinion. In cryo-ablation (or any ablation) of Barrett's Esophagus, the risks must not outweigh the potential benefits:
For Barrett's adenocarcinoma: each patient must be considered individually; the decision to use cryotherapy is a matter between patient and physician.
For Barrett's high-grade dysplasia: the lifetime risk of developing AdCa is high; cryoablation therapy is one of several modalities that may prevent the development of cancer.
For Barrett's low-grade dysplasia or less: The lifetime risk of developing adenocarcinoma is low; aside from selected cases, routine cryoablation therapy cannot be justified.
Concise summaries.
Spray (noncontact) cryotherapy delivered to the esophagus via standard upper endoscope induces necrosis extending into the submucosa and inflammation into the muscularis propria at doses currently being used clinically. For Barrett's high-grade dysplasia, the lifetime risk of developing adenocarcinoma is high, and cryo-ablation therapy is one of several modalities that may prevent the development of cancer.
The clinical experience to date seems to suggest that 10–15 sec freeze times may be adequate for short-term efficacy in ablation of Barrett's esophagus (BE), but the optimum number of freeze-thaw cycles for tissue ablation is still unclear. However, recent work presented at the 2010 OESO World Congress suggests an ability to accomplish this with less than three treatments, on average, and this may reflect advantages gained by the shift toward a dosimetry of 2 × 20 sec, yielding a more robust treatment response than 4 × 10 sec sprays.
There are very limited data available to compare the two cryotherapy methods, but high efficacy in elimination of early neoplasia in BE and excellent safety profiles appear similar at this time.
Cryotherapy is probably no better than other ablation modalities for palliation of bulky esophageal cancers, but experience is limited. It appears to cause tumor regression for extended periods even if eradication is not possible.
When sizing and ablation catheter selection are performed according to instructions, circumferential ablation using radiofrequency ablation (RFA) technology is a safe, effective procedure with a <0.02% perforation rate and no patient deaths. The possibility of even larger devices, and “one-size fits all” balloon devices that unfurl or unroll to accommodate individual esophageal sizes and shapes, are also in the development pipeline, and these would obviate the need for balloon staging.
With both RFA and cryotherapy, the strictures that have been reported have for the most part been responsive to single balloon dilation. Overall, the safety profile with RFA and cryotherapy is significantly improved over PDT with RFA currently with the most data in support of its safety profile.
Endoscopic ablation appears to achieve excellent rates of ablation of Barrett epithelium and Barrett-associated neoplasia; however, longer follow-up is needed to identify risk factors for persistence and recurrence, using regular and careful biopsy surveillance strategies.
Much remains unknown regarding the utility of ablative therapy in Barrett's. Although preliminary data suggest that in both dysplastic and nondysplastic Barrett's, the neosquamous epithelium is durable in mid-term results, further work will define the long-term durability of the neosquamous reversion. The cancer risk in treated subjects requires further definition.
There is some variability in recommendations for dealing with low-grade dysplasia (LGD). These recommendations vary from continuing endoscopic biopsy surveillance every six months for LGD for an indefinite period of time, to antireflux therapy followed by endoscopic surveillance every one to three years.
Concerning the progression of BE to HGD and adenocarcinoma, the role of acid in increasing or decreasing proliferation is still controversial and uncertain. The results of the 10-year prospective controlled trial Aspirin Esomeprazole Chemoprevention Trial (AspECT), to be reported in 2014, will be of great importance to making decisions concerning the use of proton pump inhibitors (PPIs) on a continuous basis to specifically decrease the risk of high-grade dysplasia (HGD) and adenocarcinoma in Barrett's patients.
Footnotes
Conflicts of interest: The authors declare no conflicts of interest.
References
- 1.Johnston MH, Schoenfeld P, Mysore JV, Dubois A. Endoscopic spray cryotherapy: a new technique for mucosal ablation in the esophagus. Gastrointest Endosc. 1999;50:86–92. doi: 10.1016/s0016-5107(99)70352-4. [DOI] [PubMed] [Google Scholar]
- 2.Pasricha PJ, Hill S, Wadwa, et al. Endoscopic cryotherapy: experimental results and first clinical use. Gastrointest Endosc. 1999;49:627–631. doi: 10.1016/s0016-5107(99)70393-7. [DOI] [PubMed] [Google Scholar]
- 3.Raju GS, Ahmed I, Xiao SY, et al. Graded esophageal mucosal ablation with cryotherapy, and the protective effects of submucosal saline. Endoscopy. 2005;37:523–526. doi: 10.1055/s-2005-861312. [DOI] [PubMed] [Google Scholar]
- 4.Unpublished data, kindly provided by Afonso Ribeiro, MD. University of Miami; Miami, FL: [Google Scholar]
- 5.Greenwald BD, Dumot JA, Horwhat JD, et al. Safety, tolerability, and efficacy of endoscopic low-pressure liquid nitrogen spray cryotherapy in the esophagus. Dis Esoph. 2010;23:13–19. doi: 10.1111/j.1442-2050.2009.00991.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen AM, Pasricha PJ. Cryotherapy for Barrett's esophagus: who, how, and why? Gastrointest Endosc Clin North Am. 2011;21:111–118. doi: 10.1016/j.giec.2010.09.007. [DOI] [PubMed] [Google Scholar]
- 7.Shaheen NJ, Greenwald BD, Peery AF, et al. Safety and efficacy of endoscopic spray cryotherapy for Barrett's esophagus with high-grade dysplasia. Gastrointest Endosc. 2010;71:680–685. doi: 10.1016/j.gie.2010.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Greenwald BD, Dumot JA, Abrams JA, et al. Endoscopic spray cryotherapy for esophageal cancer: safety and efficacy. Gastrointest Endosc. 2010;71:686–693. doi: 10.1016/j.gie.2010.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Canto MI, Gorospe EC, Shin EJ, et al. Carbon dioxide (CO2) cryotherapy is a safe and effective treatment of Barrett's esophagus (BE) with HGD/intramucosal carcinoma.(Abstract) Gastrointest Endosc. 2009;69:AB341. [Google Scholar]
- 10.Johnston CM, Schoenfeld LP, Mysore JV, Dubois A. Endoscopic spray cryotherapy: a new technique for mucosal ablation in the esophagus. Gastrointest Endosc. 1999;50:86–92. doi: 10.1016/s0016-5107(99)70352-4. [DOI] [PubMed] [Google Scholar]
- 11.Pasricha PJ, Hill S, Wadwa KS, et al. Endoscopic cryotherapy: experimental results and first clinical use. Gastrointest Endosc. 1999;49:627–631. doi: 10.1016/s0016-5107(99)70393-7. [DOI] [PubMed] [Google Scholar]
- 12.Johnston MH, Eastone JA, Horwhat JD, et al. Cryoablation of Barrett's esophagus: a pilot study. Gastrointest Endosc. 2005;62:842–848. doi: 10.1016/j.gie.2005.05.008. [DOI] [PubMed] [Google Scholar]
- 13.Ribiero AC. Poster Session Presented at the 10th World Congress, OESO; Boston, MA. 2010. [Google Scholar]
- 14.Roorda AK, Marcus SN, Triadafilopoulos G, et al. Early experience with radiofrequency energy ablation therapy for Barrett's esophagus with and without dysplasia. Dis Esoph. 2007;20:516–520. doi: 10.1111/j.1442-2050.2007.00728.x. [DOI] [PubMed] [Google Scholar]
- 15.Cash BD, Johnston LR, Johnston MH, et al. Cryospray ablation (CSA) in the palliative treatment of squamous cell carcinoma of the esophagus. World J Surg Oncol. 2007;5:34. doi: 10.1186/1477-7819-5-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dumot JA, Vargo JJ, 2nd, Falk GW, et al. An open-label, prospective trial of cryospray ablation for Barrett's esophagus high-grade dysplasia and early esophageal cancer in high-risk patients. Gastrointest Endosc. 2009;70:635–644. doi: 10.1016/j.gie.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 17.Basu KK, Pick B, Bale R, et al. Efficacy and one year follow up of argon plasma coagulation therapy for ablation of Barrett's oesophagus: factors determining persistence and recurrence of Barrett's epithelium. Gut. 2002;51:776–780. doi: 10.1136/gut.51.6.776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kelty CJ, Ackroyd R, Brown NJ, et al. Endoscopic ablation of Barrett's oesophagus: a randomized-controlled trial of photodynamic therapy vs. argon plasma coagulation. Aliment Pharmacol Ther. 2004;20:1289–1296. doi: 10.1111/j.1365-2036.2004.02277.x. [DOI] [PubMed] [Google Scholar]
- 19.Ragunath K, Krasner N, Raman VS, et al. Endoscopic ablation of dysplastic Barrett's oesophagus comparing argon plasma coagulation and photodynamic therapy: a randomized prospective trial assessing efficacy and cost-effectiveness. Scand J Gastroenterol. 2005;40:750–758. doi: 10.1080/00365520510015737. [DOI] [PubMed] [Google Scholar]
- 20.Hage M, Siersema PD, van Dekken H, et al. 5-aminolevulinic acid photodynamic therapy versus argon plasma coagulation for ablation of Barrett's oesophagus: a randomised trial. Gut. 2004;53:785–790. doi: 10.1136/gut.2003.028860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bronner MP, Overholt BF, Taylor SL, et al. Squamous overgrowth is not a safety concern for photodynamic therapy for Barrett's esophagus with high-grade dysplasia. Gastroenterology. 2009;136:56–64. doi: 10.1053/j.gastro.2008.10.012. [DOI] [PubMed] [Google Scholar]
- 22.Sharma P, Bhattacharyya A, Garewal HS, et al. Durability of new squamous epithelium after endoscopic reversal of Barrett's esophagus. Gastrointest Endosc. 1999;50:159–164. doi: 10.1016/s0016-5107(99)70218-x. [DOI] [PubMed] [Google Scholar]
- 23.van Laethem JL, Peny MO, Salmon I, et al. Intramucosal adenocarcinoma arising under squamous re-epithelialisation of Barrett's oesophagus. Gut. 2000;46:574–577. doi: 10.1136/gut.46.4.574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mino-Kenudson M, Ban S, Ohana M, et al. Buried dysplasia and early adenocarcinoma arising in barrett esophagus after porfimer-photodynamic therapy. Am J Surg Pathol. 2007;31:403–409. doi: 10.1097/01.pas.0000213407.03064.37. [DOI] [PubMed] [Google Scholar]
- 25.Fleischer DE, Overholt BF, Sharma VK, et al. Endoscopic ablation of Barrett's esophagus: a multicenter study with 2.5-year follow-up. Gastrointest Endosc. 2008;68:867–876. doi: 10.1016/j.gie.2008.03.008. [DOI] [PubMed] [Google Scholar]
- 26.Fleischer DE, Overholt BF, Sharma VK, et al. Endoscopic radiofrequency ablation for Barrett's esophagus: 5-year outcomes from a prospective multicenter trial. Endoscopy. 2010;42:781–789. doi: 10.1055/s-0030-1255779. [DOI] [PubMed] [Google Scholar]
- 27.Shaheen NJ, Sharma P, Overholt BF, et al. Radiofrequency ablation in Barrett's esophagus with dysplasia. N Engl J Med. 2009;360:2277–2288. doi: 10.1056/NEJMoa0808145. [DOI] [PubMed] [Google Scholar]
- 28.Hernandez JC, Reicher S, Chung D, et al. Pilot series of radiofrequency ablation of Barrett's esophagus with or without neoplasia. Endoscopy. 2008;40:388–392. doi: 10.1055/s-2007-995747. [DOI] [PubMed] [Google Scholar]
- 29.Pouw RE, Gondrie JJ, Sondermeijer CM, et al. Eradication of Barrett esophagus with early neoplasia by radiofrequency ablation, with or without endoscopic resection. J Gastrointest Surg. 2008;12:1627–1636. doi: 10.1007/s11605-008-0629-1. [DOI] [PubMed] [Google Scholar]
- 30.Sharma VK, Kim HJ, Das A, et al. A prospective pilot trial of ablation of Barrett's esophagus with low-grade dysplasia using stepwise circumferential and focal ablation (HALO system) Endoscopy. 2008;40:380–387. doi: 10.1055/s-2007-995587. [DOI] [PubMed] [Google Scholar]
- 31.Ganz RA, Overholt BF, Sharma VK, et al. Circumferential ablation of Barrett's esophagus that contains high-grade dysplasia: a U. S. multicenter registry. Gastrointest Endosc. 2008;68:35–40. doi: 10.1016/j.gie.2007.12.015. [DOI] [PubMed] [Google Scholar]
- 32.Lyday WD, Corbett FS, Kuperman DA, et al. Radiofrequency ablation of Barrett's esophagus: outcomes of 429 patients from a multicenter community practice registry. Endoscopy. 2010;42:272–278. doi: 10.1055/s-0029-1243883. [DOI] [PubMed] [Google Scholar]
- 33.Eldaif SM, Lin E, Singh KA, et al. Radiofrequency ablation of Barrett's esophagus: short-term results. Ann Thorac Surg. 2009;87:405–410. doi: 10.1016/j.athoracsur.2008.11.043. [DOI] [PubMed] [Google Scholar]
- 34.Vassiliou MC, von Renteln D, Wiener DC, et al. Treatment of ultralong-segment Barrett's using focal and balloon-based radiofrequency ablation. Surg Endosc. 2010;24:786–791. doi: 10.1007/s00464-009-0639-4. [DOI] [PubMed] [Google Scholar]
- 35.O'Connell K, Velanovich V. Effects of Nissen fundoplication on endoscopic endoluminal radiofrequency ablation of Barrett's esophagus. Surg Endosc. 2010 doi: 10.1007/s00464-010-1270-0. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 36.Sharma VK, Kim HJ, Das A, et al. Circumferential and focal ablation of Barrett's esophagus containing dysplasia. Am J Gastroenterol. 2009;104:310–317. doi: 10.1038/ajg.2008.142. [DOI] [PubMed] [Google Scholar]
- 37.Herrero LA, van Vilsteren FG, Pouw RE, et al. Endoscopic radiofrequency ablation combined with endoscopic resection for early neoplasia in Barrett's esophagus longer than 10 cm. Gastrointest Endosc. 2011 doi: 10.1016/j.gie.2010.11.016. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 38.Van Vilsteren FG, Pouw RE, Seewald S, et al. Stepwise radical endoscopic resection versus radiofrequency ablation for Barrett's oesophagus with high-grade dysplasia or early cancer: a multicentre randomised trial. Gut. 2011;60:765–773. doi: 10.1136/gut.2010.229310. [DOI] [PubMed] [Google Scholar]
- 39.Zehetner J, Demeester SR, Hagen JA, et al. Endoscopic resection and ablation versus esophagectomy for high-grade dysplasia and intramucosal adenocarcinoma. J Thorac Cardiovasc Surg. 2011;141:39–47. doi: 10.1016/j.jtcvs.2010.08.058. [DOI] [PubMed] [Google Scholar]
- 40.Ganz RA, Utley DS, Stern RA, et al. Complete ablation of esophageal epithelium with a balloon-based bipolar electrode: a phased evalulation in the procine and in the humanesophagus. Gastrointest Endosc. 2004;60:1002–1010. doi: 10.1016/s0016-5107(04)02220-5. [DOI] [PubMed] [Google Scholar]
- 41.Sharma VK, Wang KK, Overholt BF, et al. Balloon-based, circumferential, endoscopic radiofrequency ablation of Barrett's esophagus: 1-year follow-up of 100 patients. Gastrointest Endosc. 2007;65:185–195. doi: 10.1016/j.gie.2006.09.033. [DOI] [PubMed] [Google Scholar]
- 42.Shaheen NJ, Sharma P, Overholt BF, et al. Radiofrequency ablation in Barrett's esophagus with dysplasia. N Engl J Med. 2009;360:2277–2288. doi: 10.1056/NEJMoa0808145. [DOI] [PubMed] [Google Scholar]
- 43.Van Vilsteren FGI, Bergman JGHM. Endoscopic therapy using radiofrequency ablation for esophageal dysplasia and carcinoma in Barrett's esophagus. Gastrointest Endoscopy Clin North Am. 2010;20:55–74. doi: 10.1016/j.giec.2009.07.007. [DOI] [PubMed] [Google Scholar]
- 44.Overholt BF, Wang KK, Burdick JS, et al. Five-year efficacy and safety of photodynamic therapy with Photofrin in Barrett's high-grade dysplasia. Gastrointest Endosc. 2007;66:460–468. doi: 10.1016/j.gie.2006.12.037. [DOI] [PubMed] [Google Scholar]
- 45.Rees JRE, Lao-Sirieix P, Wong A, et al. Treatment for Barrett's oesophagus. Cochrane Data Base Systemic. 2010 Review. [Google Scholar]
- 46.Shaheen NJ, Peery AF, Overholt BF, et al. AIM Dysplasia Investigators. 2010. Biopsy depth after radiofrequency ablation of dysplastic Barrett's esophagus. Gastrointest Endosc. 2010;72:490–496. doi: 10.1016/j.gie.2010.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Halsey KD, Chang JW, Greenwald BD, et al. Recurrent disease following endoscopic ablation of Barrett's neoplasia. Gastroenterology. 138:S–16. doi: 10.1055/s-0030-1256649. [DOI] [PubMed] [Google Scholar]
- 48.Finkelstein SD, Lyday WD. The molecular pathology of radiofrequency mucosal ablation of Barrett's esophagus. Gastroenterology. 2008;134:A436. [Google Scholar]
- 49.Montgomery E, Bronner MP, Goldblum JR, et al. Reproducibility of the diagnosis of dysplasia in Barrett esophagus: a reaffirmation. Hum Pathol. 2001;32:368–378. doi: 10.1053/hupa.2001.23510. [DOI] [PubMed] [Google Scholar]
- 50.Wang KK, Sampliner RE. Updated guidelines. 2008. for the diagnosis, surveillance and therapy of Barrett's esophagus. Am J Gastroenterol. 2008;103:788–797. doi: 10.1111/j.1572-0241.2008.01835.x. [DOI] [PubMed] [Google Scholar]
- 51.Curvers WL, ten Kate FJ, Krishnadath KK, et al. Low-grade dysplasia in Barrett's esophagus: overdiagnosed and underestimated. Am J Gastroenterol. 2010;105:1523–1530. doi: 10.1038/ajg.2010.171. [DOI] [PubMed] [Google Scholar]
- 52.Sharma P, Falk GW, Weston AP, et al. Dysplasia and cancer in a large multicenter cohort of patients with Barrett's esophagus. Clin Gastroenterol Hepatol. 2006;4:566–572. doi: 10.1016/j.cgh.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 53.Fitzgerald RC, Omary MB, Triadafilopoulos G. Dynamic effects of acid on Barrett's esophagus. An ex vivo proliferation and differentiation model. J Clin Invest. 1996;98:2120–2128. doi: 10.1172/JCI119018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Feagins LA, Zhang HY, Hormi-Carver K, et al. Acid has antiproliferative effects in nonneoplastic Barrett' s epithelial cells. Am J Gastroenterol. 2007;102:10–20. doi: 10.1111/j.1572-0241.2006.01005.x. [DOI] [PubMed] [Google Scholar]
- 55.Lao-Sirieix P, Roy A, Worrall C, et al. Effect of acid suppression on molecular predictors for esophageal cancer. Cancer Epidemiol Biomarkers Prev. 2006;15:288–293. doi: 10.1158/1055-9965.EPI-05-0528. [DOI] [PubMed] [Google Scholar]
- 56.Cooper BT, Chapman W, Neumann CS, et al. Continuous treatment of Barrett's oesophagus patients with proton pump inhibitors up to 13 years: observations on regression and cancer incidence. Aliment Pharmacol Ther. 2006;23:727–733. doi: 10.1111/j.1365-2036.2006.02825.x. [DOI] [PubMed] [Google Scholar]
- 57.DeJonge PF, van Blankenstein M, Looman CWN, et al. Risk of malignant progression in patients with Barrett's oesophagus: a Dutch nationwide cohort study. Gut. 2010;59:1030–1036. doi: 10.1136/gut.2009.176701. [DOI] [PubMed] [Google Scholar]
- 58.Pohl H, Welch HG. The role of overdiagnosis and reclassification in the marked increase of esophageal adenocarcinoma incidence. J Natl Cancer Inst. 2005;97:142–146. doi: 10.1093/jnci/dji024. [DOI] [PubMed] [Google Scholar]
- 59.Dulai GS, Jensen DM, Cortina G, et al. Randomized trial of argon plasma coagulation vs. multipolar electrocoagulation for ablation of Barrett's esophagus. Gastrointest Endosc. 2005;61:232–240. doi: 10.1016/s0016-5107(04)02576-3. [DOI] [PubMed] [Google Scholar]
- 60.Sampliner RE, Fennerty B, Garewal HS, et al. Reversal of Barrett's esophagus with acid suppression and multipolar electrocoagulation: preliminary results. Gastrointest Endosc. 1996;44:532–535. doi: 10.1016/s0016-5107(96)70004-4. [DOI] [PubMed] [Google Scholar]
- 61.Overholt BF, Panjehpour M, Haydek JM, et al. Photodynamic therapy for Barrett's esophagus: follow-up in 100 patients. Gastrointest Endos. 1999;49:1–7. doi: 10.1016/s0016-5107(99)70437-2. see the comments. [DOI] [PubMed] [Google Scholar]
- 62.Sharma VK, Wang KK, Overholt BF, et al. Balloon-based, circumferential, endoscopic radiofrequency ablation of Barrett's esophagus: 1-year follow-up of 100 patients. Gastrointest Endosc. 2007;65:185–195. doi: 10.1016/j.gie.2006.09.033. [DOI] [PubMed] [Google Scholar]
- 63.Overholt BF, Lightdale CJ, Wang KK, et al. Photodynamic therapy with porfimer sodium for ablation of high-grade dysplasia in Barrett's esophagus: international, partially blinded, randomized phase III trial. Gastrointest Endosc. 2005;62:488–498. doi: 10.1016/j.gie.2005.06.047. [DOI] [PubMed] [Google Scholar]
- 64.Inadomi JM, Somsouk M, Madanick RD, et al. A cost-utility analysis of ablative therapy for Barrett's esophagus. Gastroenterology. 2009;136:613–623. doi: 10.1053/j.gastro.2009.02.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Schnell TG, Sontag SJ, Chejfec G, et al. Long-term nonsurgical management of Barrett's esophagus with high-grade dysplasia. Gastroenterology. 2001;120:1607–1619. doi: 10.1053/gast.2001.25065. [DOI] [PubMed] [Google Scholar]
- 66.Wang KK, Sampliner RE. Updated Guidelines 2008 for the Diagnosis, Surveillance and Therapy of Barrett's esophagus. Am J Gastroenterol. 2008;103:788–797. doi: 10.1111/j.1572-0241.2008.01835.x. [DOI] [PubMed] [Google Scholar]
- 67.Shaheen NJ, Fleischer DE, Eisen GM, et al. Durability of epithelial reversion after radiofrequency ablation: follow-up of the AIM dysplasia trial. Gastroenterology. 2010;138(Suppl. 1):92. Ref Type: Abstract. [Google Scholar]
- 68.Gondrie JJ, Pouw RE, Sondermeijer CM, et al. Stepwise circumferential and focal ablation of Barrett's esophagus with high–grade dysplasia: results of the first prospective series of 11 patients. Endoscopy. 2008;40:359–369. doi: 10.1055/s-2007-995567. [DOI] [PubMed] [Google Scholar]
- 69.Gondrie JJ, Pouw RE, Sondermeijer CM, et al. Effective treatment of early Barrett's neoplasia with stepwise circumferential and focal ablation using the HALO system. Endoscopy. 2008;40:370–379. doi: 10.1055/s-2007-995589. [DOI] [PubMed] [Google Scholar]
- 70.Pouw RE, Wirths K, Eisendrath P, et al. Efficacy of radiofrequency ablation combined with endoscopic resection for Barrett's esophagus with early neoplasia. Clin Gastroenterol Hepatol. 2010;8:23–29. doi: 10.1016/j.cgh.2009.07.003. [DOI] [PubMed] [Google Scholar]
- 71.Pouw RE, Bisschops R, Pech O, et al. Safety Outcomes of Balloon-Based Circumferential Radiofrequency Ablation after Focal Endoscopic Resection of Early Barrett's Neoplasia in 118 Patients: Results of an Ongoing European Multicenter Study. Gastrointest Endosc. 2010;71:AB126. [Google Scholar]
- 72.Velanovich V. Endoscopic endoluminal radiofrequency ablation of Barrett's esophagus: initial results and lessons learned. Surg Endosc. 2009;23:2175–2180. doi: 10.1007/s00464-009-0364-z. [DOI] [PubMed] [Google Scholar]
- 73.Shaheen NJ, Overholt BF, Sampliner SE, et al. Durability of Radiofrequency Ablation in Barrett's Esophagus with Dysplasia. Gastroenterology. 2011 May 6; doi: 10.1053/j.gastro.2011.04.061. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]