Abstract
The bed nucleus of the stria terminalis (BST) is a brain structure located at the interface of the cortex and the cerebrospinal trunk. The BST is a cluster of nuclei organized in a complex intrinsic network that receives inputs from cortical and subcortical sources, and that sends a widespread top-down projection. There is growing evidence that the BST is a key component in the neurobiological basis of substance abuse. In the present study, the regulation of excitatory inputs onto identified neurons in the BST was examined in rats treated chronically with morphine. Neurons projecting to the ventral tegmental area (VTA) were identified by retrograde transport of fluorescent microspheres and recorded in the whole-cell voltage clamp configuration in brain slices. Selective excitatory inputs to these neurons were electrically evoked with electrodes placed in the medial and lateral aspects of the dorsal BST. The chronic morphine treatment selectively increased AMPA-dependent excitatory postsynaptic currents in a subset of inputs activated by dorso-lateral stimulation in the BST. Inputs activated by medial stimulation were not affected by morphine. Likewise, the inputs to neurons that did not project to the VTA were not changed by morphine. Altogether, these results extend the understanding of neuronal circuits intrinsically sensitive to drugs of abuse within the BST.
Keywords: bed nucleus of the stria terminalis, BST, electrophysiology, morphine, AMPA, NMDA
The bed nucleus of the stria terminalis (BST) is a cluster of 11 nuclei surrounding the caudal part of the anterior commissure. An increasing number of anatomical and behavioral observations suggest that the BST is part of a system that coordinates autonomic, neuroendocrine, and behavioral functions, particularly with regards to energy balance, defense, and reproduction (Dong et al., 2000, 2001a,b; Dong and Swanson, 2003, 2004a,b, 2006a,b,c). The projection pattern of the BST is widespread but primarily directed toward the cerebrospinal trunk with more limited projections to cortical, striatal and pallidal targets. The BST projects to the nucleus accumbens and the ventral tegmental area (VTA), suggesting a role in goal-directed behaviors. In fact, plasticity at excitatory synapses within the BST correlates with operant learning, supporting a role of the BST in goal-directed behaviors toward natural rewards (Dumont et al., 2005). Likewise the BST may be part of the neurocircuitry underlying addiction since it contributes to the reinforcing properties of several drugs of abuse (Walker et al., 2000; Fudge and Haber, 2001; Georges and Aston-Jones, 2002; Dumont et al., 2005). In addition, the BST is a key contributor in negative reinforcement, where it is thought to mediate aversive aspects of drug withdrawal resulting in drug seeking in dependent animals (Delfs et al., 2000; Aston-Jones and Harris, 2004; Georges et al., 2005).
The lateral region of the anterior bed nucleus of the stria terminalis (alBST) is an important component of the brain motivation pathways. The alBST sends a monosynaptic excitatory output to dopamine neurons in the VTA (Georges and Aston-Jones, 2002; Dumont and Williams, 2004); excitatory synapses onto this subgroup of VTA-projecting neurons show an increase in strength in rats trained to self-administer cocaine (Dumont et al., 2005). This suggests that the regulation of synaptic inputs onto alBST projection neurons can influence the reward pathways, perhaps by synaptic activation of dopamine neurons.
The BST receives bottom-up and top-down information respectively from the cerebrospinal trunk and the cortex. Cortical inputs to the BST are particularly intriguing since they are topographically organized (McDonald, 1998). Specifically, afferents from the insula and the amygdalopiriform area enter the laterodorsal aspect of the BST whereas inputs from the ventral entorhinal, ventral subiculum, and infralimbic regions reach the BST medially (McDonald, 1998). Given that cortical regions, such as the insula and infralimbic cortex, are circuits that mediate components of addiction, the BST could serve as an interface between the cortex and lower regions of the brain (Bechara and Damasio, 2002; Kalivas, 2004; Contreras et al., 2007).
The objective of the present study was to determine if excitatory inputs to the BST were altered in morphine-dependent rats. Whole-cell patch-clamp recordings were made from alBST neurons in brain slices and excitatory post-synaptic currents (EPSCs) were evoked by stimulation of distinct afferent inputs. Morphine selectively strengthened a population of excitatory fibers entering the dorso-lateral BST and making synapses on a subpopulation of alBST neurons projecting to the VTA. This circuit-specific effect of morphine in the BST extends our understanding of brain pathways involved in the addictive properties of drugs of abuse such as opioids.
EXPERIMENTAL PROCEDURES
Identification of BST neurons projecting to the VTA
The alBST is a complex region of the brain with at least three distinct subpopulations of neurons (Dumont and Williams, 2004; Hammack et al., 2007). In order to study BST neurons intrinsic to the neurocircuitry of goal-directed behaviors, fluorescent microspheres were stereotaxically microinjected (10 nl, 0.04 µm, 488/560 EX/EM, www.invitrogen.com) into the VTA (−5.3 mm from bregma, 0.8 mm lateral, vertical 7.8 mm vertical (Swanson, 2005)) of 20 rats (Sprague–Dawley, 38–51 days, 126–150 g, www.criver.com). During the procedure, the rats were deeply anesthetized with isoflurane (5% isoflurane V/V with O2, 100 ml/min, www.bensonmedical.ca). Pain and inflammation were minimized with Anafen™ (1 mg/kg, s.c., www.cdmv.com) pre-operatively and twice daily for 2 days following surgery. In addition, topical Xylocaine-jelly™ (lidocaine-HCl 2%, www.cdmv.com) was applied on the surgical incision immediately post-operatively. Animal protocols were approved by the Queen’s University Animal Care Committee in accordance with the guidlines set by the Canadian Council on Animal Care. All efforts were made to ensure that the number of animals used and suffering was kept to a minimum.
Morphine treatment
Thirty rats (including those with intra-cranial micro-injections) were randomly assigned to placebo or morphine groups. The rats were anesthetized with isoflurane (5% isoflurane V/V with O2, 100 ml/min) and received placebo- or morphine-containing s.c. pellet (75 mg morphine, National Institute on Drug Abuse) implants. The morphine treatment consisted of one pellet on day 1 and two pellets on days 3 and 5; experiments were done on days 6 or 7.
Brain slices preparation
Twenty-four hours after the last treatment, the rats were deeply anesthetized (isoflurane) and killed for brain slice preparation. Coronal slices (250 µm) containing the alBST were prepared in a physiological solution containing (in mM) 126 NaCl, 2.5 KCl, 1.2 MgCl2, 2.5 CaCl2, 1.2 NaH2PO4, 25 NaHCO3, 11 d-glucose, and morphine sulfate 0.001 at 15 °C. The slices were incubated at 34 °C for 30 min and transferred to a chamber constantly irrigated (1.5 ml/min) with the physiological solution maintained at 34 °C and equilibrated with 95% O2/5% CO2. Whole-cell voltage-clamp recordings were made in the ventral part of the alBST (Bregma −0.26 mm, Fig. 1A), using glass microelectrodes (2–5 MΩ) filled with a solution containing (in mM) 130 Cs+MeSO3−, 4 NaCl, 1 MgCl2, 1 EGTA, 10 Hepes, 2 MgATP, and 0.3 GTP. The recordings were done under visual guidance using an upright transmitted light microscope (BX-51W1, www.olympus.com) equipped with Dodt-Gradient-Contrast-System (www.Luigs-Neumann.com). The microscope was also equipped with an X-Cite 120 fluorescence illumination system (EXFO Life Sciences & Industrial Division, www.exfo-lifesciences.com) to visualize neurons labeled with fluorescent beads. Recordings were made using a Multiclamp 700B amplifier and a Digidata 1440A (www.mds.com). Data were acquired and analyzed with Axograph X (www.axographx.com) running on an Apple computer (www.apple.com).
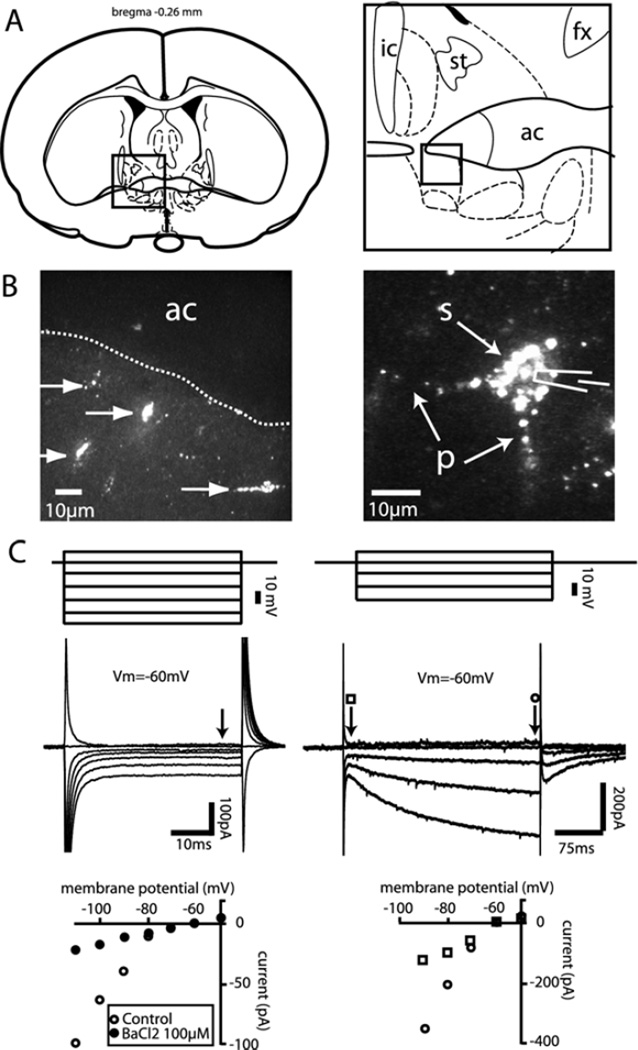
Fig. 1.
Identification and characterization of alBST neurons projecting to the VTA. (A) Schematics representing the anterior region of the BST where neurons labeled with a retrograde tracer were observed following microinjection of fluorescent microspheres in the VTA. (B) Photomicrographs showing alBST filled with fluorescent microspheres. (C) Superimposed current responses (middle traces) to graded series of voltage pulses (top) in alBST neurons filled (left) or not filled with fluorescent microspheres (right). Graphs at the bottom represent the current responses as a function of membrane voltage in alBST neurons filled (left) or not filled (right) with fluorescent microspheres. Vm: membrane voltage; ic: internal capsule; ac: anterior commissure; st: stria terminalis; fx: fornix; s: soma; p: processes.
Evoked EPSCs
EPSCs were evoked by local stimulation (two pulses at 40 Hz, 0.1ms duration, 0.1 Hz) using bipolar tungsten electrodes in the presence of picrotoxin (100 µM) while neurons were voltage-clamped at −60 mV. Stimulation intensity was adjusted to obtain approximately 80% of the maximum response. Neurons were then voltage-clamped at +40 mV to release Mg2+-induced blockade of NMDA receptors. After a stabilization period of 5–15 min, the NMDA receptor antagonist D-AP5 (5 min, 50 µM) was bath-applied to isolate AMPA currents. Isolated AMPA currents were digitally subtracted offline from the total EPSC to obtain the NMDA currents and compute AMPA to NMDA ratios. The addition of naloxone (1 µM) during the recordings precipitated morphine withdrawal. At the end of each experiment, NBQX (5 µM) and D-AP5 (50 µM) were added to completely abolish all EPSCs.
Isolation of multiple excitatory inputs to the alBST
To stimulate multiple excitatory inputs to the alBST, multiple bipolar stimulating electrodes were placed in the dorso-medial (±1.5 mm from recording), dorso-lateral (±1.5 mm from recording), and ventro-lateral (±0.5 mm from recording) aspects of the anterior BST. With this configuration, two electrical pulses could be evoked and paired-pulse ratios (PPR) calculated from a single or from two different stimulation sites during recordings from a single neuron. We hypothesized that fiber stimulation in the immediate vicinity of the recorded neuron recruited a mixed population of excitatory inputs. Stimulation from remote sites in the dorsal alBST produced EPSCs with longer latencies. Short-term plasticity of excitatory synapses was measured using a paired-pulse protocol (two stimuli applied at 40 Hz) in an attempt to isolate various excitatory inputs to the alBST. Two stimuli from a single stimulation site always produced facilitation (PPR >1 when stimulus two was divided by stimulus 1). The absence of cross short-term plasticity between two stimuli (40 Hz) from two different stimulation sites reflected the recruitment of independent excitatory inputs to the alBST.
Drugs
Stock solution of NBQX (50 mM) was prepared in DMSO (100%) and further diluted in the physiological solution at the desired concentration. DMSO concentration never exceeded 0.001%. Stock solutions of morphine (10 mM), naloxone (10 mM), picrotoxin (100 mM), and AP-5 (10 mM) were prepared in water and dissolved in the physiological solution at the desired concentration. All drugs were obtained from Sigma-Aldrich (www.sigmaaldrich.com).
RESULTS
Retrograde tracer from the VTA labeled a population of anterior BST neurons
Consistent with previously published observations (Dumont and Williams, 2004), microinjections of fluorescent microspheres into the VTA produced retrograde labeling in the alBST (Fig. 1B). Eighteen out of 20 microinjections were restricted to the VTA and in all cases produced retrograde labeling in the BST (Fig. 1B). In the remaining two cases, the microinjections were above the VTA and did not produce labeling in the BST. As previously reported, BST neurons projecting to the VTA shared common electrophysiological properties when submitted to a series of voltage-steps in the whole-cell voltage clamp configuration, including a time-independent and barium-sensitive inwardly rectifying conductance at hyperpolarized membrane potentials (Fig. 1C, left). These neurons were similar to type III alBST neurons of Hammack et al. (2007). As previously reported, we also recorded from a subpopulation of alBST neurons that were never labeled with the tracer. These neurons displayed a time-dependent sag (Ih) in the inward direction at hyperpolarized membrane voltages (Fig. 1C, right) and likely correspond to type I and/or type II of Hammack et al. (2007).
Chronic morphine increases AMPA currents in VTA-projecting alBST neurons
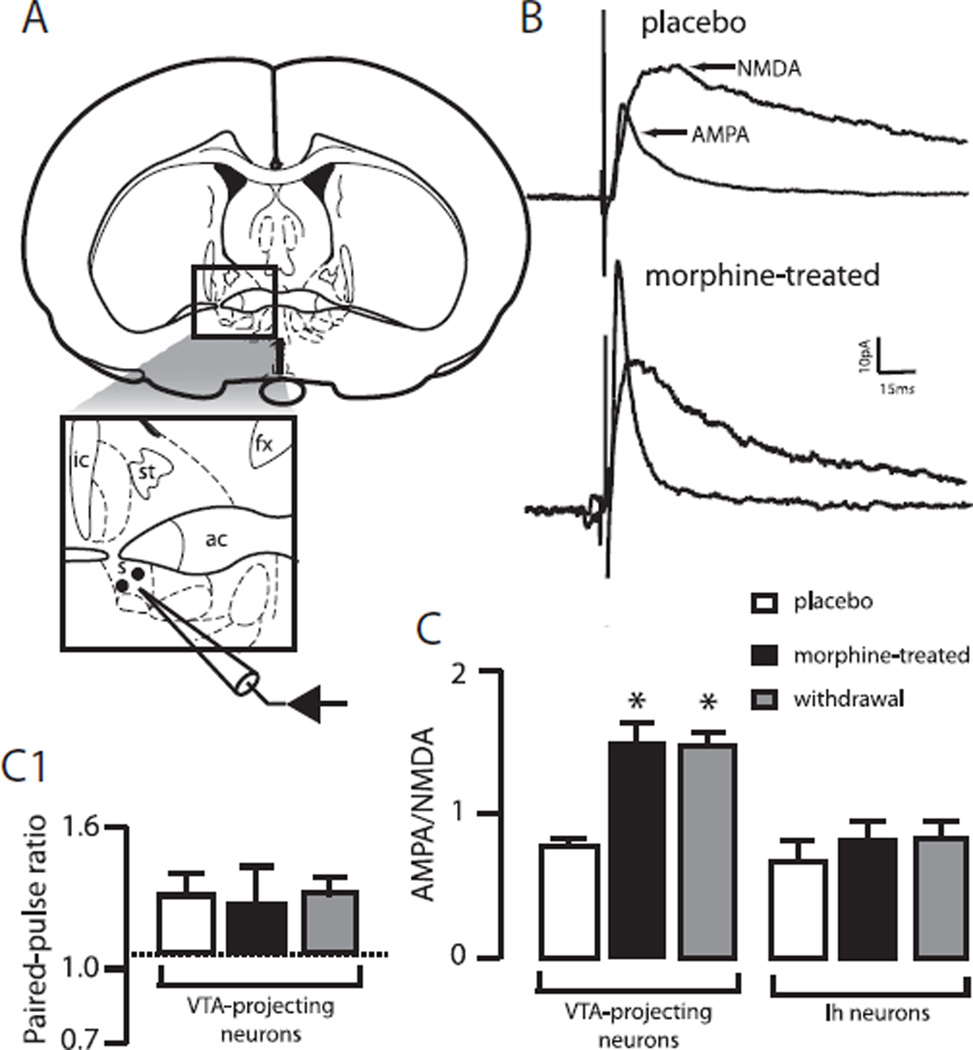
Local stimulation in the alBST evoked fast EPSCs with both AMPA and NMDA glutamate ionotropic receptor-mediated components in both subpopulations of neurons (Fig. 2B). In VTA-projecting neurons, chronic morphine doubled AMPA EPSCs (81±8 pA vs. 39±3pA, P<0.05, n=10 neurons per group) whereas NMDA EPSCs remained unchanged (53±8 pA vs. 51±3pA, P>0.05, n=10 neurons per group). As a result, the ratio of AMPA to NMDA currents increased significantly after the chronic morphine treatment (1.57±0.1 vs. 0.76±0.04, P<0.05, n=10 neurons per group, Fig. 2B, C). The PPR was not affected by chronic morphine suggesting that the increase in AMPA EPSCs resulted from a postsynaptic mechanism (Fig. 2C1).
Fig. 2.
Effect of a chronic morphine treatment on excitatory synaptic transmission in the alBST. (A) Schematic representing the placement of recording and stimulating electrodes for whole-cell voltage clamp recordings of evoked excitatory postsynaptic currents in the alBST. (B) Application of the NMDA receptors antagonist AP-5 (50 µM, 5 min) revealed both AMPA- and NMDA-mediated excitatory synaptic transmission when neurons were voltage-clamped at +40 mV, in brain slices prepared from non-treated/placebo (upper traces) and morphine-treated rats (lower traces). Each trace is the average of 5 to 10 evoked EPSCs. (C) The bar chart displays the ratio of AMPA to NMDA components of the evoked EPSCs in alBST neurons projecting (left bars) or not projecting (right bars) to the VTA in placebo (open bars), morphine-treated (closed bars) or after acute withdrawal produced by a 10-min bath-application of naloxone (1 µM). (C1) Bar chart representing the PPRs of two sequentially applied stimuli at 40 Hz in VTA-projecting neurons in placebo (open bars), morphine-treated (closed bars) or after acute withdrawal produced by a 10-min bath-application of naloxone (1 µM). * P<0.05, n=10 neurons per group. s: Stimulation site; st: stria terminalis; ic: internal capsule; fx: fornix; ac: anterior commissure.
In contrast with VTA-projecting neurons, morphine did not alter AMPA or NMDA EPSCs in the second subpopulation of alBST neurons (Fig. 2C). There was no change in the kinetics of AMPA or NMDA-dependent EPSCs. Rise-times of AMPA (2.44±0.4 and 2.55±0.4 ms, placebo and morphine-treated, respectively) and NMDA (16.6±0.9 and 12±1.1 ms, placebo and morphine-treated, n=10 neurons per group) EPSCs were statistically comparable. Similarly, the decay to 90% of the peak was not different between the placebo and morphine-treated groups for both AMPA (8.1±1.0 and 7.5±1.1 ms, placebo and morphine-treated, n=10 neurons per group) and NMDA EPSCs (108.5±21 and 89±17 ms, placebo vs. morphine-treated, n=10 neurons per group). Finally, the morphine-induced increase in AMPA EPSC measured in VTA-projecting neurons was not changed when acute morphine withdrawal was precipitated by bath-application of naloxone (AMPA/NMDA=1.51±0.1, P>0.05, n=10 neuron per group, Fig. 1C).
Excitatory inputs onto projection neurons were distinguished
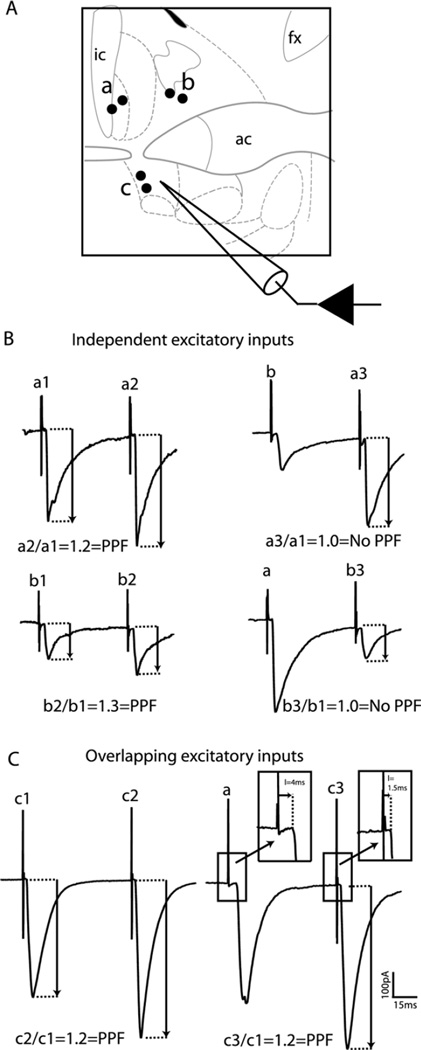
Placing stimulating electrodes in various regions of the BST allowed isolation of independent excitatory inputs to alBST VTA-projecting neurons (Fig. 3). Consistent EPSCs could be evoked through local stimulation in the ventral aspect of the alBST, close to the recorded neurons, as well as by stimulation in the lateral or medial regions of the dorsal BST (Fig. 3). Paired-pulse facilitation was observed with stimulation at all three stimulation sites (two pulses at 40 Hz to the same site, Fig. 3, left traces). However, pairing two pulses (at 40 Hz) from two different sites revealed evidence of independence between excitatory inputs entering the BST in its lateral and medial dorsal aspects. Pairing a lateral with a medial stimulation (or vice versa) did not produce short-term plasticity (facilitation or depression) suggesting independent populations of synapses (Fig. 3B, right traces). In contrast, paired-pulse facilitation was measured when a stimulus from the lateral BST was paired with a local stimulation in the ventral alBST (Fig. 3C, right traces). This suggests that stimulation of local fibers in the alBST recruited a mix of excitatory inputs to the BST, including those entering the lateral part of the dorsal BST.
Fig. 3.
The alBST receives multiple independent excitatory inputs. (A) Schematic representing the placement of multiple bipolar stimulating electrodes in (a) the latero-dorsal BST, (b) the medio-dorsal BST, or (c) the ventral alBST, to evoke EPSCs in the ventral alBST of non-treated rats. (B) Pairing two stimuli (40 Hz) produced short-term plasticity (paired-pulse facilitation) in alBST EPSCs evoked in both the latero-dorsal (top left traces) and medio-dorsal (bottom left traces) BST. No paired-pulse facilitation was observed when stimuli were paired between both dorsal BST stimulation sites (top and bottom right traces). Each trace is the average of 5 to 10 evoked EPSCs. Numbers (1, 2, or 3) next to site symbols indicate a single stimulus from a particular site. (C) Pairing two stimuli also produced paired-pulse facilitation in the alBST when EPSCs were evoked by local fiber stimulation (left traces). Pairing local alBST stimulation with an evoked EPSC from a remote site in the latero-dorsal BST also produced paired-pulse facilitation (right traces). Insets demonstrate that stimulation in remote sites produced longer latencies than local stimulation in the alBST. ic: Internal capsule; ac: anterior commissure; fx: fornix.
Chronic morphine increased excitatory inputs activated by dorso-lateral stimulation
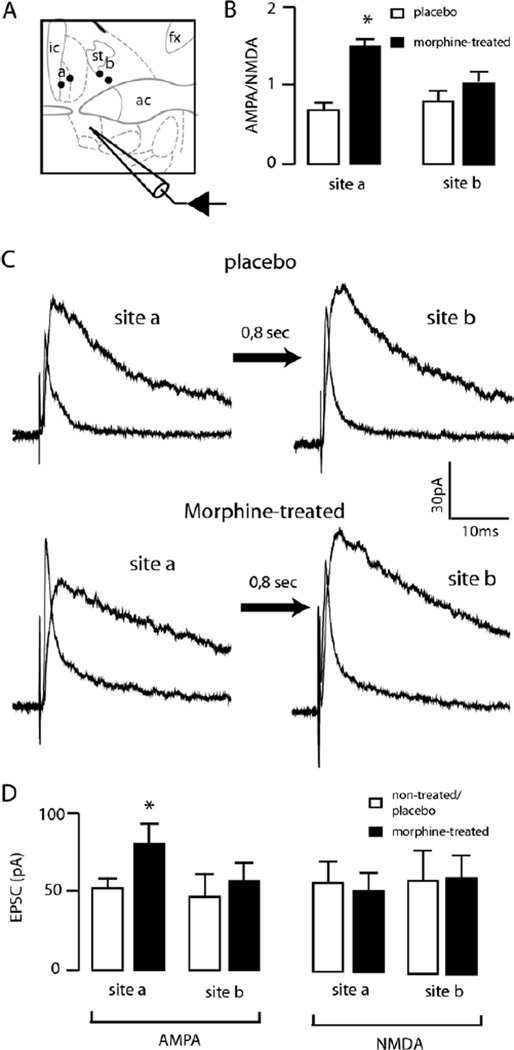
With the ability to distinguish separate inputs to projection neurons in the BST, the regulation of these inputs was examined after the chronic morphine treatment. EPSCs evoked from both dorso-lateral and dorso-medial stimulations were sequentially evoked (1.6 Hz) while neurons were voltage-clamped at +40 mV. In slices from morphine-treated rats, dorso-lateral stimulation resulted in an increase in the AMPA to NMDA ratio (1.5±0.06 vs. 0.81±0.05, P<0.05, morphine and placebo, respectively, n=8 neurons per group, Fig. 4). This change resulted from an increase in AMPA currents (80±11 pA vs. 51±5, P<0.05, morphine vs. placebo, respectively, n=8 neurons per group) and not from a decrease in NMDA currents (50±10 pA vs. 53±11, morphine vs. placebo, n=8 neurons per group). There was no change in the kinetics of the AMPA or NMDA EPSCs (AMPA rise: placebo 2.18±0.9, morphine 2.21±0.6 ms, decay to 90%: placebo 10.1±0.7, morphine 8.5±0.9 ms; NMDA rise: placebo 9.85±1.2, morphine 10.9±1.3 ms; decay to 90%: placebo 101.1±22, morphine 92±14 ms).
Fig. 4.
Chronic morphine selectively increased the alBST excitatory inputs entering the latero-dorsal BST. (A) Schematic representing the placement of multiple bipolar stimulating electrodes in the lateral (a) and medial dorsal BST (b) to evoke EPSCs in the ventral alBST. (B) Bar chart representing AMPA to NMDA ratios measured in VTA-projecting alBST neurons after stimulation in the dorso-lateral (left) and dorso-medial BST (right), in both placebo (open bars) and morphine-treated (closed bars) conditions. (C) AMPA and NMDA EPSCs recorded in VTA-projecting alBST neurons after stimulation in the latero-dorsal (left traces) and medio-dorsal (right traces) BST, in placebo (top traces) and morphine-treated (bottom traces) conditions. The interval between both stimuli was increased to 800 ms to avoid short-term plasticity. Each trace is the average of five evoked EPSCs. (D) Bar chart summarizing the amplitudes of AMPA (left) and NMDA (right) EPSCs measured in VTA-projecting alBST neurons in the dorso-lateral (site a) and dorso-medial BST (site b), in placebo (open bars) or morphine-treated (closed bars) conditions. * P<0.05, n=8 neurons per group. ic: Internal capsule; st: stria terminalis; ac: anterior commissure; fx: fornix.
Stimulation from the dorso-medial position evoked EPSCs that were not different in slices from placebo- and morphine-treated rats. The AMPA to NMDA ratios were the same in slices from placebo and morphine-treated rats (morphine 0.9±0.1, placebo 1.0±0.1, n=8 neurons per group, Fig. 4). AMPA and NMDA currents were comparable (Fig. 4D). The kinetics of AMPA and NMDA EPSCs were also not modified by the morphine treatment.
DISCUSSION
In the present study, we observed that a five-day morphine treatment produced plasticity of alBST excitatory synapses. This neuroplasticity was both input- and output-specific: only a subset of excitatory inputs entering the latero-dorsal BST (making synapses onto a subpopulation of VTA-projecting alBST neurons) was strengthened by the morphine treatment.
Plasticity at excitatory synapses is a primary mechanism for memory formation in many areas of the brain, including the motivation and reward pathways. Natural rewards and drugs of abuse can both produce plasticity of excitatory synapses in multiple sites within the brain reward pathways (Wolf, 2002; Gerdeman et al., 2003; Kauer, 2004; Kelley, 2004; Robinson and Kolb, 2004; Jones and Bonci, 2005; Hyman et al., 2006). Drug-induced plasticity is, however, a pathological form of memory formation that may contribute to the addictive properties of these drugs. Psychostimulants (Ungless et al., 2001; Dumont et al., 2005), opioids (Saal et al., 2003), cannabinoids (Mato et al., 2004), and alcohol (Melis et al., 2002; Weitlauf et al., 2004) all produce plasticity at excitatory synapses in various structures of the brain reward pathways. The VTA (Ungless et al., 2001; Melis et al., 2002), the nucleus accumbens (Thomas et al., 2001; Mato et al., 2004; Kourrich et al., 2007), and the BST (Weitlauf et al., 2004; Dumont et al., 2005) are all structures of brain reward pathways where drugs of abuse produce plasticity at excitatory synapses. The BST is a key brain structure within the reward pathways since it contributes to both the rewarding and the negative affective states related to substance abuse (Delfs et al., 2000; Walker et al., 2000; Erb et al., 2001; Leri et al., 2002; Harris and Aston-Jones, 2003; Dumont and Williams, 2004; Dumont et al., 2005). Chronic treatments with psychostimulants or alcohol produce plasticity at excitatory synapses in the BST, further suggesting that this structure is part of the circuitry involved in drug-associated neuroadaptations (Weitlauf et al., 2004; Dumont et al., 2005). The BST may also be involved in incentive learning since there is a clear increase in AMPA EPSCs in the BST of rats that self-administer rewards (cocaine or palatable food) but not in those receiving rewards passively (yoked controls) (Dumont et al., 2005). In the present study, a chronic morphine treatment also strengthened excitatory synapses in the BST although the morphine was administered passively. This disparity between the effects of opioids and psychostimulants is currently unknown but may be secondary to different mechanisms of action, the various routes of administration of the drugs, or a combination of both factors. Nonetheless, the morphine-induced BST neuroplasticity herein measured supports a role for long-term associative memory in the pathology of addiction. The morphine-induced increase in the peak amplitude of AMPA EPSCs was a postsynaptic effect since NMDA-dependent EPSCs were not affected. The exact mechanism of drug-induced changes in AMPA currents is not known although there were no changes in the kinetics of AMPA currents, which is consistent with other observations (Ungless et al., 2001; Borgland et al., 2004; Kourrich et al., 2007). This suggests the recruitment of more AMPA receptors to the membrane rather than intrinsic changes in the activity of AMPA channels, although this necessitates further investigation.
A key finding of the present study was the specificity of the effects of morphine on excitatory transmission in the BST. The effect of chronic morphine treatment was specific to excitatory synapses innervating a subpopulation of alBST neurons that project to dopamine-rich regions in the VTA. In contrast, excitatory synapses innervating a separate group of anatomically and physiologically identifiable neurons in the alBST were insensitive to the morphine treatment. Evidence suggests that alBST neurons projecting to the VTA are glutamatergic, sending an excitatory signal to dopamine neurons (Georges and Aston-Jones, 2002). Thus, the alBST neurons projecting to the VTA would be an intrinsic part of an excitatory circuit contributing to morphine-induced activation of dopamine neurons (Nowycky et al., 1978; Johnson and North, 1992; Grant and Sonti, 1994).
In addition, the effect of chronic morphine on excitatory transmission in the BST was input-specific. The BST receives multiple excitatory inputs from various brain regions (e.g. cortex, thalamus, amygdala) (McDonald, 1998; Dong et al., 2001a; Li and Kirouac, 2008). Furthermore, excitatory inputs are topographically organized in the BST and in some cases, clearly delineated in the medio-lateral axes in the anterior BST (McDonald, 1998; Dong et al., 2001a). This topographical organization of excitatory inputs was exploited by placing stimulating electrodes in both the medial and lateral regions of the dorsal BST in order to study independent excitatory inputs. Medial and lateral excitatory inputs were independent because no cross-short-term plasticity was detected. In contrast, pairing either of the dorsal stimulation sites (medial or lateral) with local stimulation in the ventral alBST produced paired-pulse facilitation. This suggests that inputs entering the lateral and the medial BST ultimately converge on alBST neurons. Examples of excitatory inputs specific to the lateral part of the BST include cortical afferents from the insula, a cortical region critically involved in addiction (McDonald, 1998; Contreras et al., 2007; Naqvi et al., 2007). Our results showed a functional dissociation between excitatory fibers entering the lateral and medial aspects of the BST that correlates anatomical observations. However, further experimentation is required to unequivocally identify the origin of these inputs. For example, sagittal or horizontal preparation might allow stimulating directly within cortical areas and record excitatory transmission in the BST.
The mechanism(s) underlying the selective effect of morphine on the latero-dorsal inputs to alBST projection neurons is currently unknown. However, the dorso-lateral BST receives a very dense dopamine projection as demonstrated by intense TH-positive fibers (Deutch et al., 1988; Freedman and Cassell, 1994; Hasue and Shammah-Lagnado, 2002). This suggests a potential feed-forward excitatory loop between the BST and the VTA that could result in activity-dependent increased strength of excitatory synapses in the presence of morphine. Furthermore, D1 dopamine receptors are present in the dorso-lateral BST and it is well documented that these receptors contribute to plasticity at excitatory synapses (Kerr and Wickens, 2001; Wolf et al., 2003; Sun et al., 2005).
CONCLUSION
In conclusion, our results show that chronic morphine treatment facilitated an input–output specific excitatory transmission in the BST. This confirms previous observations that drugs of abuse selectively target a neurocircuit that includes a subpopulation of presumably glutamatergic alBST neurons that project to the VTA. Selective stimulation of afferent excitatory inputs entering the BST revealed that a dorso-lateral pathway was selectively facilitated after chronic morphine. These results extend the understanding of neuronal circuits sensitive to drugs of abuse and further strengthen the suggestion that the BST plays a role in addiction.
Acknowledgments
Work supported by the Canadian Institutes of Health Research (MOP-79277 to ECD) and NIH-NIDA (DA08163 to J.T.W.). E.C.D. was a fellow of the Canadian Institutes of Health Research (MFE-49878).
Abbreviations
- alBST
lateral region of the anterior bed nucleus of the stria terminalis
- BST
bed nucleus of the stria terminalis
- EPSC
excitatory post-synaptic current
- PPR
paired-pulse ratio
- VTA
ventral tegmental area
REFERENCES
- Aston-Jones G, Harris GC. Brain substrates for increased drug seeking during protracted withdrawal. Neuropharmacology. 2004;47(Suppl 1):167–179. doi: 10.1016/j.neuropharm.2004.06.020. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio H. Decision-making and addiction (part I): impaired activation of somatic states in substance dependent individuals when pondering decisions with negative future consequences. Neuropsychologia. 2002;40:1675–1689. doi: 10.1016/s0028-3932(02)00015-5. [DOI] [PubMed] [Google Scholar]
- Borgland SL, Malenka RC, Bonci A. Acute and chronic cocaine-induced potentiation of synaptic strength in the ventral tegmental area: electrophysiological and behavioral correlates in individual rats. J Neurosci. 2004;24:7482–7490. doi: 10.1523/JNEUROSCI.1312-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contreras M, Ceric F, Torrealba F. Inactivation of the interoceptive insula disrupts drug craving and malaise induced by lithium. Science. 2007;318:655–658. doi: 10.1126/science.1145590. [DOI] [PubMed] [Google Scholar]
- Delfs JM, Zhu Y, Druhan JP, Aston-Jones G. Noradrenaline in the ventral forebrain is critical for opiate withdrawal-induced aversion. Nature. 2000;403:430–434. doi: 10.1038/35000212. [DOI] [PubMed] [Google Scholar]
- Deutch AY, Goldstein M, Baldino F, Jr, Roth RH. Telencephalic projections of the A8 dopamine cell group. Ann N Y Acad Sci. 1988;537:27–50. doi: 10.1111/j.1749-6632.1988.tb42095.x. [DOI] [PubMed] [Google Scholar]
- Dong H, Petrovich GD, Swanson LW. Organization of projections from the juxtacapsular nucleus of the BST: a PHAL study in the rat. Brain Res. 2000;859:1–14. doi: 10.1016/s0006-8993(99)02246-5. [DOI] [PubMed] [Google Scholar]
- Dong HW, Petrovich GD, Swanson LW. Topography of projections from amygdala to bed nuclei of the stria terminalis. Brain Res Brain Res Rev. 2001a;38:192–246. doi: 10.1016/s0165-0173(01)00079-0. [DOI] [PubMed] [Google Scholar]
- Dong HW, Petrovich GD, Watts AG, Swanson LW. Basic organization of projections from the oval and fusiform nuclei of the bed nuclei of the stria terminalis in adult rat brain. J Comp Neurol. 2001b;436:430–455. doi: 10.1002/cne.1079. [DOI] [PubMed] [Google Scholar]
- Dong HW, Swanson LW. Projections from the rhomboid nucleus of the bed nuclei of the stria terminalis: implications for cerebral hemisphere regulation of ingestive behaviors. J Comp Neurol. 2003;463:434–472. doi: 10.1002/cne.10758. [DOI] [PubMed] [Google Scholar]
- Dong HW, Swanson LW. Organization of axonal projections from the anterolateral area of the bed nuclei of the stria terminalis. J Comp Neurol. 2004a;468:277–298. doi: 10.1002/cne.10949. [DOI] [PubMed] [Google Scholar]
- Dong HW, Swanson LW. Projections from bed nuclei of the stria terminalis, posterior division: implications for cerebral hemisphere regulation of defensive and reproductive behaviors. J Comp Neurol. 2004b;471:396–433. doi: 10.1002/cne.20002. [DOI] [PubMed] [Google Scholar]
- Dong HW, Swanson LW. Projections from bed nuclei of the stria terminalis, anteromedial area: Cerebral hemisphere integration of neuroendocrine, autonomic, and behavioral aspects of energy balance. J Comp Neurol. 2006a;494:142–178. doi: 10.1002/cne.20788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong HW, Swanson LW. Projections from bed nuclei of the stria terminalis, dorsomedial nucleus: implications for cerebral hemisphere integration of neuroendocrine, autonomic, and drinking responses. J Comp Neurol. 2006b;494:75–107. doi: 10.1002/cne.20790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong HW, Swanson LW. Projections from bed nuclei of the stria terminalis, magnocellular nucleus: implications for cerebral hemisphere regulation of micturition, defecation, and penile erection. J Comp Neurol. 2006c;494:108–141. doi: 10.1002/cne.20789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumont EC, Mark GP, Mader S, Williams JT. Self-administration enhances excitatory synaptic transmission in the bed nucleus of the stria terminalis. Nat Neurosci. 2005;8:413–414. doi: 10.1038/nn1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumont EC, Williams JT. Noradrenaline triggers GABAA inhibition of bed nucleus of the stria terminalis neurons projecting to the ventral tegmental area. J Neurosci. 2004;24:8198–8204. doi: 10.1523/JNEUROSCI.0425-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erb S, Salmaso N, Rodaros D, Stewart J. A role for the CRF-containing pathway from central nucleus of the amygdala to bed nucleus of the stria terminalis in the stress-induced reinstatement of cocaine seeking in rats. Psychopharmacology (Berl) 2001;158:360–365. doi: 10.1007/s002130000642. [DOI] [PubMed] [Google Scholar]
- Freedman LJ, Cassell MD. Distribution of dopaminergic fibers in the central division of the extended amygdala of the rat. Brain Res. 1994;633:243–252. doi: 10.1016/0006-8993(94)91545-8. [DOI] [PubMed] [Google Scholar]
- Fudge JL, Haber SN. Bed nucleus of the stria terminalis and extended amygdala inputs to dopamine subpopulations in primates. Neuroscience. 2001;104:807–827. doi: 10.1016/s0306-4522(01)00112-9. [DOI] [PubMed] [Google Scholar]
- Georges F, Aston-Jones G. Activation of ventral tegmental area cells by the bed nucleus of the stria terminalis: a novel excitatory amino acid input to midbrain dopamine neurons. J Neurosci. 2002;22:5173–5187. doi: 10.1523/JNEUROSCI.22-12-05173.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georges F, Le Moine C, Aston-Jones G, Stinus L. Clonidine intra-vBNST blocks opiate-withdrawal-induced aversion: involvement of midbrain dopaminergic neurons. SFN 35th Annual Meeting; Washington, DC. 2005. [Google Scholar]
- Gerdeman GL, Partridge JG, Lupica CR, Lovinger DM. It could be habit forming: drugs of abuse and striatal synaptic plasticity. Trends Neurosci. 2003;26:184–192. doi: 10.1016/S0166-2236(03)00065-1. [DOI] [PubMed] [Google Scholar]
- Grant SJ, Sonti G. Buprenorphine and morphine produce equivalent increases in extracellular single unit activity of dopamine neurons in the ventral tegmental area in vivo. Synapse. 1994;16:181–187. doi: 10.1002/syn.890160303. [DOI] [PubMed] [Google Scholar]
- Hammack SE, Mania I, Rainnie DG. Differential expression of intrinsic membrane currents in defined cell types of the anterolateral bed nucleus of the stria terminalis. J Neurophysiol. 2007;98:638–656. doi: 10.1152/jn.00382.2007. [DOI] [PubMed] [Google Scholar]
- Harris GC, Aston-Jones G. Enhanced morphine preference following prolonged abstinence: association with increased Fos expression in the extended amygdala. Neuropsychopharmacology. 2003;28:292–299. doi: 10.1038/sj.npp.1300037. [DOI] [PubMed] [Google Scholar]
- Hasue RH, Shammah-Lagnado SJ. Origin of the dopaminergic innervation of the central extended amygdala and accumbens shell: a combined retrograde tracing and immunohistochemical study in the rat. J Comp Neurol. 2002;454:15–33. doi: 10.1002/cne.10420. [DOI] [PubMed] [Google Scholar]
- Hyman SE, Malenka RC, Nestler EJ. Neural mechanisms of addiction: the role of reward-related learning and memory. Annu Rev Neurosci. 2006;29:565–598. doi: 10.1146/annurev.neuro.29.051605.113009. [DOI] [PubMed] [Google Scholar]
- Johnson SW, North RA. Opioids excite dopamine neurons by hyperpolarization of local interneurons. J Neurosci. 1992;12:483–488. doi: 10.1523/JNEUROSCI.12-02-00483.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones S, Bonci A. Synaptic plasticity and drug addiction. Curr Opin Pharmacol. 2005;5:20–25. doi: 10.1016/j.coph.2004.08.011. [DOI] [PubMed] [Google Scholar]
- Kalivas PW. Glutamate systems in cocaine addiction. Curr Opin Pharmacol. 2004;4:23–29. doi: 10.1016/j.coph.2003.11.002. [DOI] [PubMed] [Google Scholar]
- Kauer JA. Learning mechanisms in addiction: synaptic plasticity in the ventral tegmental area as a result of exposure to drugs of abuse. Annu Rev Physiol. 2004;66:447–475. doi: 10.1146/annurev.physiol.66.032102.112534. [DOI] [PubMed] [Google Scholar]
- Kelley AE. Memory and addiction: shared neural circuitry and molecular mechanisms. Neuron. 2004;44:161–179. doi: 10.1016/j.neuron.2004.09.016. [DOI] [PubMed] [Google Scholar]
- Kerr JN, Wickens JR. Dopamine D-1/D-5 receptor activation is required for long-term potentiation in the rat neostriatum in vitro. J Neurophysiol. 2001;85:117–124. doi: 10.1152/jn.2001.85.1.117. [DOI] [PubMed] [Google Scholar]
- Kourrich S, Rothwell PE, Klug JR, Thomas MJ. Cocaine experience controls bidirectional synaptic plasticity in the nucleus accumbens. J Neurosci. 2007;27:7921–7928. doi: 10.1523/JNEUROSCI.1859-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leri F, Flores J, Rodaros D, Stewart J. Blockade of stress-induced but not cocaine-induced reinstatement by infusion of noradrenergic antagonists into the bed nucleus of the stria terminalis or the central nucleus of the amygdala. J Neurosci. 2002;22:5713–5718. doi: 10.1523/JNEUROSCI.22-13-05713.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Kirouac GJ. Projections from the paraventricular nucleus of the thalamus to the forebrain, with special emphasis on the extended amygdala. J Comp Neurol. 2008;506:263–287. doi: 10.1002/cne.21502. [DOI] [PubMed] [Google Scholar]
- Mato S, Chevaleyre V, Robbe D, Pazos A, Castillo PE, Manzoni OJ. A single in-vivo exposure to delta9THC blocks endocan-nabinoid-mediated synaptic plasticity. Nat Neurosci. 2004;7:585–586. doi: 10.1038/nn1251. [DOI] [PubMed] [Google Scholar]
- McDonald AJ. Cortical pathways to the mammalian amygdala. Prog Neurobiol. 1998;55:257–332. doi: 10.1016/s0301-0082(98)00003-3. [DOI] [PubMed] [Google Scholar]
- Melis M, Camarini R, Ungless MA, Bonci A. Long-lasting potentiation of GABAergic synapses in dopamine neurons after a single in vivo ethanol exposure. J Neurosci. 2002;22:2074–2082. doi: 10.1523/JNEUROSCI.22-06-02074.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naqvi NH, Rudrauf D, Damasio H, Bechara A. Damage to the insula disrupts addiction to cigarette smoking. Science. 2007;315:531–534. doi: 10.1126/science.1135926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowycky MC, Walters JR, Roth RH. Dopaminergic neurons: effect of acute and chronic morphine administration on single cell activity and transmitter metabolism. J Neural Transm. 1978;42:99–116. doi: 10.1007/BF01675349. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Kolb B. Structural plasticity associated with exposure to drugs of abuse. Neuropharmacology. 2004;47(Suppl 1):33–46. doi: 10.1016/j.neuropharm.2004.06.025. [DOI] [PubMed] [Google Scholar]
- Saal D, Dong Y, Bonci A, Malenka RC. Drugs of abuse and stress trigger a common synaptic adaptation in dopamine neurons. Neuron. 2003;37:577–582. doi: 10.1016/s0896-6273(03)00021-7. [DOI] [PubMed] [Google Scholar]
- Sun X, Zhao Y, Wolf ME. Dopamine receptor stimulation modulates AMPA receptor synaptic insertion in prefrontal cortex neurons. J Neurosci. 2005;25:7342–7351. doi: 10.1523/JNEUROSCI.4603-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson LW, editor. Brain maps: structure of the rat brain. San Diego: Elsevier; 2005. [Google Scholar]
- Thomas MJ, Beurrier C, Bonci A, Malenka RC. Long-term depression in the nucleus accumbens: a neural correlate of behavioral sensitization to cocaine. Nat Neurosci. 2001;4:1217–1223. doi: 10.1038/nn757. [DOI] [PubMed] [Google Scholar]
- Ungless MA, Whistler JL, Malenka RC, Bonci A. Single cocaine exposure in vivo induces long-term potentiation in dopamine neurons. Nature. 2001;411:583–587. doi: 10.1038/35079077. [DOI] [PubMed] [Google Scholar]
- Walker JR, Ahmed SH, Gracy KN, Koob GF. Microinjections of an opiate receptor antagonist into the bed nucleus of the stria terminalis suppress heroin self-administration in dependent rats. Brain Res. 2000;854:85–92. doi: 10.1016/s0006-8993(99)02288-x. [DOI] [PubMed] [Google Scholar]
- Weitlauf C, Egli RE, Grueter BA, Winder DG. High-frequency stimulation induces ethanol-sensitive long-term potentiation at glutamatergic synapses in the dorsolateral bed nucleus of the stria terminalis. J Neurosci. 2004;24:5741–5747. doi: 10.1523/JNEUROSCI.1181-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf ME. Addiction: making the connection between behavioral changes and neuronal plasticity in specific pathways. Mol Interv. 2002;2:146–157. doi: 10.1124/mi.2.3.146. [DOI] [PubMed] [Google Scholar]
- Wolf ME, Mangiavacchi S, Sun X. Mechanisms by which dopamine receptors may influence synaptic plasticity. Ann N Y Acad Sci. 2003;1003:241–249. doi: 10.1196/annals.1300.015. [DOI] [PubMed] [Google Scholar]