Abstract
Objective
This study utilized a combination of HPV self-sampling, iFTA elute specimen cards, and long distance transport for centralized processing of specimens to determine the feasibility of large-scale screening in remote and transient populations.
Methods
This study was performed in two locations in Peru (Manchay and Iquitos). The “just for me” cervico-vaginal brush and iFTA elute cards were used for collection and transport of specimens. Samples were shipped via FedEx to China and tested for 14 types of high-risk HPV using PCR based MALDI-TOF. HPV positive women were treated with cryotherapy after VIA triage, and followed-up with colposcopy, biopsy, ECC, and repeat HPV testing at 6 months.
Results
Six hundred and forty three women registered, and 632 returned a sample over a 10 day period. Within 2 weeks, specimens were shipped, samples tested, and results received by study staff. Sixty-eight women (10.8%) tested positive, and these results were delivered over 4 days. Fifty-nine HPV positive women (87%) returned for evaluation and treatment, and 2 had large lesions not suitable for cryotherapy. At 6 months, 42 women (74%) returned for follow-up, and 3 had CIN 2 (all positive samples from the endocervical canal). Ninety eight percent of participants reported that they would participate in this type of program again.
Conclusions
Utilizing HPV self-sampling, solid media specimen cards for long distance transport, and centralized high throughput processing, we achieved rapid delivery of results, high satisfaction levels, and low loss to follow-up for cervical cancer screening in remote and transient populations.
I. INTRO
World-wide, cervical cancer is the third most common cancer in women, accounting for 13% of women’s cancers in the developing world. There were 275,000 deaths from cervical cancer in 2008, and more than 85% of these deaths occurred in developing countries [1]. In Peru, the incidence of cervical cancer is in GLOBOCAN’s highest category (34.5/100,000), and the disease has an extremely high mortality rate (16.3/100,000) [2].
Cervical cancer is the only human cancer for which we know the necessary cause [3] and which can be prevented; however, in the developing world women often do not have access to life-saving screening techniques to prevent advanced stage disease. The public health application of some newer technologies can improve our ability to reach remote populations most in need of screening, and leads to far superior prevention strategies when compared to simpler forms of screening. Denny et al. in South Africa showed that high-risk human papillomavirus (HR-HPV) testing followed by cryotherapy proved to be a highly effective screen-and-treat strategy, with significant reduction of cervical intraepithelial neoplasia grade 2 or worse (CIN2+) when compared to visual inspection with ascetic acid (VIA) [4,5]. In addition, this study showed that the addition of colposcopy actually decreased the effectiveness of the algorithm. The negative predictive value of HR-HPV testing affords the advantage of a longer safe screening interval [6, 7, 8], and combined with self-sampling eliminates some critical obstacles, such as the need for medical facilities and personnel, as well as providing convenience and acceptability for women [9, 10, 11].
Over the past 2 decades, there have been significant advances related to self-sampling technology. One of the greatest advances is the demonstration that by utilizing PCR technology, a self-collected sample tested for the presence of HR-HPV can acheive a sensitivity equal to that of a physician-collected direct endocervical sample (94+ %) [5, 12]. Additionally, the processing of samples with high through-put (4500/day) allows for more rapid delivery of results at a cost per case applicable to public health programs [12]; furthermore, the development of, solid media specimen cards (iFTA elute, GE Healthcare Piscataway, N.J.) have demonstrated reliability for transportation of HPV specimens for PCR analysis, eliminating the need for careful handling of liquid-based specimens [13]. Combining all of these technologies may achieve transport over long distances with rapid return of results, which can facilitate effective cervical cancer screening of isolated populations.
We studied this combination of technologies in 2 sequential screening protocols in Peru, where cervical cancer screening has not reached a significant percentage of the population. One site was a shantytown outside of Lima called Manchay, and the other, Iquitos, is a city with several surrounding remote villages in the Amazonian rainforest. Both studies were conducted as mother-child screen, treat, and vaccinate prevention studies grounded in Community Based Participatory Research (CBPR). The results of the CBPR strategies and our progressive development of a preventive healthcare model are reported individually for Manchay and Iquitos in 2 separate manuscripts. The present manuscript focuses specifically on the medical interventions applied in these studies. To our knowledge this is the first clinical example using a combination of HPV self-collection and solid media specimen transport cards to achieve real-time long distance transport of specimens in difficult to reach populations.
II. METHODS
International Review Board approval for the studies was achieved through the Instituto Nacional de Enfermedades Neoplásticas (INEN) 1, the Peruvian NIH, and the Cleveland Clinic. The projects were registered with the US National Institutes of Health as number NCT01338051. Funding for the projects was obtained through the Merck Inc. “Investigator Initiated Studies Program”, Preventive Oncology International, and the NIH Fogarty Fellowship Program.
CBPR concepts were used to determine strategies for advertising, recruitment, distribution and collection of specimens as well as reporting of results. The ultimate goal of the model was to allow the community to manage the screening program and identify the individuals at risk involving the healthcare system for those who screened positive for HPV. The inclusion and exclusion criteria for participants can be seen in Figure 1.
Figure 1.
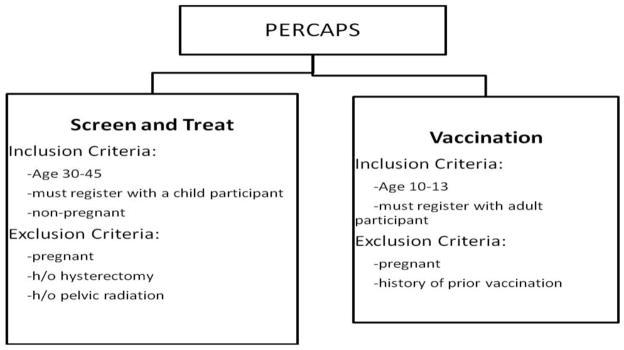
Exclusion and Inclusion criteria for the study
Screening: HPV Self-Sampling, Solid Media, and Long Distance Transport
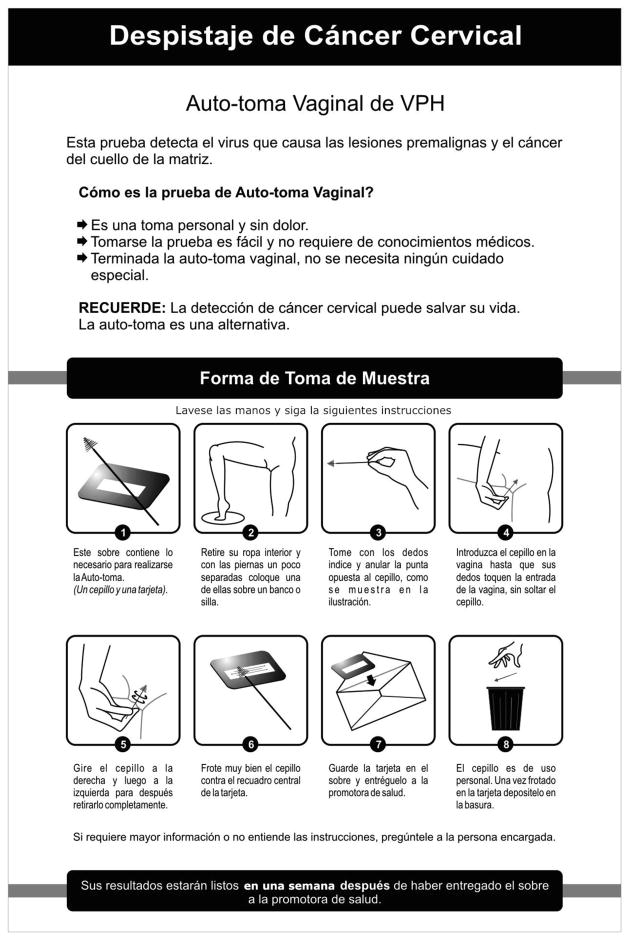
The self-sampling technology used for these studies included a simple nylon brush specifically designed for self-collecting a cervico-vaginal sample (“Just for Me” Preventive Oncology International Inc., Cleveland Heights, Ohio, USA), and a specimen card (iFTA-Elute, GE Healthcare, Piscataway N.J.) to transport the specimen. The brush, specimen card and simple illustrated instructions were delivered to all of the participants by the community health workers (CHW). Each filter paper card was labeled with an identification number unique to each subject (Figure 2). In addition to the illustrated instruction sheet, the CHWs also contained written instructions for collecting the sample. The brushes were discarded after sampling, and filter-paper cards were either collected by the CHWs or delivered by participants to the CHWs at a central location. Each sample was recorded on a de-identified Excel spreadsheet, which was printed out and shipped by air with all the samples in a group to Hong Kong then to BGI2 Shenzhen, in Shenzhen China. There, samples were tested for 14 types of high-risk HPV (16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, 68) with a PCR based multiplex high-risk HPV assay developed by BGI, integrated with the mass spectrometry system MALDI-TOF (Mass Array Matrix-Assisted Laser Desorption/Ionization Time-Of-Flight)3 [14, 15]. Results were sent to the POI epidemiology and bio-statistical center in Chicago and then forwarded to a research team member in Peru, who matched results to participants and created written documents for the CHWs to deliver to the women.
Figure 2.
HPV Self-Sampling Instruction Sheet
Treatment and Follow-up
Women testing HR- HPV positive were evaluated and treated at health clinics in each region a few days after HPV results were distributed. The women were triaged by unaided visual inspection with acetic acid (VIA) to rule out large pre-cancerous lesions (unable to be covered by the 1.9cm cryotherapy probe) or cancer. Women who qualified for immediate treatment were treated with cryotherapy using a Medi-Gyn cryotherapy unit with a 1.9cm/3mm nipple tip. The treatment protocol was 3 minute freeze – 5 minute thaw – 3 minute freeze. Patients received verbal and written post treatment instructions from the research team. Follow-up for all women treated with cryotherapy was planned at 6 months, at which time the women underwent colposcopy, biopsy, endocervical curettage, and repeat HPV testing (in this case a direct endocervical sample assayed using the PCR based MALDI-TOF).
Analyses
While much of the analysis for this study is descriptive in nature, all quantitative analysis was performed using STATA 10.0 software (College Station, TX). Pearson chi-square analysis and Fisher exact tests were performed for goodness of fit and association amongst HR-HPV groups.
III. RESULTS
A total of 643 women were registered. The average age of all participants was 37 in Manchay and 36 in Iquitos, and the average parity was 3. Neither of these demographics differed significantly between HR-HPV+ and − groups or between the two sites. Approximately 58% of participants from Manchay had a Pap smear in the past 5 years and had received a report of the results, compared with only 29% in Iquitos. While there was no statistically significant difference between HR-HPV+ and HR-HPV − groups, there was a trend towards higher likelihood of recent Pap smear among HR-HPV − participants (60% vs. 46% in Manchay). In Iquitos, there was also a non- statistically significant trend towards higher likelihood of recent Pap smear among HR-HPV −, percentages were 46 and 37 percent respectively [Table 1].
Table 1.
Demographics
| MANCHAY | IQUITOS | MANCHAY AND IQUITOS | |
|---|---|---|---|
| Average Age | 37 | 36 | 36 |
| Average Parity | 3 | 3 | 3 |
| Pap smear in past 5 years | |||
| HR-HPV + | 46% | 28% | 37% |
| HR-HPV − | 60% | 29% | 46% |
| Total | 58% | 29% | 44% |
Screening Technology: HPV Self-Sampling, Solid Media, Long Distance Transport
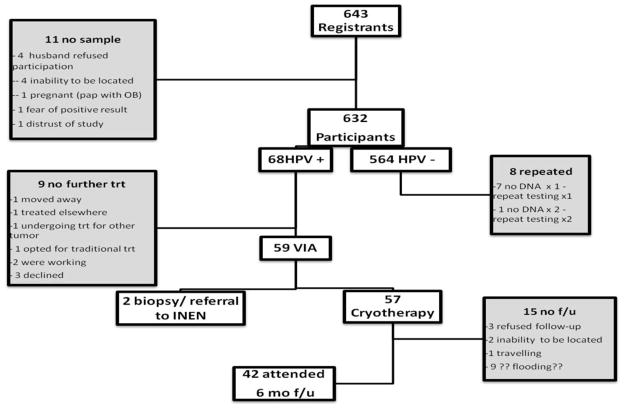
Six-hundred and thirty two (98.3%) of the women who registered for the study returned a self-sample to the CHW. Five-hundred and eighty-six (92.7%) of these women were subsequently interviewed, and 68 (11.6%) reported some discomfort or pain with insertion. Five patients (less than 1%) reported difficulty obtaining the sample, and 1 patient reported nausea. Four-hundred and seventy-seven patients (81.4%) reported that they would prefer to do a self-sample than have an examination performed in the clinic, 36 (6.1%) reported that they did not have a preference, and 107 (16.9%) preferred to have a sample taken in the clinic setting. Of those women who did not return a sample, reasons included: 4 were unable to be located after registering, 6 subsequently refused to participate, and 1 became pregnant and received a Pap smear from her obstetrician (Figure 3).
Figure 3.
Overall participation flowsheet for both Manchay and Iquitos
In Manchay, specimen cards with self-collection specimens were collected quickly, over a 2-day period, while in Iquitos, samples were collected over a 10-day period due to more difficult geographic limitations. CHWs transported these cards to the research team and all samples were immediately mailed via FedEx to China for PCR analysis in accordance with the study protocol. Within 2 weeks in Iquitos and 1 week in Manchay, the results were received by study staff in Peru and subsequently delivered to participants over a 4 day period. Therefore, the time span between screening for HR-HPV and delivery of results was no more than 3 weeks in either location. Actual laboratory processing for each site’s cases took only one day but depended on the queue of research projects at the BGI laboratory.
Significantly, upon receipt of results, it became apparent that eight of the filter-paper cards did not have sufficient DNA to test the sample for HR-HPV (these cases were all in Manchay). Therefore these samples were all repeated. Upon repeat, 7 out of the 8 specimens had sufficient DNA while 1 again required repeat testing. The third sample from the participant was obtained in the presence of a member of the research staff and this sample resulted in sufficient DNA for HPV testing.
During the study evaluation, both HPV positive and HPV negative participants reported high levels of study satisfaction, with 574 out of 585 women who were interviewed (98.0%) reporting that they would participate in this type of program in the future.
HPV results and management
Out of the 632 specimens collected, 68 (10.8%) tested positive for HR-HPV [Figure 1]. A total of 19 (27.9%) women had either HPV 16 (14 women) or HPV 18 (5 women). HPV 16 was the most prevalent type in both communities.
Fifty-nine (86.8%) of 68 women who tested positive for HR-HPV returned for evaluation and treatment. Upon VIA triage (utilized for the purpose of excluding large lesions), 2 women were believed to have a large pre-cancerous or cancerous lesion not suitable for cryotherapy, and were referred to INEN. One was diagnosed with invasive cervical cancer and completed radiation, chemotherapy and brachytherapy at INEN. After some difficulty re-locating the other patient, she was found to have CIN3 on cone biopsy. Fifty-seven (57/59) women therefore underwent treatment with immediate cryotherapy.
Approximately 6 months after the initial treatment, all participants who received cryotherapy were re-contacted, and 42 women (73.7%) returned for further evaluation (samples were obtained for 41 women as 1 woman was pregnant). Twenty-six out of 41 women (63.4%) who had repeat HPV testing at 6 months were found to be negative for HR-HPV, 5 women (12.2%) were found to have a different HPV type on follow-up, and 10 (24.4%) women had the same type of HPV that was initially detected. Three of these women (7.1%) were also found to have CIN2+; all 3 of these cases were in the endocervical canal (found on ECC), had type specific persistence, and all were p16 positive on immune histo-chemistry.
DISCUSSION
Despite our knowledge of the pathogenesis of cervical cancer and techniques for managing pre-cursor lesions, cervical cancer continues to cause significant morbidity and mortality in the developing world. While advances in screening technologies continue to improve our ability to detect precursor lesions, we are just beginning to see efforts to implement these technologies in the populations most in need, a necessary step for reducing cervical cancer prevalence in large populations [16]. To help reach this goal, we have studied the effectiveness of a screening protocol that combines self-sampling for HR-HPV, solid media specimen transport cards, and centralized high through-put processing that can achieve high sensitivity with good quality control. Our findings suggest that by instituting this protocol, we can rapidly screen remote populations of women at high risk for cervical cancer.
HPV testing has been shown to provide superior sensitivity and negative predictive value compared to VIA and cytology based screening. Self-sampling is convenient and highly accepted by most women with minimal disruption to their daily lives [9, 17, 18, 19, 20]. The use of iFTA-elute cards provides several key advantages for self-screening; 1) The cards indicate to both the women and to the laboratory that the sample is on the paper by a color change; 2) The cells are lysed and the DNA is stabilized creating a non-infectious sample; 3) A punch of the card, heated in water, allows elution of the DNA which eliminates the need for extraction prior to PCR; and 4) The absence of liquids (other transport media are often alcohol based) is a safety factor for the women who are unlikely to be familiar with the proper handling of such solutions. International transport of DNA samples on iFTA-elute cards has been safely done for years with specimens transferred to centralized testing facilities [21]. Our samples were integrated into the daily research work of the MALDI-TOF technology at BGI. Once the specimens arrived in the laboratory from Peru (3 days), BGI was easily able to meet whatever time requirement we requested. Results were, therefore, able to be delivered to women quickly, which is a critical element of screening in at risk populations. Quick turnaround allows the screening event to be fresh in the participants’ minds when results are available; because at-risk populations are often transient, rapid delivery of results is vital to both high rates of satisfaction and low loss to follow-up.
Our study shows that by integrating self-sampling for HPV, iFTA elute for specimen transport (long distance if needed) and centralized testing, we can effectively achieve comprehensive screening for cervical cancer with rapid delivery of results. However, there are limitations to consider. First, the sample sizes for each study site were small (approximately 330 women each in Manchay and Iquitos), which does not accurately reflect the goals of large-scale screening. However, all aspects of this protocol can easily be scaled up. This includes the community based screening events as well as the assay technology, which allows one machine to process up to 4500 samples per day. These studies represent considerable progress towards the final design of a community based screening model for large populations, with the major rate limiting factor being the ability of the healthcare system to effectively manage the positives.
While CBPR also played a critical role in the study’s success, the documentation of the feasibility of combining the technologies with long distance transport is independently significant. We fully recognize the need to combine this protocol with effective community based delivery of the screening technologies, as well as having an evaluation and treatment plan capable of managing the HR-HPV positive women in a manner appropriate for a public health program. While each element of a public health strategy is critical, it is the combination of these elements that can lead to successful cervical cancer control.
Although this manuscript focuses on screening technology, treatment is a critical aspect of any prevention program and therefore must be considered in program evaluation. While the efficacy of treatment with cryotherapy is well-established in the literature [8], few studies have focused on the clearance of HPV by cryotherapy. In one study by Davidov et al, 62% of patients who underwent cryotherapy were found to be HPV negative following treatment [22]. Similarly, Aerssens et al. examined HPV detected by polymerase chain reaction (PCR) (as used in our study) after cryotherapy, and found that 65.4% of patients cleared the HPV. Both of these results are consistent with our finding of 63.4% clearance. Of those who were HPV positive, more women in our studies were found to have the same type of HPV (24.4% vs. 11% in the Aerssens study) rather than a different type of HPV (12.2% vs. 23.6% in the Aerssens study). While the cause for these differences is unclear, persistence and/or reinfection were consistent overall. Importantly, Aerssens et al. also demonstrated, via extended follow-up, that HPV infection continues to decline even 2 years after cryotherapy treatment [23]. While there were 3 women in our studies who were found to have CIN2+ after cryotherapy, all 3 cases were in the endocervical canal, where cryotherapy cannot reach. Therefore, the findings from our study support previously published data that show the efficacy of this treatment strategy. Of note, it is unclear how the assay for HPV impacts the findings of persistent infection since PCR based methods have a much lower threshold for detection and therefore may detect clinically irrelevant presence of the virus [24].
To our knowledge, this is the first study that combines a community-based model with highly efficacious technology, long distance transport, and rapid delivery of results. These elements allowed for high levels of satisfaction and minimal loss to follow-up. We continue to work to refine our community-based model and its training work-shop, as well as to study treatment alternatives easily applied in the local community settings (often quite rural).
Highlights.
Combining HPV self-sampling, FTA cards, transport, and central processing is useful
This combination allows for efficient screening in remote and transient populations
Rapid result delivery, high satisfaction, and low loss to follow-up were achieved
Acknowledgments
Funding:
Preventive Oncology International
Supported in part by a research grant from the Investigator-Initiated Studies Program of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc. The opinions expressed in this paper are those of the authors and do not necessarily represent those of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc.
This work was supported by the National Institutes of Health Office of the Director, Fogarty International Center, Office of AIDS Research, National Cancer Center, National Eye Institute, National Heart, Blood, and Lung Institute, National Institute of Dental and Craniofacial Research, National Institute on Drug Abuse, National Institute of Mental Health, National Institute of Allergy and Infectious Diseases, and National Institutes of Health Office of Women’s Health and Research through the Fogarty International Clinical Research Scholars and Fellows Program at Vanderbilt University (R24 TW007988) and the American Relief and Recovery Act.
Footnotes
English Translation : National Institute of Neoplastic Diseases
Originally named The Beijing Genomic Institute, BGI is the largest sequencing center in the world
This assay uses a method of mass spectrometry for multiple polymerase chain reaction (PCR), with several primers of HPV (GPR5+/6+), targeting specific bp to the L1 region of the viral genome of 14 HPV types, followed by amplification with specific primer for each genotype.
Conflict of Interest Statement:
Preventive Oncology International has received support in kind (reagents and testing) and/or funds for direct support and research from Hologic Inc., Qiagen, Gen-Probe, Merck Inc., BGI Shenzhen, and GE Healthcare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Kimberly L. Levinson, Email: kimlynnlev@gmail.com.
Carolina Abuelo, Email: princesa_carolina@yahoo.com.
Jorge Salmeron, Email: jsalme@prodigy.net.mx.
Eunice Chyung, Email: eunice.chyung@gmail.com.
Jing Zou, Email: zoujing@genomics.cn.
Suzanne E Belinson, Email: seb@poiinc.org.
Guixiang Wang, Email: wgx828@yahoo.com.
Carlos (Santos Ortiz), Email: c_santos_o@yahoo.com.
Carlos Santos Vallejos, Email: cvallejos@oncosalud.pe.
Jerome L. Belinson, Email: jlb@poiinc.org.
References
- 1.Globocan 2008 Cancer Fact Sheet. Globocan 2008 (IARC) [cited Nov 19, 2011]. Available at: http://globocan.iarc.fr/factsheets/cancers/cervix.asp.
- 2.Globocan 2008 Fast Stats: Peru. Globocan 2008 (IARC) [cited Nov 19, 2011]. Available at: http://globocan.iarc.fr/factsheets/populations/factsheet.asp?uno=604#WOMEN.
- 3.Walboomers JM, et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol. 1999;189(1):12–9. doi: 10.1002/(SICI)1096-9896(199909)189:1<12::AID-PATH431>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 4.Denny L, Kuhn L, Hy C, Tsai W, Wright TC. Human Papillomavirus-Based Cervical Cancer Prevention: Long-term Results of a Randomized Screening Trial. JNCI. 2010;102:1557–1567. doi: 10.1093/jnci/djq342. [DOI] [PubMed] [Google Scholar]
- 5.Zhao F, Lewkowitz AK, Chen F, Lin M, Hu S, Zhang X, et al. Pooled Analysis of a Self-Sampling HPV DNA Test as a Cervical Cancer Primary Screening Method. JNCI. 2012;104(3):178–88. doi: 10.1093/jnci/djr532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sherman ME, Lorincz AT, Scott DR, et al. Baseline cytology, human papillomavirus testing, and risk for cervical neoplasia: a 10-year cohort analysis. JNCI. 2003;95(1):46–52. doi: 10.1093/jnci/95.1.46. [DOI] [PubMed] [Google Scholar]
- 7.Shi JF, Belinson JL, Zhao FH, et al. Human papillomavirus testing for cervical cancer screening: results from a 6-year prospective study in rural China. Am J Epidemiol. 2009;170(6):708–16. doi: 10.1093/aje/kwp188. [DOI] [PubMed] [Google Scholar]
- 8.Dillner J, Rebolj M, Birembaut P, et al. Long term predictive values of cytology and human papillomavirus testing in cervical cancer screening: jounr European cohort study. BMJ. 2008;337:a1754. doi: 10.1136/bmj.a1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arriba LN, Enerson CL, Belinson S, Loyd N, Belinson J. Mexican Cervical Cancer Screening Study II: Acceptability of Human Papillomavirus Self-Sampler. IJGC. 2010;20(8):1415–23. doi: 10.1111/IGC.0b013e3181f58678. [DOI] [PubMed] [Google Scholar]
- 10.Barvee L, Kobetz E, Menard J, Cook N, Blanco J, Barton B, Auguste P, McKenzie N. Assessing the acceptability of self-sampling for HPV among Haitian immigrant women: CBPR in action. Cancer Causes Control. 2010;21:421–31. doi: 10.1007/s10552-009-9474-0. [DOI] [PubMed] [Google Scholar]
- 11.Belinson JL, Du H, Wang GP, Wu RS, Zhang W, Liu Y, Belinson SE, Yang B, Huang QX, Wu RF. Evaluation of the POI/NIH cervico-vaginal self-sampler for HPV from the SHENCCAST II trial. Abstract - Presented AOGIN; March 2010; Delhi, India. [Google Scholar]
- 12.Belinson JL, Du H, Yang B, Wu R, Belinson SE, Qu X, Pretorius RG, Yi X, Castle PE. Imrpoved sensitivity of vaginal self-collection and high-risk human papillomavirus testing. Int J Cancer. 2011;130:1855–60. doi: 10.1002/ijc.26202. [DOI] [PubMed] [Google Scholar]
- 13.Gustavsson I, Sanner K, Lindell M, Strand A, Olovsson M, Wikstrom I, Wilander E, Gyllensten U. Type-Specific detection of high-risk human papillomavirus (hPV) in self-sampled cervicovaginal cells applied to FTA elute cartridge. Journal of Clinical Virology. 2011;51:251–54. doi: 10.1016/j.jcv.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 14.Yi X, Li J, Yu S, et al. A new PCR-based mass spectrometry system for high-risk HPV, part I: methods. Am J Clin Pathol. 2011;136 (6):913–9. doi: 10.1309/AJCPWTZDT0Q7DOVI. [DOI] [PubMed] [Google Scholar]
- 15.Du H, Yi J, Wu R, et al. A new PCR-based mass spectrometry system for high-risk HPV, part II: clinical trial. Am J Clin Pathol. 2011;136 (6):920–3. doi: 10.1309/AJCPJDAORUY4EYR6. [DOI] [PubMed] [Google Scholar]
- 16.Sankaranarayanan R, Nene BM, Shastri SS, et al. HPV screening for cervical cancer in rural India. N Engl J Med. 2009;360(14):1385–1394. doi: 10.1056/NEJMoa0808516. [DOI] [PubMed] [Google Scholar]
- 17.Anhang R, Nelson JA, Telerant R, Chiasson MA, Wright TC. Acceptability of self-collection of specimens for HPV DNA testing in an urban population. J Women Health. 2005;14:721–8. doi: 10.1089/jwh.2005.14.721. [DOI] [PubMed] [Google Scholar]
- 18.Dzuba IG, Díaz EY, Allen B, et al. The acceptability of self-collected samples for HPV testing vs. the Pap test as alternatives in cervical cancer screening. J Womens Health Gend Based Med. 2002 Apr;11(3):265–75. doi: 10.1089/152460902753668466. [DOI] [PubMed] [Google Scholar]
- 19.Mitchell S, Ogilvie G, Steinberg M, et al. Assessing women’s willingness to collect their own cervical samples for HPV testing as part of the ASPIRE cervical cancer screening project in Uganda. Int J Gynaecol Obstet. 2011 Aug;114(2):111–5. doi: 10.1016/j.ijgo.2011.01.028. Epub 2011 Jun 12. [DOI] [PubMed] [Google Scholar]
- 20.Tisci SM, Shen YHM, Fife DR, et al. Patient acceptance of self-sampling for human papillomavirus in rural China. J Low Genit Tract Dis. 2003;7:107–16. doi: 10.1097/00128360-200304000-00007. [DOI] [PubMed] [Google Scholar]
- 21.Beck IA, Drennan KD, Melvin AJ, et al. Simple, sensitive, and specific detection of human immunodeficiency virus type 1 subtype B DNA in dried blood samples for diagnosis in infants in the field. J Clin Microbiol. 2001;39(1):29–33. doi: 10.1128/JCM.39.1.29-33.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Davidov A, Rajupet SR, Maiman MD, et al. LEEP versus Cryotherapy: Rates of HPV detection after treatment for CIN II/III. Supplement to J Lower Gen Tract Dis (ASCCP Abstracts) 2012 Apr;:S7. [Google Scholar]
- 23.Aerssens A, Claeys P, Garcia A, et al. Natural history and clearance of HPB after treatment of precancerous cervical lesions. Histopathology. 2008;52:381–386. doi: 10.1111/j.1365-2559.2007.02956.x. [DOI] [PubMed] [Google Scholar]
- 24.Snijders PJ, van den Brule AJ, Meijer CJ. The clinical relevance of human papillomavirus testing: relationship between analytical and clinical sensitivity. J Pathol. 2003;201(1):1–6. doi: 10.1002/path.1433. [DOI] [PubMed] [Google Scholar]