Abstract
Vesicular monoamine transporter type 2 (VMAT2) is a newly emerging target for both diagnostic and therapeutic applications in diabetes mellitus. In pursuit of novel VMAT2 antagonists, we identified a potent hypoglycemic agent with a novel dihydropyridone scaffold. Several analogs were designed and synthesized. A preliminary structure activity relationship (SARs) showed that the dihydropyridone scaffold is required for the activity.
Keywords: VMAT2, hypoglycemic, SARs, dihydropyridone
Diabetes mellitus is a growing epidemic affecting hundreds of millions worldwide.1 Despite a recent explosion of new classes of hypoglycemic agents, the medical need remains largely unmet and innovative diagnostics and therapeutics are still urgently needed. We have been particularly interested in the vesicular monoamine transporter type 2 (VMAT2) as a potential diagnostic and therapeutic target for diabetes.
VMAT is a member of the vesicular transporter family responsible for the uptake and secretion of monoamine neurotransmitters in neurons and endocrine cells.2 Two isoforms of VMAT (type 1 and 2) have been cloned, and interestingly, the insulin-producing beta cells in the pancreas only express the VMAT2 isoform.3 We recently have demonstrated the feasibility of noninvasive measurement of beta cell mass both in humans and rodents by positron emission tomography (PET) using VMAT2 as the biomarker and its specific antagonist dihydrotetrabenazine (DTBZ) as the tracer.4,5 More strikingly, our studies have further shown that VMAT2 plays an important functional role in the regulation of insulin secretion in beta cells.6 VMAT2 antagonist tetrabenazine (TBZ) and its active metabolite DTBZ (Figure 1) are potent hypoglycemic agents that stimulate insulin secretion in vitro and improve glucose tolerance in normal and diabetic rats.6 VMAT2 antagonists, therefore, have both diagnostic and therapeutic potential in the management of diabetes mellitus.
Figure 1.

The structure of TBZ, DTBZ and compound 1
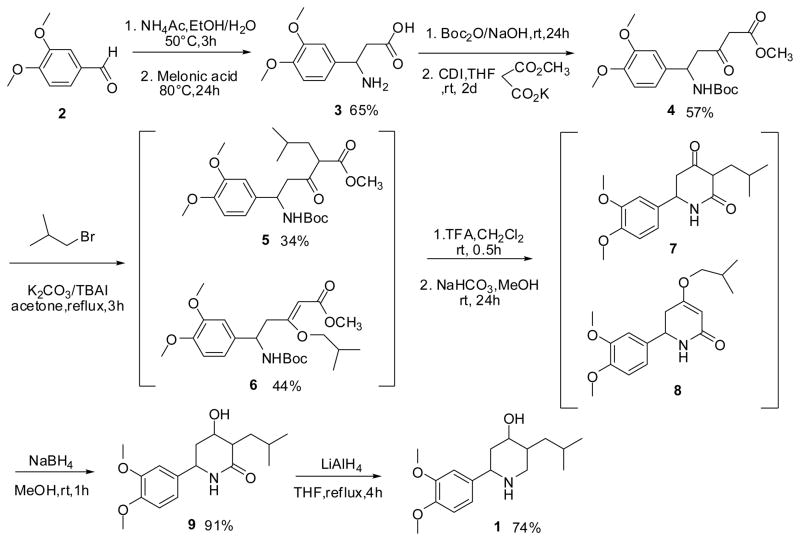
In an effort to generate novel VMAT2 antagonists, we attempted to synthesize compound 1(Figure 1), a simplified analog of DTBZ. As shown in Scheme 1, veratraldehyde 2 was treated with ammonium acetate and converted into β-amino acid 3 by condensation with malonic acid. Protection with Boc anhydride and subsequent condensation with potassium malonate methyl ester led to β-keto ester 4. Alkylation with isobutyl bromide in the presence of potassium carbonate afforded a mixture of 5 and 6 in moderate yield. After removal of the Boc group, the mixture was treated with sodium bicarbonate in methanol to yield the cyclized products 7 and 8 quantitatively. Compound 9 were obtained from reduction of 7 with sodium borohydride and then further converted to 1 and its diasteroisomers with lithium aluminum hydride.
Scheme 1.
The synthesis of Compound 1
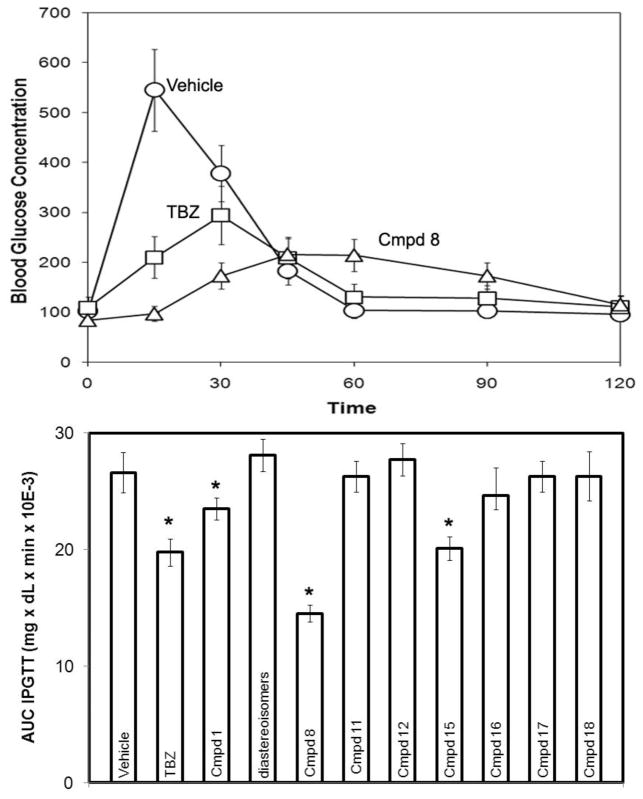
Racemic compound 1 and its diastereoisomers were evaluated for their ability to improve glucose tolerance by intraperitoneal glucose tolerance tests (IPGTT)7 in rats. The new analogs were less potent than TBZ possibly due to diminished affinity for VMAT2 (Figure 2). This poor result halted our further study of compound 1. However, during random screens of intermediates generated in the course of the synthesis of 1, we found that compound 8, a novel dihydropyridone resulted from the competing O-versus C-alkylation of enolic β-keto ester 4 followed by cyclization, showed potent hypoglycemic effect. As illustrated in Figure 2, compound 8 decreased by 45% the AUC IPGTT (the area under the blood glucose concentration-time curve) at the dose of 2mg/kg compared to 26% for TBZ.
Figure 2.
Glucose tolerance tests of novel hypoglycemic compounds. 6h fasted Lewis rats were administered the drugs intravenously (−30min, 2mg/kg) followed by intraperitoneal glucose injection (0min, 2g/kg), and blood glucose levels were monitored for 120mins. Top panel: The blood glucose versus time curves are presented for three repeat experiments with Vehicle, TBZ and Compound 8 performed in a single rodent. Bottom Panel: Results are presented as area under the curve. The area under the curve (AUC IPGTT) was calculated by the trapezoidal rule. Error bars indicate SEM (n=5). The statistical significance of the difference between drug and vehicle was calculated by the method of Student. A p value of less than 0.005 is shown by an asterisk. The p value of the difference in between average AUC IPGTT of TBZ and Compound 8 was 0.030.
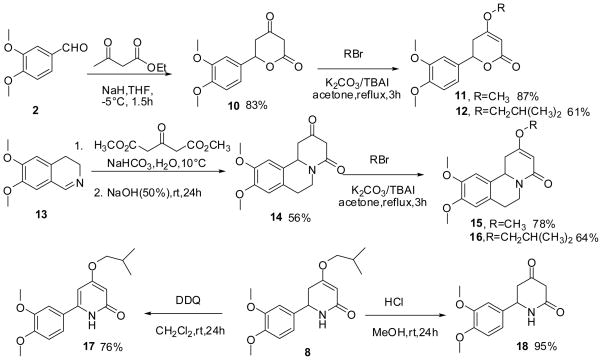
Prompted by this surprising result, we designed and synthesized several analogs of 88. As outlined in Scheme 2, veratraldehyde 2 was first condensed with ethyl acetoacetate and spontaneous cyclization yielded lactone 10. Using potassium carbonate as the base, O-alkylation of 10 with methyl bromide or isobutyl bromide provided 11 and 12. Similarly, 15 and 16 were prepared from dihydroisoquinoline 13 via condensation with dimethyl 1,3-acetonedicarboxylate followed by cyclization and alkylation. DDQ-induced aromatization and acidic hydrolysis of 8 afforded 17 and 18 respectively. Analogs prepared as above were tested for their hypoglycemic activities in rats using the IPGTT protocol. Interestingly, the hypoglycemic effects of these compounds were only seen following glucose stimulation. Results in Figure 2 demonstrated that the dihydropyridone scaffold in 8 is essential to the hypoglycemic activity. Replacement with dihydropyrone (11, 12), oxidation or hydrolysis of 8 (17, 18) resulted in total loss of activities. Interestingly, the rigid derivatives 15 and 16 were active but less potent in opposition to the SAR trend seen in compound 1 and DTBZ.
Scheme 2.
The synthesis of analogs of compound 8
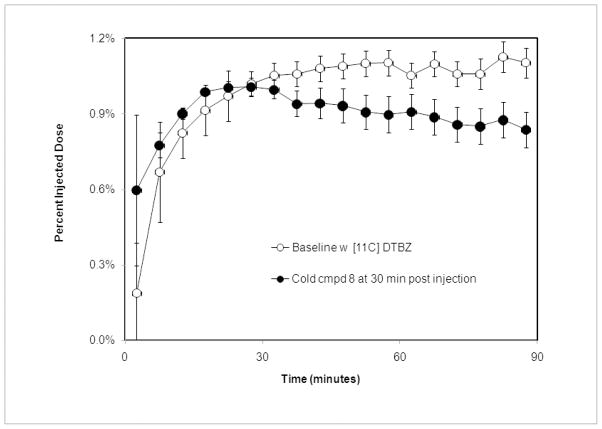
To test whether the strong hypoglycemic effect of 8 is conferred by binding to VMAT2 in beta cells, we performed a PET study in rats.9 The animals were treated with radiolabeled DTBZ and the uptake of [11C] DTBZ in pancreas was monitored by PET scan in the presence of excess of 8. Contrary to our expectation, 8 did not significantly displace [11C]DTBZ in the endocrine pancreas (Figure 3), suggesting a weak binding of 8 to VMAT2 relative to DTBZ. VMAT2 is known to possess multiple binding sites.10 Our results don’t rule out the possibility that 8 binds to VMAT2 at different binding site. We have previously shown that TBZ and DTBZ act directly on beta cells of the endocrine pancreas to increase insulin secretion following glucose challenge 6, and give rise to a hypoglycemic activity, but due to abundant expression of VMAT2 in brain, these compounds also cause central nervous system side effects. Interestingly, we observed that the treatment with 8 (at 2 mg/Kg) was devoid of tonic seizure commonly associated with equal doses of TBZ or DTBZ, supporting that compound 8 works through a different mechanism. Another possibility consistent with our observations is that 8 may act as a competitive inhibitor of dopamine at VMAT2 on beta cell vesicles. DTBZ and its analogs are structurally similar to a class of quinolizine alkaloids previously shown to inhibit dipeptidyl peptidase IV (DPP-IV),11 but 8 tested negative against DPP-IV in vitro at concentrations up to 10μM (data not shown).12 Therefore the mechanism underlying the anti-hyperglycemic effect of 8 remains to be determined.
Figure 3.
Effect of post injection of compound 8 on biodistribution of [11C]DTBZ in pancreas. PET scans were performed on anesthetized 12–14 week old Lewis male rats injected with radioligand [11C]DTBZ (0 min, 0.5–1.0μCi/gm body weight, specific activity > 2000 mCi/mole) and cold compound 8(30mins, 2mg/kg). The fraction of radioligand in pancreas relative to the total amount of injected was calculated and plotted versus time after injection. Error bars indicate SEM (n=3). The area under the curve (AUC) from thirty minutes to ninety was calculated by the trapezoidal rule for each experiment. The statistical significance of difference between the average AUC baseline and AUC Compound 3 was p >0.05 as calculated by the method of Student.
In summary, we have completed the synthesis of a novel analog of VMAT2 antagonist DTBZ (Compound 1). Despite the disappointing results of 1, a highly potent hypoglycemic agent (compound 8) was serendipitously discovered. Subsequent modification revealed the requirement of a novel dihydropyridone core structure for the activity. Mechanism studies show that compound 8 may not work through VMAT2 and DPP-IV. More detailed studies of 8 are currently being pursued.
Acknowledgments
This work was supported by grants from the PHS, NIH, NIDDK 2 RO1 DK63567-05 (PEH) and the Columbia University DERC (5 P30 DK063608-05).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and Notes
- 1.Zimmet PZ, Alberti KGMM, Shaw J. Nature. 2001;414:782. doi: 10.1038/414782a. [DOI] [PubMed] [Google Scholar]
- 2.Zheng G, Dwoskin LP, Crooks PA. AAPS J. 2006;8(4):689. doi: 10.1208/aapsj080478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anlauf M, eissele R, Schafer MK. J Histochem Cytochem. 2003;51:1027. doi: 10.1177/002215540305100806. [DOI] [PubMed] [Google Scholar]
- 4.Souza F, Simpson N, Raffo A, Saxena C, Maffei A, Hardy M, Kibourn M, Goland R, Leibel R, Mann JJ, Van heertum R, Harris PE. J Clin Invest. 2006;116(6):1506. doi: 10.1172/JCI27645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Murthy R, Harris PE, Simpson N, Van Heertum R, Leibel R, Mann JJ, Parsey R. Eur J Nucl Med Mol Imaging. 2008;35(4):790. doi: 10.1007/s00259-007-0648-2. [DOI] [PubMed] [Google Scholar]
- 6.Raffo A, Hancock K, Polito T, Xie Y, Andan G, Witkowski P, Hardy M, Barba P, Ferrara C, Maffei A, Freeby M, Goland R, Leibel RL, Sweet L, Harris PE. JOE. 2008;198:1. doi: 10.1677/JOE-07-0632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.All animal studies were reviewed and approved by the Institutional Animal Care and Use Committee (IACUC) at Columbia University’s College of Physicians and Surgeons. All experiments were performed in accordance with ‘Principles of laboratory animal care’ (NIH publication no. 85–23, revised 1985). IPGTT was performed as described in reference 5.
- 8.Analytical data for active compounds: 8: 1H NMR (300 MHz, CDCl3) δ 6.90-6.85 (m, 2H), 6.84 (s, 1H), 5.45 (br, 1H), 5.07(s, 1H), 4.69-4.63(dd, 1H), 3.87(s, 3H), 3.86(s, 3H), 3.64-3.60(m, 2H), 2.71-2.47 (m, 2H), 2.02-1.96 (m, 1H), 0.96-0.93 (dd, 6H); 13C NMR (75 MHz, CD3OD) δ 170.0, 168.9, 149.4, 149.1, 133.5, 119.0, 111.4, 109.3, 93.8, 75.1, 56.3, 56.2, 55.0, 37.3, 28.1, 19.5, 19.4; ESI-MS(M+ + H): 306.3. 15: 1H NMR (300 MHz, CDCl3) δ 6.58 (s, 1H), 6.54 (s, 1H), 5.15 (s, 1H), 4.71-4.65 (m, 2H), 3.80(s, 3H), 3.79(s, 3H), 3.67 (s, 3H), 2.81-2.60(m, 4H), 2.48-2.37 (m, 1H); 13C NMR (75 MHz, CD3OD) δ 168.3, 167.4, 148.0(2C), 127.4, 127.3, 111.3, 108.6, 94.6, 56.3, 56,2, 56.0, 54.1, 38.4, 37.2, 29.4; ESI-MS(M+ + H): 290.1.
- 9.PET study protocol: PET scans were performed on 12–14 week old Lewis Male rats. Prior to imaging, the animals were anesthetized by isoflurane inhalation. After a transmission scan of the area of interest had been acquired (used to perform attenuation correction of the emission data), the radioligand [11C]DTBZ was administered (0.5 – 1.0 μCi / gm) in saline as bolus injection via the penile vein. PET scans of the animals were acquired dynamically to 90 min postinjection on a Concorde microPET-R4 (CTI Molecular Imaging, Knoxville, TN, USA). The scanner provided a 100 x 80 mm field of view with a reconstructed resolution of 2.25 mm in the central 40 mm of the field of view. At thirty minutes post injection of [11C]DTBZ, animals received a second injection via the penile vein of cold analog. PET data were processed using attenuation correction matrix obtained by transmission scans and images were reconstructed using Fourier rebinning, followed by two-dimensional, filtered back projection using microPET manager software (CTI Molecular Imaging).
- 10.Scherman D, Henry JP. Mol Pharmacol. 1984;25:113–122. [PubMed] [Google Scholar]
- 11.Lubbers A, Bohringer M, Gobbi L, Henning M, Hunziker D, Kuhn B, Loffler B, Matei P, Narquizian R, Peters J, Ruff Y, Wessel HP, Wyss P. Biorg Med Chem Lett. 2007;17:2966. doi: 10.1016/j.bmcl.2007.03.072. [DOI] [PubMed] [Google Scholar]
- 12.The DPP-IV assay was provided by BPS Bioscience, Inc. (San diego, CA)