PEDIATRIC TBI GUIDELINES: HISTORY AND METHODOLOGY
In March 2000, the process ultimately culminating in the first edition of the Guidelines for Medical Management of Severe Traumatic Brain Injury for Infants, Children, and Adolescents (referred to as the Guidelines from this point forward) was initiated during the fifth Annual Aspen Neurobehavioral Conference. Participants in the conference from the Evidence-Based Practice Center (EPC) of Oregon Health and Science University agreed to form a panel of experts to develop guidelines for the medical management of children with severe traumatic brain injury (TBI).1-19 Using the previously completed guidelines developed for adult victims of TBI as a guide,20 the panel identified topics that are relevant to pediatric TBI needs; specifically, topics that are widely believed to be related to outcomes and TBI systems for children with severe injuries. A reference librarian searched Medline (1966–2001) for articles related to the various topics using an inclusive search strategy. Abstracts were reviewed by primary and secondary authors identified for each topic and then articles were reviewed for possible inclusion. The basic requirement for inclusion was the ability (1) to identify children with severe TBI and (2) to discern outcomes within the article. Data were classified into 3 categories of evidence: class I from randomized controlled trials; class II from clinical studies with prospective data collection; class III from retrospective data, case reports, and expert opinion. Altogether the information available for these first guidelines included data from virtually all sources, including all forms of pediatric studies, expert opinions, and even suggestions from adult studies. Ultimately, recommendations were made regarding the strength of the evidence: standards for accepted principles that “reflect a high degree of clinical certainty,” guidelines that “reflect a moderate clinical certainty,” and options that reflect an “unclear clinical certainty.”
The resulting articles were watershed works for pediatric neurotrauma and pediatric critical care as a whole, serving as a virtual template on issues that were related to the severely brain-injured child. As the first evidenced-based guideline for a pediatric critical care illness or syndrome, it was divided into sections regarding systems of care, thresholds for therapies, and specific treatments. The first 3 chapters focused on the system of caring for children with severe TBI, including the trauma systems, prehospital airway management, and resuscitation. The next 4 chapters focused on intracranial hypertension, including indications for implementing monitoring modalities, thresholds for treatments, technology related to the devices themselves, and management of cerebral perfusion pressure (CPP; a measure derived from the arithmetical difference between the mean arterial blood pressure and intracranial pressure [ICP]). The remaining chapters focused on therapies for children with severe TBI, including those for intracranial hypertension (cerebrospinal fluid drainage, hyperosmolar therapies, hyperventilation, barbiturates, decompressive surgery, temperature control, corticosteroids) and supportive care (sedatives/neuromuscular antagonists and nutrition). Finally, a suggested algorithm for management of acute intracranial hypertension was developed based on the expert opinions of the Committee. The overall tenor of this revolutionary document is a combination of a comprehensive review of the literature combined with expert opinions to act as a user’s guide for pediatric TBI. Within the various chapters, a wide variety of quality of evidence was shown (Table 1). Of note, there were no topics with sufficient evidence for the expert panel to define a standard, thus showing that the state of the literature is insufficient to compel clinicians toward various specific aspects of TBI care. Nevertheless, valuable information can be gleaned from the Guidelines and options that are outlined.
Table 1.
Summary of standards, guidelines, and options generated from the 2003 pediatric TBI guidelines
| Topic | Level of Evidence | Recommendation |
|---|---|---|
| Trauma systems, pediatric trauma centers, and the neurosurgeon | Guideline | “In a metropolitan area, pediatric patients with severe TBI should be transported directly to a pediatric trauma center, if available” |
| Option | “…should be treated in a pediatric trauma center or in an adult trauma center with added qualifications to treat children in preference to a level I or II adult trauma center without added qualifications…” | |
| Prehospital airway management | Guideline | “Hypoxia must be avoided … and attempts made to correct it immediately. Supplemental oxygen should be administered … no evidence to support an advantage of endotracheal intubation over bag-valve-mask ventilation…” |
| Option | “If prehospital endotracheal intubation is instituted…, then specialized training and use of end-tidal CO2 detectors is necessary” | |
| Resuscitation of blood pressure and oxygenation | Guideline | “Hypotension should be identified and corrected … defined as systolic blood pressure below fifth percentile for age or by clinical signs of shock…” |
| Option | “Airway control should be obtained in children with a GCS ≤8 to avoid hypoxemia, hypercarbia and aspiration … hypoxia should be identified and corrected … blood pressure should be monitored frequently and accurately…” | |
| Indications for intracranial pressure monitoring | Option | “ICP monitoring is appropriate … (GCS ≤8). The presence of an open fontanelle and/or sutures does not preclude the development of intracranial hypertension or negate the utility of ICP monitoring” |
| Threshold for treatment of intracranial hypertension | Option | “Treatment for intracranial hypertension … should begin at an ICP ≥20 mm Hg” |
| Intracranial pressure monitoring technology | Option | “…a ventricular catheter or an external strain gauge transducer or catheter tip pressure transducer device is an accurate and reliable method of monitoring ICP” |
| Cerebral perfusion pressure | Guideline | “A CPP >40 mm Hg … should be maintained” |
| Option | “A CPP between 40 and 65 mm Hg probably represents an age-related continuum for optimal treatment threshold … Hypotension should be avoided” | |
| Sedation and neuromuscular blockade | Option | “…the choice and dosing of sedatives, analgesics and neuromuscular blocking agents … should be left to the treating physician…” |
| Hyperosmolar therapies | Option | “Hypertonic saline is effective at control of increased ICP … Mannitol is effective for control of increased ICP…” |
| Hyperventilation | Option | “…Prophylactic hyperventilation … should be avoided. Mild hyperventilation (Paco2 30–35 mm Hg) may be considered for longer periods of intracranial hypertension … Aggressive hyperventilation (Paco2 <30 mm Hg) may be considered as a second tier option in the setting of refractory intracranial hypertension…” |
| Barbiturates | Option | “High-dose barbiturate therapy may be considered in hemodynamically stable patients with refractory intracranial hypertension … appropriate hemodynamic monitoring and cardiovascular support are essential…” |
| Temperature control | Option | “…Hyperthermia should be avoided…” |
| Surgical treatment of intracranial hypertension | Option | “Decompressive craniectomy should be considered….” |
| Corticosteroids | Guideline | “The use of steroids significantly reduces endogenous cortisol production … may have an associated increased risk of complications of infection” |
| Option | “The use of steroids is not recommended for improving outcome or reducing ICP…” | |
| Nutritional support | Option | “Replace 130%–160% of resting metabolism expenditure … nutritional support should begin by 72 h with full replacement by 7 d” |
A total of 5 guidelines were identified concerning trauma systems, hypoxia/airway management, hypotension, CPP thresholds, and corticosteroid usage. Based on a study by Johnson and Krishnamurthy,21 the benefits of a pediatric trauma center were demonstrated. In their prospective study of 225 children, children who were directly transferred to a Level 1 Pediatric Trauma Center had improved mortality compared with those who had been “indirectly” transferred (1.9% vs 4.7% mortality). This study, in concert with other supporting data from retrospective studies of patients from Pennsylvania, Oregon, and Washington,22,23 led to the guideline that children within urban centers should be transferred directly to specialized pediatric trauma centers. Two guidelines generated from this document involved prehospitalization management of hypoxia and hypotension. In a series of 4 studies regarding airway management, it was determined that although hypoxia was to be avoided, a firm recommendation regarding the superiority of tracheal intubation or bag-valve-mask ventilation could not be discerned.24-27 In 4 other studies from the 1980s and 1990s, the effect of hypoxia and hypotension was measured. Based on data from the Traumatic Coma Databank project and including more than 1900 children, Luerssen and colleagues28 demonstrated that hypotension was independently associated with an increased mortality rate in children with TBI, although hypoxia was not measured in this cohort. In single-center experiences within the United States, Michaud and colleagues29 found an increased mortality rate in hypotensive children with severe TBI in a study of 75 children, and Pigula and colleagues30 reported improved survival without hypotension and hypoxia in 58 children. In a study from Kuala Lumpur,31 hypotension and hypoxia were associated with increased poor outcome, with hypoxia increasing the risk of poor outcome by 2- to 4-fold. The fourth guideline regarding a CPP threshold was generated from a study by Downard and colleagues,32 who reported no survivors in a total of 118 patients, with a mean CPP of less than 40 mm Hg. Finally, the guideline recommending against corticosteroid use to either decrease ICP or improve outcome was generated from 2 small randomized studies from several decades ago.33,34
In addition to these guidelines, a large number of optional therapies were outlined. Of particular significance are: (1) 14 studies providing evidence that ICP monitoring is helpful for children with Glasgow Coma Scale (GCS) score of 8 or less; (2) 5 studies that provided support for the ICP threshold of 20 mm Hg; (3) 4 studies that demonstrated some efficacy of hypertonic saline solutions in lowering ICP; and (4) 3 small studies demonstrating that decompressive surgery can lead to decreased ICP and some improvement in outcomes. However, in this document a significant proportion of these optional recommendations were from expert opinion. For instance, the recommendation that high-dose barbiturate therapy be used in conjunction with hemodynamic monitoring is valuable information for practitioners, yet there are obviously no studies randomizing children receiving barbiturates to varying levels in invasive hemodynamic monitoring. Obviously this represents prudent advice from the experts who have decades of experience in caring for such children. Similarly, the recommendations regarding hyperventilation were based on a very limited data set of approximately 36 patients.35,36 However, the advice generated—avoid prophylactic hyperventilation when ICP is not a problem, judicious use of hyperventilation as an adjuvant therapy for ICP when first-tier measures are operational, and aggressive use of it during times of crisis—is a thoroughly reasonable approach for this maneuver. Nonetheless, these optional recommendations rest on the vast experience of the expert panel and not on the experience of large clinical trials. The culmination of the guidelines and options within the guideline, along with the expertise of the panel, is a treatment algorithm for intracranial hypertension (Fig. 1), which has served as a template for clinical protocols for patient care and research.
Fig. 1.
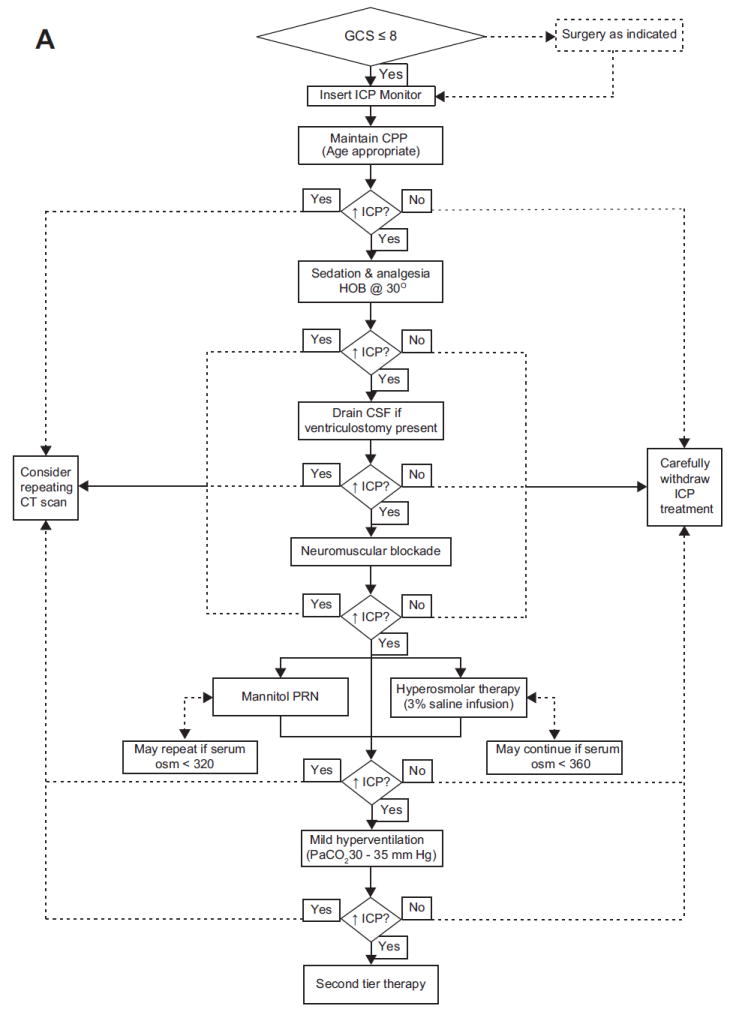
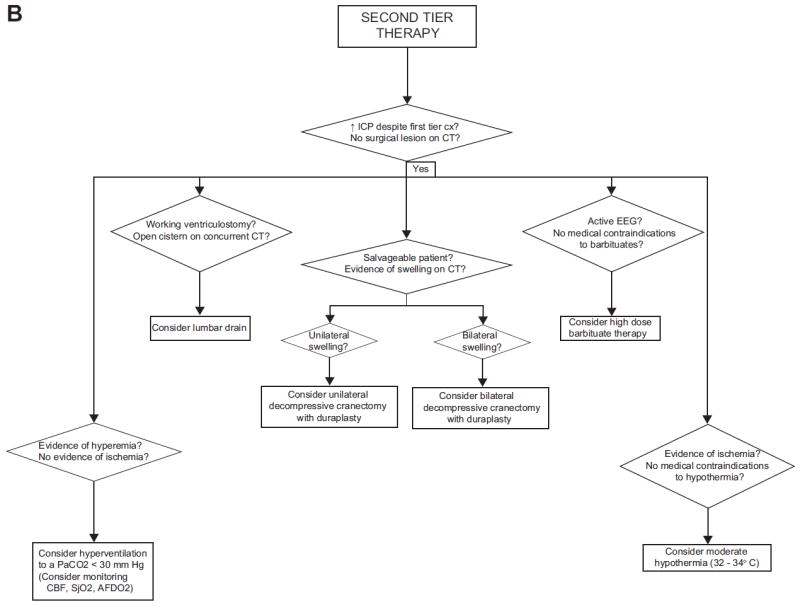
Algorithm generated by the Brain Trauma Foundation Committee for the first edition of the Guidelines for the Medical Management of Severe Traumatic Brain Injury in Infants, Children, and Adolescents for first-tier therapies (A) and second-tier therapies (B). AFDO2, arteriovenous difference in oxygen; CBF, cerebral blood flow; CPP, cerebral perfusion pressure; CSF, cerebrospinal fluid; CT, computed tomography; EEG, electroencephalogram; GCS, Glasgow Coma Scale; HOB, head of bed; ICP, intracranial pressure; PaCO2, partial pressure of carbon dioxide; PRN, as needed; SjO2, jugular bulb venous oxygen saturation. (From Adelson, PD, Bratton SL, Carney NA, et al. Guidelines for the acute medical management of severe traumatic brain injury in infants, children, and adolescents. Chapter 17. Critical pathway for the treatment of established intracranial hypertension in pediatric traumatic brain injury. Pediatr Crit Care Med 2003;4(Suppl 3):S65–7; with permission.)
NEW GUIDELINES: A DIFFERENT APPROACH LEADS TO NEW RESULTS
Almost a decade after the generation of the first edition of the Guidelines, the Brain Trauma Foundation convened another panel of experts to begin the process of reexamining the literature to generate new recommendations in 2009. In the interval between the reviews of the literature, a large body of work had been performed including the largest randomized controlled trial of therapeutic hypothermia in children, the implementation of monitoring for brain-tissue oxygen pressure, a randomized controlled trial of immune-enhanced nutrition, and many reports regarding fundamental aspects of pediatric TBI care. Therefore, several topics were added (advanced neuro-monitoring, neuroimaging, cerebrospinal fluid drainage, antiseizure prophylaxis) and others were eliminated (trauma systems, prehospital airway management, resuscitation of blood pressure and oxygenation, intracranial pressure monitoring technology, and the critical pathway). The result is that the new guidelines are considerably more focused on therapies for improvements in outcomes (either overall outcomes or surrogate outcomes) and hospital-based procedures rather than on issues related to prehospital or system-based approaches. In addition, the standards for evidenced-based medicine guidelines had evolved in the intervening time, decreasing the emphasis on expert opinion and relying on rigorous definitions of inclusion/exclusion criteria for articles that are included within these types of guidelines. These advances led to a quite different guideline document, which was published in early 2012.37
Methodologically there were several significant changes within the procedure that generated the new Guidelines. Although the methods of gathering articles for consideration were largely similar (scanning of search engines using keywords specific for the topic by a librarian as well as from the knowledge of the committee members), a priori inclusion criteria for articles within the new guidelines were agreed to include: severe TBI (GCS <9), human subjects only, English-language articles only, pediatric patients (age ≤18 years), randomized controlled trials (N≥25), cohort studies (prospective or retrospective, N≥25), case-control studies (N≥25), and case series (N≥5). Articles were excluded for exclusively including penetrating brain injuries, animal studies, cadaver studies, and those including adult subjects (>15% of adult subjects) or those with brain injuries other than TBI (>15% of subjects with conditions other than TBI). Case studies, editorials, letters to the editors, and commentary works were also excluded. The intervention needed to be specific to the topic and the outcome must be a relevant health outcome (normally mortality, favorable outcome, or a surrogate outcome specific for the intervention).
There were also significant changes in the assessments of the quality of the evidence. For this version of the Guidelines, epidemiologists and staff from the Evidence-Based Practice Center of Oregon Health and Science University developed criteria and procedures for the quality assessments of individual studies and assigned a level of evidence for each topic. Specifically, criteria for classification of evidence were derived from several international sources of other evidence-based methodologies, and assignment of evidence to levels I, II, and III was adjudicated by members of the staff. Overall, Class I evidence was defined as having been derived from high-quality randomized controlled trials, with adequate randomization, allocation concealment, blinded assessments, adequate statistical power, and follow-up rates greater than 85%. Class II evidence was defined as having been derived from clinical studies in which data were either prospectively collected or retrospectively collected with clearly reliable data, representing observational, cohort, prevalence, and case-control studies, along with randomized controlled trials of lower quality. These studies required unbiased selection of subjects, statistical analysis for confounders, clear differentiation of treatment groups, and blinded assessments of outcomes. Lastly, Class III evidence was defined as data derived from purely observational studies including registries, case series, or databases. These articles lacked some of the more rigorous controls outlined for the other classifications. The strength of the overall evidence within a given topic was assessed as high, moderate, or low, based on a grading system adopted by the Agency for Healthcare Research and Quality,38 including the risk of bias from individual studies, consistency of findings across studies, directness of the evidence, and precision of the findings.
As a result of these changes and of the new review of the literature, 27 new articles were added to the Guidelines. However, following the procedures outlined here, 25 articles that were included in the first version of the Guidelines were excluded from this revised version. The reasons for exclusion of articles varied, but centered around lack of clarity regarding admission GCS score (thereby leaving the diagnosis of the severity of TBI in doubt), inclusion of subjects with diagnoses other than TBI, inclusion of adult subjects (without the ability to discern the effect of the therapy on children only), and lack of a relevant outcome as defined here. In contrast to the previous guideline, the classification of standards, guidelines, and options were replaced with evidence Class I, II, and III, respectively. These criteria were generally regarded as therapies that “must be done,” “should be considered,” and “may be considered,” respectively.
Overall, again, there were no topics that generated data of sufficient quality to generate Class I evidence. There were 4 Class II recommendations generated; disappointingly, only one of these recommendations was supportive of a therapeutic approach (Table 2 summarizes the recommendations). For hyperosmolar therapies, 2 studies were judged to be of Class II quality.39,40 Fisher and colleagues39 performed a randomized controlled trial including 18 children of 3% saline compared with 0.9% saline during a 2-hour trial period. The group receiving the hypertonic saline had lower ICP and decreased need for other ICP-related therapies. Simma and colleagues40 performed a small randomized controlled trial comparing the utility of hypertonic saline solution (1.7% saline) with that of isotonic solutions (lactated Ringer solution) over a 3-day period after TBI. Although there was no difference in overall outcomes, the hypertonic saline group required fewer ICP-related interventions during the study period. Both studies were judged to be of moderate quality.
Table 2.
Summary of evidence generated from the 2012 pediatric TBI guidelines
| Topic | Level of Evidence | Recommendation |
|---|---|---|
| Indications for ICP monitoring | Level III | “Use of ICP monitoring may be considered…” |
| Threshold for treatment of intracranial hypertension | Level III | “Treatment of ICP may be considered at a threshold of 20 mm Hg” |
| Cerebral perfusion pressure thresholds | Level III | “A minimum CPP of 40 mm Hg may be considered … A CPP threshold of 40–50 mm Hg may be considered…” |
| Advanced neuromonitoring | Level III | “If brain oxygenation monitoring is used, maintenance of a partial pressure of brain-tissue oxygen (Pbto2) ≥10 mm Hg may be considered” |
| Neuroimaging | Level III | “In the absence of neurologic deterioration … routine repeat CT scan … may not be indicated…” |
| Hyperosmolar therapy | Level II | “Hypertonic saline should be considered … for intracranial hypertension … effective doses … range between 6.5 and 10 mL/kg” |
| Level III | “Hypertonic saline should be considered … effective doses as a continuous infusion of 3% saline range between 0.1 and 1.0 mL/kg/h administered on a sliding scale…” | |
| Temperature control | Level II | “Moderate hypothermia … for only 24 h duration should be avoided … moderate hypothermia starting within 8 h after injury and lasting for 48 h duration should be considered to reduce ICP … rewarming at a rate of 0.5°C/h should be avoided” |
| Level III | “Moderate hypothermia … for 48 h duration may be considered” | |
| Cerebrospinal fluid drainage | Level III | “CSF drainage through an externalized ventricular drain … may be considered … The addition of a lumbar drain may be considered…” |
| Barbiturates | Level III | “High-dose barbiturate therapy may be considered in hemodynamically stable patients with refractory intracranial hypertension … continuous arterial blood pressure monitoring and cardiovascular support to maintain adequate CPP are required” |
| Decompressive craniectomy for the treatment of intracranial hypertension | Level III | “Decompressive craniectomy with duraplasty … may be considered for pediatric patients … showing early signs of neurologic deterioration or herniation or are developing intracranial hypertension refractory to medical management…” |
| Hyperventilation | Level III | “Avoidance of prophylactic severe hyperventilation to a Paco2 <30 mm Hg may be considered within the first 48 h … If hyperventilation is used … advanced neuromonitoring for evaluation of cerebral ischemia may be considered” |
| Corticosteroids | Level II | “The use of corticosteroids is not recommended to improve outcome or lower ICP…” |
| Glucose and nutrition | Level II | “The evidence does not support the use of an immune-modulating diet … to improve outcome” |
| Level III | “…glycemic control … should be left to the treating physician” | |
| Antiseizure prophylaxis | Level III | “Prophylactic treatment with phenytoin may be considered to reduce the incidence of early posttraumatic seizures…” |
Abbreviations: CPP, cerebral perfusion pressure (mean arterial blood pressure minus intracranial pressure); CSF, cerebrospinal fluid; CT, computed tomography; ICP, intracranial pressure.
Data from Kochanek PK, et al. Guidelines for the acute medical management of severe traumatic brain injury in infants, children, and adolescents: second edition. Pediatr Crit Care Med 2012;13(Suppl 1):S1–82.
The other 3 Class II recommendations involved avoiding therapies. In perhaps the most important clinical trial in pediatric neurotrauma and neurocritical care to date, Hutchison and colleagues41 completed the Hyp-HIT study, a randomized controlled trial of early, moderate hypothermia for pediatric TBI. In this landmark study, 225 children were randomized (205 were available for follow-up) to an experimental group who were cooled to 32° to 33°C for 24 hours (initiation of cooling within 8 hours, rewarming at 0.5°C per hour). The study was confounded in a few ways (the normothermia group had greater exposure to hypertonic saline therapies, both groups received aggressive hyperventilation [Paco2 <30 mm Hg] at frequencies >40%, decreases in CPP were noted in the hypothermia group), but the experimental group failed to demonstrate a benefit from the treatment and even trended toward a worse outcome at 6 months after injury (P = .08). These findings led to a Class II recommendation to avoid the use of therapeutic hypothermia to improve outcome, and along with the stopping of the Pediatric Traumatic Brain Injury Consortium: Hypothermia (the “Cool Kids” trial) for reasons of futility, this leaves the future of the use of therapeutic hypothermia to improve overall outcome after TBI in doubt. Despite these findings, there are also data of Class II quality demonstrating that therapeutic hypothermia is associated with decreased ICP in some cases.42 The second Class II recommendation is also generated from a new randomized controlled trial, studying the use of an immune-modulating diet to improve outcomes. Briassoulis and colleagues43 randomized 40 children to receive an immune-modulated diet (with increased glutamine, arginine, antioxidants, and ω-3 fatty acids) using a protocol that standardized the amount of support within both groups. Unfortunately, there were no significant differences in mortality or other outcomes measures tested, thereby generating the Class II evidence that this form of immune-modulated diet could not be recommended at this time. Moreover, the fourth Class II recommendation regarded the avoidance of corticosteroids to improve outcome or reduce ICP that were described in the previous guideline.
Several of the optional therapies from the previous guideline were included in the new version as Class III evidence, including the indication for ICP monitoring, threshold for ICP treatment, threshold for CPP, utility of barbiturates, and decompressive surgery for intracranial hypertension. However, several new Class III recommendations were also generated. Within the new topic of advanced neuromonitoring, a Class III recommendation was generated, based on studies from the groups of Figaji and Narotam44,45 establishing that a threshold of 10 mm Hg may be considered when brain-tissue oxygen partial pressure (PbO2) is used. For neuroimaging, Figg and colleagues46 found that repeated computed tomography scans without neurologic deterioration were not fruitful in an observational cohort from a single center performed over a decade. For antiseizure prophylaxis, a retrospective study of 31 children demonstrated that prophylactic administration of phenytoin reduced the incidence of posttraumatic seizures, leading to the Class III recommendation.47 For a variety of other topics, including glycemic control, cerebrospinal fluid drainage, and analgesics/sedatives/neuromuscular blockade, definitive recommendations could not be made based on the available literature.
In their totality, the newest version of the Guidelines represents a synthesis of a body of literature spanning more than 40 years into a workable document to guide clinical practice and research protocols. Literature of the highest quality in these topics is desirable for a condition that is responsible for the largest number of deaths of children every year in the United States, and the pediatric neurotrauma community is frustrated by the lack of definitive evidence to generate high-level recommendations. In this version of the Guidelines, suggestions for future studies are made at the end of each topic in an effort to spur the pediatric neurotrauma community to add to the existing foundation of the literature. It is only with new data that better and more comprehensive recommendations can be generated.
NEW STEPS FORWARD: COMMON DATA ELEMENTS FOR PEDIATRIC TBI
A reasonable conclusion to reach after reviewing the Guidelines is that there is insufficient high-quality evidence to make specific treatment plans for children with severe TBI. One reason for this is the inadequate sample sizes of clinical studies, likely because of both the relatively recent focus on pediatric neurocritical care and the relatively small numbers of severe patients seen at individual institutions. As an example, there were 8 randomized controlled trials outlined within the revised Guidelines that included only 469 children. The small sample sizes of these trials obviously hinder the statistical power to prove their primary hypothesis (especially if it is related to overall outcomes) as well as the performance of secondary analyses. Combining data derived from these trials and, possibly, adding other data from well-performed observational studies, might prove valuable in increasing the sample size so that other analyses of secondary hypotheses might be possible. A tremendous impediment to this possibility is the lack of firm criteria identifying data elements that are essential for clinical trials in pediatric TBI.
An effort to identify such key data elements for children with severe TBI was initiated for just this purpose by the National Institute of Neurological Disorders and Stroke (NINDS) in 2009, and completed in 2012. Panels of experts in pediatric TBI were convened to determine data elements essential for (1) demographics and clinical assessment, (2) neurologic imaging, (3) outcome assessments, and (4) biomarkers.48-51 These panels worked via e-mail, phone conferences, and an in-person meeting to identify which elements were essential to all TBI studies (core elements), elements that might be useful in a subset of TBI studies (supplemental elements), and those that may have some future use (emerging elements). In the demographics and clinical assessment effort, 44 separate data elements were identified and precisely defined, while the neuroimaging group identified several overlapping elements along with stressing the “proper age-dependent interpretation” of radiologic findings as a necessary next step in the process. Meanwhile, the outcomes group painstakingly reviewed the potential outcome tests within the literature and determined age-specific tests within 18 different domains of functioning after TBI, and the biomarker group made recommendations regarding how biological samples from children with TBI should be processed and analyzed. The work done by these panels of experts will need to be implemented into new trials and then undoubtedly revised into newer versions of these important data points, to ultimately allow comparison and recombining of TBI studies to expand data sets with common definitions and completeness of data. For adult TBI studies, this process has ultimately led to electronic data-collection forms that can be used for all studies, ultimately allowing thousands of subjects to be integrated into a common database. More information regarding this constantly evolving effort can be found at the Web site of the Federal Interagency Traumatic Brain Injury Research (FITBIR) (http://fitbir.nih.gov).
SUMMARY
By any measure, there has been an enormous effort within the pediatric neurotrauma community to identify the optimal practices that can lead to improved outcomes for TBI, a disorder that is the leading killer of children. It is equally obvious that the current state of TBI literature is such that a wide variety of clinical approaches can fall “within” the guidelines because of the lack of data regarding the superiority of clinical decisions made every day for children with severe injuries. With the development of common data elements, it is possible that some of these questions may be answered by combining clinical trials in the future for secondary analyses. Alternatively, the time may be right for an observational study of pediatric TBI that uses novel statistical methods to compare the effectiveness of these commonly used TBI therapies as they are currently used in clinical practice.
KEY POINTS.
By any measure, there has been an enormous effort within the pediatric neurotrauma community to identify the optimal practices that can lead to improved outcomes for traumatic brain injury (TBI), a disorder that is the leading killer of children.
It is equally obvious that the current state of TBI literature is such that a wide variety of clinical approaches can fall “within” the guidelines because of the lack of data regarding the superiority of clinical decisions that are made every day for children with severe injuries.
With the development of common data elements, it is possible that some of these questions may be answered by combining clinical trials in the future for secondary analyses.
Alternatively, the time may be right for an observational study of pediatric TBI that uses novel statistical methods for comparing the effectiveness of these commonly used TBI therapies as they are currently used in clinical practice.
Acknowledgments
Dr Kochanek is supported by several Federal grants (U44 NS070324, T32HD040686 from the NIH and W81XWH-09-2-0187 and W81XWH-10-0623 from the US Army), as is Dr Bell (U01 HD049981, R01 NS069247, R01 NS072308 from the NIH).
Footnotes
Disclosures: Both Drs Bell and Kochanek were selected by the Brain Trauma Foundation to develop the Guidelines for the Medical Management of Severe Traumatic Brain Injury for Infants, Children and Adolescents but were not compensated for their work. In addition, Dr Bell was selected by the National Institute of Neurological Disorders and Stroke to participate in development of the Common Data Elements for Pediatric TBI, but he was not compensated for his work.
References
- 1.Adelson PD, Bratton SL, Carney NA, et al. Guidelines for the acute medical management of severe traumatic brain injury in infants, children, and adolescents. Chapter 3. Prehospital airway management. Pediatr Crit Care Med. 2003;4(Suppl 3):S9–11. [PubMed] [Google Scholar]
- 2.Adelson PD, Clyde B, Kochanek PM, et al. Guidelines for the acute medical management of severe traumatic brain injury in infants, children, and adolescents. Chapter 4. Resuscitation of blood pressure and oxygenation and prehospital brain-specific therapies for the severe pediatric traumatic brain injury patient. Pediatr Crit Care Med. 2003;4(Suppl 3):S12–8. [PubMed] [Google Scholar]
- 3.Adelson PD, Bratton SL, Carney NA, et al. Guidelines for the acute medical management of severe traumatic brain injury in infants, children, and adolescents. Chapter 18. Nutritional support. Pediatr Crit Care Med. 2003;4(Suppl 3):S68–71. [PubMed] [Google Scholar]
- 4.Adelson PD, Bratton SL, Carney NA, et al. Guidelines for the acute medical management of severe traumatic brain injury in infants, children, and adolescents. Chapter 16. The use of corticosteroids in the treatment of severe pediatric traumatic brain injury. Pediatr Crit Care Med. 2003;4(Suppl 3):S60–4. [PubMed] [Google Scholar]
- 5.Adelson PD, Bratton SL, Carney NA, et al. Guidelines for the acute medical management of severe traumatic brain injury in infants, children, and adolescents. Chapter 15. Surgical treatment of pediatric intracranial hypertension. Pediatr Crit Care Med. 2003;4(Suppl 3):S56–9. [PubMed] [Google Scholar]
- 6.Adelson PD, Bratton SL, Carney NA, et al. Guidelines for the acute medical management of severe traumatic brain injury in infants, children, and adolescents. Chapter 14. The role of temperature control following severe pediatric traumatic brain injury. Pediatr Crit Care Med. 2003;4(Suppl 3):S53–5. [PubMed] [Google Scholar]
- 7.Adelson PD, Bratton SL, Carney NA, et al. Guidelines for the acute medical management of severe traumatic brain injury in infants, children, and adolescents. Chapter 13. The use of barbiturates in the control of intracranial hypertension in severe pediatric traumatic brain injury. Pediatr Crit Care Med. 2003;4(Suppl 3):S49–52. [PubMed] [Google Scholar]
- 8.Adelson PD, Bratton SL, Carney NA, et al. Guidelines for the acute medical management of severe traumatic brain injury in infants, children, and adolescents. Chapter 12. Use of hyperventilation in the acute management of severe pediatric traumatic brain injury. Pediatr Crit Care Med. 2003;4(Suppl 3):S45–8. [PubMed] [Google Scholar]
- 9.Adelson PD, Bratton SL, Carney NA, et al. Guidelines for the acute medical management of severe traumatic brain injury in infants, children, and adolescents. Chapter 11. Use of hyperosmolar therapy in the management of severe pediatric traumatic brain injury. Pediatr Crit Care Med. 2003;4(Suppl 3):S40–4. [PubMed] [Google Scholar]
- 10.Adelson PD, Bratton SL, Carney NA, et al. Guidelines for the acute medical management of severe traumatic brain injury in infants, children, and adolescents. Chapter 10. The role of cerebrospinal fluid drainage in the treatment of severe pediatric traumatic brain injury. Pediatr Crit Care Med. 2003;4(Suppl 3):S38–9. [PubMed] [Google Scholar]
- 11.Adelson PD, Bratton SL, Carney NA, et al. Guidelines for the acute medical management of severe traumatic brain injury in infants, children, and adolescents. Chapter 9. Use of sedation and neuromuscular blockade in the treatment of severe pediatric traumatic brain injury. Pediatr Crit Care Med. 2003;4(Suppl 3):S34–7. [PubMed] [Google Scholar]
- 12.Adelson PD, Bratton SL, Carney NA, et al. Guidelines for the acute medical management of severe traumatic brain injury in infants, children, and adolescents. Chapter 8. Cerebral perfusion pressure. Pediatr Crit Care Med. 2003;4(Suppl 3):S31–3. [PubMed] [Google Scholar]
- 13.Adelson PD, Bratton SL, Carney NA, et al. Guidelines for the acute medical management of severe traumatic brain injury in infants, children, and adolescents. Chapter 7. Intracranial pressure monitoring technology. Pediatr Crit Care Med. 2003;4(Suppl 3):S28–30. [PubMed] [Google Scholar]
- 14.Adelson PD, Bratton SL, Carney NA, et al. Guidelines for the acute medical management of severe traumatic brain injury in infants, children, and adolescents. Chapter 6. Threshold for treatment of intracranial hypertension. Pediatr Crit Care Med. 2003;4(Suppl 3):S25–7. [PubMed] [Google Scholar]
- 15.Adelson PD, Bratton SL, Carney NA, et al. Guidelines for the acute medical management of severe traumatic brain injury in infants, children, and adolescents. Chapter 5. Indications for intracranial pressure monitoring in pediatric patients with severe traumatic brain injury. Pediatr Crit Care Med. 2003;4(Suppl 3):S19–24. [PubMed] [Google Scholar]
- 16.Adelson PD, Bratton SL, Carney NA, et al. Guidelines for the acute medical management of severe traumatic brain injury in infants, children, and adolescents. Chapter 2: trauma systems, pediatric trauma centers, and the neurosurgeon. Pediatr Crit Care Med. 2003;4(Suppl 3):S5–8. [PubMed] [Google Scholar]
- 17.Adelson PD, Bratton SL, Carney NA, et al. Guidelines for the acute medical management of severe traumatic brain injury in infants, children, and adolescents. Chapter 1: introduction. Pediatr Crit Care Med. 2003;4(Suppl 3):S2–4. doi: 10.1097/01.CCM.0000066600.71233.01. [DOI] [PubMed] [Google Scholar]
- 18.Adelson PD, Bratton SL, Carney NA, et al. Guidelines for the acute medical management of severe traumatic brain injury in infants, children, and adolescents. Chapter 17. Critical pathway for the treatment of established intracranial hypertension in pediatric traumatic brain injury. Pediatr Crit Care Med. 2003;4(Suppl 3):S65–7. [PubMed] [Google Scholar]
- 19.Adelson PD, Bratton SL, Carney NA, et al. Guidelines for the acute medical management of severe traumatic brain injury in infants, children, and adolescents. Chapter 19. The role of anti-antiseizure prophylaxis following severe pediatric traumatic brain injury. Pediatr Crit Care Med. 2003;4(Suppl 3):S72–5. [PubMed] [Google Scholar]
- 20.Bullock R, Chesnut RM, Clifton G, et al. Guidelines for the management of severe head injury. Brain Trauma Foundation. Eur J Emerg Med. 1996;3(2):109–27. doi: 10.1097/00063110-199606000-00010. [DOI] [PubMed] [Google Scholar]
- 21.Johnson DL, Krishnamurthy S. Send severely head-injured children to a pediatric trauma center. Pediatr Neurosurg. 1996;25(6):309–14. doi: 10.1159/000121145. [DOI] [PubMed] [Google Scholar]
- 22.Hulka F, Mullins RJ, Mann NC, et al. Influence of a statewide trauma system on pediatric hospitalization and outcome. J Trauma. 1997;42(3):514–9. doi: 10.1097/00005373-199703000-00020. [DOI] [PubMed] [Google Scholar]
- 23.Potoka DA, Schall LC, Gardner MJ, et al. Impact of pediatric trauma centers on mortality in a statewide system. J Trauma. 2000;49(2):237–45. doi: 10.1097/00005373-200008000-00009. [DOI] [PubMed] [Google Scholar]
- 24.Cooper A, DiScala C, Foltin G, et al. Prehospital endotracheal intubation for severe head injury in children: a reappraisal. Semin Pediatr Surg. 2001;10(1):3–6. doi: 10.1053/spsu.2001.19379. [DOI] [PubMed] [Google Scholar]
- 25.Gausche M, Lewis RJ, Stratton SJ, et al. Effect of out-of-hospital pediatric endotracheal intubation on survival and neurological outcome: a controlled clinical trial. JAMA. 2000;283(6):783–90. doi: 10.1001/jama.283.6.783. [DOI] [PubMed] [Google Scholar]
- 26.Murray JA, Demetriades D, Berne TV, et al. Prehospital intubation in patients with severe head injury. J Trauma. 2000;49(6):1065–70. doi: 10.1097/00005373-200012000-00015. [DOI] [PubMed] [Google Scholar]
- 27.Nakayama DK, Gardner MJ, Rowe MI. Emergency endotracheal intubation in pediatric trauma. Ann Surg. 1990;211(2):218–23. doi: 10.1097/00000658-199002000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Luerssen TG, Klauber MR, Marshall LF. Outcome from head injury related to patient’s age. A longitudinal prospective study of adult and pediatric head injury. J Neurosurg. 1988;68(3):409–16. doi: 10.3171/jns.1988.68.3.0409. [DOI] [PubMed] [Google Scholar]
- 29.Michaud LJ, Rivara FP, Longstreth WT, et al. Predictors of survival and severity of disability after severe brain injury in children. Neurosurgery. 1992;31(2):254–64. doi: 10.1227/00006123-199208000-00010. [DOI] [PubMed] [Google Scholar]
- 30.Pigula FA, Wald SL, Shackford SR, et al. The effect of hypotension and hypoxia on children with severe head injuries. J Pediatr Surg. 1993;28(3):310–4. doi: 10.1016/0022-3468(93)90223-8. discussion: 315–6. [DOI] [PubMed] [Google Scholar]
- 31.Ong L, Selladurai BM, Dhillon MK, et al. The prognostic value of the Glasgow Coma Scale, hypoxia and computerised tomography in outcome prediction of pediatric head injury. Pediatr Neurosurg. 1996;24(6):285–91. doi: 10.1159/000121057. [DOI] [PubMed] [Google Scholar]
- 32.Downard C, Hulka F, Mullins RJ, et al. Relationship of cerebral perfusion pressure and survival in pediatric brain-injured patients. J Trauma. 2000;49(4):654–8. doi: 10.1097/00005373-200010000-00012. discussion: 658–9. [DOI] [PubMed] [Google Scholar]
- 33.Fanconi S, Meuli M, Zaugg H, et al. Dexamethasone therapy and endogenous cortisol production in severe pediatric head injury. Intensive Care Med. 1988;14(2):163–6. doi: 10.1007/BF00257471. [DOI] [PubMed] [Google Scholar]
- 34.Kloti J, Fanconi S, Zachmann M, et al. Dexamethasone therapy and cortisol excretion in severe pediatric head injury. Childs Nerv Syst. 1987;3(2):103–5. doi: 10.1007/BF00271134. [DOI] [PubMed] [Google Scholar]
- 35.Skippen P, Seear M, Poskitt K, et al. Effect of hyperventilation on regional cerebral blood flow in head-injured children. Crit Care Med. 1997;25(8):1402–9. doi: 10.1097/00003246-199708000-00031. [DOI] [PubMed] [Google Scholar]
- 36.Stringer WA, Choi SC, Fatouros P, et al. Hyperventilation-induced cerebral ischemia in patients with acute brain lesions: demonstration by xenon-enhanced CT. AJNR Am J Neuroradiol. 1993;14(2):475–84. [PMC free article] [PubMed] [Google Scholar]
- 37.Kochanek PM, Carney N, Adelson PD, et al. Guidelines for the acute medical management of severe traumatic brain injury in infants, children and adolescents: second Edition. Pediatr Crit Care Med. 2012;13(Suppl 1):S1–82. doi: 10.1097/PCC.0b013e31823f435c. [DOI] [PubMed] [Google Scholar]
- 38.Owens DK, Lohr KN, Atkins D, et al. AHRQ series paper 5: grading the strength of a body of evidence when comparing medical interventions–agency for healthcare research and quality and the effective health-care program. J Clin Epidemiol. 2010;63(5):513–23. doi: 10.1016/j.jclinepi.2009.03.009. [DOI] [PubMed] [Google Scholar]
- 39.Fisher B, Thomas D, Peterson B. Hypertonic saline lowers raised intracranial pressure in children after head trauma. J Neurosurg Anesthesiol. 1992;4(1):4–10. doi: 10.1097/00008506-199201000-00002. [DOI] [PubMed] [Google Scholar]
- 40.Simma B, Burger R, Falk M, et al. A prospective, randomized, and controlled study of fluid management in children with severe head injury: lactated Ringer’s solution versus hypertonic saline. Crit Care Med. 1998;26(7):1265–70. doi: 10.1097/00003246-199807000-00032. [DOI] [PubMed] [Google Scholar]
- 41.Hutchison JS, Ward RE, Lacroix J, et al. Hypothermia therapy after traumatic brain injury in children. N Engl J Med. 2008;358(23):2447–56. doi: 10.1056/NEJMoa0706930. [DOI] [PubMed] [Google Scholar]
- 42.Adelson PD. Phase II clinical trial of moderate hypothermia after severe traumatic brain injury in children. Neurosurgery. 2005;56(4):740–54. doi: 10.1227/01.neu.0000156471.50726.26. discussion: 740–54. [DOI] [PubMed] [Google Scholar]
- 43.Briassoulis G, Filippou O, Kanariou M, et al. Temporal nutritional and inflammatory changes in children with severe head injury fed a regular or an immune-enhancing diet: a randomized, controlled trial. Pediatr Crit Care Med. 2006;7(1):56–62. doi: 10.1097/01.pcc.0000192339.44871.26. [DOI] [PubMed] [Google Scholar]
- 44.Figaji AA, Zwane E, Thompson C, et al. Brain tissue oxygen tension monitoring in pediatric severe traumatic brain injury. Part 1: relationship with outcome. Childs Nerv Syst. 2009;25(10):1325–33. doi: 10.1007/s00381-009-0822-x. [DOI] [PubMed] [Google Scholar]
- 45.Narotam PK, Burjonrappa SC, Raynor SC, et al. Cerebral oxygenation in major pediatric trauma: its relevance to trauma severity and outcome. J Pediatr Surg. 2006;41(3):505–13. doi: 10.1016/j.jpedsurg.2005.11.069. [DOI] [PubMed] [Google Scholar]
- 46.Figg RE, Stouffer CW, Vander Kolk WE, et al. Clinical efficacy of serial computed tomographic scanning in pediatric severe traumatic brain injury. Pediatr Surg Int. 2006;22(3):215–8. doi: 10.1007/s00383-005-1560-0. [DOI] [PubMed] [Google Scholar]
- 47.Lewis RJ, Yee L, Inkelis SH, et al. Clinical predictors of post-traumatic seizures in children with head trauma. Ann Emerg Med. 1993;22(7):1114–8. doi: 10.1016/s0196-0644(05)80974-6. [DOI] [PubMed] [Google Scholar]
- 48.Adelson PD, Pineda J, Bell MJ, et al. Common data elements for pediatric traumatic brain injury: recommendations from the working group on demographics and clinical assessment. J Neurotrauma. 2012;29(4):639–53. doi: 10.1089/neu.2011.1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Berger RP, Goyal A, Carter M, et al. Common data elements for pediatric traumatic brain injury: recommendations from the biospecimens and biomarkers workgroup. J Neurotrauma. 2012;29(4):672–7. doi: 10.1089/neu.2011.1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Duhaime AC, Berger RP, Beers SR, et al. Common data elements for neuroimaging of traumatic brain injury: pediatric considerations. J Neurotrauma. 2012;29(4):629–33. doi: 10.1089/neu.2011.1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.McCauley SR, Wilde EW, Miller ER, et al. Recommendations for the use of common outcome measures in pediatric traumatic brain injury research. J Neurotrauma. 2012;29(4):678–705. doi: 10.1089/neu.2011.1838. [DOI] [PMC free article] [PubMed] [Google Scholar]


