Abstract
Ribosomal stress is an important, yet poorly understood, mechanism that results in activation of the p53 tumour suppressor. We present a mutation in the ribosomal protein Rpl27a gene (sooty foot ataxia mice), isolated through a sensitized N-ethyl-N-nitrosourea (ENU) mutagenesis screen for p53 pathway defects, that shares striking phenotypic similarities with high p53 mouse models, including cerebellar ataxia, pancytopenia and epidermal hyperpigmentation. This phenocopy is rescued in a haploinsufficient p53 background. A detailed examination of the bone marrow in these mice identified reduced numbers of haematopoietic stem cells and a p53-dependent c-Kit down-regulation. These studies suggest that reduced Rpl27a increases p53 activity in vivo, further evident with a delay in tumorigenesis in mutant mice. Taken together, these data demonstrate that Rpl27a plays a crucial role in multiple tissues and that disruption of this ribosomal protein affects both development and transformation.
Keywords: ribosomal stress, p53, development, tumorigenesis, stem cells, c-Kit
Introduction
Cellular defects that alter ribosomal protein levels or ‘ribosomal stress’ are often capable of invoking a powerful p53 response. In 1994, Levine and co-workers first suggested that p53 protein levels are coupled to ribosomal protein levels, when they showed that a ribosomal protein, RPL5, forms a ternary protein complex with p53 and its major inhibitor, Mdm2 [1]. Thereafter, other ribosomal proteins have been shown to directly interact with Mdm2 to affect p53 levels [1–7]. Loss-of-function/expression of some ribosomal proteins decreased p53 levels [4,5,7–10], while for others p53 levels increased [6,11–16]. Conversely, over-expression of some ribosomal proteins raised p53 levels [3,5–10,17]. Both reduction and over-expression of RPL23 induced p53 [6]. In mice, ribosomal protein mutations or heterozygous deletions affecting their expression have been associated with various p53-dependent pathologies, including embryonic lethality during gastrulation [11] (Rps6 ; partial rescue on p53-null background), T cell-specific lineage defects [13] (Rpl22 ), hyperpigmentation and mild anaemia [12] (Rps19 and Rps20 ), hypopigmentation and skeletal and retinal abnormalities [14] (Rpl24 ). The diversity of these phenotypes may be due to differences in basal levels of certain ribosomal proteins in different tissues and may reflect the developmental stage and differentiation-specific expression of these ribosomal proteins. Moreover, the absence of obvious phenotypes that recapitulate p53 pathway mouse models indicates that p53 activation in these mutant mice originated in differentiated cells rather than in undifferentiated stem-like cells, which would have resulted in graver and more diverse pathologies.
Through a p53-sensitized mutagenesis N-ethyl-N-nitrosourea (ENU) screen, we isolated a model of ribosomal stress that uniquely phenocopies mice with reduced levels of p53 inhibitors, such as Mdm2puro/Δ7–12 [18], Mdm4+/− [19,20] and Mdm2+/−:Mdm4+/− [20] mice. This model provides evidence of a link between a deficiency in the Rp127a ribosomal protein and p53-dependent developmental defects, tumour development and progression.
Materials and methods
Positional cloning
The ENU-induced mutation was generated on a C57BL/6J background and affected mice carrying the mutation were backcrossed for two generations to the 129S6SvEvTAC background for meiotic mapping (see Supporting information, Supplementary Materials and Methods).
Mice and colony maintenance
The SFA/+ mouse was isolated from a previously described mutagenesis screen [21] and maintained on a C57BL/6J background (for genotyping and maintenance, see Supporting information, Supplementary materials and methods). p53+/− mice [22] were purchased from the Jackson laboratory. Mdm2+/Δ7–12 mice [18] were obtained from Mouse models of human cancers consortium, while Mdm2+/−, Mdm4+/− [19], and Mdm4+/Δ2 [23] mice were provided by Dr G. Lozano. Dopachrome Tautomerase (DCT)-LacZ transgenic mice [24] were provided by Dr P. Overbeek.
Bacterial artificial chromosome (BAC) transgenics were made using RP23–74E7 and RP23–341B3 clones, obtained from the Children’s Hospital, Oakland Research Institute, and containing the entire Rpl27a genomic locus and its immediate 5′ regulatory sequences.
All animal protocols were approved by the Institutional Animal Care and Use Committee.
Complete blood counts (CBCs)
Peripheral blood was collected by retro-orbital sinus puncture of P21–25 anesthetized mice into Microtainer tubes containing dipotassium–EDTA (Becton-Dickinson). CBCs were performed using the Cell-Dyn 3500R haematology analyser (Abbott Diagnostics).
Histology and immunostaining
Tissues were fixed for 24 h in 4% formalin, processed and then embedded in paraffin. Immunostaining for p53 (1 : 400; Novocastra Laboratories, UK) and cleaved caspase-3 (1 : 200; Cell Signalling) were performed as described [20] and according to the respective manufacturers’ recommendations. For proliferation, P3 pups were injected intraperitoneally with BrdU (1 ml/100 g body weight) and tissues were harvested 2 h later and processed for immunofluorescence (IF) [20], using anti-BrdU–FITC antibody (BD Biosciences). Propidium iodide (PI) was used as nuclear stain. Nissl and haematoxylin and eosin (H&E) staining were performed by the Baylor College of Medicine (BCM) in situ hybridization (ISH) core facility.
Bone marrow proliferation, apoptosis and HSC counts by flow cytometry
Bone marrows (BM) of postnatal day 21–25 (P21–25), wild-type (WT) and SFA/+ mice were flushed from both femurs and haematopoietic stem cells (HSCs) were isolated as described previously [25]. Proliferation and apoptosis were assessed by BrdU/PI and annexin V staining, respectively, as described [25].
RNA isolation, expression assays and 3′ RACE
P3 skin and cerebellums were snap-frozen in liquid nitrogen and whole RNA was extracted for cDNA synthesis and expression assays and as a template for 3′ Rapid Amplification of cDNA 3′ Ends (3′ RACE) of the Rpl27a locus to identify any alternatively spliced RNAs (see Supporting information, Supplementary Materials and Methods).
In situ hybridization
ISH for Mdm2, Mdm4, p53 and Cdkn1a was performed using digoxigenin-labelled probes on 20 μm mid-sagittal brain sections, as described [26], by the BCM ISH core facility.
IR experiment
Five week-old WT and SFA/+ littermates were subjected to a sublethal 6 Gy dose of γ-rays (γ-IR) from a 137cesium source. After 5 h thymi were collected for IF. Ten mice of each genotype were also followed for survival.
Statistical and survival anaylsis
To compare the means of two datasets, we used the unpaired Student’s t-test. To compare the survival of two cohorts, we performed a Log-rank test and Kaplan–Meier analysis. Differences between observed and expected genotypes in Table S4 (see Supporting information) were assessed by χ2 analysis. Statistical differences were considered significant if they had a two-sided p value of <0.05 (see Supporting information, Supplementary Materials and Methods).
Rotarod assay
The rotarod assay is a performance test based on forced motor activity of an experimental animal, usually rodents. We measured the riding time (s) or endurance of mice running on an accelerating rotating rod (Ugo Basile). Latency to fall was recorded in four successive five minute trials on the first day, followed by a second set of four trials on the second day. The last trial was plotted.
Results
A mouse mutagenesis screen for p53 pathway modifiers
A large-scale random ENU mutagenesis screen that was the first to use a balancer chromosome in a mouse screen was performed in a previous study [21]. As part of the breeding scheme, mutant mice were crossed to mice that inherited an inversion allele of chromosome 11 with breakpoints at the p53 and Wnt3 loci [27]. This 23 centiMorgan (cM) chromosomal inversion, Inv(11)8Brdp53–Wnt3, is homozygous lethal, due to disruption of the Wnt3 locus, and is haploinsufficient for p53 in the heterozygous state [28]. We identified that this mutagenesis screen doubles as a sensitized screen for p53 and Wnt pathway defects, since it included breeding all mutagenized progeny with mice carrying the Inv(11)8Brdp53–Wnt3 inversion allele. In 230 isolated mutations, we identified the ‘sooty foot ataxia’ phenotype that demonstrated a unique inheritance pattern that suggested genetic interaction with either p53 or Wnt3. This phenotype, mainly characterized by excessive shaking (ataxia) and hyperpigmentation of the footpads, was noticeably absent when inherited together with the Inv(11)8Brdp53–Wnt3 allele, but strikingly penetrant when the inversion allele was absent.
The sooty foot ataxia phenotype and identification of the mutation
The sooty foot ataxia (SFA) phenotype has a semidominant mode of inheritance, with embryonic lethality of homozygotes prior to E7.5 (see Supporting information, Table S1). The SFA mutation was localized to a large interval on mouse chromosome 7, using a meiotic mapping approach. Fine mapping in this interval identified a region that included 12 odorant receptor genes which we did not consider as candidates, and 13 additional genes with at least 120 exons that were subjected to sequence analysis in C57BL/6J and mice heterozygous for the SFA mutation (SFA/+ mice) (Figure 1A). The scan for mutations identified an A > G point mutation at position −15 in the 3′ splice acceptor of intron 4 (IVS4-15A > G) within the Rpl27a gene, which is a large subunit ribosomal protein of 148 amino acids encoded by five exons [29] (Figure 1B). This mutation was the only one found in 82 kb of sequenced genomic DNA.
Figure 1.
Meiotic mapping and identification of the mutation responsible for the SFA phenotype. (A) Meiotic mapping of the SFA locus performed after a two-generation backcross onto the 129S6/SvEv background from the C57BL/6J parent strain. C57BL/6J genotypes are represented by black boxes and 129S6/SvEv by white boxes. Polymorphic microsatellite marker designation is shown on the left, while physical location is shown on the right of the box genotype diagram. Numbers of both SFA/+ and WT mice of each genotype combination for the nine microsatellite markers are shown underneath the box genotype diagram. (B) The first two panels are sequence chromatograms of mice of indicated genotypes. The bottom panel represents the intron–exon structure of the Rpl27a gene. The white boxes illustrate the untranslated exons and the red boxes illustrate the coding exons. The ENU-induced point mutation (IVS4 -15A > G) within the intron four-splice acceptor is represented by a red star. (C) Expression levels of Rpl27a (mean + SEM) in SFA/+ P3 cerebellum (n = 8, p = 0.0388), P23-25 BM (n = 6, p = 0.0426) and P3 footpad skin (n = 9, p = 0.0283) in comparison to levels in WT tissue indicated by the dotted line (set at 1; n = 6 for cerebellum, n = 7 for BM and n = 5 for skin). (d) Variably penetrant low body weight and size in P35 SFA/+ mice. (E) Postnatal growth and survival curves of WT and SFA/+ mice.
To validate the cloning of the putative SFA mutation, we used the RP23-74E7 and RP23-341B3 BAC clones to generate transgenic mice that carried an extra copy of the Rpl27a gene and its genomic regulatory sequences (see Supporting information, Figure S1A). An intercross between these transgenics and SFA/+ mice showed that the extra Rpl2 7a genomic copy was capable of restoring the WT phenotype in these mice (see Supporting information, Table S2). Accordingly, Rpl2 7a expression levels appeared to be restored on average in the SFA/+: BAC footpads compared to SFA/+ mice (see Supporting information, Figure S1B).
Analysis of the SFA phenotype
Since the SFA mutation is situated at the intron–exon junction, we checked for alternatively spliced products of the Rpl27a gene by 3′ RACE analysis in E14.5 whole embryos, or in either cerebellums or footpad skins of P3 mice. We could not detect any splice variants in SFA/+ : versus WT mice. However, lower levels of Rpl27a transcripts were observed by quantitative PCR (qPCR) in P3 mice. Rpl27a expression was reduced in SFA/+ : cerebellum (59.5%), BM (72.7%) and skin (79.2%) compared to WT tissues (Figure 1C).
Low body weight and anaemia
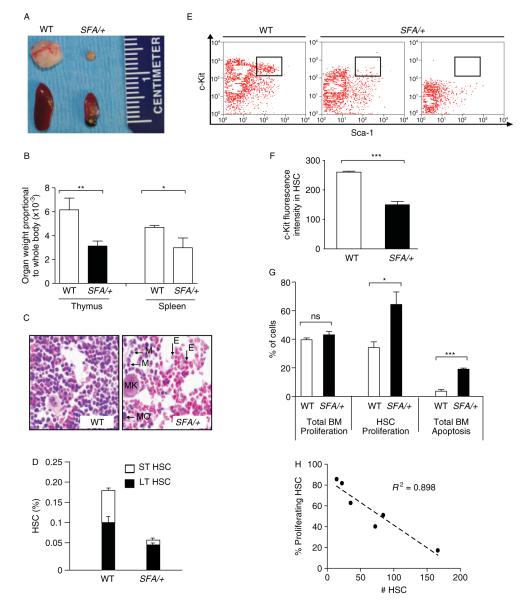
SFA/+ : mice presented with variably penetrant low body weight and anaemia (Figure 1D). Seriously affected SFA/+ : mice were unable to undertake the rapid growth spurt that normally occurs 19–30 days after birth, indicating that normal body weight is dependent on a basal expression level of Rpl27a. Approximately 20% of affected animals looked anaemic and died in early postnatal development (Figure 1E). The mutant thymi and spleens were also significantly reduced in size in comparison to WT tissues (Figure 2A, B). Blood was drawn for CBCs from a number of severely affected P21–23 SFA/+ : mice. These mice had thin, watery blood and suffered from pancytopenia, a condition in which the numbers of all blood cells were reduced. Thus, affected mice had 14% white blood cells, 46% red blood cells and 30% platelets compared to WT littermates (see Supporting information, Table S3), confirming the histopathological examination of affected SFA/+ : BM (Figure 2C) and indicating that all types of blood cells were affected by sub-physiological levels of Rpl27a.
Figure 2.
Blood phenotype in SFA/+ mice. (A). A representative picture of thymi and spleens from a severely affected SFA/+ and WT littermate (B) Histogram (mean + SEM, n = 3) of thymus and spleen weights relative to whole body weights in WT and SFA/+ littermates. p = *0.0079 and p = **0.001. (C) H&tE stain of sections from upper femurs of P21-P23 WT and SFA/+ littermates, showing BM with all subtypes of blood cells present, such as megakaryocyte (MK), macrophage (MO), myeloid (M) and erythroid (E) cells. (D) Histogram presenting flow cytometry analysis of short-term (ST) and long-term (LT) HSC numbers (mean + SEM) in flushed BM of P21-P23 mice (n = 6 for WT; n = 10 for severely affected SFA/+), measured by Lin−/lo c-Kit+ Sca-1+ gated cells, which are sorted into Flk-2+ (LT) and FLK-2− (ST). p = 0.0114 for LT and p = 0.001 for ST. (E) Representative flow cytometric analysis of HSCs from flushed BM of P21-P23 WT and severely affected SFA/+ mice. Lin−/lo cells were gated on c-Kit and Sca-1. Small quadrant (R3) represents Sca-1+ c-Kit+ cells which contain the stem cells population. The second panel is representative of staining from most of the affected SFA/+ mice. The third panel shows staining from one of the most affected mice. (F) Quantitation of fluorescence intensity of c-Kit+ cells (mean + SEM) in the R3 HSC quadrant of (E) (n = 6 mice/genotype). ***p < 0.0001. (G) Total BM and HSCs (%) undergoing proliferation (BrdU+ cells) [n = 6/genotype; p = 0.3130 (ns) and *0.0136], and total BM cells (%) undergoing apoptosis (annexin V staining, n = 3/genotype; ***p = 0.0001) measured by flow cytometry (mean + SEM) in WT andseverely affected SFA/+ mice. (H) The relationship between HSC numbers in whole BM and percentage proliferating HSCs (BrdU+ cells) is plotted. A ‘best-fit’ straight dotted line is drawn through the data (linear regression). R2 = square of correlation coefficient.
To determine whether this defect was due to a decrease in the number of HSCs, we performed a count using standard lin−/lo/c-Kit/Sca-1 staining for flow cytometry [25] in combination with the Flk-2 antibody to differentiate between short-term (ST) and long-term (LT) HSCs [30]. Severely affected SFA/+ : mice had ~40% total HSC counts compared to WT littermates, with a sharp reduction in both ST and LT HSCs (Figure 2D). HSC detection by flow cytometry using the c-Kit antibody (see Supporting information, Figure S2) identified a strong reduction (~42%) of cell surface c-Kit receptors on BM cells in all severely affected mice compared to WT mice (Figure 2E, F), confirming the loss of HSCs. We were able to detect a significant increase in apoptosis in three of eleven SFA/+ : whole BMs (~20% versus ~3% in WT mice; Figure 2G). However, a significant increase in HSC proliferation was observed in SFA/+ : mice, while whole BM proliferation was not significantly different from WT tissues (Figure 2G). Furthermore, it was notable that SFA/+ : mice with the lowest HSC numbers had the highest proportion of proliferating HSCs, as shown by the linear trend in Figure 2H.
The hyperpigmentation phenotype
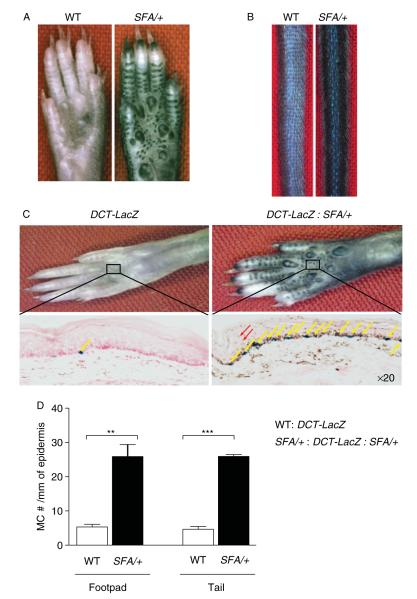
SFA/+ : mice exhibited fully penetrant hyperpigmentation of the tail, feet, ears and genital areas (Figure 3A, B and data not shown). To further examine this phenotype, we crossed the SFA/+ mouse to the DCT –LacZ transgenic mouse, which expresses the β-galactosidase reporter gene under the melanocyte-specific tyrosinase-related protein-2 promoter [24,31], permitting us to easily visualize and count the melanocytes. A dramatic increase in epidermal melanocyte numbers with increased deposition of melanin granules was noted in DCT–LacZ : SFA/+ footpads and tails compared to DCT–LacZ mice [32] (Figure 3C, D).
Figure 3.
Hyperpigmentation in SFA/+ mice. (A) Hyperpigmentation of adult WT footpads in comparison to age-matched SFA/+ mice. (B) Hyperpigmentation of WT and SFA/+ tails. (C) DCT–LacZ transgenic mouse enables specific identification of melanocytes by β-galactosidase staining (stained in blue). Top panels represent footpads of indicated genotypes. Bottom panels represent β-galactosidase staining of the above corresponding panel. Blue melanocytes are indicated by yellow arrows. The red arrow points to representative melanin granules in SFA/+ skin. (D) Melanocyte counts/10 mm of linear epidermis [visualized by the blue LacZ-stained cells in (C)] in WT and SFA/+ footpads and tails (n = 3/genotype/tissue). p = **0.0024 and ***p < 0.0001.
Ataxia
Cerebellar ataxia is the third major defect observed in SFA/+ mice that suffered from an unsteady gait and consistent falling (see Supporting information, Figure S3). Nissl staining for neurons in 12–14 week-old adult mice revealed a pronounced reduction in cell numbers in the cerebellar granule cell layer (Figure 4A). Furthermore, Calbindin-1D28K IF staining revealed a severe disruption of the layers of the cerebellum (the molecular, Purkinje and granule layers), where Purkinje neurons were disorganized and found ectopically located throughout the cerebellum (Figure 4B). We also examined P0 and P3 cerebellums because much of normal development occurs in the first postnatal week, when granule neurons rapidly proliferate in a transient structure called the external granule layer (EGL). An abnormally small EGL was observed in SFA/+ mice, starting at P2. Quantification of BrdU+ staining of proliferating cells at P3 showed that 51% of WT EGL was proliferating versus 21% in SFA/+ mice (Figure 4C, D). We also detected increased levels of apoptosis by caspase-3 IHC staining in the SFA/+ EGL (Figure 4C). In conclusion, these data suggest that abnormally low proliferation and high apoptosis levels in developing granule neurons significantly contribute to the SFA/+ defect, resulting in ataxia.
Figure 4.
Cerebellar ataxia in SFA/+ mice. (A) Nissl stains of mid-sagittal sections of WT and SFA/+ adult (P56) cerebellum. (B) Calbindin-1D28K IF staining of the Purkinje cells. Disorganization of Purkinje neurons found ectopically located throughout the cerebellum of P56 SFA/+ mouse. M, molecular layer showing staining of Purkinje dendrites; P, Purkinje cell bodies; G, granule layer. (C) Top panel, proliferation measured by BrdU (green)/PI (red) IF on mid-sagittal sections of P3 cerebellums; white line delineates the granule layer; bottom panel, apoptosis assayed by caspase-3 IHC staining; apoptotic cells are indicated by black arrows. (D) Quantitation of % BrdU+ cells/field of P3 WT and SFA/+ cerebellums (n = 3/genotype). *p = 0.0315.
SFA/+ mouse phenocopies and other high p53 mouse models
Based on the observation that the SFA phenotypes were rescued by the Inv(11)8Brdp53–Wnt3 balancer chromosome used in the original mutagenesis screen [21], we hypothesized that these phenotypes were p53-dependent. In support of this, we observed that the BM depletion and cerebellar granule neuron deficiency found in affected SFA/+ mice bore a striking histological similarity (Figure 5A, B) to the Mdm2+/−:Mdm4+/− high p53 mouse model [20] and to Mdm2-null mice carrying the p53 R172P mutation (Mdm2−/−:p53R172P/P mice [33]). Histological sections of BMs show that SFA/+, Mdm2+/−:Mdm4+/− and Mdm2−/−:p53R172P/P mice have a hypocellularity indicative of aplastic anaemia (Figure 2C, 5A), as in the Mdm2 hypomorphic mouse (Mdm2puro/Δ7–12), another high p53 mouse model [18]. Also, all three SFA/+ Mdm2+/−:Mdm4+/− and Mdm2−/−:p53R172P/P cerebellums were small compared to WT, with considerably reduced numbers of granule neurons and disorganized distribution of Purkinje neurons (Figure 4B, 5B). Interestingly, Mdm2+/−:Mdm4+/− mice had heavily pigmented footpads, detectable first at P4, identical to those observed in SFA/+ mice (Figure 5C). We carried out a closer examination of other p53 pathway mouse models, such as Mdm2+/− [34], two different loss-of-function alleles of Mdm4: Mdm4+/− (deletion of part of introns 2–5) [19], Mdm4+/Δ2 (deletion of exon 2) [35], and Mdm2+/−:Mdm4+/Δ2 mice. We identified a mild footpad hyperpigmentation beginning at around 4–6 months of age in Mdm2+/− and Mdm4+/Δ2 mice, and a stronger hyperpigmentation at around 3–4 weeks in Mdm4+/− mice and in early postnatal development in Mdm2+/−:Mdm4+/Δ2 mice (Figure 5C and not shown). In conclusion, all high p53 mouse models that we examined had hyperpigmented extremities.
Figure 5.
Low Rpl27a mouse phenocopies high p53 mouse models. (A) H&tE of femur showing the BM in P15 WT, severely affected SFA/+ and Mdm2+/−:Mdm4+/− mouse (high p53). (B) H&tE of cerebellum in WT, severely affected SFA/+ and Mdm2+/−:Mdm4+/− mice. (C) Footpads from adult mice of p53 pathway models. *Mdm4-null allele with deletion of exon 2. $Mdm4-null allele with deletion of part of introns 2–5.
Since it has been shown that hypomorphic Mdm2 (Mdm2puro/Δ7–12), Mdm2+/Δ7–12, Mdm2+/− and Mdm4+/− mice are highly sensitive to γ-IR [18,20], we hypothesized that SFA/+ mice would be similarly radiosensitive. A cohort of WT and least affected SFA/+ littermates (near-normal body weight and minimal signs of ataxia) were subjected to a sub-lethal dose of γ-IR (6 Gy. SFA/+ mice were highly sensitive to irradiation and died <20 days post-treatment, whereas WT littermates survived (see Supporting information, Figure S4A). A more than two-fold increase in p53-positive cells was seen in the SFA/+ versus WT thymi after irradiation (see Supporting information, Figure S4B, C), indicating that the SFA mutation may synergize with DNA damage to stabilize p53, contributing to this radiosensitivity.
The SFA phenotype is rescued by haploinsufficient p53
To directly test the contribution of p53 to the SFA phenotype, we crossed SFA/+ mice to p53+/− mice. We compared the degree of ataxia in 10 week-old SFA/+, p53+/− and p53+/−:SFA/+ littermates, using the rotarod assay (Figure 6A), which tests for balance and motor coordination. We noticed that the SFA/+ mice lacked motor coordination and were generally underweight, whereas p53+/−:SFA/+ mice performed almost identically to the p53+/− group (Figure 6A). In addition, we observed that the smaller the animal, the stronger the ataxia and the more likely it was to have anaemia and early death.
Figure 6.
Genetic interaction between Rpl27a and p53 and impact of low Rpl27a on survival. (A) Motor coordination ranges of mice of designated genotypes, measured by the rotarod assay. Latency to fall on the final rotarod trial for each 10 week-old mouse is plotted versus the weight of the mouse. Boxes designate the ranges for weight and scores/genotype. (B) The impact of different p53 genotypes on pigmentation of SFA/+ footpads. (C) Kaplan–Meier survival curve (Log-rank test, p = 0.001) between p53+/−:SFA/ and p53+/− curves.
Histological examination of p53+/−:SFA/+ mice showed no deficiencies in either the BM or cerebellar compartments. However, the hyperpigmentation phenotype was not fully rescued and a complete rescue was only observed when both p53 alleles were lost (Figure 6B). Thus, pigmentation is strongly influenced by sub-physiological levels of Rpl27a and is a p53-dependent phenotype, as seen in Rps19 and Rps20 mice [12].
Given that all major phenotypes of the SFA/+ mouse are p53-dependent, we investigated whether deletion of both p53 alleles could rescue the embryonic lethality of SFA/SFA mice. We intercrossed p53+/−:SFA/+ mice, and no p53−/−:SFA/SFA mice were observed+out of over 100 offspring (see Supporting information, Table S4), indicating that a homozygous reduction in levels of Rpl27a resulted in p53-independent lethality.
Low Rpl27a increases survival of p53+/− mice, yet promotes metastasis
Given that SFA/+ mice have increased p53 activity compared to WT mice, we examined the role of low Rpl27a in tumorigenesis by monitoring p53+/−:SFA/+ and p53+/− mice. Reduced Rpl27a levels significantly delayed the tumour onset and increased survival in the p53+/− background (Figure 6C). Low Rpl27a increased the median survival of p53+/− mice (538 days) compared to p53+/− mice (487 days). Moreover, we observed tumours starting at ~13 months in the p53+/−:SFA/+ cohort, while p53+/− tumours started at ~9 months of age. Examining the tumour spectrum, we noticed that the percentage of sarcomas and carcinomas were similar in both cohorts, while lymphomas slightly decreased (8.5%) and tumour type multiplicity increased (9.9%; Table 1) in p53+/−:SFA/+ mice. Further, metastasis greatly increased from 3.7% (p53+/−) to 22% in p53+−:SFA/+ tumours. If we only considered osteosarcoma, this increase was further elevated (33.3%; Table 1).
Table 1.
Tumour spectrum in p53+/− and p53+/−:SFA/+ mice
| p53+/− (n = 29) |
p53+/−:SFA/+ (n = 36) |
|
|---|---|---|
| Lymphoma | 9 (23.7%) | 9 (15.2%) |
| Sarcoma | 21 (55.3%) | 30 (50.8%) |
| Unclassified | 8 (21.08%) | 8*1 (13.5%) |
| Osteosarcoma | 12*1 (31.6%) | 21*7 (35.6%) |
| Rhabdomyosarcoma | 1 (2.6%) | 1 (1.7%) |
| Carcinoma | 6 (15.8%) | 11 (18.6%) |
| Unclassified | 0 | 1 (1.7%) |
| Squamous cell | 5 (13.2%) | 7 (11.8%) |
| Adenocarcinoma | 1 (2.6%) | 3*1 (5.1%) |
| Other | 2 (5.3%) | 9 (15.2%) |
| Adenoma | 2 (5.3%) | 3 (5.1%) |
| Lipoma | 0 | 2 (3.4%) |
| Leiomyoma | 0 | 1 (1.7%) |
| Plasmacytoma | 0 | 2 (3.4%) |
| Haemangioma | 0 | 1 (1.7%) |
| Tumours total | 38 | 59 |
| Tumours/mouse | 1.31 | 1.64 |
| Mice with multiple primaries | 8 (27%) | 12 (33%) |
| Metastasis | 1 (3.7%) | 9 (22%) |
| Metastasis from osteosarcoma | 1 (8.3%) | 8 (33.3%) |
Number of tumours that metastasized.
p53 pathway signalling in SFA/+ mice
We checked p53 protein levels by IHC staining in SFA/+ P14 BM (n = 2) and P3 cerebellums (n = 3). We observed a widespread but variable stabilization of p53 in the BM (see Supporting information, Figure S5) and a more localized increase in p53 protein in the EGL (Figure 7A). ISH on P3 brain sections of the p53 pathway members Mdm2, Mdm4, p53 and Cdkn1a were performed, and we found that Mdm2 and Mdm4 were broadly expressed in the cerebellum, hampering reliable detection of expression differences (Figure 7B). Interestingly, the EGL showed the highest levels of p53 in both WT and SFA/+ brains, suggesting that SFA phenotypic defects involve sites of elevated p53 expression during development (Figure 7B). Moreover, Cdkn1a was elevated in the SFA/+ EGL (Figure 7B). qPCR quantification revealed a significant increased expression of p53 by 1.4-fold, Mdm2 by 3.5-fold, Cdkn1a by ~2.1-fold and Puma by 2.5-fold in SFA/+ compared to WT mice (Figure 7C). Since Mdm2 was elevated concurrently with p53 in the SFA/+ cerebellum, it appears that, in the context of low Rpl27a, Mdm2 is less efficient in degrading p53.
Figure 7.
p53 pathway signalling in P3 SFA/+ cerebellums. (A) Detection of p53 immunopositivity in mid-sagittal SFA/+ cerebellum by IHC. (B) ISH of p53, its negative regulators Mdm2 and Mdm4, and target gene Cdkn1a on WT and SFA/+ cerebellum. (C) Histogram expression of fold expression of indicated genes (mean + SEM, n = 6/genotype) in SFA/+ cerebellums, measured in triplicate in comparison to levels in WT cerebellums (indicated by the dotted line that is set at 1). p = 0.017 (p53); p = 0.0240 (Mdm2); p = 0.0002 (Cdkn1a); and p = 0.0168 (Puma).
To examine the interplay of Rpl27a with the p53 pathway, we checked whether haploinsufficiency of either Mdm2 or Mdm4 may increase the severity of the SFA/+ phenotype. We crossed the SFA/+ mice with Mdm2+/Δ7–12 [18] or Mdm4+/− mice [19]. Of 40 progeny from each cross, no Mdm2+/Δ7–12:SFA/+ (see Supporting information, Table S5) or Mdm4+/−+:SFA/+ progeny were born (see Supporting information, Table S6), showing the genetic interaction between Rpl27a and Mdm2 and Mdm4 during development.
Discussion
This study identifies a new high p53 mouse model characterized by low Rpl27a expression and bearing the four known p53-dependent phenotypes: hyperpigmentation [12], anaemia [12,18,20,33], cerebellar ataxia [20,33] and radiosensitivity [18,20]. In this mouse, the reduction in Rpl27a expression is due to a mutation located in the final splice acceptor of the gene. The mode of action of this mutation was comparable to other ribosomal nonsense destabilizing mutations [36]. Low Rpl27a resulted in raised p53 levels, driving the system closer to a survival threshold that is crossed with HSC depletion in severely affected mice. While this resulted in pancytopenia, the p53-dependent red blood cell anaemia in mutant mice of Rps19 and Rps20 (dominant loss-of-function) was reported as an erythrocyte specific lineage defect [12], rather than a stem cell shortage.
In addition to HSC depletion, significant down-regulation of c-Kit expression was noted in residual SFA/+ HSCs, indicating that c-Kit may be a transcriptional target of p53 in the BM, as previously been observed for melanocytes [37]. Interestingly, a c-Kit loss-of-function mutant mouse had significantly reduced HSCs, causing early postnatal lethality [38], suggesting that p53-dependent down-regulation of c-Kit may be the mechanism causing early postnatal lethality in severely affected SFA/+ mice. We also observed an increase in HSC proliferation with a significant increase in apoptosis in severely affected mice. Interestingly, as HSC numbers decreased, HSC proliferation increased, suggesting a compensatory mechanism as seen in mice lacking Mdm2 or Mdm4 in the intestinal epithelium with elevated p53 levels [39,40]. Nevertheless, we have not directly examined the cell autonomous nature of this effect in our mouse model. It is of interest to note that a p53-dependent effect on stem cells or proliferating progenitor cells is an emerging paradigm, explaining developmental defects associated with high p53 mouse models [12,18,20,33], and is concordant with increased HSC numbers observed in p53-null mouse BM [25,41,42].
In contrast to the low penetrant defect of the BM, the hyperpigmentation is fully penetrant and is attributed to the stabilization of keratinocyte p53, resulting in the release of Kitlg and other paracrine factors, inducing an increase in melanocyte numbers ( [12] and unpublished observations). Thus, p53 may regulate members of the c-Kit signalling pathway differently, depending on the tissue context. On the other hand, in the developing SFA/+ cerebellum, stabilized p53 in progenitor EGL neurons induced Puma and Cdkn1a expression, resulting in apoptosis and cell cycle arrest. A p53-dependent involvement of c-Kit or Kitlg in the ataxia is less likely, especially since c-Kit appears to be primarily expressed in the molecular layer [43].
Together, these phenotypes are due to a developmental interruption of the negative regulation of p53, suggesting that normal Rpl27a levels are required to efficiently maintain viable p53 levels during development. Moreover, the variable degree of severity of SFA/+ defects is likely due to different expression levels of Rpl27a, depending on the affected tissue type and developmental stage. This also suggests that a number of cellular and biochemical mechanisms operate to couple the level of this ribosomal protein with p53 levels. We observed no effect on ribosome biogenesis, since low Rpl27a phenotypes are completely rescued by homozygous p53 deletion.
The strong phenocopy of low Rpl27a mice with p53 pathway models [18,20,33] and its synthetic lethality with haploinsufficient Mdm2/Mdm4 suggests that Rpl27a might well be acting through Mdm2/Mdm4 to influence p53 protein levels, as is the case of ribosomal proteins RPL5, RPS7, RPL11 and RPL23 [1,3,6,7,9].
Since alterations in ribosomal protein levels (RNA/protein) or stoichiometry are an obligate event in cellular transformation, where many ribosomal proteins are over-expressed to facilitate rapid cell cycle progression, p53 may be recruited in mammalian ribosomal surveillance to suppress transformation. Thus, we sought to test the in vivo impact of reduced Rpl27a levels on transformation. In p53+/−:SFA/+ mice, we observed a statistically significant delay+in tumour onset, and an extended survival compared to p53+/− mice. This improvement in survival constitutes another functional evidence of high p53 levels in SFA/+ mice, as found in Eμ-myc-driven Mdm2 or Mdm4 haploin-sufficient mice [20,44]. In contrast, p53+/−:Mdm4+/− mice developed tumours sooner than p53+/− mice, which is believed to be through a p53-independent role of Mdm4 [45]. Surprisingly, reduced Rpl27a levels in p53+/−:SFA/+ mice also inherently conferred a higher risk for metastatic conversion, concomitant with the extended survival (~18% increase in metastasis from overall tumours and ~25% from osteosarcomas). The fact that no difference in tumour onset, spectrum or metastasis was reported for Rpl24-deficient mice on a p53+/− background suggests that the role of Rpl27a in transformation and prevention of malignant conversion may not be a generalized feature of ribosomal proteins [46]. This metastasis promoter effect of Rpl27a underlines a p53-independent role of this protein that is more complex and will require further investigation, especially in light of RPL27A being over-expressed/amplified in a number of tumour types [47–49] and cancer cell lines [50].
In conclusion, the SFA/+ mouse offers a unique opportunity to study the effects + of the p53 gradient in many tissue systems, with a particular interest in the role of Rpl27a in ageing, stem cell function and tumour development.
Supplementary Material
Acknowledgment
Dr Paul Overbeek generously provided the DCT–LacZ transgenic mouse. We thank Dr Lynne Bemis for technical help, advice and reagents on 3′ RACE, and Dr Chris Hogan for helpful discussions. We are grateful to Victoria Gonzalez and Regan Stiegmann for technical help. We thank Christopher Threeton and Karen Helm for their expert technical assistance in flow cytometry. We also thank Steven Robinson and Irene Choi for critical reading of the manuscript. This study was supported by a Dermatology Foundation RCDA and an American Skin Association CDA to NB. LAD was funded by the Ellison Medical Foundation and MJJ by grants U01 HD39372 and R01 CA115503 from NIH/NCI respectively. DRR was supported by NIH/NCI (# CA52607-18 and CA105491-05).
Abbreviations
- BM
bone marrow
- BrdU
bromodeoxyuridine
- ENU
N-ethyl-N-nitrosourea
- HSC
haematopoetic stem cells
Footnotes
Author contributions TT performed experiments, analysed data, prepared figures and wrote the paper; MD performed HSC analysis by FACS; CT performed ISH and Nissl staining; LAD contributed to financial support and in FACS analysis assay design; FA performed the histopathology; GL provided mice and contributed to the phenotypic assay; MJJ provided mice and financial support; DRR provided financial support and helpful discussions; NB conceived and performed experiments, analysed data, provided financial support and contributed to writing. All authors had final approval of the submitted and published versions.
SUPPORTING INFORMATION ON THE INTERNET The following supporting information may be found in the online version of this article:
Supplementary materials and methods Table S1. No SFA/SFA genotype observed in progeny from the cross SFA/+ × SFA/+. Embryonic lethality of SFA homozygous mice.
Table S2. Phenotypes observed in progeny of intercross SFA/+ mice with two BAC transgenics expressing Rpl27a from its genomic locus.
Table S3. Complete blood count of WT and SFA/+ mice from peripheral blood.
Table S4. Genotypes of mice born from a p53+/− × SFA/+ intercross.
Table S5. No Mdm2+/Δ7–12:SFA/+ genotype observed in progeny from SFA/+ × Mdm2+/Δ7–12 cross.
Table S6. No Mdm4+/−:SFA/+ genotype observed in progeny from SFA/+ × Mdm4+/− cross.
Figure S1. Coverage area of the Rpl27a gene by the two BACs used and levels of Rpl27a in transgenic footpad skin in comparison to WT tissues.
Figure S2. An example of gating for HSCs in a WT mouse. HSCs sorting of cells by lineage (c-Kit/Sca-1) and PI staining of BMs of mice.
Figure S3. SFA/+ mice suffer from an unsteady gait in comparison to WT mice. Record of SFA/+ walking with ink footprints.
Figure S4. SFA/+ mice are sensitive to γ-IR compared to WT mice. Kaplan–Meier survival curve of SFA/+ and WT mice after 6 Gy of IR and IF staining for p53 on irradiated and non-irradiated thymi.
Figure S5. p53 immunopositivity in SFA/+ bone marrow. p53 IHC staining in WT and affected SFA/+ tissues.
No conflicts of interest were declared.
References
- 1.Marechal V, Elenbaas B, Piette J, et al. The ribosomal L5 protein is associated with mdm-2 and mdm-2–p53 complexes. Mol Cell Biol. 1994;14:7414–7420. doi: 10.1128/mcb.14.11.7414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Walker G, Box N. Ribosomal stress, p53 activation and the tanning response. Exp Rev Dermatol. 2008;3:649–656. doi: 10.1586/17469872.3.6.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen D, Zhang Z, Li M, et al. Ribosomal protein S7 as a novel modulator of p53-MDM2 interaction: binding to MDM2, stabilization of p53 protein, and activation of p53 function. Oncogene. 2007;26:5029–5037. doi: 10.1038/sj.onc.1210327. [DOI] [PubMed] [Google Scholar]
- 4.Yadavilli S, Mayo LD, Higgins M, et al. Ribosomal protein S3: a multi-functional protein that interacts with both p53 and MDM2 through its KH domain. DNA Repair (Amst) 2009;8:1215–1224. doi: 10.1016/j.dnarep.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang Y, Wolf GW, Bhat K, et al. Ribosomal protein L11 negatively regulates oncoprotein MDM2 and mediates a p53-dependent ribosomal-stress checkpoint pathway. Mol Cell Biol. 2003;23:8902–8912. doi: 10.1128/MCB.23.23.8902-8912.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jin A, Itahana K, O’Keefe K, et al. Inhibition of HDM2 and activation of p53 by ribosomal protein L23. Mol Cell Biol. 2004;24:7669–7680. doi: 10.1128/MCB.24.17.7669-7680.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dai MS, Lu H. Inhibition of MDM2-mediated p53 ubiquitination and degradation by ribosomal protein L5. J Biol Chem. 2004;279:44475–44482. doi: 10.1074/jbc.M403722200. [DOI] [PubMed] [Google Scholar]
- 8.Bhat KP, Itahana K, Jin A, et al. Essential role of ribosomal protein L11 in mediating growth inhibition-induced p53 activation. EMBO J. 2004;23:2402–2412. doi: 10.1038/sj.emboj.7600247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lohrum MA, Ludwig RL, Kubbutat MH, et al. Regulation of HDM2 activity by the ribosomal protein L11. Cancer Cell. 2003;3:577–587. doi: 10.1016/s1535-6108(03)00134-x. [DOI] [PubMed] [Google Scholar]
- 10.Castro ME, Leal JF, Lleonart ME, et al. Loss-of-function genetic screening identifies a cluster of ribosomal proteins regulating p53 function. Carcinogenesis. 2008;29:1343–1350. doi: 10.1093/carcin/bgm302. [DOI] [PubMed] [Google Scholar]
- 11.Panic L, Tamarut S, Sticker-Jantscheff M, et al. Ribosomal protein S6 gene haploinsufficiency is associated with activation of a p53-dependent checkpoint during gastrulation. Mol Cell Biol. 2006;26:8880–8891. doi: 10.1128/MCB.00751-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McGowan KA, Li JZ, Park CY, et al. Ribosomal mutations cause p53-mediated dark skin and pleiotropic effects. Nat Genet. 2008;40:963–970. doi: 10.1038/ng.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Anderson SJ, Lauritsen JP, Hartman MG, et al. Ablation of ribosomal protein L22 selectively impairs αβ T cell development by activation of a p53-dependent checkpoint. Immunity. 2007;26:759–772. doi: 10.1016/j.immuni.2007.04.012. [DOI] [PubMed] [Google Scholar]
- 14.Barkic M, Crnomarkovic S, Grabusic K, et al. The p53 tumor suppressor causes congenital malformations in Rpl24-deficient mice and promotes their survival. Mol Cell Biol. 2009;29:2489–2504. doi: 10.1128/MCB.01588-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Danilova N, Sakamoto KM, Lin S. Ribosomal protein S19 deficiency in zebrafish leads to developmental abnormalities and defective erythropoiesis through activation of p53 protein family. Blood. 2008;112:5228–5237. doi: 10.1182/blood-2008-01-132290. [DOI] [PubMed] [Google Scholar]
- 16.Panic L, Montagne J, Cokaric M, et al. S6-haploinsufficiency activates the p53 tumor suppressor. Cell Cycle. 2007;6:20–24. doi: 10.4161/cc.6.1.3666. [DOI] [PubMed] [Google Scholar]
- 17.Takagi M, Absalon MJ, McLure KG, et al. Regulation of p53 translation and induction after DNA damage by ribosomal protein L26 and nucleolin. Cell. 2005;123:49–63. doi: 10.1016/j.cell.2005.07.034. [DOI] [PubMed] [Google Scholar]
- 18.Mendrysa SM, McElwee MK, Michalowski J, et al. mdm2 Is critical for inhibition of p53 during lymphopoiesis and the response to ionizing irradiation. Mol Cell Biol. 2003;23:462–472. doi: 10.1128/MCB.23.2.462-473.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Parant J, Chavez-Reyes A, Little NA, et al. Rescue of embryonic lethality in Mdm4-null mice by loss of Trp53 suggests a nonoverlapping pathway with MDM2 to regulate p53. Nat Genet. 2001;29:92–95. doi: 10.1038/ng714. [DOI] [PubMed] [Google Scholar]
- 20.Terzian T, Wang Y, Van Pelt CS, et al. Haploinsufficiency of Mdm2 and Mdm4 in tumorigenesis and development. Mol Cell Biol. 2007;27:5479–5485. doi: 10.1128/MCB.00555-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kile BT, Hentges KE, Clark AT, et al. Functional genetic analysis of mouse chromosome 11. Nature. 2003;425:81–86. doi: 10.1038/nature01865. [DOI] [PubMed] [Google Scholar]
- 22.Jacks T, Remington L, Williams BO, et al. Tumor spectrum analysis in p53-mutant mice. Curr Biol. 1994;4:1–7. doi: 10.1016/s0960-9822(00)00002-6. [DOI] [PubMed] [Google Scholar]
- 23.Grier JD, Xiong S, Elizondo-Fraire AC, et al. Tissue-specific differences of p53 inhibition by Mdm2 and Mdm4. Mol Cell Biol. 2006;26:192–198. doi: 10.1128/MCB.26.1.192-198.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhao S, Overbeek PA. Tyrosinase-related protein 2 promoter targets transgene expression to ocular and neural crest-derived tissues. Dev Biol. 1999;216:154–163. doi: 10.1006/dbio.1999.9480. [DOI] [PubMed] [Google Scholar]
- 25.Dumble M, Moore L, Chambers SM, et al. The impact of altered p53 dosage on hematopoietic stem cell dynamics during aging. Blood. 2007;109:1736–1742. doi: 10.1182/blood-2006-03-010413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yaylaoglu MB, Titmus A, Visel A, et al. Comprehensive expression atlas of fibroblast growth factors and their receptors generated by a novel robotic in situ hybridization platform. Dev Dyn. 2005;234:371–386. doi: 10.1002/dvdy.20441. [DOI] [PubMed] [Google Scholar]
- 27.Zheng B, Sage M, Cai WW, et al. Engineering a mouse balancer chromosome. Nat Genet. 1999;22:375–378. doi: 10.1038/11949. [DOI] [PubMed] [Google Scholar]
- 28.Zheng B, Vogel H, Donehower LA, et al. Visual genotyping of a coat color tagged p53 mutant mouse line. Cancer Biol Ther. 2002;1:433–435. doi: 10.4161/cbt.1.4.24. [DOI] [PubMed] [Google Scholar]
- 29.Kusuda J, Hirai M, Tanuma R, et al. Genomic structure and chromosome location of RPL27A/Rpl27a, the genes encoding human and mouse ribosomal protein L27A. Cytogenet Cell Genet. 1999;85:248–251. doi: 10.1159/000015303. [DOI] [PubMed] [Google Scholar]
- 30.Christensen JL, Weissman IL. Flk-2 is a marker in hematopoietic stem cell differentiation: a simple method to isolate long-term stem cells. Proc Natl Acad Sci USA. 2001;98:14541–14546. doi: 10.1073/pnas.261562798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mackenzie MA, Jordan SA, Budd PS, et al. Activation of the receptor tyrosine kinase Kit is required for the proliferation of melanoblasts in the mouse embryo. Dev Biol. 1997;192:99–107. doi: 10.1006/dbio.1997.8738. [DOI] [PubMed] [Google Scholar]
- 32.Fitch KR, McGowan KA, van Raamsdonk CD, et al. Genetics of dark skin in mice. Genes Dev. 2003;17:214–228. doi: 10.1101/gad.1023703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu G, Terzian T, Xiong S, et al. The p53–Mdm2 network in progenitor cell expansion during mouse postnatal development. J Pathol. 2007;213:360–368. doi: 10.1002/path.2238. [DOI] [PubMed] [Google Scholar]
- 34.Montes de Oca Luna R, Wagner DS, Lozano G. Rescue of early embryonic lethality in mdm2-deficient mice by deletion of p53. Nature. 1995;378:203–206. doi: 10.1038/378203a0. [DOI] [PubMed] [Google Scholar]
- 35.Xiong S, Van Pelt CS, Elizondo-Fraire AC, et al. Synergistic roles of Mdm2 and Mdm4 for p53 inhibition in central nervous system development. Proc Natl Acad Sci USA. 2006;103:3226–3231. doi: 10.1073/pnas.0508500103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mitrovich QM, Anderson P. Unproductively spliced ribosomal protein mRNAs are natural targets of mRNA surveillance in C. elegans. Genes Dev. 2000;14:2173–2184. doi: 10.1101/gad.819900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Terzian T, Torchia EC, Dai D, et al. p53 prevents progression of nevi to melanoma predominantly through cell cycle regulation. Pigment Cell Melanoma Res; 23:781–794. doi: 10.1111/j.1755-148X.2010.00773.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Takeda S, Shimizu T, Rodewald HR. Interactions between c-kit and stem cell factor are not required for B-cell development in vivo. Blood. 1997;89:518–525. [PubMed] [Google Scholar]
- 39.Valentin-Vega YA, Box N, Terzian T, et al. Mdm4 loss in the intestinal epithelium leads to compartmentalized cell death but no tissue abnormalities. Differentiation. 2009;77:442–449. doi: 10.1016/j.diff.2009.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Valentin-Vega YA, Okano H, Lozano G. The intestinal epithelium compensates for p53-mediated cell death and guarantees organismal survival. Cell Death Differ. 2008;15:1772–1781. doi: 10.1038/cdd.2008.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu Y, Elf SE, Asai T, et al. The p53 tumor suppressor protein is a critical regulator of hematopoietic stem cell behavior. Cell Cycle. 2009:8. doi: 10.4161/cc.8.19.9627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen J, Ellison FM, Keyvanfar K, et al. Enrichment of hematopoietic stem cells with SLAM and LSK markers for the detection of hematopoietic stem cell function in normal and Trp53-null mice. Exp Hematol. 2008;36:1236–1243. doi: 10.1016/j.exphem.2008.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Morii E, Hirota S, Kim HM, et al. Spatial expression of genes encoding c-kit receptors and their ligands in mouse cerebellum as revealed by in situ hybridization. Brain Res Dev Brain Res. 1992;65:123–126. doi: 10.1016/0165-3806(92)90016-p. [DOI] [PubMed] [Google Scholar]
- 44.Alt JR, Greiner TC, Cleveland JL, et al. Mdm2 haplo-insufficiency profoundly inhibits Myc-induced lymphomagenesis. EMBO J. 2003;22:1442–1450. doi: 10.1093/emboj/cdg133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Matijasevic Z, Steinman HA, Hoover K, et al. MdmX promotes bipolar mitosis to suppress transformation and tumorigenesis in p53-deficient cells and mice. Mol Cell Biol. 2008;28:1265–1273. doi: 10.1128/MCB.01108-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Barna M, Pusic A, Zollo O, et al. Suppression of Myc oncogenic activity by ribosomal protein haploinsufficiency. Nature. 2008;456:971–975. doi: 10.1038/nature07449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li R, Wang H, Bekele BN, et al. Identification of putative oncogenes in lung adenocarcinoma by a comprehensive functional genomic approach. Oncogene. 2006;25:2628–2635. doi: 10.1038/sj.onc.1209289. [DOI] [PubMed] [Google Scholar]
- 48.Stronach EA, Sellar GC, Blenkiron C, et al. Identification of clinically relevant genes on chromosome 11 in a functional model of ovarian cancer tumor suppression. Cancer Res. 2003;63:8648–8655. [PubMed] [Google Scholar]
- 49.Varis A, Wolf M, Monni O, et al. Targets of gene amplification and overexpression at 17q in gastric cancer. Cancer Res. 2002;62:2625–2629. [PubMed] [Google Scholar]
- 50.Ross DT, Scherf U, Eisen MB, et al. Systematic variation in gene expression patterns in human cancer cell lines. Nat Genet. 2000;24:227–235. doi: 10.1038/73432. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.