Abstract
Background
Previous studies suggest that semicircular canal dehiscences (SCDs) have a developmental origin.
Objective
We hypothesized that if SCDs originate during development, incidence of radiographic SCDs in young children will be higher than in adults.
Materials and methods
Thirty-four temporal bone HRCTs of children younger than 2 years and 40 temporal bone HRCTs of patients older than 18 years were reformatted and re-evaluated for presence of SCD or canal thinning. Results were compared with indications for HRCT and clinical information.
Results
SCDs were detected in 27.3% of children younger than 2 years of age (superior, 13.8%; posterior, 20%) and in 3% of adults (P≤0.004). Of children with one radiographic dehiscence, 55.6% had multiple and 44% had bilateral SCDs on HRCT. No lateral canal SCDs were present. Thinning of bone overlying the semicircular canals was found in 44% of children younger than 2 years and 2.5% of adults (P<0.0001).
Conclusion
SCDs are more common on HRCTs of very young children. This supports the hypothesis that SCDs originate from discontinuation of bone deposition/maturation. However, SCDs on imaging do not necessarily correlate with canal dehiscence syndrome and should therefore be interpreted carefully.
Keywords: Superior semicircular canal dehiscence, Posterior semicircular canal dehiscence, Semicircular canal dehiscence syndrome, High-resolution CT, Pediatric
Introduction
Initially described by Minor in 1998 [1], superior semicircular canal dehiscence (superior SCD) syndrome results from absence of bone overlying the superior semicircular canal. Superior SCD syndrome is characterized by vertigo following loud noises (Tullio phenomenon) and autophony; additional symptoms include hearing loss, imbalance and pulsatile tinnitus. A postmortem survey of more than 1,000 temporal bones reported the prevalence of superior SCD syndrome in adults at 0.7% [2, 3]. Similar symptoms have been attributed to posterior SCDs [4–6].
The etiology of SCD syndrome is unknown. Rarely, causative insults can be attributed to direct head injury. More frequently, dehiscences are hypothesized to result from pressure from overlying tissues or from pulsations of cerebrospinal fluid, arachnoid granulations, the sigmoid or superior petrosal sinuses [1, 2, 7]. Alternatively, SCDs may result from anomalies in bone deposition during childhood and adolescence [2].
The most commonly accepted imaging study for diagnosis of SCD syndrome is high-resolution computed tomography (HRCT) of the temporal bone [8, 9]. Due to volume averaging and resolution limits, HRCT overestimates the incidence of SCDs, yielding higher numbers than those found in pathological studies [10, 11]. Adult HRCT surveys have found a combined incidence of 2.1–8.6% of posterior and superior SCDs [4, 12]. In patients presenting with symptoms of SCD syndrome, the incidence is greater than 34% [4].
A few imaging studies have identified SCDs in children [9, 13–23]. More recently, cases of patients with lifelong histories of symptoms and documentation of SCDs on imaging, vestibular evoked myogenic potentials (vEMPs) and operative exploration have been reported [20, 24–26]. Two cases of children (one 6 years of age, the other 15 years old) with superior SCD syndrome whose symptoms improved following canal plugging demonstrate that not all cases of SCD are due to prolonged exposure to erosive forces [20, 26].
To further evaluate the possibility that SCDs have a developmental origin, we evaluated HRCTs of children ages 0–24 months and HRCTs of adults for SCDs or thinning of bone over the semicircular canals.
Materials and methods
This study was approved by the Institutional Review Board of our medical center (#11-00599). Due to its retrospective nature and low risk for patients, this study was granted exempt status from patient informed consent.
Patients
A retrospective study was performed examining high-resolution temporal bone CT scans of patients ages 0–24 months and 40 randomly selected HRCTs of patients ages 18 years and older, imaged between Jan. 1, 2006, and Nov. 1, 2010. The patients were identified via an age-and scan- search of the radiology departmental database. Thirty-four pediatric HRCTs matched these criteria (20 girls and 14 boys). For these 34 children, data including the patient’s age and indication for the scan were extracted from CT request forms. Clinic charts and audiograms of 32 of these children were also reviewed for general otological history, and, in particular, signs and symptoms consistent with SCD syndrome (two charts were not available). HRCTs of the 40 randomly selected patients 18 years and older who received HRCTs of the temporal bone during the same time period were identified (20 men and 20 women). The HRCTs of these adults were re-examined by the same protocol used for children younger than 2 years of age, and their ages and indications for scans extracted. The charts of the adults were also reviewed for general otological history and signs and symptoms consistent with SCD syndrome when available (n = 8). Inclusion criteria for this study included temporal bone HRCTs of sufficient quality to meet standards for reconstruction (as detailed below) and belonging to patients younger than 2 years or older than 18 years, performed within the described time period. Exclusion criteria included absence or atypia of the semicircular canals, presence of erosive tumor or cholesteatoma, or other pathologies affecting the anatomy of the inner ear other than semicircular canal dehiscence or enlarged vestibular aqueduct.
Imaging
CT was performed using either a Siemens Somatom Sensation 40 or Siemens Somatom Definition AS+ scanner (Siemens AG, Erlangen, Germany). Axial plane images were acquired with a section thickness of 0.6 mm, collimation of 0.5 mm, pitch of 0.6, 120 kilovolts, rotation time 1 s, field of view 16 cm and a 512 × 512 matrix. Prior to 2008, all HRCTs were performed at 200 mA and a CTDI of 52.7; all HRCTs on children since January 2008 have been performed at 154 mA and a CTDI of 23.52. The raw data was then reconstructed at 0.1 mm intervals. Reconstructions were performed in this manner to provide accurate reformation of the obtained data in the coronal plane [27, 28]. From this data, individual temporal bone images were reformatted in both the axial and coronal planes using a display field of 7 cm and a slice thickness of 0.6 mm. All images were also postprocessed to include 45° oblique Pöschl and Stenvers views to allow for optimal visualization of the semicircular canals [29, 30]. Some patients previously had HRCT images reformatted from the raw dataset in the Pöschl and Stenvers planes. If the raw dataset was no longer available, Pöschl and Stenvers views were reconstructed on the electronic picture archiving and communication system (PACS), using the submitted reformatted axial or coronal images. However, this technique was only performed if submillimeter scans were available from the original images, thus providing adequate image quality for further reassessment. Reconstructions were performed using 3-D software on the iSite Philips PACS system (iSite View Forum Applications; Philips Medical Systems, Amsterdam, Netherlands). Images were reviewed using iSite PACS.
HRCT interpretation
All collected images were evaluated independently by two attending neuroradiologists, one with 10 years of experience and the other with 5 years of experience in head and neck radiology. The neuroradiologists were blinded to patient name, age, previous radiologic evaluation and clinical history. Each canal was scored for the presence of radiographic dehiscence and thinning or normal bone thickness. In this study, dehiscence was defined as a defect in the bony covering of the semicircular canal, and thinning was defined as a barely detectable layer of bone overlying the canal. A radiographic dehiscence or thin area had to be clearly present on more than one CT view (Stenvers, Pöschl, axial or coronal) to be scored as present. Normal bone thickness was defined as clearly and easily detectable bone overlying the semicircular canal. All patients were also assessed for cochlear and/or inner ear malformations, trauma, active infection, ossicular anomalies and canal atresia. Results were tabulated and compared between the two readings for reliability. Patients with abnormalities of the inner ear (cochlear or vestibular) other than SCD were excluded from further study.
Data analysis
Pearson chi-square test was used to determine whether indications for CT, sex of the patient, or ear (left vs. right) correlated with the presence of radiographic canal dehiscence or thinning. Fisher exact test was used to determine significance of increased frequency of radiographic canal dehiscence and thinning in patients younger than 24 months of age vs. adults. Within the group of very young children, Fisher exact test was used to determine if the frequencies of apparent dehiscence on HRCT were significantly different between two groups (divided by mean or median age), and logistical regression was conducted to determine the correlation between age in months and presence of SCD. Interrater reliability for the two radiologists reading scans was determined using Cohen’s kappa. All calculations were performed using SAS 9.2 (SAS, Cary, NC) by a statistician.
Results
From January 2006–November 2010, temporal bone HRCTs were performed on 34 children ages 0–24 months at our institution (20 girls and 14 boys, average age 10 months). Indications for HRCT in children younger than 2 years included hearing loss (n = 15), preoperative for cochlear implant (n = 5), preoperative for hearing loss, procedure not specified (n = 2), preoperative with neither condition nor procedure specified (n = 3), sensorineural hearing loss (n = 1), aural atresia (n = 1), conductive hearing loss (n = 1), fever (n = 1), rule-out mastoiditis (n = 1), otitis media (n = 1), fluid around ear (n = 1) and none provided (n = 2). Review of clinic charts and audiograms was also performed for these children. Two children’s charts were not available. For the remaining 32 children, the average age at last follow-up examination was 3.4 years and the mean length of follow-up since HRCT was 16 months. Twenty-five had severe-profound sensorineural hearing loss and had unilateral or bilateral cochlear implants performed, two had microtia, aural atresia, and a 60 dB unilateral or bilateral hearing loss (one had conductive hearing loss as the indication for HRCT), one had chronic otitis media, and one had retroauricular swelling and suspicion of mastoiditis, which was ruled out on HRCT. Thus, none of the children’s hearing loss was consistent with that caused by SCDs. Only two of the children’s charts mentioned any symptoms of dizziness. One child’s dizziness consisted of three episodes of benign paroxysmal positional vertigo, while the other child’s single episode occurred while the child was on Benadryl. Neither of the children with a history of dizziness was ultimately found to have a radiographic SCD. No other signs or symptoms that could be construed as resulting from canal dehiscence syndrome were present within the children’s charts, tympanograms or audiograms [1, 4–6, 31, 32].
Forty randomly selected adults (older than 18 years of age) who had HRCTs during the same time period were included as a comparison group (20 women and 20 men, average age 53.9 years). Indications for HRCT in adults included hearing loss (n = 13), cochlear implant (n = 1), conductive hearing loss (n = 3), tinnitus (n = 2), pain (n = 2), headache (n = 1), aural fullness (n = 1), vertigo (n = 1), eight for chronic infections(cholesteatoma, n = 5; otorrhea, n = 2; tympanic membrane perforation, n = 1), five for mass/lesion/neoplasm/glomus jugulare and none provided (n = 2). Clinic charts for eight of the adults were available for further review. Five of these adults ultimately received cochlear implants for severe-profound sensorineural hearing loss, one had episodic imbalance, and one had a fluctuating hearing loss on the right and mixed hearing loss on the left. None of the adults with available clinic charts ultimately was found to have a radiographic SCD.
HRCTs were reformatted and evaluated for presence of SCDs in axial, coronal, 45° oblique, Stenvers and Pöschl views (Figs. 1 and 2). One child had absent posterior semicircular canals and was excluded from tabulation in the results. Another child’s left temporal bone images could not be found. Thus, 65 temporal bones (33 right, 32 left) of children younger than 24 months were evaluated. All adult temporal bones were evaluated (n = 80). Representative CT scans of both the posterior and superior canals are shown in Figs. 1 and 2.
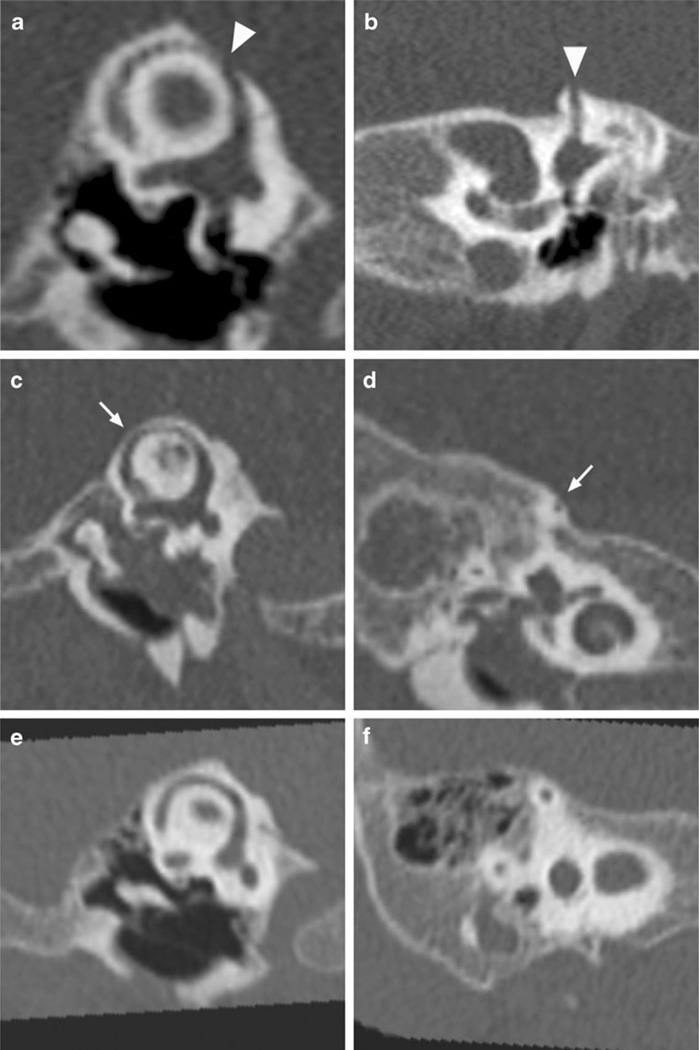
Fig. 1.
Representative high-resolution CTs of the temporal bone focusing on the superior semicircular canal demonstrate superior semicircular canal dehiscence (arrowheads) in a 9-month-old patient (a, b), superior semicircular canal thinning (thin arrow) in a 12-month-old patient (c, d) and normal bone thickness (e, f) in a 7-month-old patient as defined in this study. Shown are Pöschl (a, c, e) and Stenvers (b, d, f) views
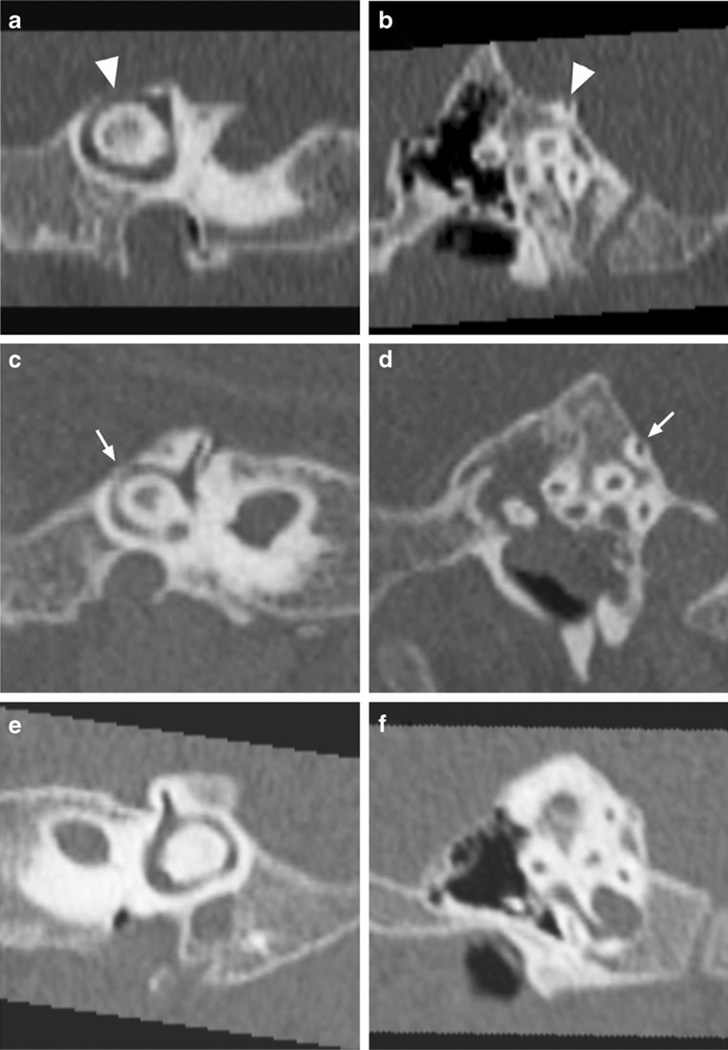
Fig. 2.
High-resolution CT scans of the temporal bone examine the posterior semicircular canal. Shown are representative posterior semicircular canal dehiscence (arrowheads) in a 9-month-old (a, b), posterior semicircular canal thinning (thin arrows) (c, d) in a 12-month-old and normal bone thickness (e, f) in a 7-month-old as measured in this study. Shown are 45° oblique (a, c, e) and sagittal (b, d, f) views
Five children younger than 2 years had significant findings other than semicircular canal anomalies. One had bilateral cochlear implants, one had unilateral aural atresia and three had fluid within the middle ear/mastoid without coalescent mastoiditis. Fourteen adults had other anomalies present on their HRCTs. Seven patients had findings of active or chronic otitis media (cholesteatoma, n = 2; soft tissue in the middle ear, n = 1; otomastoiditis, n = 2; tympanic membrane perforation, n = 1; previous mastoidectomy and TORP, n = 1). Two adults had otosclerosis and one had bilateral cochlear implants. Other findings included petrous apex effusion (n = 1), glomus jugulare (n = 1), exostoses (n = 1), soft tissue within the external auditory canal (n = 1) and high-riding jugular bulb (n = 1).
Nine children younger than 2 years of age had at least one SCD (27.3%). Superior SCDs were present in 9 (13.8%) (3 right, 6 left), while posterior SCDs were found in 13 (20%) (6 right, 7 left). Three very young children had radiographic bilateral superior and posterior canal dehiscences, and one had bilateral posterior canal dehiscences on HRCT. One child had left superior and posterior radiographic SCDs, while another had both right posterior and left superior canal SCDs on HRCT. Thus, children with one SCD were more likely to have subsequent SCDs (P< 0.0001, permutation test/10,000 permutations). Results for children younger than 2 years are summarized in Table 1.
Table 1.
Semicircular canals affected by dehiscence and thinning in children younger than 2 years of age
| Subject | Age (months) | R SSC | R PSC | L SSC | L PSC |
|---|---|---|---|---|---|
| dehiscent | |||||
| 2 | 4 | + | + | + | + |
| 5 | 6 | − | + | − | − |
| 6 | 7 | − | + | + | + |
| 10 | 8 | − | − | − | + |
| 13 | 9 | − | − | + | − |
| 14 | 9 | + | + | + | + |
| 19 | 11 | − | + | − | + |
| 20 | 13 | − | − | + | + |
| 28 | 15 | + | + | + | + |
| thinned | |||||
| 3 | 6 | + | − | + | + |
| 4 | 6 | + | − | − | − |
| 8 | 7 | + | − | + | − |
| 11 | 8 | + | + | − | − |
| 12 | 8 | + | − | − | − |
| 13 | 8 | − | + | n/a | n/a |
| 14 | 9 | + | − | − | − |
| 16 | 10 | + | − | − | − |
| 18 | 10 | − | + | − | − |
| 19 | 10 | + | − | − | − |
| 20 | 11 | + | − | + | − |
| 21 | 13 | + | + | − | − |
| 22 | 13 | + | − | − | − |
| 25 | 14 | + | − | + | − |
n/a not available
Of the 65 temporal bones of children younger than 2 years of age that were evaluated, 9 had radiographic superior SCDs (13.8%), 13 had posterior SCDs (18.4%), and 7 had dehiscences of both canals (10.8%) (Table 1). There were no horizontal SCDs. Distributions of SCDs on HRCT by side were fairly equivalent: 14% of right semicircular canals had dehiscences, while 20% of left canals had dehiscences. There was no significant correlation between side and presence of SCD (P=0.296; chi-square).
There was a trend toward decreasing frequency of radiographic SCD with increasing age for children younger than 2 years. Using logistic regression, a 1-month increase in age in very young children had a multiplicative effect of 0.87 on the odds of SCD. However, the P value was 15.23% (not significant). Similarly, if children younger than 2 years were divided into equal older and younger groups by median (10 months) or mean (11 months) age, there was a decreasing frequency of SCD with increasing age that was not significant (P=0.241, Fisher exact test).
There was no relationship between indication for HRCT and radiographic SCDs in very young children (P =.287, chi-square). This lack of correlation was maintained even when the children younger than 24 months were divided into two groups by indication for HRCT: hearing loss vs. all other indications (P=0.3500, chi-square). There was also no correlation with sex and SCDs (P=0.1164; pooled t-test).
Comparison of prevalence of SCDs between children younger than 2 years (27.3%) and adults (2.5%) revealed a significant difference between the two groups (P≤0.004, Fisher exact test; power=0.72). Only 1/40 adults had an SCD. The patient was a 62-year-old woman who presented with complaints of right pulsatile tinnitus; she had a left superior SCD.
Patients were also evaluated for thickness of the bone overlying superior and posterior semicircular canals. Of 33 children younger than 2 years, 14 (42.4%) had a very thin layer of bone overlying their superior or posterior semicircular canals. Of the 65 temporal bones of these very young children, 21 had evidence of thinning (32.3%). Thinning of right posterior and superior canals was more common than left-sided thinning, with 24% of right canals affected compared with 8% of left canals (P<0.005, chi-square).
Bilateral findings were common in children younger than 2 years of age. Four of nine children with a radiographic SCD had a dehiscence of the contralateral semicircular canal on HRCT (44%). Four of 14 children with thin bone overlying one posterior or superior semicircular canal had thinned bone overlying a contralateral canal (28%) on HRCT. Ten children younger than 2 years with thinned or dehiscent bone overlying one semicircular canal had a contralateral canal with either thin or dehiscent bone (76.9%).
There was no correlation between age and canal thinning (P=0.1127) for children ages 0–24 months. Similarly, there was no relationship between sex and canal thinning (P=0.7267).
For adults, semicircular canal thinning was extremely rare, and was noted in only one patient (2.5%). This 79-year-old man had an HRCT indication of left conductive hearing loss. He had thinning of both his left and right superior semicircular canals. Thus, there was a significantly higher rate of canal thinning in children younger than 24 months (42.4%) than in adults (2.5%) (P<0.0001, Fis'sher exact test).
Inter-evaluator reliability was excellent for the two radiologists who evaluated the scans (agreement=99.2% for children ages 0–24 months, 100% for adults), with 100% concordance for assessment of radiographic SCD in both age groups. Inter-radiologist reliability was lower but still in the good-excellent range for evaluation of canal thinning. Assessment of agreement between radiologists’ assessment of semicircular canals for both dehiscence and thinning yielded a Cohen’s kappa of 0.739 (95% confidence limits 0.626—0.853).
Discussion
The etiology of SCD syndrome is unknown. While dehiscences have been hypothesized to result from repetitive trauma from cerebrospinal fluid or vascular pulsations, they may result from a congenital or developmental etiology [1, 2, 7, 15, 16, 33–35].
Fetal temporal bone studies have found bone deposition overlying the superior semicircular canal by 23 weeks’ gestation and overlying the posterior canal by 24 weeks [3, 36]. At 24 weeks, lacunae connect perilymph with the meninges and persist until 30 weeks [3]. Normally, density of bone overlying the semicircular canals increases throughout fetal development [36]. Bone deposition is not complete until later in life [2]. Thus, SCD syndrome may result from interruption of bone deposition or maturation overlying the semicircular canals. Trauma may cause dehiscence later in life and symptoms of SCD syndrome [1, 2, 15].
As a necessary corollary of developmental etiological hypotheses, SCDs should be present in at least some children. A thinner layer of bone overlying the semicircular canals should also be present in infants and children. A few case reports and series have documented SCDs in children [9, 13–16, 18, 20]. In children with enlarged vestibular aqueduct syndrome, SCDs have been documented as early as 3 months of age by HRCT [19]. Chen et al. [18] in (1993) found a 13.7% rate of superior SCD in children ages 3–17 years. Others have not identified increased numbers of SCDs in younger patients. Nadgir et al. [37] studied HRCTs of patients ages 7 months-89 years and noted increasing incidence of superior SCDs as patient age increased. The authors interpreted this increasing incidence with age as evidence that SCDs are not the result of a congenital or developmental process [37]. There are several potential reasons that the authors have come to different conclusions than those found in this study. In the Nadgir et al. paper [37], patients were divided into separate age categories in 20-year intervals for analysis, and the numbers of patients of any given age within these age groups is not mentioned. Thus, although the authors included HRCTs of 46 patients within the 0- to 20-year category, they do not mention how many of these are children, toddlers and infants. Therefore, it is possible that the radiographic SCDs that we see with greater frequency in our patient population would not have been measured in this study because younger children have not been included in sufficient number. Also, the authors did not examine their HRCTs for evidence of dehiscences of the other semicircular canals [37].
Within our series, we have found more frequent SCDs and bony thinning of semicircular canals in children younger than 2 years of age than adults. In our series of 33 children ages 2–23 months, 27.3% had evidence of either superior and/or posterior SCDs. In comparison, only 2.5% of adults had SCDs. Thus, the prevalence of SCDs in very young children is much higher than adults in our study (P≤0.004). The rate of SCDs in children younger than 24 months is also significantly higher than the rate of posterior and superior canal dehiscences as measured by HRCT previously reported in adults (8.6 %) (x2 = 14.26, P<0.001) and older children (13 .7%) (x2 = 4.86, P<0.05) [4, 18]. We found multiple radiographic SCDs in 67% of children younger than 2 years who had a first canal dehiscence, with 44% of these SCDs present bilaterally. Furthermore, we noted a significantly higher prevalence of radiographic semicircular canal thinning in very young children (42.4%) vs. adults (2.5%) (P<0.0001). Thus, our results provide further support for the hypothesis that arrest of normal bone development over the semicircular canals may underlie SCD syndrome.
One interpretation of these results is that the increased prevalence of radiographic SCDs in children younger than 24 months of age is due to the inability of HRCT to visualize immature bone. This bone could then later form the bone coverage found in normal adults by HRCT. This possibility does not undermine the hypothesis that discontinuation of bone deposition/maturation during childhood predisposes to SCD syndrome. However, this possibility highlights the lack of equivalence between radiographic SCD, a true bony dehiscence of the semicircular canals, and canal dehiscence syndrome [2, 10, 11]. This is supported by our review of the children’s clinic charts and audiograms, which revealed no evidence of symptoms of canal dehiscence syndrome or audiological signatures of this syndrome [31, 32, 38]. In particular, overinterpretation of radiographic SCDs in children should be avoided, as presence of radiographic SCD may result from bone immaturity rather than bony dehiscence per se. Many of these radiographic dehiscences may resolve as the patient ages.
If SCDs result from a failure of bony coverage over superior or posterior semicircular canals, it remains unclear why patients typically present in adulthood [38, 39]. One possibility is that children do not present with complaints of dizziness, but instead present with ataxia, fatigue or delayed walking [26]. A second is that symptoms typical of SCD syndrome cannot be identified in children. Reports of patients with complaints of lifelong symptoms of SCD syndrome and of a child with superior SCD syndrome corrected by superior semicircular canal plugging make these arguments more probable [20, 24, 25]. Recent articles describing children with SCD syndrome presenting with hearing loss without vestibular symptoms also support this hypothesis [21, 22]. Another possibility is that while SCD of the affected semicircular canal is necessary, it is not sufficient for development of SCD syndrome [18]. Thus, potentially, a later event would be necessary to result in SCD syndrome [2, 15].
The results of our study should be interpreted in light of the study’s limitations. The number of children younger than 24 months evaluated in this study is relatively low due to our strict inclusion of temporal bone HRCT of very high quality. If submillimeter reconstructions were not available, patients were not included in our study. Other head CTs had insufficient resolution to appropriately examine the semicircular canals, and HRCTs acquired before 2006 could not be reconstructed with sufficient resolution. However, our study contains the largest population of children younger than 24 months who have been studied for SCD prevalence. Second, this is an HRCT study of SCDs. Multiple studies have noted that HRCT overcalls SCDs due to volume averaging and limitations of resolution. The clinical follow-up period was relatively short (16 months) for the children in this study. Finally, we were unable to monitor patients identified for potential closure of their SCDs, as it would be unethical to expose otherwise normal children to the radiation required for repetitive CT imaging; also, vEMP testing is impossible in these patients. Future developments in the use of MRI or ocular vestibular evoked myogenic potentials will make longitudinal studies of children with SCDs identified, via HRCT possible.
Conclusion
We have reported the largest series of very young children evaluated by temporal bone HRCT for the presence of radiographic SCDs. Compared with HRCT studies of adults, children younger than 2 years have a higher incidence of superior and posterior SCDs and a high incidence of thin bone overlying the semicircular canals. This suggests that SCDs may not exclusively result from repetitive trauma. Instead, at least some SCDs may result from persistent bony dehiscences overlying the affected semicircular canals. Symptomatic SCD syndrome may later result from the combination of bony dehiscence or thinning with other, as yet unknown, factors. However, HRCT SCDs are not equivalent to SCD syndrome and should be interpreted with caution. Particularly in children younger than 2 years of age, SCDs on HRCT should be interpreted carefully, as semicircular canal dehiscences on imaging are common at this age but decrease in prevalence during later development.
Acknowledgement
The authors would like to acknowledge Lorelei Wilkens for her assistance with this study.
Footnotes
Conflicts of interest We have no conflicts of interest to declare.
Contributor Information
Mari Hagiwara, Department of Radiology, New York University School of Medicine, New York, NY, USA.
Jamil A. Shaikh, Department of Otolaryngology, New York University School of Medicine, New York, NY, USA
Yixin Fang, Department of Otolaryngology, New York University School of Medicine, New York, NY, USA; Division of Biostatistics, Department of Environmental Medicine, New York University School of Medicine, New York, NY, USA.
Girish Fatterpekar, Department of Radiology, New York University School of Medicine, New York, NY, USA.
Pamela C. Roehm, Email: pamela.roehm@nyumc.org, Department of Otolaryngology, New York University School of Medicine, New York, NY, USA; Department of Otolaryngology, Division of Otology/Neurotology, New York University School of Medicine, 530 First Ave., Suite 7S, New York, NY 10016, USA.
References
- 1.Minor LB, Solomon D, Zinreich JS, et al. Sound- and/or pressure-induced vertigo due to bone dehiscence of the superior semicircular canal. Arch Otolaryngol Head Neck Surg. 1998;124:249–258. doi: 10.1001/archotol.124.3.249. [DOI] [PubMed] [Google Scholar]
- 2.Carey JP, Minor LB, Nager GT. Dehiscence or thinning of bone overlying the superior semicircular canal in a temporal bone survey. Arch Otolaryngol Head Neck Surg. 2000;126:137–147. doi: 10.1001/archotol.126.2.137. [DOI] [PubMed] [Google Scholar]
- 3.Crovetto de la Torre MA, Whyte Orozco J, Cisneros Gimeno AI, et al. Superior semicircular canal dehiscence syndrome. Embryological and surgical consideration. Acta Otorrinolaringol Esp. 2005;56:6–11. doi: 10.1016/s0001-6519(05)78562-9. [DOI] [PubMed] [Google Scholar]
- 4.Krombach GA, DiMartino E, Schmitz-Rode T, et al. Posterior semicircular canal dehiscence: a morphologic cause of vertigo similar to superior semicircular canal dehiscence. Eur Radiol. 2003;13:1444–1450. doi: 10.1007/s00330-003-1828-5. [DOI] [PubMed] [Google Scholar]
- 5.Gopen Q, Zhou G, Poe D, et al. Posterior semicircular canal dehiscence: first reported case series. Otol Neurotol. 2010;31:339–344. doi: 10.1097/MAO.0b013e3181be65a4. [DOI] [PubMed] [Google Scholar]
- 6.Chien WW, Carey JP, Minor LB. Canal dehiscence. Curr Opin Neurol. 2011;24:25–31. doi: 10.1097/WCO.0b013e328341ef88. [DOI] [PubMed] [Google Scholar]
- 7.Minor LB. Clinical manifestations of superior semicircular canal dehiscence. Laryngoscope. 2005;115:1717–1727. doi: 10.1097/01.mlg.0000178324.55729.b7. [DOI] [PubMed] [Google Scholar]
- 8.Belden CJ, Weg N, Minor LB, et al. CT evaluation of bone dehiscence of the superior semicircular canal as a cause of sound-and/or pressure-induced vertigo. Radiology. 2003;226:337–343. doi: 10.1148/radiol.2262010897. [DOI] [PubMed] [Google Scholar]
- 9.Krombach GA, Di Martino E, Martiny S, et al. Dehiscence of the superior and/or posterior semicircular canal: delineation on T2-weighted axial three-dimensional turbo spin-echo images, maximum intensity projections and volume-rendered images. Eur Arch Otorhinolaryngol. 2006;263:111–117. doi: 10.1007/s00405-005-0970-x. [DOI] [PubMed] [Google Scholar]
- 10.Cloutier JF, Belair M, Saliba I. Superior semicircular canal dehiscence: positive predictive value of high-resolution CT scanning. Eur Arch Otorhinolaryngol. 2008;265:1455–1460. doi: 10.1007/s00405-008-0672-2. [DOI] [PubMed] [Google Scholar]
- 11.Sequeira SM, Whiting BR, Shimony JS, et al. Accuracy of computed tomography detection of superior canal dehiscence. Otol Neurotol. 2011;32:1500–1505. doi: 10.1097/MAO.0b013e318238280c. [DOI] [PubMed] [Google Scholar]
- 12.Loke SC, Goh JP. Incidence of semicircular canal dehiscence in Singapore. Br J Radiol. 2009;82:371–373. doi: 10.1259/bjr/32471003. [DOI] [PubMed] [Google Scholar]
- 13.Faure A, Masse H, Gayet-Delacroix M, et al. What is the arcuate eminence? Surg Radiol Anat. 2003;25:99–104. doi: 10.1007/s00276-003-0102-5. [DOI] [PubMed] [Google Scholar]
- 14.Sheykholeslami K, Schmerber S, Habiby Kermany M, et al. Vestibular-evoked myogenic potentials in three patients with large vestibular aqueduct. Hear Res. 2004;190:161–168. doi: 10.1016/S0378-5955(04)00018-8. [DOI] [PubMed] [Google Scholar]
- 15.Modugno G, Brandolini C, Savastio G, et al. Superior semicircular canal dehiscence: a series of 13 cases. ORL J Otorhinolaryngol Relat Spec. 2005;67:180–184. doi: 10.1159/000086573. [DOI] [PubMed] [Google Scholar]
- 16.Zhou G, Ohlms L, Liberman J, et al. Superior semicircular canal dehiscence in a young child: implication of developmental defect. Int J Pediatr Otorhinolaryngol. 2007;71:1925–1928. doi: 10.1016/j.ijporl.2007.08.009. [DOI] [PubMed] [Google Scholar]
- 17.Paladin AM, Phillips GS, Raske ME, et al. Labyrinthine dehiscence in a child. Pediatr Radiol. 2008;38:348–350. doi: 10.1007/s00247-007-0696-6. [DOI] [PubMed] [Google Scholar]
- 18.Chen EY, Paladin A, Phillips G, et al. Semicircular canal dehiscence in the pediatric population. Int J Pediatr Otorhinolaryngol. 2009;73:321–327. doi: 10.1016/j.ijporl.2008.10.027. [DOI] [PubMed] [Google Scholar]
- 19.Ma X, Yang Y, Xia M, et al. Computed tomography findings in large vestibular aqueduct syndrome. Acta Otolaryngol. 2009;129:700–708. doi: 10.1080/00016480802412813. [DOI] [PubMed] [Google Scholar]
- 20.Wackym PA, Black FO, Siker DA. Dehiscent superior semicircular canal patients: Phenotypes, surgical findings, and outcomes. American Otological Society 144th Annual Meeting; Chicago, IL. 2011. [Google Scholar]
- 21.Kanaan AA, Raad RA, Hourani RG, et al. Bilateral superior semicircular canal dehiscence in a child with sensorineural hearing loss and without vestibular symptoms. Int J Pediatr Otorhinolaryngol. 2011;75:877–879. doi: 10.1016/j.ijporl.2011.03.019. [DOI] [PubMed] [Google Scholar]
- 22.Lee GS, Zhou G, Poe D, et al. Clinical experience in diagnosis and management of superior semicircular canal dehiscence in children. Laryngoscope. 2011;121:2256–2261. doi: 10.1002/lary.22134. [DOI] [PubMed] [Google Scholar]
- 23.Brandolini C, Modugno GC. Superior semicircular canal dehiscence and enlarged vestibular aqueduct. Int J Pediatr Otorhinolaryngol. 2011;75:861–863. doi: 10.1016/j.ijporl.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 24.Hegemann SC, Carey JP. Is superior canal dehiscence congenital or acquired? A case report and review of the literature. Otolaryngol Clin North Am. 2011;44:377–382. doi: 10.1016/j.otc.2011.01.009. [DOI] [PubMed] [Google Scholar]
- 25.Goebel JA, Carey JP, Shepard NT, et al. Challenging diagnostic dizzy dilemmas–Clinical exam and lab testing pearls. American Neurotology Society, 46th Annual Spring Meeting; Chicago, IL. 2011. [Google Scholar]
- 26.McCall AA, McKenna MJ, Merchant SN, et al. Superior canal dehiscence syndrome associated with the superior petrosal sinus in pediatric and adult patients. Otol Neurotol. 2011;32:1312–1319. doi: 10.1097/MAO.0b013e31822e5b0a. [DOI] [PubMed] [Google Scholar]
- 27.Venema HW, Phoa SS, Mirck PG, et al. Petrosal bone: coronal reconstructions from axial spiral CT data obtained with 0.5-mm collimation can replace direct coronal sequential CT scans. Radiology. 1999;213:375–382. doi: 10.1148/radiology.213.2.r99nv11375. [DOI] [PubMed] [Google Scholar]
- 28.Honda O, Johkoh T, Yamamoto S, et al. Comparison of quality of multiplanar reconstructions and direct coronal multidetector CT scans of the lung. AJR. 2002;179:875–879. doi: 10.2214/ajr.179.4.1790875. [DOI] [PubMed] [Google Scholar]
- 29.Ceylan N, Bayraktaroglu S, Alper H, et al. CT imaging of superior semicircular canal dehiscence: added value of reformatted images. Acta Otolaryngol. 2010;130:996–1001. doi: 10.3109/00016481003602108. [DOI] [PubMed] [Google Scholar]
- 30.Lane JI, Lindell EP, Witte RJ, et al. Middle and inner ear: improved depiction with multiplanar reconstruction of volumetric CT data. Radiographics. 2006;26:115–124. doi: 10.1148/rg.261055703. [DOI] [PubMed] [Google Scholar]
- 31.Hullar TE. Vascular pulsations on impedance audiometry as a sign of a third-mobile window lesion. Otol Neurotol. 2010;31:565–566. doi: 10.1097/MAO.0b013e3181db7324. [DOI] [PubMed] [Google Scholar]
- 32.Sone M, Katayama N, Naganawa S, et al. Audiological signs in pediatric cases with dehiscence of the bony labyrinth caused by a high jugular bulb. Int J Pediatr Otorhinolaryngol. 2012;76:447–451. doi: 10.1016/j.ijporl.2011.12.007. [DOI] [PubMed] [Google Scholar]
- 33.Hirvonen TP, Weg N, Zinreich SJ, et al. High-resolution CT findings suggest a developmental abnormality underlying superior canal dehiscence syndrome. Acta Otolaryngol. 2003;123:477–481. doi: 10.1080/0036554021000028099. [DOI] [PubMed] [Google Scholar]
- 34.Tsunoda A, Terasaki O. Dehiscence of the bony roof of the superior semicircular canal in the middle cranial fossa. J Laryngol Otol. 2002;116:514–518. doi: 10.1258/002221502760132377. [DOI] [PubMed] [Google Scholar]
- 35.Potyagaylo VL, Della Santina CC, Minor LB, et al. Superior canal dehiscence is not due to cephalic displacement of the labyrinth. Ann N Y Acad Sci. 2005;1039:498–502. doi: 10.1196/annals.1325.053. [DOI] [PubMed] [Google Scholar]
- 36.Richard C, Laroche N, Malaval L, et al. New insight into the bony labyrinth: a microcomputed tomography study. Auris Nasus Larynx. 2010;37:155–161. doi: 10.1016/j.anl.2009.04.014. [DOI] [PubMed] [Google Scholar]
- 37.Nadgir RN, Ozonoff A, Devaiah AK, et al. Superior semicircular canal dehiscence: congenital or acquired condition? AJNR. 2011;32:947–949. doi: 10.3174/ajnr.A2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Minor LB, Cremer PD, Carey JP, et al. Symptoms and signs in superior canal dehiscence syndrome. Ann N Y Acad Sci. 2001;942:259–273. doi: 10.1111/j.1749-6632.2001.tb03751.x. [DOI] [PubMed] [Google Scholar]
- 39.Zhou G, Gopen Q, Poe DS. Clinical and diagnostic characterization of canal dehiscence syndrome: a great otologic mimicker. Otol Neurotol. 2007;28:920–926. [PubMed] [Google Scholar]