Abstract
Inflammation is critical for atherosclerosis development and may be a target for risk-reduction therapy. In experimental studies, activation of the inflammatory regulator, nuclear factor κB (NFκB), contributes to endothelial activation and reduced nitric oxide production. We treated patients with coronary artery disease with sulfasalazine, an inhibitor of NFκB, and placebo in a randomized, double-blind, crossover design. Brachial artery flow-mediated dilation (FMD) and digital vascular function were measured at baseline and after each 6 week treatment period. Of the 53 patients enrolled in the crossover study, 32 (age 60±10, 22% female) completed all the visits, with a high-rate of study withdrawal due to gastrointestinal side-effects. In a subset of 10 participants, we compared the effects of four days of sulfasalazine treatment (n=5) to no treatment (n=5) on NFkB-regulated gene expression in peripheral blood mononuclear cells. Tumor necrosis factor α-stimulated expression of CD69 and NFκB subunit p50 was significantly blunted after 4 days of sulfasalazine treatment but not after no treatment. However, FMD and digital vasodilator response did not significantly change from baseline with long-term sulfasalazine treatment. Short-term sulfasalazine inhibited NFκB activity; however long-term treatment was poorly tolerated and did not improve endothelial function. Our findings suggest that sulfasalazine therapy is not the optimal anti-inflammatory treatment for reversing endothelial dysfunction in cardiovascular disease. Further studies are warranted to investigate the potential for NFκB inhibition to reduce cardiovascular risk.
Keywords: vascular, inflammation, endothelial function, coronary artery disease
Abnormal endothelial function contributes to the pathogenesis of cardiovascular events in patients with clinical atherosclerosis.1–3 Systemic inflammation is a central mediator of all phases of atherogenesis including disruption of endothelial function.4,5 Novel anti-inflammatory therapies may have promise as a treatment strategy for cardiovascular risk reduction.6
Prior evidence links inflammatory activation to altered endothelial phenotype leading to loss of nitric oxide bioactivity.5,7 Inflammatory cytokines induce endothelial expression of pro-inflammatory and prothrombotic factors that may contribute to cardiovascular risk.8 There is considerable interest in the transcription factor, nuclear factor kappa B (NFκB), as a regulator of endothelial function.9,10 In animal models and in humans with cardiovascular risk factors, endothelial NFκB activation is associated with impaired vasodilator function.11–13
Sulfasalazine is a well-established anti-inflammatory drug that has been shown to inhibit the activation of NFκB.14 A related compound, salsalate, has been shown to increase flow-mediated dilation in obese humans13; however, the effects of NFκB inhibition in the setting of established clinical atherosclerotic disease remain unknown. Therefore, we conducted a randomized, placebo-controlled, cross-over study of sulfasalazine treatment to test the hypothesis that NFκB inhibition would improve vascular function in patients with stable coronary artery disease.
Methods
Study Participants
We enrolled patients with stable coronary artery disease diagnosed by coronary angiography or a history of myocardial infarction. We excluded patients with unstable angina or myocardial infarction within two weeks of enrollment, allergy to sulfa containing drugs, treatment with drugs that may interact with sulfasalazine (coumadin, digoxin, phenytoin, methenamines, probenecid, sulfinpyrazones, oral hypoglycemic agents), immunosuppressive therapy, glucose-6-phosphate dehydrogenase deficiency, or active medical illness. The study protocol was approved by the Boston Medical Center Institutional Review Board and all participants provided written informed consent. The randomized portion of the study was registered on line (http://www.clinicaltrials.gov/, NCT00554203).
Sulfasalazine treatment and study design
We employed a double-blind, placebo-controlled, crossover study design. Treatment order was assigned using computer-generated randomization. As shown in Figure 1, after an initial screening visit, participants made four study visits. After the baseline study visit, participants were treated with sulfasalazine (Azulfidine EN, Pharmacia, NY) or placebo gelatin capsules for six weeks. During each active treatment period, participants were treated with one capsule of sulfasalazine or placebo twice daily for one week (1000mg/day) then two capsules twice daily (2000mg/day) for the remaining five weeks. The selected dose of sulfasalazine has been shown to have anti-inflammatory properties in rheumatoid arthritis.15 After the initial treatment period, there was a two-week rest period between treatments and then participants crossed over to the second treatment (sulfasalazine or placebo).
Figure 1.
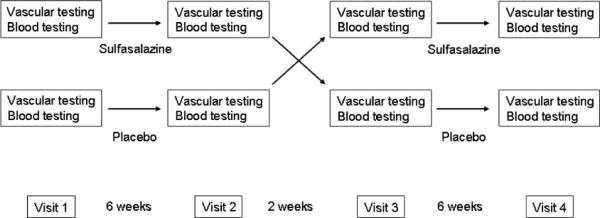
Study Design. For the crossover study, participants were randomized to sulfasalazine treatment first (top row) or placebo first (bottom row) and received the assigned treatment for 6 weeks (one 500mg or placebo pill twice daily for one week, then two 500mg or placebo pills twice daily for 5 weeks). After a two-week rest period, the participants crossed over to the alternate treatment.
Participants refrained from smoking and fasted after midnight the morning of each study. Study participants were also instructed to stop vasoactive medications for the 24 hours prior to each study visit. Participants took the study medications twice daily with the last dose on the morning of the study visit. We performed vascular testing and collected blood for evaluation of biomarkers at each study visit.
Vascular Testing
We measured conduit vessel vasodilator function by assessing flow-mediated and nitroglycerin-mediated dilation of the brachial artery as previously described.16 Briefly, high resolution ultrasound was used to measure brachial artery diameter and Doppler flow at rest and following a 5-minute cuff occlusion of the upper arm to induce reactive hyperemia. To evaluate microvessel function in the finger vessels, we simultaneously performed digital pulse amplitude tonometry (Endo-PAT, Itamar Medical Ltd., Caesarea, Israel). As we have previously described, we expressed the PAT hyperemic response as the log-transformed ratio of the pulse amplitude in the test finger in the 90 to 120 second interval after cuff release to the resting pulse amplitude divided by the corresponding ratio in the contralateral control finger (PAT ratio).17 We measured endothelium-independent brachial artery vasodilation following sublingual administration of nitroglycerin (0.4mg). The nitroglycerin portion of the testing was omitted if the participant had a prior adverse reaction to nitrates, systolic blood pressure less than 100mmHg, or use of phosphodiesterase type-5 medications.
Biochemical and inflammatory marker testing
Total cholesterol, HDL cholesterol, triglycerides and glucose were measured using enzymatic methods in the Boston Medical Center Clinical Laboratory. LDL cholesterol was calculated using the Friedewald formula.18 Insulin was measured using immunochemiluminometric methodology. Serum C-reactive protein was determined with a high-sensitivity nephelometric method.19 Serum interleukin-6, p-selectin, monocyte chemotactic protein-1, and soluble intracellular adhesion molecule 1 were measured using commercially available kits (R&D Systems, Inc.).
Assessment of NFKB activity in peripheral blood mononuclear cells
To examine the effect of sulfasalazine treatment on NFKB activation in peripheral leukocytes, we enrolled an additional 10 participants with coronary artery disease. We treated 5 participants for 4 days with sulfasalazine 1000mg twice daily and 5 participants received no treatment. Blood was collected before and after the 4 days in a Ficoll separation tube (CPT, Becton Dickinson, Franklin Lakes, NJ) and centrifuged at 1650g for 30 minutes. Isolated mononuclear cells were washed in Hank's Balanced Salt Solution (HBSS) and treated with 0, 1, or 10ng/ml TNFα (R&D Systems) in HBSS for two hours in a rotating incubator at 37°C. Total RNA was isolated using the RNeasy Mini kit (Qiagen, Inc.) and RNA was reverse transcribed to complimentary DNA using TaqMan Reverse Transcription Reagents (Applied Biosystems). Quantitative real-time PCR (Applied Biosystems' Taqman Gene Expression Assays) was performed to measure expression of genes regulated by NFκB including NFκB subunit p50 (NFKB1, Hs00765730_m1) and CD69 (Hs00934033_m1). Results were interpreted by the relative quantity method (ΔΔCt) using GAPDH as a loading control
Statistical Analysis
Analyses were completed using SPSS for Windows, version 12.1 (SPSS Inc.) and a two-sided P value<0.05 was considered statistically significant. Values are reported as mean±SD, unless otherwise indicated. We compared the baseline clinical characteristics of the two treatment order groups (sulfasalazine first and placebo first) using unpaired t-test or chi-square test as appropriate. We evaluated the effect of treatment order on each endpoint by 2-way repeated-measures analysis of variance (ANOVA). We evaluated the effect of treatment by paired t-test comparing the after sulfasalazine to the after placebo conditions. We evaluated the gene expression response to TNFα before and after sulfasalazine treatment using 2-way repeated-measures ANOVA.
Based on prior data from our laboratory, the sample size of 32 participants who completed all four study visits in a cross-over design provides greater than 90% power (alpha = 0.05) to detect a 2.0 percentage point change (e.g. 7 to 9 %) in brachial artery flow –mediated dilation, the primary endpoint of the study.
Results
Study Participants
As shown in Figure 2, 53 participants qualified for the study and were randomized. There was a high withdrawal rate (n=21, 40%) predominantly related to gastrointestinal symptoms during active treatment (n=11). The clinical characteristics of the participants are shown in Table 1. The participants in the two treatment order groups (sulfasalazine first and placebo first) were similar with a high prevalence of cardiovascular risk factors and high rate of concurrent cardiovascular medications. There were no differences in baseline clinical characteristics between the participants who completed the study and the participants who withdrew.
Figure 2.

Consolidated Standards of Reporting Trials (CONSORT) flow diagram.
Table 1.
Baseline Clinical Characteristics - Randomized Crossover Study
| Sulfasalazine first | Placebo first* | Participants who withdrew† | |
|---|---|---|---|
| Characteristic | n=18 | n=14 | n=21 |
| Age (years) | 62±8 | 57±12 | 61±9 |
| Female Gender n (%) | 5 (28) | 2 (14) | 4 (19) |
| Black Race n (%) | 3 (18) | 4 (29) | 2 (10) |
| History of Hypertension n (%) | 12 (67) | 11 (79) | 14 (67%) |
| History of diabetes mellitus n (%) | 5 (27) | 4 (29) | 8 (38) |
| History of smoking n (%) | 17 (94) | 14 (100) | 15 (71) |
| Lipid lowering therapy n (%) | 14 (78) | 13 (93) | 20 (95) |
| ACE inhibitor therapy n (%) | 13 (72) | 7 (50) | 9 (43) |
| Body mass index (kg/m2) | 30.2±6.1 | 29.7±6.9 | 30.0±5.0 |
| Total cholesterol (mg/dl) | 153±27 | 164±47 | 156±44 |
| LDL cholesterol (mg/dl) | 76±16 | 85±46 | 76±24 |
| HDL cholesterol (mg/dl) | 40±6 | 45±10 | 43±9 |
| Triglycerides (mg/dl) | 184±118 | 170±149 | 182±190 |
| Fasting glucose (g/dl) | 109±16 | 142±85 | 129±69 |
| Fasting insulin (μIU/dl) | 13.8±13.3 | 14.1±9.2 | 16.1±10.4 |
Mean±SD or number with percentage.
All P values=NS for comparison with sulfasalazine first group
All P values=NS for comparison with participants who completed all four study visits
Effect of Sulfasalazine on Vascular Function
We evaluated whether sulfasalazine treatment has an effect on vascular function in coronary artery disease patients. As shown in Table 2, we found no effect of sulfasalazine on brachial artery flow-mediated dilation, a measure of conduit vessel endothelial function, or on PAT hyperemic response, a measure of small vessel vasodilator function. In addition, we found no effect of sulfasalazine on blood pressure, hyperemic response, or arterial diameter. There was no evidence of an effect of treatment order for any of the vascular measures.
Table 2.
Effects of Sulfasalazine on Vascular Function
| Vascular Function Variable | Before Sulfasalazine | After Sulfasalazine | Before Placebo | After Placebo | P* |
|---|---|---|---|---|---|
| Systolic blood pressure (mmHg) | 135±18 | 140±18 | 138±15 | 138±22 | 0.54 |
| Diastolic blood pressure (mmHg) | 74±7 | 75±8 | 75±8 | 74±9 | 0.59 |
| Heart Rate (bpm) | 66±11 | 69±12 | 66±11 | 65±10 | 0.06 |
| Flow-mediated dilation (%) | 6.8±4.0 | 7.5±3.9 | 7.2±3.6 | 7.4±4.2 | 0.83 |
| Baseline diameter (mm) | 4.80±0.82 | 4.85±0.83 | 4.82±0.77 | 4.88±0.81 | 0.99 |
| Dilation to nitroglycerin (%) | 9.0±6.2 | 9.2±5.6 | 8.4±5.8 | 10.8±7.0 | 0.11 |
| Hyperemic flow (ml/min) | 1669±1526 | 1517±622 | 1547±621 | 1424±508 | 0.16 |
| PAT ratio (au) | 1.52±1.03 | 1.80±1.29 | 1.54±0.72 | 1.82±1.04 | 0.98 |
| lnPAT ratio (au) | 0.29±0.44 | 0.43±0.53 | 0.35±0.38 | 0.48±0.46 | 0.61 |
Mean±SD.
P value for paired t-test comparing after sulfasalazine to after placebo
In secondary analyses, we evaluated the effect of sulfasalazine on flow-mediated dilation in selected subgroups of participants. We found no effect of sulfasalazine in subgroups including: participants with HOMA-IR below the group median, participants without diabetes, participants with C-reactive protein less than 10mg/L, participants with flow-mediated dilation below the median at the baseline visit, men, women, or white race (data not shown). In the group of 7 black participants, flow-mediated dilation was lower with sulfasalazine treatment compared to placebo (5.4±4.9% compared to 8.7±5.8%, P=0.015). The apparent difference in this subgroup likely reflects the small number of individuals included.
Effect of Sulfasalazine on Biomarkers
As shown in Table 3, we observed no effect of sulfasalazine treatment on serum lipids, glucose, or insulin levels. There were no effects of sulfasalazine on multiple systemic markers of inflammation.
Table 3.
Effects of Sulfasalazine on Biochemical Profile
| Blood Marker | Before Sulfasalazine | After Sulfasalazine | Before Placebo | After Placebo | P* |
|---|---|---|---|---|---|
| Total Cholesterol (mg/dl) | 157±37 | 166±37 | 159±38 | 162±35 | 0.25 |
| LDL Cholesterol (mg/dl) | 81±30 | 94±31 | 83±34 | 89±29 | 0.09 |
| HDL Cholesterol (mg/dl) | 41±8 | 42±7 | 43±9 | 42±8 | 0.98 |
| Triglycerides (mg/dl) | 171±111 | 151±66 | 167±105 | 164±114 | 0.48 |
| Glucose (mg/dl) | 123±60 | 128±68 | 131±62 | 129±56 | 0.80 |
| Insulin (μIU/dl) | 13.0±8.1 | 10.7±5.0 | 13.2±9.3 | 11.9±5.4 | 0.25 |
| HOMA-IR(au) | 3.9±2.6 | 3.3±1.9 | 4.5±4.1 | 3.8±2.5 | 0.18 |
| C-reactive protein (mg/L) | 5.4±13.2 | 5.2±10.9 | 3.5±6.3 | 2.3±1.9 | 0.16 |
| P-selectin | 56±11 | 53±13 | 59±19 | 55±14 | 0.44 |
| Interleukin-6 | 2.7±2.0 | 2.6±1.8 | 2.5±1.5 | 2.6±2.0 | 0.99 |
| MCP-1 | 365±120 | 374±139 | 375±113 | 366±115 | 0.58 |
| Soluble-ICAM-1 | 272±72 | 283±82 | 293±84 | 281±90 | 0.85 |
Mean±SD.
P value for paired t-test comparing after sulfasalazine to after placebo HOMA-IR = Homeostasis model assessment of insulin resistance; ICAM-1 = intercellular adhesion molecule-1.
Effect of Sulfasalazine on NFκB Activation
To gain support of an effect of sulfasalazine on NFκB activity in patients with coronary artery disease (n=10), we collected peripheral leukocytes before and after 4 days with (n=5) or without (n=5) sulfasalazine treatment. As shown in Figure 3, we observed that sulfasalazine treatment markedly blunted the induction of NFκB-regulated gene expression in response to TNFα, consistent with inhibition of NFκB activation. Participants who received no sulfasalazine treatment had a similar response to TNFα stimulation at baseline compared to after 4 days for both NFκB1 (P=0.13) and CD69 (P=0.14). There was a significant difference in the effect of sulfasalazine as compared to no intervention on TNFα stimulated NFκB1 and CD69 gene expression (P=0.026 and P=0.009, comparing sulfsalazine to no treatment groups by repeated-measures ANOVA).
Figure 3.
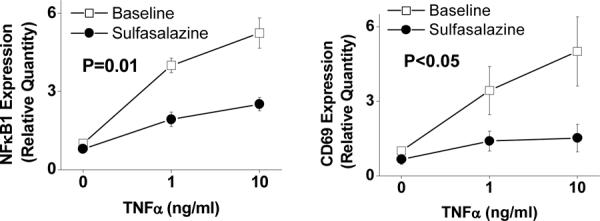
Effect of sulfasalazine on NFκB mediated inflammatory activity. Peripheral blood mononuclear cells were isolated as described in the methods section before and after 4 days of sulfasalazine treatment (1000mg twice daily) in 5 patients with coronary artery disease. Expression levels of NFκB-regulated genes were assessed with quantitative PCR after two hours of treatment with TNFα at 0, 1, and 10ng/ml. P value shown for comparison of overall gene expression before and after sulfasalazine treatment.
Discussion
The present crossover study investigated whether NFκB inhibition with sulfasalazine has a favorable effect on vascular function in stable coronary artery disease. Short-term treatment with sulfasalazine reduced NFκB activity as evidenced by a blunted induction of NFκB-regulated genes following TNFα stimulation of freshly isolated peripheral blood mononuclear cells. In contrast, we observed no effect of long-term sulfasalazine treatment on systemic inflammatory markers. Inhibition of NFκB with sulfasalazine was not associated with an improvement of endothelial function as measured in both conduit and microvessels. Sulfasalazine was poorly tolerated in our group of patients with established atherosclerosis as the study withdrawal rate was greater than 40%. Taken together, our findings do not support a vascular benefit of sulfasalazine in patients with chronic coronary artery disease.
Activation of NFκB contributes to inflammation in coronary artery disease. NFκB is a transcription factor that controls the expression of proinflammatory genes relevant to atherogenesis including cytokines, chemokines and cell adhesion molecules.20,21 Human atherosclerotic plaques contain activated NFκB and in animal models, NFκB activity localizes to atherosclerosis-prone regions.22,23 Patients with unstable coronary syndromes have evidence of NFκB activation in circulating blood monocytes that is associated with a higher risk of future cardiovascular events.24,25 Thus, inhibition of NFκB-mediated inflammatory responses has been proposed as a strategy to ameliorate risk in coronary artery disease.25,26
Prior studies establish nonacetylated salicylic acids, including sulfasalazine, as inhibitors of NFκB activation. Sulfasalazine acts by preventing phosphorylation and subsequent degradation of the NFκB inhibitory subunit, IκB.14,27–29 In cultured cells, salicylates inhibit endothelial activation and reduce intracellular adhesion molecule expression.30 We provide evidence that short-term oral sulfasalazine administration blunts NFκB activity in patients with coronary artery disease. In freshly isolated peripheral blood mononuclear cells, there was a marked reduction in TNFα-induced expression of inflammatory genes regulated by NFκB following sulfasalazine treatment. Interestingly, the anti-inflammatory effect of sulfasalazine observed in peripheral blood mononuclear cells did not correspond with a change in circulating markers of inflammation. In this regard, our findings are consistent with two previous human studies in HIV patients and obese adults that showed no effect of salsalate treatment on systemic inflammatory markers.13,31 Thus, salicylates appear to reduce intracellular inflammatory responses without altering circulating inflammatory biomarker levels.
Several lines of evidence implicate inflammation in the pathogenesis of endothelial dysfunction.5 In experimental models, inflammatory mediators limit nitric oxide bioavailability, in part through reduced endothelial nitric oxide synthase expression.32–34 In human studies, systemic inflammatory markers associate with impaired endothelial vasodilator function.5,35 Acute inflammatory states induce the development of transient endothelial dysfunction.36,37 There is evidence that NFκB activation may have particular relevance to impaired vascular function observed in the presence of cardiovascular risk factors. Endothelial cells isolated from older and obese individuals demonstrate higher NFκB activation that is associated with reduced flow-mediated dilation.11,13
The current study showed no favorable effects of sulfasalazine treatment on endothelial function. In coronary artery disease patients, both conduit artery and digital vessel flow-mediated dilation were unchanged by sulfasalazine treatment. Our findings are discordant with prior work indicating beneficial effects of salicylates on endothelial function in select samples. In a study of older obese adults without diabetes or clinical cardiovascular disease, four days of salsalate therapy improved brachial artery flow-mediated dilation and reduced endothelial cell NFκB activation.13 In a uncontrolled, pilot study, salsalate treatment for 8 weeks improved flow-mediated dilation in patients with HIV disease.31
Multiple potential explanations exist for the apparent discrepancy. Clinical characteristics of our study participants may have reduced the efficacy of anti-inflammatory treatment. Our study sample included patients in a more advanced phase of disease with established clinical cardiovascular disease. It is possible that endothelial dysfunction in chronic disease may be more resistant to improvement with NFκB inhibition. Alternatively, our participants were intensively treated with secondary prevention interventions as evidenced by well-controlled lipid levels. Sulfasalazine therapy may have limited effect in medically optimized patients. In addition, it is possible that salsalate has greater efficacy for improving endothelial function than sulfasalazine. The limited effect of sulfasalazine may relate to poor tolerability due to frequent gastrointestinal side effects.
Study Limitations
The current study has a number of limitations. The high rate of participant withdrawal reduced our overall sample size for analysis. However, the available sample size provided adequate power to detect a change in flow-mediated dilation similar in magnitude to that observed in prior intervention studies. It is possible that concurrent medication use may have modulated the effect of sulfasalazine; though the study design with a crossover methodology limits the influence of confounding. We did not measure serum levels of sulfasalazine or its metabolites limiting our ability to confirm adherence. The selected sulfasalazine dose was based on studies in rheumatoid arthritis and we did demonstrate and effect on circulating cells; however, the effective dose required to influence the endothelium may be higher. Given the poor tolerability at the dose used in the present study, using a higher dose would have limited clinical utility. It is possible that the dissociation of the NFκB activation results and the vascular function and serum markers relate to the different duration of treatment. Sulfasalazine is a pharmacologic inhibitor of NFκB that may have other nonspecific anti-inflammatory effects. We used an upper arm cuff placement to induce hyperemia; the results may differ with a forearm cuff placement. Finally, the short-term study evaluating NFκB activation in leukocytes was not randomized but all analyses were performed in blinded to study condition.
Conclusions
In summary, treatment with sulfasalazine reduced NFκB activation but did not improve endothelial function in patients with coronary artery disease. Sulfasalazine was poor tolerated in our sample of older individuals taking multiple concurrent medications. Our findings counter the hypothesis that sulfasalazine might have the potential to reverse endothelial dysfunction in established atherosclerotic disease. The optimal strategy for mitigating the adverse effects of chronic inflammation in patients with atherosclerosis remains an ongoing area of investigation. Further studies are needed to evaluate the clinical efficacy of NFκB inhibition as a strategy to lower cardiovascular risk.
Acknowledgments
We wish to thank Dr. Peter Merkel for serving as the safety monitor for this study.
This work was supported by NIH grants HL074097, HL084213, and HL083269. Drs. Hamburg, Vita and Tabit are supported by the Boston University Medical Center Leadership Program in Vascular Medicine (K12 HL083781). Dr. Vita is supported by grants from the National Institutes of Health (HL083801, HL081587, and HL75795). Dr. Hamburg is supported by grants from the National Institutes of Health (HL102299).
Footnotes
The authors have no conflicts of interest.
References
- 1.Gokce N, Keaney JF, Jr, Menzoian JO, et al. Risk stratification for postoperative cardiovascular events via noninvasive assessment of endothelial function. Circulation. 2002;105(13):1567–72. doi: 10.1161/01.cir.0000012543.55874.47. [DOI] [PubMed] [Google Scholar]
- 2.Huang AL, Silver AE, Shvenke E, et al. Predictive value of reactive hyperemia for cardiovascular events in patients with peripheral arterial disease undergoing vascular surgery. Arterioscler Thromb Vasc Biol. 2007;27(10):2113–9. doi: 10.1161/ATVBAHA.107.147322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Widlansky ME, Gokce N, Keaney JF, Jr, Vita JA. The clinical implications of endothelial dysfunction. J Am Coll Cardiol. 2003;42:1149–60. doi: 10.1016/s0735-1097(03)00994-x. [DOI] [PubMed] [Google Scholar]
- 4.Libby P, Ridker PM, Maseri A. Inflammation and atherosclerosis. Circulation. 2002;105:1135–43. doi: 10.1161/hc0902.104353. [DOI] [PubMed] [Google Scholar]
- 5.Huang AL, Vita JA. Effects of systemic inflammation on endothelium-dependent vasodilation. Trends Cardiovasc Med. 2006 Jan;16(1):15–20. doi: 10.1016/j.tcm.2005.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ridker PM. Testing the inflammatory hypothesis of atherothrombosis: scientific rationale for the cardiovascular inflammation reduction trial (CIRT) J Thromb Haemost. 2009 Jul;7(Suppl 1):332–9. doi: 10.1111/j.1538-7836.2009.03404.x. [DOI] [PubMed] [Google Scholar]
- 7.Prasad A, Zhu J, Halcox JP, Waclawiw MA, Epstein SE, Quyyumi AA. Predisposition to atherosclerosis by infections: role of endothelial dysfunction. Circulation. 2002 Jul 9;106(2):184–90. doi: 10.1161/01.cir.0000021125.83697.21. [DOI] [PubMed] [Google Scholar]
- 8.Pasceri V, Willerson JT, Yeh ET. Direct proinflammatory effect of C-reactive protein on human endothelial cells. Circulation. 2000 Oct 31;102(18):2165–8. doi: 10.1161/01.cir.102.18.2165. [DOI] [PubMed] [Google Scholar]
- 9.Read MA, Whitley MZ, Williams AJ, Collins T. NF-kappa B and I kappa B alpha: an inducible regulatory system in endothelial activation. J Exp Med. 1994 Feb 1;179(2):503–12. doi: 10.1084/jem.179.2.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Collins T, Read MA, Neish AS, Whitley MZ, Thanos D, Maniatis T. Transcriptional regulation of endothelial cell adhesion molecules: NF-kappa B and cytokine-inducible enhancers. FASEB J. 1995 Jul;9(10):899–909. [PubMed] [Google Scholar]
- 11.Silver AE, Beske SD, Christou DD, et al. Overweight and obese humans demonstrate increased vascular endothelial NAD(P)H oxidase-p47(phox) expression and evidence of endothelial oxidative stress. Circulation. 2007 Feb 6;115(5):627–37. doi: 10.1161/CIRCULATIONAHA.106.657486. [DOI] [PubMed] [Google Scholar]
- 12.Kim F, Pham M, Maloney E, et al. Vascular inflammation, insulin resistance, and reduced nitric oxide production precede the onset of peripheral insulin resistance. Arterioscler Thromb Vasc Biol. 2008 Nov;28(11):1982–8. doi: 10.1161/ATVBAHA.108.169722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pierce GL, Lesniewski LA, Lawson BR, Beske SD, Seals DR. Nuclear factor-{kappa}B activation contributes to vascular endothelial dysfunction via oxidative stress in overweight/obese middle-aged and older humans. Circulation. 2009 Mar 10;119(9):1284–92. doi: 10.1161/CIRCULATIONAHA.108.804294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wahl C, Liptay S, Adler G, Schmid RM. Sulfasalazine: a potent and specific inhibitor of nuclear factor kappa B. J Clin Invest. 1998;101:1163–74. doi: 10.1172/JCI992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Smolen JS, Kalden JR, Scott DL, et al. Efficacy and safety of leflunomide compared with placebo and sulphasalazine in active rheumatoid arthritis: a double-blind, randomised, multicentre trial. European Leflunomide Study Group. Lancet. 1999 Jan 23;353(9149):259–66. doi: 10.1016/s0140-6736(98)09403-3. [DOI] [PubMed] [Google Scholar]
- 16.McMackin CJ, Vita JA. Update on nitric oxide-dependent vasodilation in human subjects. Methods Enzymol. 2005;396:541–53. doi: 10.1016/S0076-6879(05)960-16-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hamburg NM, Keyes MJ, Larson MG, et al. Cross-sectional relations of digital vascular function to cardiovascular risk factors in the Framingham Heart Study. Circulation. 2008 May 13;117(19):2467–74. doi: 10.1161/CIRCULATIONAHA.107.748574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 19.Ridker PM, Cushman M, Stampfer MJ, Tracy RP, Hennekens CH. Inflammation, aspirin, and the risk of cardiovascular disease in apparently healthy men. N Engl J Med. 1997;336(14):973–9. doi: 10.1056/NEJM199704033361401. [DOI] [PubMed] [Google Scholar]
- 20.Collins T, Cybulsky MI. NF-kappaB: pivotal mediator or innocent bystander in atherogenesis? J Clin Invest. 2001 Feb;107(3):255–64. doi: 10.1172/JCI10373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.de Winther MP, Kanters E, Kraal G, Hofker MH. Nuclear factor kappaB signaling in atherogenesis. Arterioscler Thromb Vasc Biol. 2005 May;25(5):904–14. doi: 10.1161/01.ATV.0000160340.72641.87. [DOI] [PubMed] [Google Scholar]
- 22.Brand K, Page S, Rogler G, Bartsch A, Brandl R. Activated transcription factor nuclear factor-kappa B is present in the atherosclerotic lesion. J Clin Invest. 1996;97(7):1715–22. doi: 10.1172/JCI118598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hajra L, Evans AI, Chen M, Hyduk SJ, Collins T, Cybulsky MI. The NF-kappa B signal transduction pathway in aortic endothelial cells is primed for activation in regions predisposed to atherosclerotic lesion formation. Proc Natl Acad Sci U S A. 2000 Aug 1;97(16):9052–7. doi: 10.1073/pnas.97.16.9052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ritchie ME. Nuclear factor-kB is selectively and markedly activated in humans with unstable angina pectoris. Circulation. 1998;98:1707–13. doi: 10.1161/01.cir.98.17.1707. [DOI] [PubMed] [Google Scholar]
- 25.Liuzzo G, Santamaria M, Biasucci LM, et al. Persistent activation of nuclear factor kappa-B signaling pathway in patients with unstable angina and elevated levels of C-reactive protein evidence for a direct proinflammatory effect of azide and lipopolysaccharide-free C-reactive protein on human monocytes via nuclear factor kappa-B activation. J Am Coll Cardiol. 2007 Jan 16;49(2):185–94. doi: 10.1016/j.jacc.2006.07.071. [DOI] [PubMed] [Google Scholar]
- 26.Monaco C, Paleolog E. Nuclear factor kappaB: a potential therapeutic target in atherosclerosis and thrombosis. Cardiovasc Res. 2004 Mar 1;61(4):671–82. doi: 10.1016/j.cardiores.2003.11.038. [DOI] [PubMed] [Google Scholar]
- 27.Kopp E, Ghosh S. Inhibition of NF-kappa B by sodium salicylate and aspirin. Science. 1994 Aug 12;265(5174):956–9. doi: 10.1126/science.8052854. [DOI] [PubMed] [Google Scholar]
- 28.Yin MJ, Yamamoto Y, Gaynor RB. The anti-inflammatory agents aspirin and salicylate inhibit the activity of I(kappa)B kinase-beta. Nature. 1998 Nov 5;396(6706):77–80. doi: 10.1038/23948. [DOI] [PubMed] [Google Scholar]
- 29.Lappas M, Yee K, Permezel M, Rice GE. Sulfasalazine and BAY 11-7082 interfere with the nuclear factor-kappa B and I kappa B kinase pathway to regulate the release of proinflammatory cytokines from human adipose tissue and skeletal muscle in vitro. Endocrinology. 2005 Mar;146(3):1491–7. doi: 10.1210/en.2004-0809. [DOI] [PubMed] [Google Scholar]
- 30.Pierce JW, Read MA, Ding H, Luscinskas FW, Collins T. Salicylates inhibit I kappa B-alpha phosphorylation, endothelial-leukocyte adhesion molecule expression, and neutrophil transmigration. J Immunol. 1996 May 15;156(10):3961–9. [PubMed] [Google Scholar]
- 31.Gupta SK, Johnson RM, Saha C, et al. Improvement in HIV-related endothelial dysfunction using the anti-inflammatory agent salsalate: a pilot study. AIDS. 2008 Mar 12;22(5):653–5. doi: 10.1097/QAD.0b013e3282f470d2. [DOI] [PubMed] [Google Scholar]
- 32.Vallance P, Collier J, Bhagat K. Infection, inflammation, and infarction: does acute endothelial dysfunction provide a link? Lancet. 1997;349:1391–2. doi: 10.1016/S0140-6736(96)09424-X. [DOI] [PubMed] [Google Scholar]
- 33.Verma S, Wang CH, Li SH, et al. A self-fulfilling prophecy: C-reactive protein attenuates nitric oxide production and inhibits angiogenesis. Circulation. 2002 Aug 20;106(8):913–9. doi: 10.1161/01.cir.0000029802.88087.5e. [DOI] [PubMed] [Google Scholar]
- 34.Teoh H, Quan A, Lovren F, et al. Impaired endothelial function in C-reactive protein overexpressing mice. Atherosclerosis. 2008 Dec;201(2):318–25. doi: 10.1016/j.atherosclerosis.2008.02.034. [DOI] [PubMed] [Google Scholar]
- 35.Vita JA, Keaney JF, Jr, Larson MG, et al. Brachial artery vasodilator function and systemic inflammation in the Framingham Offspring Study. Circulation. 2004;110:3604–9. doi: 10.1161/01.CIR.0000148821.97162.5E. [DOI] [PubMed] [Google Scholar]
- 36.Hingorani AD, Cross J, Kharbanda RK, et al. Acute systemic inflammation impairs endothelium-dependent dilatation in humans. Circulation. 2000 Aug 29;102(9):994–9. doi: 10.1161/01.cir.102.9.994. [DOI] [PubMed] [Google Scholar]
- 37.Pleiner J, Schaller G, Mittermayer F, et al. Simvastatin prevents vascular hyporeactivity during inflammation. Circulation. 2004 Nov 23;110(21):3349–54. doi: 10.1161/01.CIR.0000147774.90396.ED. [DOI] [PubMed] [Google Scholar]


