Abstract
The Neuronal Ceroid Lipofuscinoses (NCLs) are lysosomal storage diseases (LSDs) affecting the central nervous system (CNS), with generally with recessive inheritance. They are characterized by pathological lipofuscin-like material accumulating in cells. The clinical phenotypes at all onset ages show progressive loss of vision, decreasing cognitive and motor skills, epileptic seizures and premature death, with dementia without visual loss prominent in the rarer adult forms. Eight causal genes, CLN10/CTSD, CLN1/PPT1, CLN2/TPP1, CLN3, CLN5, CLN6, CLN7/MFSD8, CLN8, with more than 269 mutations and 49 polymorphisms (http://www.ucl.ac.uk/ncl) have been described. Other NCL genes are hypothesized, including CLN4 and CLN9; CLCN6, CLCN7 and possibly SGSH are under study. Some therapeutic strategies applied to other LSDs with significant systemic involvement would not be effective in NCLs due to the necessity of passing the blood brain barrier to prevent the neurodegeneration, repair or restore the CNS functionality. There are therapies for the NCLs currently at preclinical stages and under phase 1 trials to establish safety in affected children. These approaches involve enzyme replacement, gene therapy, neural stem cell replacement, immune therapy and other pharmacological approaches. In the next decade, progress in the understanding of the natural history and the biochemical and molecular cascade of events relevant to the pathogenesis of these diseases in humans and animal models will be required to achieve significant therapeutic advances.
Keywords: Enzyme replacement, gene, immune, lysosomal storage diseases, Neuronal ceroid lipofuscinoses, pharmacological, stem cell, therapies
INTRODUCTION
The Neuronal Ceroid Lipofuscinoses (NCLs) are a family of lysosomal storage diseases (LSDs), usually with recessive inheritance, characterized by autofluorescence of the accumulating material [1]. They are amongst the most common progressive encephalopathies of childhood, with ages of onset ranging from birth to adulthood [2,3]. Affected children and infants are characterized clinically by progressive loss of vision, decreasing cognitive and motor skills, epileptic seizures and premature death, while dementia without visual loss is prominent in the rarer adult forms [4]. All types of NCLs share patho-morphological characteristics including staining of the stored materials with Sudan Black B and Periodic Acid-Schiff positive, and its resistance to lipid solvents [5,6]. The cytoplasm of most nerve cells and cells of peripheral tissues such as skin, blood, retina, conjunctiva and muscle, have osmiophilic deposits with various and distinctive fine structural forms detected under electron microscopy [7]. Neuronal degeneration may commence in the dendritic tree and may proceed to final neuronal loss which predominates in cortical regions of the cerebrum and the cerebellum [8,9].
Historically, the NCL have been classified according to age of onset (e.g. congenital, infantile, late infantile, juvenile and adult), with some forms appearing more common in certain countries or peoples of shared ethnic origin [10]. However, the advent of molecular genetics now allows this group of diseases to be distinguished genetically, bringing in a new gene-based classification system. It is now recognized that mutations in all genes are distributed across many countries, albeit with higher incidence in some ethnic groups due to founder effects. Among the many recognized variants of NCL human forms (recent reviews in [3,6,11]), eight causal genes have been identified: CLN10/CTSD [12-14], CLN1/PPT1 [3,6,15-18], CLN2/TPP1 [6,18-21], CLN3 [22-25], CLN5 [26-30], CLN6 [31-34], CLN7/MFSD8 [35-39], CLN8 [40-42]. The genes CLCN6 [43], and CLCN7 [44], and possibly SGSH [45], may also contribute to rare forms of NCL. The international NCL database of mutations currently has more than 269 mutations and 49 polymorphisms registered (http://www.ucl.ac.uk/ncl). Genes for two further forms, ‘CLN4’ and ‘CLN9’, are still not identified (review in [3]). We summarize the most relevant aspects of each of the thirteen different NCL diseases in Table 1.
Table 1. Current NCL Genotype-Phenotype Classification [3,6,11].
| Disease Former Eponym [Ref. N°] |
OMIM Number |
Clinical Subtype |
Morphologycal Phenotype |
Chromosome | Gene | Gene Product |
Stored Protein |
|---|---|---|---|---|---|---|---|
| CLN1 Haltia-Santavuori [15-18] |
256730 | infantile, late infantile, juvenile, adult |
GROD | 1p32 | CLN1 | PPT1 | SAP |
| CLN2 Janský-Bielschowsky [18-21] |
204500 | late infantile, classic juvenile |
CB, CB+FP | 11p15 | CLN2 | TPP1 | SCMAS |
| CLN3 Batten-Spielmeyer-Vogt- Sjögren [22-25] |
204200 | classic juvenile | FP, vacuolated lymphocytes |
16p12 | CLN3 | CLN3 | SCMAS |
| CLN4 A Kufs [46-48] |
204300 | adult | mixed | ? | ? | ? | SCMAS |
| CLN5 Finnish variant [26-30] |
256731 | late infantile, juvenile,adult |
FP, various | 13q22 | CLN5 | CLN5 | SCMAS |
| CLN6 Lake-Cavanagh [31-34] |
601780 | late infantile variant |
FP | 15q21-23 | CLN6 | CLN6 | SCMAS |
| CLN7 Turkish variant [35-39] |
610951 | late infantile variant |
FP or mixed | 4q28.1- q.28.2 |
MFSD8 | MFSD8 | SCMAS |
| CLN8 Northern epilepsy [40-42] |
600143 | late infantile variant |
CB or GROD-like |
8p32 | CLN8 | CLN8 | SCMAS |
| CLN9 [49,50] | 609055 | juvenile variant | GROD, CB, or FP- like |
? | ? | ? | ? |
| CLN10 Congenital variant [12-14] |
610127 | congenital, late infantile, juvenile, adult |
GROD, myelin-like lamellar structures |
11p15.5 | CLN10 | CTSD | SAP |
| CLCN6 [43] | 602726 | adult variant* | FP, CB | 1p36 | CLCN6 | CLC-6 | SAP D SCMAS |
| CLCN7 [44] | 259700 | I Osteopetrosis | ? | 16p3 | CLCN7 | CLC-7 | ? |
| CLN4 B Parry [51-53] |
162350 | adult | GROD | ? | ? | ? | SAP |
| SGSH MPSIII |
adult | SGSH | SGSH |
Morphological phenotype: GROD, granular osmiophilic deposits; CB: curvilinear bodies; FP: fingerprints. Gene product: PPT1, Palmitoyl Protein Thioesterase 1; TPP1, Tripeptidyl Peptidase 1; CTSD: Cathepsin D. Stored protein: SAPs, sphingolipid activator proteins; SCMAS, subunit c of mitochondrial ATPase.
mutations found only on one disease allele.
Although inflammation may not typically represent an initiating factor in neurodegenerative diseases, there is emerging evidence in animal models that sustained inflammatory responses involving microglia and astrocytes contribute to disease progression at least in some LSDs, through the concept that glia-induced inflammation is an amplifier of the neuropathology [54].
Distinct hydrophobic proteins were shown to be major protein components of the storage cytosomes in different NCL types: SCMAS and/or the SAPs A and D [3,6]. A proteomic approach was recently developed for individuals that were diagnosed with apparent LSD based upon clinical criteria, but where the gene defect remained elusive [45]. This strategy was instrumental in the identification or validation of mutations in two lysosomal proteins CLN5 and SGSH in adult forms of NCL [45].
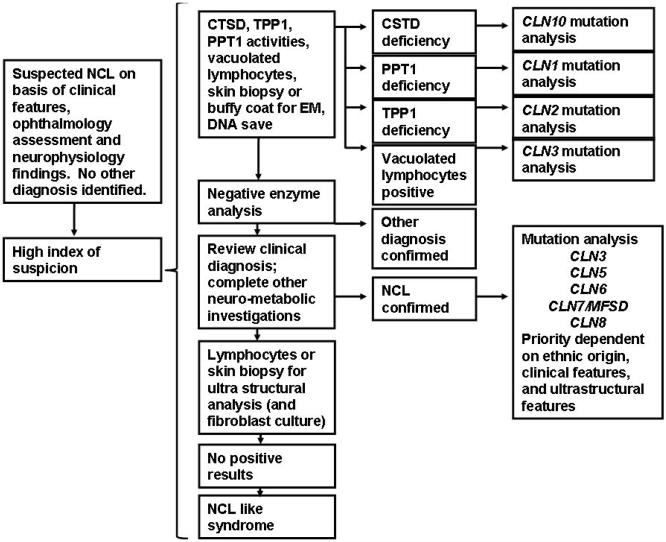
A significant question is whether some therapeutic approaches can be applied to all NCL diseases, or whether particular therapies are more appropriate to each type or even to special types of mutations. The variety of genotype-phenotype combinations means a major challenge of NCL therapies so that the most appropriate and effective therapy can be directed for each NCL type. A new diagnostic algorithm consensus was reached during the 12th International Congress on NCLs in the frame of the Rare NCL Gene Consortium-RNGC and NCL Disease registry Fig. (1).
Fig. (1).
Strategy for NCL study: Diagnostic algorithm. Consensus reached at the 12th Congress on NCLs held in Hamburg-Germany 2009, coordinated by Dr. R. Williams. EM, Electron Microscopy.
Treatment of LSDs affecting the CNS was addressed in some recent reviews [55-60]. There are some approaches effective in LSDs with significant systemic involvement. However, the greatest challenge for the NCLs is in treatment capable of passing the blood brain barrier (BBB) to repair or prevent the neurodegeneration and restore the CNS functionality [55,56]. Several strategies to transfer genes/gene products directly into the CNS have been proposed, and some NIH clinical trials are in progress. Currently, the US National Library of Medicine (http://www.clinicaltrials.gov) lists six studies on NCLs: the already completed phase I: 1) Study of Human Neural Stem Cells (HuNS-SC) in patients with LINCL (Robert Steiner- Oregon Health and Science University. ClinicalTrials.gov number NCT00337636); 2) Observational Study on Genotype-Phenotype correlations of LINCL (NCT00151268) [61,62]; 3) the currently recruiting participants Safety Study of a Gene Transfer Vector AAV2CUhCLN2 (NCT00151216) [62]; 4) the phase II Study on a Combination Therapy with Cystagon and N-Acetylcysteine to treat INCL (Eunice Kennedy Shriver National Institute of Child Health and Human Development Center, NCT00028262); 5) the not yet open Genotype-Phenotype Correlation Study of LINCL-2 (NTC01035424); 6) the active but not yet recruiting participants Stem Cell Transplant for Inborn Errors of Metabolism Study (NCT00176904).
The present paper reviews the promising therapies on preclinical assays and the fundamentals of the safe clinical studies in humans under development.
Clinical Assessment
Recent advances in the recognition of phenotype variations (Table 1) are relevant for the necessary assessment of changes induced in the disease course by the potential therapies.
CLN1 disease
The classical infantile CLN1 disease manifests itself in the second half of the first year of life and progresses dramatically with seizures, mental decay, loss of vision, microcephaly and brain atrophy. In the final stages children are blind with spastic and myoclonus, and death occurs at 8-13 years [11,63]. Ocular examination shows atrophy of the papilla and retinitis pigmentosa. Some mutations cause manifestation at a later age, including adulthood [16,63]. The underlying defect is the lack of activity of the lysosomal PPT1 which can be measured for diagnosis.
CLN2 disease
Classical late infantile CLN2 disease starts around the third year of life with seizures and a stand-still of mental development, ataxia and myoclonus, with a retinopathy occurring later that may be missed after progression to more generalized deficits (the visual loss begins to become evident after 4-6 years, evolving to blindness) [11]. Some mutations cause manifestation at a later age. The basic defect is the lack of activity of the lysosomal TPP1. CLN2 disease, classic late infantile is the most common form of NCL and has a broad distribution all over the world [64].
CLN3 disease
Classical juvenile CLN3 disease caused by the common 1 kb deletion starts at the age of four to six years with a progressive loss of vision due to retinal degeneration. After several years, dementia, epilepsy, and motor disturbances follow. The basic defect is in a membrane protein of unknown function (CLN3p). Hypotheses offered for the function of the CLN3p are varied, with a focus on its role in lysosomal metabolism, vesicular transport, autophagy, apoptosis, proteolipid modifications, and on other mechanisms [3].
CLN5 disease
This late infantile variant CLN5 disease develops symptoms somewhat later than classical CLN2 disease. Initially regarded as a Finnish disease, this type of NCL has now been observed in the Netherlands [65], Colombia [66], Portugal [29], Italy [30], Afghanistan-Pakistan [67], and Argentina [27,28]. The basic defect concerns a soluble protein, apparently localized in lysosomes, whose function may be linked to that of other NCL proteins.
CLN6 disease
This late infantile variant CLN6 disease is similar to CLN5 disease. Firstly observed in India, the Iberic Peninsula, in Middle and South America [11,31], it has a wider distribution, too. The visual loss and seizures are the initial symptoms in most patients with onset from 18 months to 8 years, in others the disease may start with epilepsy, ataxia and myoclonus. The CLN6 protein is a polytopic membrane protein of unknown function resident in the endoplasmic reticulum (ER) [32].
CLN7 disease
This late infantile variant CLN7 disease was originally observed in Turkish families, but is now recognized to have a wider distribution including Italy [39], Arabia Saudi [38], Czech Republic [36], Austria [37], India [68], among others. It is caused by mutations in the CLN7 gene (properly termed MFSD8) coding for a putative lysosomal transporter protein. The protein is expressed ubiquitously and localizes mainly to the lysosomal compartment [68].
CLN8 disease
The first mutation in CLN8 identified causes a phenotypic variant of CLN8 disease known in Finland as ‘Northern Epilepsy’ or Progressive Epilepsy with Mental Retardation (EPMR). Only later was CLN8 disease, variant late infantile recognized in Italy [41,42], Turkey, and Israel [69]. EPMR is characterized by progressive epilepsy associated with dementia and the visual problems may be mild and go unnoticed. However CLN8 disease, variant late infantile is similar to CLN5 disease. CLN8 encodes a membrane protein located in the ER.
CLN10 disease
Congenital CLN10 disease is characterized by primary microcephaly, neonatal (even intrauterine) epilepsy and death within a few weeks in early infancy [12]. Late-onset forms of this NCL may be seen in infants, juveniles and adults [13]. The CLN10 gene codes for CTSD, a lysosomal enzyme thought to be important for neuronal stability. Alterations in a macroautophagy-lysosomal degradation pathway seem to mediate neuron death in this NCL and possibly other diseases.
‘CLN9 disease’
This is a juvenile onset NCL that is clinically indistinguishable from CLN3 disease, classic juvenile, but can be distinguished diagnostically because the lymphocytes are not vacuolated. The underlying genetic cause is not yet known. Cells from CLN9 patients have shown multiple abnormalities when studied in vitro [49].
‘CLN4 disease’
First observed this term is sometimes used to refer to the adult form of NCL (Kufs or Parry disease) that is still defined by clinical and neuropathological findings only, since the genetic causes of most adult onset cases are undefined [46,47,51,52,68]. The signs and symptoms usually appear at around 30 years, with untreatable progressive myoclonus epilepsy, dementia, ataxia and more late pyramidal and extrapyramidal disorders, or in other patients with behavior disorders and dementia associated with motor dysfunction, cerebellar ataxia and extrapyramidal sing of commitment to the basal ganglia.
Unclassified NCLs
Patients with clinical findings suggestive of an NCL, autofluorescent storage material and EM evidence of intracellular storage material, but unable to be classified after thorough investigation, are being encountered in diagnostic institutions. Genes coding for ion channels have been suggested as candidates in such disorders [43].
Clinical manifestations in the NCLs are defined, and there are examples in the literature of imaging studies of Computational Tomography, Magnetic Resonance Images (MRI), and Magnetic Resonance Spectroscopy (MRS). Clinical rating scales have been developed over several decades to establish a range of severity and progression for the most common NCL types, CLN2 and CLN3 diseases. The German Seinfeld’s et al. rating scale [64] was developed for the clinical assessment of CLN2 disease, and more recently Worgall et al. [61] used the Weill Cornell scale using neurological aspects (feeding, gait, seizures and language) including images of MRI and MRS. The scale for each item studied ranges from 0 (most severe) to 3 (normal). Marshall et al. [70,71] developed clinical assessment tools, referred to as the Unified Batten Disease Rating Scale, to measure the severity and progression of CLN3 disease, juvenile based on motor assessment, behavior and functional capability. It is time to adopt one or two scales that will be applied internationally to fully understand NCL disease in the 21st Century and to have accurate data against which the effect of new therapeutic treatments can be assessed.
Enzyme Therapy
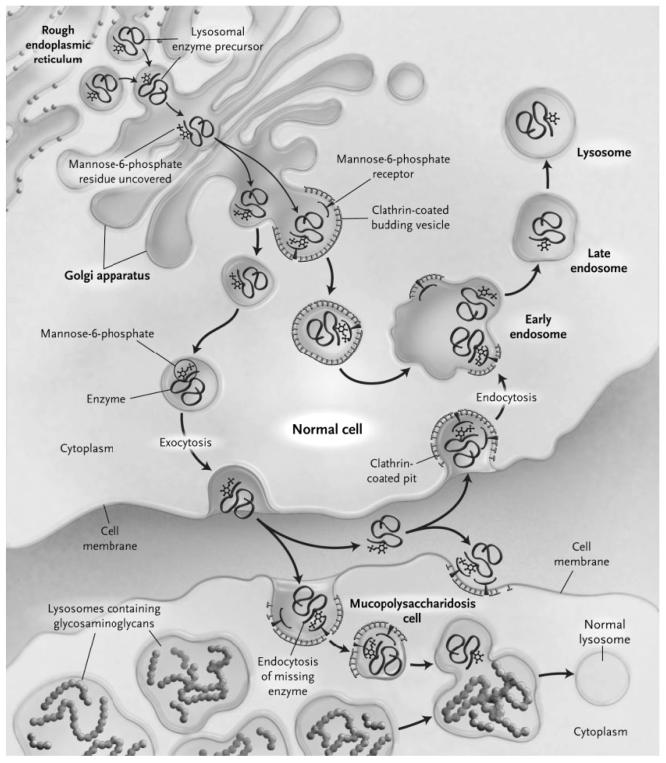
There are a number of ways the cell degrades macromolecules. One organelle, the lysosome, contains more than 50 different hydrolases that work together to degrade a variety of protein, carbohydrate, lipid, nucleic acids that are trafficked to this organelle [72,73]. The lysosomal enzymes are synthesized in the cytoplasm, traverse the rough endoplasmic reticulum (RER) and are transported intralumenally through a series of organelles to the lysosome. Within the Golgi apparatus, the soluble lysosomal hydrolases are specifically linked to the recognition marker mannose 6-phosphate (M6P) [74], which is recognized by its receptor and diverts these enzymes at the trans-Golgi network towards the lysosome. Whilst this is the main mechanism used, there are also less well understood mechanisms for entry to the lysosome that function independently of this receptor [73,75] Fig. (2). A variable portion of the newly synthesized lysosomal enzymes escape binding by the M6P receptor and are secreted, where they can be bound by M6P receptors on the plasma membrane of neighboring cells, and reach the lysosome via the endocytotic route. It is this mechanism that is a prerequisite for the effectiveness of enzyme replacement therapy (ERT) [72,76-80]. According to different authors between 1-5% [72] and 15-20% [81] of normal cellular lysosomal enzyme activity is required to correct a lysosomal enzyme metabolic defect. Thus, if enough extracellular enzymes can be trafficked to the lysosome to provide the equivalent of 10% of normal endogenous activity, this may be sufficient to rescue the underlying metabolic defect. After numerous clinical trials with promising results, the addition of intravenous purified enzymes demonstrated therapeutic activity in Gaucher, Pompe and Fabry diseases [56,82-84]. Clinical trials in other LSDs using similar ERT approaches were also successful, including Mucopolysaccharidosis I, II and VI [85-90]. Regular intravenous administration of a protein involves the risk of developing antibodies that can cause allergic reactions and/or inactivate the activity of the enzyme [91]. However, in general, ERTs proved to be well tolerated and those patients who exhibited seroconversion showed a decrease in the antibody titer during the treatment [60]. The ERTs were particularly effective in those LSDs with predominantly visceral manifestations, because the bloodstream is an appropriate vehicle for transporting enzymes to affected organs. The 25-years longitudinal analysis of treatment efficacy in inborn errors of metabolism published by Campeau et al. (2008), argues that the ERTs constituted 10% of the therapeutic modalities clinically used in patients [57].
Fig. (2).
Transport of a lysosomal enzyme in a normal cell, correction of storage in the cell of a patient with Mucopolysaccharidosis, and basis for ‘cross-correction’. The M6P recognition signal is added to the lysosomal enzyme precursor in the late-Golgi compartments, where enzyme modified by M6P binds to M6P receptors. The enzyme–receptor complex is packaged into a transport carrier vesicle and delivered to early endosomes in which low pH promotes the dissociation of the enzyme from the receptor. The enzyme is then delivered to the mature lysosome, and the M6P receptor is recycled to the Golgi apparatus. A small amount of the M6P–modified enzyme escapes capture by the M6P receptors and is released into the extra-cellular space. This enzyme can be recaptured by binding to a M6P receptor in a clathrin-coated pit on the cell surface. In a patient who has undergone hematopoietic stem-cell transplantation, enzyme released from a donor-derived stem cell can be taken up by a Mucopolysaccharidosis cell, which corrects aberrant glycosaminoglycan storage (from [Muenzer, J. & Fisher, A. (2004) Advances in the treatment of Mucopolysaccharidosis type I. New England Journal of Medicine 350(19):1932–4] Massachusetts Medical Society. All rights reserved, with permission of the editor).
It is well known that in those pathologies that affect the CNS, the ERTs are less effective due to the presence of the BBB. Since at least 75% of all LSDs have significant CNS involvement [59] this is a significant challenge. Also, it is more complex and up to now impossible to replace the function of a transmembrane protein by ERT [55]. However, Chen et al. discovered a way to turn the blood vessels surrounding brain cells into a production and delivery system for getting therapeutic molecules directly into the brain cells [92]. Peripheral injection of epitope-modified Adeno-associated viral vectors (AAVs) expressing the enzymes lacking in LSD mice reconstituted enzyme activity throughout the brain and improved disease phenotypes in two distinct disease models [92].
CLN1, CLN2 and CLN10 diseases are characterized by the loss of activity of three soluble lysosomal enzymes PPT1, TPP1 and CTSD respectively. The ERT approaches need to achieve a widespread reconstitution of the deficient enzyme activity throughout the brain. Lin and Lobel (2001) produced a cell line of Chinese-hamster ovary (CHO) that over-expressed the CLN2 protein mannose 6-phosphorylated [93]. By growing TPP1 deficient fibroblasts with different concentrations of CLN2p, they found that the recombinant protein was incorporated into the cells and that it was correctly targeted to the lysosomes. The enzymatic treatment dramatically reduced the levels of SCMAS, suggesting that CLN2p could correct the metabolic defect. However, the authors found that the process is much slower and less efficient in primary cultured rat cerebellar granule neurons. Different studies indicated that the cerebrospinal fluid might regulate the distribution of lysosomal enzymes and their subsequent incorporation into the brain parenchyma [94,95]. Chang et al. [96] verified this hypothesis in order to achieve an overall distribution of the TPP1 recombinant enzyme into the brain in a mouse model of CLN2. The infusions into the lateral ventricle showed a bilateral widespread enzyme distribution in the brain, an accumulation of mature TPP1 in the lysosomal compartment, and a TPP1 activity in treated mice that ranged from 2 to 18 times greater than that of heterozygous mice. The treated mice showed attenuated neuropathology, with a decrease in the resting tremor, as well as improvements in the neuropathological findings, including decreased gliosis in the motor cortex, decreased autofluorescence, and a partial rescue of the deep cerebellar nuclei. Sleat et al. [97] generated CLN2 mutant mice expressing different amounts of TPP1 activity to assess the reference levels required to achieve therapeutic benefits. Their results suggested that 6% of normal TPP1 at the CNS could provide the affected individuals with a significant therapeutic benefit; however, they argue that higher levels should be necessary to cure the disease.
The first ERT study for CLN1 was published by Lu et al. [98], who produced human recombinant PPT1 enzyme in a CHO cell line. The recombinant enzyme was injected intravenously in PPT1-deficient mice, and distributed to potential corrective levels in peripheral organs (kidney, liver, heart, lung and spleen), but minimally in the brain. Although the method of enzyme delivery failed to achieve a therapeutic enzyme level in the CNS, in the future alternative methods of delivery could be assessed such as intraventricular infusions, chemical modifications or chronic high-dose therapies.
The effectiveness of ERTs for NCLs is still an open therapeutic issue. However, there are other options to supplement its effect by combining ERT with enzyme enhancement, gene therapy, cell-based transplants and/or pharmacological agents that permeate the BBB [55].
Gene Therapy
Gene therapy can refer to an ex vivo genetic modification of donor cells that are reintroduced to the host or to the introduction of a gene into host cells in vivo [55,56,99]. When gene therapy involves the direct infection of cells in vivo by a viral vector, the virus is typically altered to reduce its virulence so as to avoid pathogenicity [55]. For ex vivo, white blood cells can be removed from a patient, transduced with a viral vector containing a corrected gene and reintroduced to the patient. The first clinical trial for gene therapy was performed in a child with severe combined immunodeficiency syndrome where a viral delivery system was used to transduce ex vivo lymphocytes with the normal gene for adenosine deaminase [55]. A gene therapy trial was accomplished in two patients with X-linked Adrenoleukodystrophy (ALD) [100]. Over a follow-up of 24 to 30 months, the expressing ALD protein was detected, and the progressive cerebral demyelination stopped [100].
Cell transplant therapy can include a number of approaches, involving different types of donor cells (some genetically modified) and delivery methods (review in [55]). Allogeneic stem cell therapy is based on the knowledge that bone marrow-derived stem cells can differentiate into blood monocytes that migrate to the brain to further differentiate into microglial cells [101,102]. Genetically modified cell therapy is similar to stem cell therapy, but with several advantages: it uses patient’s own cells and not embryonic stem cells, it circumvents two common problems of conventional stem cell therapy, namely, immunologic complications and ethical concerns [56,99,100]. The phenomenon of cross correction by secreted enzymes Fig. (2) obviates the need to transfer genes to all cells, allowing the possibility that a small percentage of transduced cells can be therapeutic at the organ or whole animal level [59,103]. Viral vectors are used to deliver a normal copy of the defective gene. The normal gene encoded by the viral vector results in protein synthesis and secretion. Surrounding cells internalize the secreted protein through receptor-mediated endocytosis and deliver it to the endosomal-lysosomal system [56], Fig. (2). However, because of the BBB, somatic cells that produce and secrete large enzyme proteins may not target the CNS. The injection of viral based vectors directly into the brain has been successfully achieved in vivo in LSD animal models, overcoming the accessibility block by the BBB [56,59,104,105].
A gene therapy strategy approach was effective in preclinical assays in model animals like mouse, dog, cat and primate for several LSDs [55,59,104-108]. The studies examined the feasibility of gene transfer into brain or eye using recombinant Adenovirus feline immunodeficiency virus and AAV. Thus, various vector systems were tested and the progression and effects in the animal models were evaluated for different disorders. Mouse models were invaluable for the first analyses of gene therapy, but the use of large animal models was a critical step in moving toward clinical trials. In particular, dogs and cats with LSDs (Mucopolysaccharidosis I, VI, VII, α-Mannosidosis, among others) have a heterogeneous genetic background, a suitable size for clinical evaluations, surgical manipulations and a longevity that permits assessment of the long-term expression and risks of gene therapy over years.
The introduction of functional genes to the brain for CLN1 or CLN2 patients by intracranial injection of a viral vector may remove storage material and rescue cells from dysfunction. Neurons are particularly sensitive to the lysosomal accumulation of storage materials; individuals with CLN1 or CLN2 diseases show a generalized extensive and progressive neurodegeneration, eventually resulting in a vegetative state and premature death [55,101,109]. It was assumed that successful gene therapy for these and for other LSDs that affect the CNS will require neurotropic gene delivery, expression of therapeutic level of protein for a long duration with extensive spread through the brain [106,109].
Gene therapy should restore a sufficient percentage of normal intracellular TPP1 activity to prevent the progression of the disease in the brain and in the retina [106-108]. Preclinical studies have shown that AAV2 and AAV5 vectors containing CLN2 can achieve locally high levels of TPP1 for at least 18 months, clear storage granules in the CNS of CLN2−/− mice, but with no reported improvement in performance or mortality [107,109]. The AAVrh.10 vector was also tested, and several AAV serotypes (AAV2, AAV5, AAV8, AAVrh.10) were compared for TPP1 expression following CNS administration. Until now, the non-human primate-derived serotype AAVrh.10 expressing CLN2 cDNA, produced the highest levels and broadest distribution of TPP1 expression in animal models [109]. Remarkable is that immunity to human AAV serotypes did not affect CLN2 gene transfer by AAVrh.10, an important consideration for gene delivery in human subjects who may have been exposed to AAV [109]. CNS delivery of AAVrh.10CLN2 to CLN2−/− mice restored normal levels of TPP1 and reduced the accumulated autofluorescent granules in the brain. There was also a significant improvement in behavioral activity and performance compared to controls, and most importantly, an extended survival [109].
After those preclinical experiments, a Phase I trial for testing the safety of gene transfer vectors in humans with CLN2 disease was initiated at Medical College of Cornell University by R. Crystal and collaborators (ClinicalTrials.gov number NCT00151216, http://clinicaltrials.gov) [62].
A concluding remark is that preclinical gene therapy assays in CLN2−/− mice restored normal levels of enzyme activity using AAVrh.10CLN2, being this approach successful to improve behavior and prolonged the time lapse in the knock out mice. The same has until now not be stated for humans, and that is a long process underway.
Pharmacological Therapy
Mechanisms addressed by different pharmacological approaches include production of neurotrophic factors and cytokines, expression of various cell survival-promoting proteins (e.g., protein chaperones, antioxidant enzymes, Bcl-2 and inhibitors of apoptosis proteins), preservation of genomic integrity by telomerase and DNA repair proteins, and mobilization of neural stem cells to replace damaged neurons and glia [110].
a) Diet manipulation: About 20 years ago, six patients of different ages with CLN3 disease, juvenile who were demonstrated to have abnormally low levels of membrane phospholipids were treated by dietary supplementation with polyunsaturated fatty acids (PUFAs). Clinical data suggested that PUFA supplementation may arrest the natural course of juvenile CLN3 disease if treatment is started early [111]. This was also reported upon the reversibility of induced lysosomal storage in juvenile CLN3 disease lymphoblast cell lines, when incubated with gentamycin (an agent known to damage mitochondria) and a defined mixture of PUFAs [112]. The real therapeutic significance of those early experiments together needs to be the subject of further investigations. The ketogenic diet (KD) was studied in a randomized controlled trial to reveal its efficacy to control seizures in 145 children aged 2-16 years (trial of Ruth Williams). The study showed that the KD could be a complement to drug control of seizures in CLN2 disease patients. Further clinical follow up studies in patients and in animal models of NCL may be justified [113]. PPT1 (a thioesterase/ lipoprotease) and TPP1 (a peptidase) are present in the saliva [114]; both enzymes are evolutionary well conserved proteins, that may have some kind of functionality in the digestion of food in mammals. This hypothesis should be tested in animal models.
b) Inhibition of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionate (AMPA) receptors: under normal conditions, the glutamatergic system plays a prominent role in synaptic plasticity, learning, and memory, but in pathological conditions it is known to be a potent neuronal excitotoxin, triggering either rapid or delayed neurotoxicity that ranges from atrophic changes to cell death (review in [115]). The AMPA glutamate receptor is one of the main contributors of excitatory neurotransmission, mediating the fast desensitizing excitation of many synapses. AMPA receptors are known to play roles in long-term neurotrophic/ neuroprotective effects [115]. Enhancing the activity of AMPA receptors increases the production of growth factors, such as brain derived neurotrophic factor and regulate the mechanism of neurite growth [115].
Agents that attenuate the activity of AMPA receptors, such as EGIS-8332([+/−]-7-acetyl-5-[4-aminophenyl]-7,8-dihydro-8-cyano-8-methyl-9H-1,3-dioxolo-[4,5-h]-2,3-benzodiazepine) could have therapeutic potential possibilities for juvenile CLN3 disease [116,117]. Characterizing Cln3 deficient mice revealed evidence for elevated levels of glutamate within the CNS, and a cell type selective sensitivity to AMPA glutamate receptor overactivation. EGIS-8332 was studied in the Cln3Δex1–6 mouse model of juvenile CLN3 disease. The selective sensitivity of cerebellar granule cells to AMPA receptor-mediated toxicity suggested that lack of CLN3p has a significant influence on the function of AMPA receptors, and that their dysregulation may be a major contributor to the cerebellar dysfunction [116]. Administration of EGIS-8332 in low doses aimed to attenuate AMPA receptor function avoiding a toxic complete blockade showed that inhibition of AMPA receptor activity significantly improved the motor skills. Another potent antagonist of AMPA, 2,3-dimethyl-6-phenyl-12H-[1,3]dioxolo[4,5-h]imidazo[1,2-c][2,3]benzodiazepine (ZK 187638) was tested in the mnd mice model for CLN8 disease [9]. Talampanel (TLP) is a novel anticonvulsant that acts as an allosteric inhibitor of the AMPA receptor; it has a broad spectrum of action in animal models of epilepsy, and neuroprotection. A randomized, double-blind, placebo controlled, multicenter, efficacy and safety study of TLP is underway in the US in 190 patients (18-65 years old) with refractory partial seizures (ClinicalTrials.gov number NCT00034814, http://clinicaltrials.gov).
c) Antioxidant mechanisms: The production of reactive oxygen species and reactive nitrogen during oxidative stress initiates and promotes the damage of macromolecules, resulting in CNS neurodegeneration (review in [118]). The oxidative stress also activates mechanisms that result in a glia-mediated inflammation that, in turn, causes secondary neuronal damage [118,119]. Therefore, a rational therapy for neurodegenerative diseases targeting mitochondrial functions may potentially reduce both inflammatory, and neurodegenerative events. This aim may be, at least partially, achieved in the SNC by Mildronate [3-(2,2,2-trimethylhydrazinium) propionate dihydrate] [120]. The possible antiapoptotic, anti-inflammatory and neuroprotective effects of Mildronate should be tested in mouse models of different NCLs.
Advances in the development of pharmacological chaperones and guidelines for their design were recently reviewed [121]. Pharmacological chaperones are potentially therapeutic molecules aimed to correct misfolded proteins, acting as substrates or modulators that stabilize and promote functional proteins [83,122-124]. The oxidative stress causes neuronal death by apoptosis in GM1 gangliosidosis [125] and INCL [126-128], through activation of the unfolded protein response. Experiments with Brefeldin-A stressed fibroblasts from different NCLs and GM1 patients, and cells from neurodegenerative and non- neurodegenerative LSDs, supplemented with the chaperones dihydride trimethylamine N-oxide (TMAO) and tauroursodeoxycholic acid (TUCDA), showed modified oxidative stress. Lindholm et al. applied a similar methodology to tissue cells of neural models for neuronopathic Gaucher disease [129]. The authors found no similar evidence than in human skin fibroblasts from GM1, CLN1, other neurodegenerative LSDs, Parkinson’s and Alzheimer’s diseases [130]. It was thus demonstrated that both synthetic chaperones, TMAO and TUCDA, are efficient repressors of oxidative stress depending on the type of LSD or neurodegenerative diseases cells used [126-128].
d) Reduction of substrate: Recent studies indicated that substrate deprivation therapy (or substrate reduction therapy) may be effective for the treatment of neuronopathic forms in at least some LSDs. This therapeutic approach was studied in several animal models of various diseases and in few patients [55].
Oral Miglustat (N-butyl-deoxynojirimycin) is an imino sugar which was effective in Niemann-Pick C, San Filippo, Tay-Sachs, Sandhoff and Fabry diseases [131-134]. Miglustat showed its effectiveness as inhibitor of glucosilceramide in Gaucher’s disease type I. It was still not reported experimental work in NCLs in spite of being its use approved for treatment of LSDs in general by the FDA of USA.
Cystagon and N-acetylcysteine (Mucomyst) are currently been tested in combination in a clinical trial (ClinicalTrials.gov number NCTOOO28262, http://clinicaltrials.gov) in classic infantile CLN1 disease children. Phase II presented favorable anti-apoptotic properties, reducing lipofuscin ceroid like materials and slowing of the progression of neurological symptoms.
Clenbuterol racemate (alpha adrenoceptor beta2-agonist) enhanced axon regeneration lesions of motor neurons in the mnd mouse that showed also enhanced survival and maintenance of motor neurons, with preserved motorfunction [135]. Clenbuterol had neuroprotective effects in neuronal cultures of various stroke rodent models. Only its enantiomer S(+)-clenbuterol, but not R(−)-clenbuterol, was effective in mediating the neuroprotective effects of beta-2 agonists [136]. Most importantly, only the S(+)-enantiomer clenbuterol reduced ischemic brain damage similar to the effect of the racemate. These results clearly demonstrated that S(+)-clenbuterol is the eutomer that mediates neuroprotective effects of the beta(2)-adrenoceptor agonist [55,135].
e) Readthrough therapy: Many inherited diseases are caused by nonsense mutations (between 5-70%) leading to premature termination codons (PTCs) [137-139]. Nonsense-mediated mRNA decay is one of the quality control mechanisms developed by cells to maintain the metabolic homeostasis. The cell’s objective is to destroy mRNA species that contain PTCs so that only full-length proteins are produced [140]. The pharmacological suppression of nonsense mutations by drugs has been assumed to have therapeutic potential in CLN2 disease [141].
The ability of aminoglycoside antibiotics to promote readthrough of eukaryotic cell’s stop codons has attracted interest in these drugs as potential therapeutic agents in human genetic diseases caused by nonsense mutations. Studies in tissue culture cells as well as in animal models showed that gentamicin, and PTC124 can readthrough disease-causing PTCs and partially restore the expression and/or the function of the proteins. The best known examples of this mechanism in inherited disorders belong to Cystic Fibrosis, Duchenne and Becker muscular dystrophies, Hurler disease, X linked nephrogenic diabetes insipidus, and several forms of cancer. Clinical trials in these diseases have shown to promote in vivo readthrough [137,138,142].
PTC mutations in the CLN2 gene are present in approximately half of the children diagnosed with CLN2 disease (http://www.ucl.ac.uk/ncl). The ability of gentamicin to restore TPP1 activity was tested in cultured human fibroblasts from classic late infantile disease patients with one or more PTCs within the CLN2 gene [141]. Interestingly, the levels of TPP1 activity induced by gentamicin varied with different stop codon mutations. This variability was proposed to be associated with the identity of the PTC and its sequence context, as well as with the chemical composition of the aminoglycoside, the duration of the treatment and the method of application [137,141].
f) Antiapoptotic treatments: It has been suggested that treatments that increase the antiapoptotic Bcl-2 activity would be potential therapeutic approaches. TPP1−/− mice were generated that either lacked pro-apoptotic p53 protein or had increased levels of anti-apoptotic Bcl-2. The results showed that the changes did not affect or shorten the life of the CLN2 disease mice, and were dismissed for the development of effective pharmacological therapeutics [143]. Flupirtine is a derivative of Triaminopyridine. It was assumed to prevent the death of neurons in CLN1, CLN2, CLN3 and CLN6 disease, involving the mechanism the functional antagonism of N-methyl-D-aspartate or N-methyl-D-aspartate-induced neuronal apoptosis. Flupirtine may thus be useful as a drug capable of arresting the progression of neurodegenerative diseases caused by deregulated apoptosis [144,145].
There are now a number of therapeutic resources aimed at improving the quality of life of patients and their families, without ultimately altering the inexorable course of the disease. Progress has been made on experiments with dietary management, antioxidant therapy with chaperones and other small molecules, readthrough, and antiapoptotic drugs that could influence the course of one or several of these diseases. The greatest challenge is transporting the BBB before irreversible destruction of neurons in the brain occurs.
Stem Cell Therapy
Regenerative medicine is an emerging frontier of medical therapy that holds the promise of replacing damaged tissues largely by prompting autonomous tissue repair or facilitating the implantation of engineered tissue derived from progenitors or stem cells [146,147]. It is obvious that no single approach will solve all problems; rather each tissue and each pathologic condition probably will require a different methodology to obtain optimal results [148].
Hedgehog activators are prime candidates for therapeutics to initiate or modulate tissue self-repair [149]. Hedgehog signaling through the plasma membrane receptor Smoothened (Smo) is an important process for regulating stem cell proliferation. Using a high-content screen with cells expressing Smo receptors and a β-arrestin2-GFP reporter, Wang et al. identified four FDA-approved drugs- halcinonide, fluticasone, clobetasol, and fluocinonide- as Smo agonists that activate Hedgehog signaling. These drugs provided an unprecedented opportunity to develop unique clinical strategies to treat Hedgehog-dependent illnesses [149].
Cellular regenerative medicine for NCLs has got some experimental advances in two interdependent directions related to other approaches treated in this review, like ERT and gene therapy: a) transplantation of stem cells related to the blood; b) transplantation of hCNS-SCns.
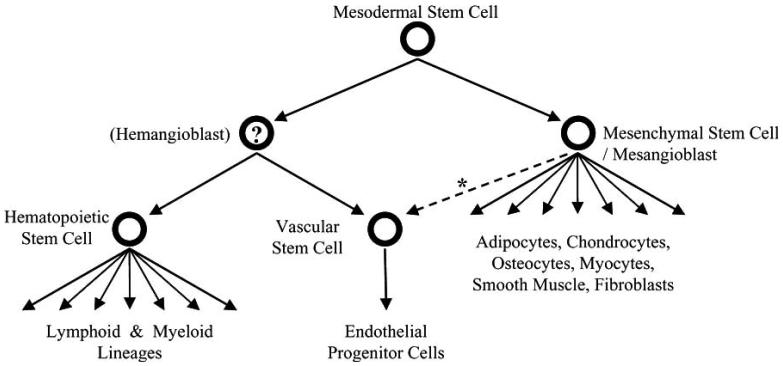
a) The blood-related stem cells can be categorized as [150]: (1) Hematopoietic stem cells (HSCs); (2) Mesenchymal stem cells; (3) Other stem cells that may not share the characteristics of either of the first two, but may contribute to functional recovery in a given model, Fig. (3). A fourth category is emerging with the successful use of Umbilical Cord Blood Cells (UCBs), increasingly banked in several parts of the world. UCBs are considered as an alternative for transplantation in Inborn Errors of Metabolism (IEM) in which allogenic hematopoietic stem cells transplantation is the standard of care when not related donors of bone marrow are available [102,151]. Children with Mucopolysaccharidosis have benefited from many advances, including the development of guidelines used worldwide for their evaluation and treatment [151], and the emission of general principles for standardized use. HSC and/or UCB transplantation is the standard care for about fifteen IEM and more than 2000 treatments are reported for Asparylgrucosaminuria, Metachromatic Leucodystrophy, Fucosidosis, Adrenoleucodystrophy, Mucopolisaccharidosis type I (severe phenotype), Mucopolisaccharidosis type IV (if ERT failed), Wolman Disease, Mucolipidosis II or I-Cell Disease, Globoid Leucodystrophy [102]. For other fifteen IEM, including NCLs, HSC and UCB transplantation are in investigational phase [152-154], and for two it is not indicated.
Fig. (3).
A model of how blood-related stem cells may be related. The authors wish to acknowledge the suggestion of Ralf Huss, MD, PhD (from [Graham C. Parker. Blood Stem Cells and non-Hematological Clinical Practice: Pragmatics Before Therapeutics. Current Pharmaceutical Biotechnology, 2007, 8, 51-56], with permission of the editor).
The purpose of HSC transplantation is to introduce hematopoietic cells from a normal donor that contains the enzyme able to get rid of the substances that have accumulated in the body of patients with storage diseases by an elucidated mechanism, called cross correction, Fig. (2) [102]. The ERT was used in conjunction with HSC transplantation in a series of 18 patients with severe Mucopolysaccharidosis type I, with a survival and engraftment rate of 89% and 93% for the patients who received full-intensity conditioning [155].
The therapeutic value of stem cell transplants was not apparent in clinical studies performed in CLN1, CLN2 and CLN3 disease patients, and even not in an animal model [55,152-154,156], and it is a contra-indication of ERTs [157-159]. Emerging stem cell sources, such as UCB, as well as improvement of transplantation techniques, and the mechanistic fact that the macrophages of the blood surpass the BBB and integrate as microglia in the brain, may extend the list of IEM with HSCT and/or UCB indication [157]. More support for the failure of HSC transplantation if CNS degeneration is advanced was provided recently by bone marrow transplantation that did not prevented the development of blindness and severe CNS degeneration in a small number of patients with CLCN7 mutations, even if the Osteopetrosis was successfully treated [3]. The reasons for HSCT failures to correct CNS damage have been hypothesized, and one of the most powerful arguments is that the cross correction mechanism does not reverse the CNS damage in advanced stages of the disease [102]; this is the main argument to extended newborn screening including IEM that will benefit on early transplantations regimes.
b) Neural stem cells: The subventricular (SVZ) zone is the source of the HuCNS-SC [160,161]. Cells with stem cell properties have been isolated from the subcallosal area and several other CNS regions, such as the cerebellum; the histological characterization of the anatomy of the human SVZ has been recently reported, showing remarklable similarities with the murine structure (review in [162]). It was reported that prospectively isolated human CNS stem cells grown as neurospheres (hCNS-SCns), survive, migrate, and express differentiation markers for neurons and oligodendrocytes after longterm engraftment in spinal cord-injured NOD-scid mice [163]. Moreover, remielinization was observed and electron microscopic evidence was consistent with synapse formation between hCNS-SCns and mouse host neurons [163]. However, the hCNS-SCns could alter the host microenvironment as an additional or alternative mechanism of recovery. New experiments of stereological quantification for lesion volume, tissue sparing, descending serotonergic host fiber sprouting, chondroitin sulfate proteoglycan deposition, glial scarring, and angiogenesis demonstrated no evidence of host modification within the mouse spinal cord as a result of hCNS-SCns transplantation [164]. Altogether, the data suggest that the locomotor improvements associated with hCNS-SCns transplantation were not due to modifications within the host microenvironment, supporting the hypothesis that human cell integration within the host circuitry mediates functional recovery following a nine day delayed transplant [164]. Thus it could be inferred that hCNS-SCns may possess therapeutic potential for CNS injury and disease.
Among the NCLs, a preclinical trial using hCNS-SCns was developed in the mice model of CLN1 disease, Ppt1 [165]. Purified non-genetically modified hCNS-SCns were transplanted into the brains of immunodeficient Ppt1−/− mice, where they engrafted robustly, migrated extensively, and produced sufficient levels of PPT1 to alter host neuropathology. Grafted mice displayed reduced autofluorescent lipofuscin, significant neuroprotection of host hippocampal and cortical neurons, and delayed loss of motor coordination. An early intervention with cellular transplants of hCNS-SCns into the brains of CLN1 disease patients may supply a continuous and long-lasting source of the missing PPT1, and provide some therapeutic benefit through protection of endogenous neurons.
The first FDA authorized use (phase I trial) of HuCNS-SC for clinical testing in CLN1 and CLN2 disease patients has recently been completed (ClinicalTrials.gov number NCT00337636, http://clinicaltrials.gov). Its goals were to evaluate the safety of HuCNS-SC, in addition to the immunosupression regimen and surgical transplantation technique, and also to evaluate the evidence of preliminary efficacy in terms of neurological and radiographic outcome after transplantation. After completion of this study, patients will be monitored for an additional four years under a separate long term follow-up protocol. The company that holds a patent on the HuCNS-SC announced that data from the trial demonstrated the clinical safety and tolerability of HuCNS-SC cells (http:www.stemcellinc.com).
As concluding remarks on stem cells therapies, we can say that according to the present state of the art, HSCT is not a therapeutic indication for NCLs, although expectation remains open since donor derived monocytes cross the BBB, and technological improvements are underway. Preclinical and clinical experiments using HuCNS-SC provided evidence that ventricular implants could be a therapeutic alternative, since useful amounts of enzyme activity could be measured after implantation, but it was not proved that the intellectual and motor disabilities are reversible in patients with advanced manifestations of the disease.
Immune Therapy
CNS inflammation is evident in all forms of NCLs [166-169], and also in other neurodegenerative disorders [170-172]. The CNS is an immunologically privileged site and circulating immune cells normally do not have access to it in the absence of inflammation or injury. The microglia exhibits a deactivated phenotype in the healthy brain and, may play important roles in maintenance of tissue homeostasis through communication with astrocytes and neurons, analogous to homeostatic roles of “alternatively activated” macrophages in other tissues. Dendritic cells with specialized antigen-presenting capabilities are not present under normal conditions, and the microglia was assumed to be the major initial sensor of danger or stranger signals recognized by Toll-like receptor 4. The response is the secretion of inflammatory mediators such as TNF-α and IL-1β that can act on astrocytes to induce the secondary inflammatory responses [173]. Although inflammation may not typically represent an initiating factor in neurodegenerative diseases, there is emerging evidence in animal models that sustained inflammatory responses involving microglia and astrocytes contribute to disease progression. A major unresolved question is whether inhibition of these responses will be a safe and effective means of reversing or slowing the neurodegeneration [54]. The emerging evidence for both protective and pathogenic roles of microglia and astrocytes and the activation of common inflammation pathways in these cells in several neurodegenerative diseases, supports the concept that glia-induced inflammation is an amplifier of pathology. Although inhibition of neuroinflammation may not alter the underlying cause of disease, it may reduce the production of factors that contribute to neurotoxicity, thereby resulting in clinical benefit [54].
The Ppt1−/− mouse, a model for CLN1 disease, mimics the disease affecting the CNS during infancy or childhood. Preliminary data suggest that reactive astrocytes play a major role in modulating a neuroimmune response in the CNS of Ppt1−/− mice. The first pathological change seen in the Ppt1−/− brain is astrocyte activation (GFAP upregulation) in the thalamus and cerebellum. These sites act as a predictor for future sites of neurodegeneration. Preventing GFAP upregulation will alter the disease course since GFAP is the main component of intermediate filaments, and is synthesized in increased amounts during reactive gliosis accompanying neurodegeneration in multiple neurodegenerative diseases including NCLs [166,174,175]. The CCL3 Ppt1−/− mice protein displayed a similar expression profile in thalamus and cortex regions, with a very high expression at one month of age, followed by a dramatic decrease from three months onwards. This findings are of potential interest because CCL3 protein, also known as macrophage inflammatory protein 1a (MIP-1α), is a chemokine involved in inflammation [176,177]. A mouse model of Sandhoff disease (Hexb−/−), demonstrated elevated levels of CCL3/MIP-1a, and loss of MIP-1 α improved disease phenotype [178]. The elevated expression of CCL3 in Ppt1−/− mice supported the hypothesis that inflammation may contribute to neurodegeneration. Lacking of a similar initially increased level of Ccl3 expression in the nclf mice (model of CLN6 disease variant late infantile) suggested that the inflammatory response is not necessarily triggered in different forms of NCLs, and that it may be informative to determine whether up regulation of Ccl3/MIP-1a occurs in the other NCL forms.
Significant differences in individual protein expression profiles were stated [179] for both Ppt1−/− mouse and Cln6 nclf models in two of the fifteen proteins examined. The voltage-dependent anion channel 1 (VDAC1) and the pituitary tumor-transforming 1 (Pttg1) displayed robust and significant changes at pre/early-symptomatic time-points in both models. This study demonstrated that synapses and axons are important early pathological targets in the NCLs and identified two proteins, VDAC1 and Pttg1, as potential in vivo biomarkers of pre/early-symptomatic axonal and synaptic vulnerability in the NCLs.
The presence of auto reactive antibodies within multiple forms of NCL was demonstrated in sera, not exclusively directed towards protein GAD65 [180]. As a matter of fact, patients and a mouse model of juvenile CLN3 disease, raised auto-antibodies against GAD65 and other brain directed antigens. Cln3−/− mice showed auto-antibodies to GAD65 in their cerebrospinal fluid and elevated levels of brain bound immunoglobulin G (IgG). IgG deposition was found within human CLN3 disease, juvenile autopsy material, a feature that became more evident with increased age in Cln3−/− mice. The lymphocyte infiltration present in human and murine CLN3 disease, juvenile occurred late in disease progression, and was not capable of central/intrathecal IgG production. In contrast, early systemic immune dysregulation was detected in Cln3−/− mice. In addition, evidence for a size-selective breach in the BBB integrity in these mice suggested that systemically produced auto-antibodies can access the CLN3 disease, juvenile CNS and contribute to a progressive inflammatory response [181].
Mice modeling CLN3 disease, juvenile that cannot make antibodies seem to do better than those that can, as do mice treated with the immunosuppressant mycophenolate (mycophenolate mofetil, Cellcept, Myfortic, mycophenolate sodium or mycophenolic acid-MPA). MPA is a selective, reversible inhibitor of inosine monophosphate dehydrogenase (IMPDH). IMPDH has an important role in the de novo purine synthesis in T and B lymphocytes, and the inhibition of this pathway causes immunosuppression [182]. A trial with MPA in CLN3 disease, juvenile patients is underway in the USA via the University of Rochester Medical Center, in conjunction with the Batten Disease Support Association-BDSRA (http://www.bdsra.org/).
A temporary positive effect of intermittent prednisolone treatment on motor symptoms was reported in CLN3 disease, juvenile homozygous and heterozygous for the common 1 kb deletion [183]. GAD65- antibodies became undetectable in these patients after initiation of the treatment, suggesting that systematically produced GAD65-antibodies worsen the motor symptoms of older patients. This is in agreement with an earlier study [181] that evaluated the deposition of antibodies in the CNS in one autopsy brain of a CLN3 disease, juvenile patient (no genotype specified). GAD65-antibodies were assumed to occur late in the disease process as result of the invasion of systematically produced antibodies into the CNS. In two of the younger patients under prednisolone therapy, the mean verbal IQ increased significantly when measured through the Reliable Change Index, with no correlation with the existence of GAD65-antibodies. The motor improvements in older patients and the increased IQ in younger patients are encouraging, but a longer, randomized, controlled follow-up study is needed to verify whether corticosteroid treatment would have a true effect on CLN3 disease progression. Furthermore, the possible benefits must be weighed against the adverse effects associated with the therapy.
The anti-inflammatory therapy with corticosteroids and other anti-inflammatory chemicals may be valuable to ameliorate some of the symptoms of CLN3 disease, juvenile, but have not shown significant clinical benefit on the progression of the disease.
CONCLUSIONS AND FUTURE PROSPECTS
The NCLs present a significant therapeutic challenge for several reasons: the main clinical manifestation is in the brain which is relatively inaccessible, disease onset for the most part is in early childhood and for treatment to be most effective it needs to begin as early as possible and preferably before there is significant cell death, and established approaches do not work for most NCLs because the mutant protein is a membrane protein located intracellularly. Nevertheless, progress is being made, particularly for those NCLs caused by defective enzymes, and the strategies being developed for the remainder could also be applicable to other diseases. The advent of several new approaches may allow significant progress to be made in the next decade. It can also be anticipated that future therapies for diseases such as juvenile CLN3 disease may be a combination of several approaches added over time designed to minimize but not initially cure the effect on the disease.
ACKNOWLEDGEMENTS
We are grateful to Ruth Williams MD for coordinating the consensus of the NCL diagnostic algorithm. We acknowledge the grants of the Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET-RA), Secretaría de Ciencia y Técnica de la Universidad Nacional de Córdoba (SE-CyT-UNC), Ministerio de Ciencia y Tecnología de la Provincia de Córdoba- Argentina, the Batten Disease Support and Research Association (BDSRA-USA) and the Batten Disease Family Association (BDFA, UK).
LIST OF ABBREVIATIONS
- NCLs
Neuronal ceroid lipofuscinoses
- LSDs
Lysosomal storage diseases
- CNS
Central nervous system
- GROD
Granular osmiophilic deposits
- CB
Curvilinear bodies
- FP
Fingerprints
- PPT1
Palmitoyl Protein Thioesterase 1
- TPP1
Tripeptidyl Peptidase 1
- CTSD
Cathepsin D
- SAPs
Sphingolipid activator proteins
- SCMAS
Subunit c of mitochondrial ATPase
- EM
Electron microscopy
- BBB
Blood brain barrier
- HuNS-SC
Human Neural Stem Cells
- ER
Endoplasmic reticulum
- EPMR
Progressive Epilpsy with Mental Retardation
- MRI
Magnetic Resonance Images
- MRS
Magnetic Resonance Spectroscopy
- RER
Rough endoplasmic reticulum
- M6P
Mannose 6-phosphate
- ERT
Enzyme replacement therapy
- AAVs
Adeno-associated viral vectors
- CHO
Chinese-hamster ovary
- PUFAs
Polyunsaturated fatty acids
- KD
Ketogenic diet
- AMPA
α-amino-3-hydroxy-5-methyl-4-isoxazolepropionate
- TLP
Talampanel
- TMAO
Dihydride trimethylamine N-oxide
- TUCDA
Tauroursodeoxycholic acid
- PTCs
Premature termination codons
- HSCs
Hematopoietic stem cells
- UCBs
Umbilical Cord Blood Cells
- IEM
Inborn Errors of Metabolism
- SVZ
Subventricular zone
- hCNS-SCns
CNS stem cells grown as neurospheres
- CC
Corpus callosum
- CTX
Cerebral cortex
- OB
Olfactory bulb
- STR
Striatum
- IgG
Immunoglobulin G
- MPA
Mycophenolic acid
- IMPDH
Inosine monophosphate dehydrogenase
REFERENCES
- [1].Goebel HH, Wisniewski KE. Current state of clinical and morphological features in human NCL. Brain Pathol. 2004;14(1):61–69. doi: 10.1111/j.1750-3639.2004.tb00499.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Williams RE, Aberg L, Autti T, Goebel HH, Kohlschutter A, Lonnqvist T. Diagnosis of the neuronal ceroid lipofuscinoses: an update. Biochim. Biophys. Acta. 2006;1762(10):865–872. doi: 10.1016/j.bbadis.2006.07.001. [DOI] [PubMed] [Google Scholar]
- [3].Jalanko A, Braulke T. Neuronal ceroid lipofuscinoses. Biochim. Biophys. Acta. 2009;1793(4):697–709. doi: 10.1016/j.bbamcr.2008.11.004. [DOI] [PubMed] [Google Scholar]
- [4].Mole SE, Williams RE, Goebel HH. Correlations between genotype, ultrastructural morphology and clinical phenotype in the neuronal ceroid lipofuscinoses. Neurogenetics. 2005;6(3):107–126. doi: 10.1007/s10048-005-0218-3. [DOI] [PubMed] [Google Scholar]
- [5].Seehafer SS, Pearce DA. You say lipofuscin, we say ceroid: defining autofluorescent storage material. Neurobiol. Aging. 2006;27(4):576–588. doi: 10.1016/j.neurobiolaging.2005.12.006. [DOI] [PubMed] [Google Scholar]
- [6].Haltia M. The neuronal ceroid-lipofuscinoses: from past to present. Biochim. Biophys. Acta. 2006;1762(10):850–856. doi: 10.1016/j.bbadis.2006.06.010. [DOI] [PubMed] [Google Scholar]
- [7].Kyttala A, Lahtinen U, Braulke T, Hofmann SL. Functional biology of the neuronal ceroid lipofuscinoses (NCL) proteins. Biochim. Biophys. Acta. 2006;1762(10):920–933. doi: 10.1016/j.bbadis.2006.05.007. [DOI] [PubMed] [Google Scholar]
- [8].Williams RE, Aberg L, Autti T, Goebel HH, Kohlschutter A, Lonnqvist T. Diagnosis of the neuronal ceroid lipofuscinoses: an update. Biochim. Biophys. Acta. 2006;1762(10):865–872. doi: 10.1016/j.bbadis.2006.07.001. [DOI] [PubMed] [Google Scholar]
- [9].Cooper JD, Russell C, Mitchison HM. Progress towards understanding disease mechanisms in small vertebrate models of neuronal ceroid lipofuscinosis. Biochim. Biophys. Acta. 2006;1762(10):873–889. doi: 10.1016/j.bbadis.2006.08.002. [DOI] [PubMed] [Google Scholar]
- [10].Goebel HH, Wisniewski KE. Current state of clinical and morphological features in human NCL. Brain Pathol. 2004;14(1):61–69. doi: 10.1111/j.1750-3639.2004.tb00499.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Kohlschutter A, Schulz A. Towards understanding the neuronal ceroid lipofuscinoses. Brain Dev. 2009;31(7):499–502. doi: 10.1016/j.braindev.2008.12.008. [DOI] [PubMed] [Google Scholar]
- [12].Siintola E, Partanen S, Stromme P, Haapanen A, Haltia M, Maehlen J, Lehesjoki AE, Tyynela J. Cathepsin D deficiency underlies congenital human neuronal ceroid-lipofuscinosis. Brain. 2006;129(Pt 6):1438–1445. doi: 10.1093/brain/awl107. [DOI] [PubMed] [Google Scholar]
- [13].Steinfeld R, Reinhardt K, Schreiber K, Hillebrand M, Kraetzner R, Bruck W, Saftig P, Gartner J. Cathepsin D deficiency is associated with a human neurodegenerative disorder. Am. J. Hum. Genet. 2006;78(6):988–998. doi: 10.1086/504159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Fritchie K, Siintola E, Armao D, Lehesjoki AE, Marino T, Powell C, Tennison M, Booker JM, Koch S, Partanen S, Suzuki K, Tyynela J, Thorne LB. Novel mutation and the first prenatal screening of cathepsin D deficiency (CLN10) Acta Neuropathol. 2009;117(2):201–208. doi: 10.1007/s00401-008-0426-7. [DOI] [PubMed] [Google Scholar]
- [15].Simonati A, Tessa A, Bernardina BD, Biancheri R, Veneselli E, Tozzi G, Bonsignore M, Grosso S, Piemonte F, Santorelli FM. Variant late infantile neuronal ceroid lipofuscinosis because of CLN1 mutations. Pediatr. Neurol. 2009;40(4):271–276. doi: 10.1016/j.pediatrneurol.2008.10.018. [DOI] [PubMed] [Google Scholar]
- [16].van Diggelen OP, Thobois S, Tilikete C, Zabot MT, Keulemans JL, van Bunderen PA, Taschner PE, Losekoot M, Voznyi YV. Adult neuronal ceroid lipofuscinosis with palmitoyl-protein thioesterase deficiency: first adult-onset patients of a childhood disease. Ann. Neurol. 2001;50(2):269–272. doi: 10.1002/ana.1103. [DOI] [PubMed] [Google Scholar]
- [17].Kalviainen R, Eriksson K, Losekoot M, Sorri I, Harvima I, Santavuori P, Jarvela I, Autti T, Vanninen R, Salmenpera T, van Diggelen OP. Juvenile-onset neuronal ceroid lipofuscinosis with infantile CLN1 mutation and palmitoyl-protein thioesterase deficiency. Eur. J. Neurol. 2007;14(4):369–372. doi: 10.1111/j.1468-1331.2007.01668.x. [DOI] [PubMed] [Google Scholar]
- [18].Kohan R, Cismondi A, Dodelson de Kremer R, Muller VJ, Guelbert N, Tapia Anzolini V, Fietz MJ, Ramírez AM, Halac IN. An integrated strategy for the diagnosis of Neuronal Ceroid Lipofuscinosis types 1 (CLN1) and 2 (CLN2) in eleven Latin American patients. Clin.Genet. 2009;76(4):372–382. doi: 10.1111/j.1399-0004.2009.01214.x. [DOI] [PubMed] [Google Scholar]
- [19].Kohan R, Muller VJ, Fietz MJ, Cismondi A, Oller Ramirez AM, Noher de Halac I. Novel human pathological mutations. Gene symbol: TPPI. Disease: Neuronal Ceroid Lipofuscinosis, Late infantile.ACCESSION HM070148. Hum. Genet. 2008;123:537–555. [PubMed] [Google Scholar]
- [20].Elleder M, Dvorakova L, Stolnaja L, Vlaskova H, Hulkova H, Druga R, Poupetova H, Kostalova E, Mikulastik J. Atypical CLN2 with later onset and prolonged course: a neuropathologic study showing different sensitivity of neuronal subpopulations to TPP1 deficiency. Acta Neuropathol. 2008;116(1):119–124. doi: 10.1007/s00401-008-0349-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Bessa C, Teixeira CA, Dias A, Alves M, Rocha S, Lacerda L, Loureiro L, Guimaraes A, Ribeiro MG. CLN2/TPP1 deficiency: the novel mutation IVS7-10A>G causes intron retention and is associated with a mild disease phenotype. Mol. Genet. Metab. 2008;93(1):66–73. doi: 10.1016/j.ymgme.2007.08.124. [DOI] [PubMed] [Google Scholar]
- [22].Hobert JA, Dawson G. A novel role of the Batten disease gene CLN3: association with BMP synthesis. Biochem. Biophys. Res. Commun. 2007;358(1):111–116. doi: 10.1016/j.bbrc.2007.04.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Haines RL, Codlin S, Mole SE. The fission yeast model for the lysosomal storage disorder Batten disease predicts disease severity caused by mutations in CLN3. Dis. Model. Mech. 2009;2(1-2):84–92. doi: 10.1242/dmm.000851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Sarpong A, Schottmann G, Ruther K, Stoltenburg G, Kohlschutter A, Hubner C, Schuelke M. Protracted course of juvenile ceroid lipofuscinosis associated with a novel CLN3 mutation (p.Y199X) Clin. Genet. 2009;76(1):38–45. doi: 10.1111/j.1399-0004.2009.01179.x. [DOI] [PubMed] [Google Scholar]
- [25].Rakheja D, Narayan SB, Bennett MJ. Juvenile neuronal ceroid-lipofuscinosis (Batten disease): a brief review and update. Curr. Mol. Med. 2007;7(6):603–608. doi: 10.2174/156652407781695729. [DOI] [PubMed] [Google Scholar]
- [26].Lyly A, von SC, Heine C, Schmiedt ML, Sipila T, Jalanko A, Kyttala A. Novel interactions of CLN5 support molecular networking between Neuronal Ceroid Lipofuscinosis proteins. BMC. Cell Biol. 2009;10:83. doi: 10.1186/1471-2121-10-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Kohan R, Cannelli N, Aiello C, Santorelli FM, Cismondi A, Milà M, Oller Ramirez AM, Noher de Halac I. Novel human pathological mutations. Gene symbol: CLN5. Disease: Neuronal Ceroid Lipofuscinosis, Finnish variant. ACCESSION HD070030. Hum. Genet. 2008;123:537–555. [PubMed] [Google Scholar]
- [28].Cismondi A, Cannelli N, Aiello C, Santorelli FM, Kohan R, Oller Ramirez AM, Noher de Halac I. Novel human pathological mutations. Gene symbol: CLN5. Disease: Neuronal Ceroid Lipofuscinosis, Finnish variant. ACCESSION HI1070015. Hum. Genet. 2008;123:537–555. [PubMed] [Google Scholar]
- [29].Bessa C, Teixeira CA, Mangas M, Dias A, Sa Miranda MC, Guimaraes A, Ferreira JC, Canas N, Cabral P, Ribeiro MG. Two novel CLN5 mutations in a Portuguese patient with vLINCL: insights into molecular mechanisms of CLN5 deficiency. Mol. Genet. Metab. 2006;89(3):245–253. doi: 10.1016/j.ymgme.2006.04.010. [DOI] [PubMed] [Google Scholar]
- [30].Cannelli N, Nardocci N, Cassandrini D, Morbin M, Aiello C, Bugiani M, Criscuolo L, Zara F, Striano P, Granata T, Bertini E, Simonati A, Santorelli FM. Revelation of a novel CLN5 mutation in early juvenile neuronal ceroid lipofuscinosis. Neuropediatrics. 2007;38(1):46–49. doi: 10.1055/s-2007-981449. [DOI] [PubMed] [Google Scholar]
- [31].Cismondi A, Kohan R, Ghio A, Oller Ramirez AM, Noher de Halac I. Novel human pathological mutations. Gene symbol: CLN6. Disease: Neuronal Ceroid Lipofuscinosis, Late infantile. ACCESSION HM080059. Hum. Genet. 2008;124:293–324. [PubMed] [Google Scholar]
- [32].Siintola E, Topcu M, Kohlschutter A, Salonen T, Joensuu T, Anttonen AK, Lehesjoki AE. Two novel CLN6 mutations in variant late-infantile neuronal ceroid lipofuscinosis patients of Turkish origin. Clin. Genet. 2005;68(2):167–173. doi: 10.1111/j.1399-0004.2005.00471.x. [DOI] [PubMed] [Google Scholar]
- [33].Cannelli N, Garavaglia B, Simonati A, Aiello C, Barzaghi C, Pezzini F, Cilio MR, Biancheri R, Morbin M, Dalla BB, Granata T, Tessa A, Invernizzi F, Pessagno A, Boldrini R, Zibordi F, Grazian L, Claps D, Carrozzo R, Mole SE, Nardocci N, Santorelli FM. Variant late infantile ceroid lipofuscinoses associated with novel mutations in CLN6. Biochem. Biophys. Res. Commun. 2009;379(4):892–897. doi: 10.1016/j.bbrc.2008.12.159. [DOI] [PubMed] [Google Scholar]
- [34].Al-Muhaizea MA, Al-Hassnan ZN, Chedrawi A. Variant late infantile neuronal ceroid lipofuscinosis (CLN6 gene) in Saudi Arabia. Pediatr. Neurol. 2009;41(1):74–76. doi: 10.1016/j.pediatrneurol.2009.01.012. [DOI] [PubMed] [Google Scholar]
- [35].Siintola E, Topcu M, Aula N, Lohi H, Minassian BA, Paterson AD, Liu XQ, Wilson C, Lahtinen U, Anttonen AK, Lehesjoki AE. The novel neuronal ceroid lipofuscinosis gene MFSD8 encodes a putative lysosomal transporter. Am. J Hum. Genet. 2007;81(1):136–146. doi: 10.1086/518902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Kousi M, Siintola E, Dvorakova L, Vlaskova H, Turnbull J, Topcu M, Yuksel D, Gokben S, Minassian BA, Elleder M, Mole SE, Lehesjoki AE. Mutations in CLN7/MFSD8 are a common cause of variant late-infantile neuronal ceroid lipofuscinosis. Brain. 2009;132(Pt 3):810–819. doi: 10.1093/brain/awn366. [DOI] [PubMed] [Google Scholar]
- [37].Stogmann E, El TS, Wagenstaller J, Gaber A, Edris S, Abdelhady A, ssem-Hilger E, Leutmezer F, Bonelli S, Baumgartner C, Zimprich F, Strom TM, Zimprich A. A novel mutation in the MFSD8 gene in late infantile neuronal ceroid lipofuscinosis. Neurogenetics. 2009;10(1):73–77. doi: 10.1007/s10048-008-0153-1. [DOI] [PubMed] [Google Scholar]
- [38].Aldahmesh MA, Al-Hassnan ZN, Aldosari M, Alkuraya FS. Neuronal ceroid lipofuscinosis caused by MFSD8 mutations: a common theme emerging. Neurogenetics. 2009;10(4):307–311. doi: 10.1007/s10048-009-0185-1. [DOI] [PubMed] [Google Scholar]
- [39].Aiello C, Terracciano A, Simonati A, Discepoli G, Cannelli N, Claps D, Crow YJ, Bianchi M, Kitzmuller C, Longo D, Tavoni A, Franzoni E, Tessa A, Veneselli E, Boldrini R, Filocamo M, Williams RE, Bertini ES, Biancheri R, Carrozzo R, Mole SE, Santorelli FM. Mutations in MFSD8/CLN7 are a frequent cause of variant-late infantile neuronal ceroid lipofuscinosis. Hum. Mutat. 2009;30(3):E530–E540. doi: 10.1002/humu.20975. [DOI] [PubMed] [Google Scholar]
- [40].Reinhardt K, Grapp M, Schlachter K, Bruck W, Gartner J, Steinfeld R. Novel CLN8 mutations confirm the clinical and ethnic diversity of late infantile neuronal ceroid lipofuscinosis. Clin. Genet. 2010;77(1):79–85. doi: 10.1111/j.1399-0004.2009.01285.x. [DOI] [PubMed] [Google Scholar]
- [41].Vantaggiato C, Redaelli F, Falcone S, Perrotta C, Tonelli A, Bondioni S, Morbin M, Riva D, Saletti V, Bonaglia MC, Giorda R, Bresolin N, Clementi E, Bassi MT. A novel CLN8 mutation in late-infantile-onset neuronal ceroid lipofuscinosis (LINCL) reveals aspects of CLN8 neurobiological function. Hum. Mutat. 2009;30(7):1104–1116. doi: 10.1002/humu.21012. [DOI] [PubMed] [Google Scholar]
- [42].Cannelli N, Cassandrini D, Bertini E, Striano P, Fusco L, Gaggero R, Specchio N, Biancheri R, Vigevano F, Bruno C, Simonati A, Zara F, Santorelli FM. Novel mutations in CLN8 in Italian variant late infantile neuronal ceroid lipofuscinosis: Another genetic hit in the Mediterranean. Neurogenetics. 2006;7(2):111–117. doi: 10.1007/s10048-005-0024-y. [DOI] [PubMed] [Google Scholar]
- [43].Poet M, Kornak U, Schweizer M, Zdebik AA, Scheel O, Hoelter S, Wurst W, Schmitt A, Fuhrmann JC, Planells-Cases R, Mole SE, Hubner CA, Jentsch TJ. Lysosomal storage disease upon disruption of the neuronal chloride transport protein ClC-6. Proc. Natl. Acad. Sci. U. S. A. 2006;103(37):13854–13859. doi: 10.1073/pnas.0606137103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Kasper D, Planells-Cases R, Fuhrmann JC, Scheel O, Zeitz O, Ruether K, Schmitt A, Poet M, Steinfeld R, Schweizer M, Kornak U, Jentsch TJ. Loss of the chloride channel ClC-7 leads to lysosomal storage disease and neurodegeneration. EMBO J. 2005;24(5):1079–1091. doi: 10.1038/sj.emboj.7600576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Sleat DE, Ding L, Wang S, Zhao C, Wang Y, Xin W, Zheng H, Moore DF, Sims KB, Lobel P. Mass spectrometry-based protein profiling to determine the cause of lysosomal storage diseases of unknown etiology. Mol. Cell Proteomics. 2009;8(7):1708–1718. doi: 10.1074/mcp.M900122-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Gdynia HJ, Sperfeld AD, Ludolph AC. [Adult-onset neuronal ceroid lipofuscinosis] Nervenarzt. 2007;78(2):139–140. doi: 10.1007/s00115-006-2222-8. [DOI] [PubMed] [Google Scholar]
- [47].Fealey ME, Edwards WD, Grogan M, Orszulak TA. Neuronal ceroid lipofuscinosis in a 31-year-old woman presenting as biventricular heart failure with restrictive features. Cardiovasc. Pathol. 2009;18(1):44–48. doi: 10.1016/j.carpath.2007.09.004. [DOI] [PubMed] [Google Scholar]
- [48].Sakajiri K, Matsubara N, Nakajima T, Fukuhara N, Makifuchi T, Wakabayashi M, Oyanagi S, Kominami E. A family with adult type ceroid lipofuscinosis (Kufs’ disease) and heart muscle disease: report of two autopsy cases. Intern. Med. 1995;34(12):1158–1163. doi: 10.2169/internalmedicine.34.1158. [DOI] [PubMed] [Google Scholar]
- [49].Schulz A, Mousallem T, Venkataramani M, Persaud-Sawin DA, Zucker A, Luberto C, Bielawska A, Bielawski J, Holthuis JC, Jazwinski SM, Kozhaya L, Dbaibo GS, Boustany RM. The CLN9 protein, a regulator of dihydroceramide synthase. J. Biol. Chem. 2006;281(5):2784–2794. doi: 10.1074/jbc.M509483200. [DOI] [PubMed] [Google Scholar]
- [50].Schulz A, Dhar S, Rylova S, Dbaibo G, Alroy J, Hagel C, Artacho I, Kohlschutter A, Lin S, Boustany RM. Impaired cell adhesion and apoptosis in a novel CLN9 Batten disease variant. Ann. Neurol. 2004;56(3):342–350. doi: 10.1002/ana.20187. [DOI] [PubMed] [Google Scholar]
- [51].Josephson SA, Schmidt RE, Millsap P, McManus DQ, Morris JC. Autosomal dominant Kufs’ disease: a cause of early onset dementia. J. Neurol. Sci. 2001;188(1-2):51–60. doi: 10.1016/s0022-510x(01)00546-9. [DOI] [PubMed] [Google Scholar]
- [52].Nijssen PC, Ceuterick C, van Diggelen OP, Elleder M, Martin JJ, Teepen JL, Tyynela J, Roos RA. Autosomal dominant adult neuronal ceroid lipofuscinosis: a novel form of NCL with granular osmiophilic deposits without palmitoyl protein thioesterase 1 deficiency. Brain Pathol. 2003;13(4):574–581. doi: 10.1111/j.1750-3639.2003.tb00486.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Nijssen PC, Brusse E, Leyten AC, Martin JJ, Teepen JL, Roos RA. Autosomal dominant adult neuronal ceroid lipofuscinosis: parkinsonism due to both striatal and nigral dysfunction. Mov. Disord. 2002;17(3):482–487. doi: 10.1002/mds.10104. [DOI] [PubMed] [Google Scholar]
- [54].Glass CK, Saijo K, Winner B, Marchetto MC, Gage FH. Mechanisms underlying inflammation in neurodegeneration. Cell. 2010;140(6):918–934. doi: 10.1016/j.cell.2010.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Pierret C, Morrison JA, Kirk MD. Treatment of lysosomal storage disorders: Focus on the neuronal ceroid-lipofuscinoses. Acta Neurobiol. Exp. (Wars. ) 2008;68(3):429–442. doi: 10.55782/ane-2008-1709. [DOI] [PubMed] [Google Scholar]
- [56].Hobert JA, Dawson G. Neuronal ceroid lipofuscinoses therapeutic strategies: past, present and future. Biochim. Biophys. Acta. 2006;1762(10):945–953. doi: 10.1016/j.bbadis.2006.08.004. [DOI] [PubMed] [Google Scholar]
- [57].Campeau PM, Scriver CR, Mitchell JJ. A 25-year longitudinal analysis of treatment efficacy in inborn errors of metabolism. Mol. Genet. Metab. 2008;95(1-2):11–16. doi: 10.1016/j.ymgme.2008.07.001. [DOI] [PubMed] [Google Scholar]
- [58].Platt FM, Lachmann RH. Treating lysosomal storage disorders: current practice and future prospects. Biochim. Biophys. Acta. 2009;1793(4):737–745. doi: 10.1016/j.bbamcr.2008.08.009. [DOI] [PubMed] [Google Scholar]
- [59].Haskins M. Gene therapy for lysosomal storage diseases (LSDs) in large animal models. ILAR. J. 2009;50(2):112–121. doi: 10.1093/ilar.50.2.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Beck M. Therapy for lysosomal storage disorders. IUBMB. Life. 2010;62(1):33–40. doi: 10.1002/iub.284. [DOI] [PubMed] [Google Scholar]
- [61].Worgall S, Kekatpure MV, Heier L, Ballon D, Dyke JP, Shungu D, Mao X, Kosofsky B, Kaplitt MG, Souweidane MM, Sondhi D, Hackett NR, Hollmann C, Crystal RG. Neurological deterioration in late infantile neuronal ceroid lipofuscinosis. Neurology. 2007;69(6):521–535. doi: 10.1212/01.wnl.0000267885.47092.40. [DOI] [PubMed] [Google Scholar]
- [62].Worgall S, Sondhi D, Hackett NR, Kosofsky B, Kekatpure MV, Neyzi N, Dyke JP, Ballon D, Heier L, Greenwald BM, Christos P, Mazumdar M, Souweidane MM, Kaplitt MG, Crystal RG. Treatment of late infantile neuronal ceroid lipofuscinosis by CNS administration of a serotype 2 adeno-associated virus expressing CLN2 cDNA. Hum. Gene Ther. 2008;19(5):463–474. doi: 10.1089/hum.2008.022. [DOI] [PubMed] [Google Scholar]
- [63].Ramadan H, Al-Din AS, Ismail A, Balen F, Varma A, Twomey A, Watts R, Jackson M, Anderson G, Green E, Mole SE. Adult neuronal ceroid lipofuscinosis caused by deficiency in palmitoyl protein thioesterase 1. Neurology. 2007;68(5):387–388. doi: 10.1212/01.wnl.0000252825.85947.2f. [DOI] [PubMed] [Google Scholar]
- [64].Steinfeld R, Heim P, von GH, Meyer K, Ullrich K, Goebel HH, Kohlschutter A. Late infantile neuronal ceroid lipofuscinosis: quantitative description of the clinical course in patients with CLN2 mutations. Am. J. Med. Genet. 2002;112(4):347–354. doi: 10.1002/ajmg.10660. [DOI] [PubMed] [Google Scholar]
- [65].Holmberg V, Lauronen L, Autti T, Santavuori P, Savukoski M, Uvebrant P, Hofman I, Peltonen L, Jarvela I. Phenotype-genotype correlation in eight patients with Finnish variant late infantile NCL (CLN5) Neurology. 2000;55(4):579–581. doi: 10.1212/wnl.55.4.579. [DOI] [PubMed] [Google Scholar]
- [66].Pineda-Trujillo N, Cornejo W, Carrizosa J, Wheeler RB, Munera S, Valencia A, gudelo-Arango J, Cogollo A, Anderson G, Bedoya G, Mole SE, Ruiz-Linares A. A CLN5 mutation causing an atypical neuronal ceroid lipofuscinosis of juvenile onset. Neurology. 2005;64(4):740–742. doi: 10.1212/01.WNL.0000151974.44980.F1. [DOI] [PubMed] [Google Scholar]
- [67].Lebrun AH, Storch S, Ruschendorf F, Schmiedt ML, Kyttala A, Mole SE, Kitzmuller C, Saar K, Mewasingh LD, Boda V, Kohlschutter A, Ullrich K, Braulke T, Schulz A. Retention of lysosomal protein CLN5 in the endoplasmic reticulum causes neuronal ceroid lipofuscinosis in Asian sibship. Hum. Mutat. 2009;30(5):E651–E661. doi: 10.1002/humu.21010. [DOI] [PubMed] [Google Scholar]
- [68].Siintola E, Topcu M, Aula N, Lohi H, Minassian BA, Paterson AD, Liu XQ, Wilson C, Lahtinen U, Anttonen AK, Lehesjoki AE. The novel neuronal ceroid lipofuscinosis gene MFSD8 encodes a putative lysosomal transporter. Am. J. Hum. Genet. 2007;81(1) doi: 10.1086/518902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Zelnik N, Mahajna M, Iancu TC, Sharony R, Zeigler M. A novel mutation of the CLN8 gene: is there a Mediterranean phenotype? Pediatr. Neurol. 2007;36(6):411–413. doi: 10.1016/j.pediatrneurol.2007.01.008. [DOI] [PubMed] [Google Scholar]
- [70].Marshall FJ, de Blieck EA, Mink JW, Dure L, Adams H, Messing S, Rothberg PG, Levy E, McDonough T, DeYoung J, Wang M, Ramirez-Montealegre D, Kwon JM, Pearce DA. A clinical rating scale for Batten disease: reliable and relevant for clinical trials. Neurology. 2005;65(2):275–279. doi: 10.1212/01.wnl.0000169019.41332.8a. [DOI] [PubMed] [Google Scholar]
- [71].Adams HR, Beck CA, Levy E, Jordan R, Kwon JM, Marshall FJ, Vierhile A, Augustine EF, de Blieck EA, Pearce DA, Mink JW. Genotype does not predict severity of behavioural phenotype in juvenile neuronal ceroid lipofuscinosis (Batten disease) Dev. Med. Child Neurol. 2010 doi: 10.1111/j.1469-8749.2010.03628.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Beck M. New therapeutic options for lysosomal storage disorders: enzyme replacement, small molecules and gene therapy. Hum. Genet. 2007;121(1):1–22. doi: 10.1007/s00439-006-0280-4. [DOI] [PubMed] [Google Scholar]
- [73].van Meel E, Klumperman J. Imaging and imagination: understanding the endo-lysosomal system. Histochem. Cell Biol. 2008;129(3):253–266. doi: 10.1007/s00418-008-0384-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Kornfeld S, Mellman I. The biogenesis of lysosomes. Annu. Rev. Cell Biol. 1989;5:483–525. doi: 10.1146/annurev.cb.05.110189.002411. [DOI] [PubMed] [Google Scholar]
- [75].Reczek D, Schwake M, Schroder J, Hughes H, Blanz J, Jin X, Brondyk W, Van PS, Edmunds T, Saftig P. LIMP-2 is a receptor for lysosomal mannose-6-phosphate-independent targeting of beta-glucocerebrosidase. Cell. 2007;131(4):770–783. doi: 10.1016/j.cell.2007.10.018. [DOI] [PubMed] [Google Scholar]
- [76].Distler J, Hieber V, Sahagian G, Schmickel R, Jourdian GW. Identification of mannose 6-phosphate in glycoproteins that inhibit the assimilation of beta-galactosidase by fibroblasts. Proc. Natl. Acad. Sci. USA. 1979;76(9):4235–4239. doi: 10.1073/pnas.76.9.4235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Kaplan A, Achord DT, Sly WS. Phosphohexosyl components of a lysosomal enzyme are recognized by pinocytosis receptors on human fibroblasts. Proc. Natl. Acad. Sci. USA. 1977;74(5):2026–2030. doi: 10.1073/pnas.74.5.2026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Natowicz MR, Chi MM, LOWRY OH, Sly WS. Enzymatic identification of mannose 6-phosphate on the recognition marker for receptor-mediated pinocytosis of beta-glucuronidase by human fibroblasts. Proc. Natl. Acad. Sci. USA. 1979;76(9):4322–4326. doi: 10.1073/pnas.76.9.4322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Fratantoni JC, Hall CW, Neufeld EF. Hurler and Hunter syndromes: mutual correction of the defect in cultured fibroblasts. Science. 1968;162(853):570–572. doi: 10.1126/science.162.3853.570. [DOI] [PubMed] [Google Scholar]
- [80].Neufeld EF, Fratantoni JC. Inborn errors of mucopolysaccharide metabolism. Science. 1970;169(941):141–146. doi: 10.1126/science.169.3941.141. [DOI] [PubMed] [Google Scholar]
- [81].Sands MS, Davidson BL. Gene therapy for lysosomal storage diseases. Mol. Ther. 2006;13(5):839–849. doi: 10.1016/j.ymthe.2006.01.006. [DOI] [PubMed] [Google Scholar]
- [82].Desnick RJ, Dean KJ, Grabowski GA, Bishop DF, Sweeley CC. Enzyme therapy XVII: metabolic and immunologic evaluation of alpha- galactosidase A replacement in Fabry disease. Birth Defects Orig. Artic. Ser. 1980;16(1):393–413. [PubMed] [Google Scholar]
- [83].Desnick RJ. Enzyme replacement and enhancement therapies for lysosomal diseases. J. Inherit. Metab Dis. 2004;27(3):385–410. doi: 10.1023/B:BOLI.0000031101.12838.c6. [DOI] [PubMed] [Google Scholar]
- [84].Breunig F, Wanner C. Update on Fabry disease: kidney involvement, renal progression and enzyme replacement therapy. J. Nephrol. 2008;21(1):32–37. [PubMed] [Google Scholar]
- [85].Kakkis ED, Muenzer J, Tiller GE, Waber L, Belmont J, Passage M, Izykowski B, Phillips J, Doroshow R, Walot I, Hoft R, Neufeld EF. Enzyme-replacement therapy in mucopolysaccharidosis I. N. Engl. J Med. 2001;344(3):182–188. doi: 10.1056/NEJM200101183440304. [DOI] [PubMed] [Google Scholar]
- [86].Wraith JE, Clarke LA, Beck M, Kolodny EH, Pastores GM, Muenzer J, Rapoport DM, Berger KI, Swiedler SJ, Kakkis ED, Braakman T, Chadbourne E, Walton-Bowen K, Cox GF. Enzyme replacement therapy for mucopolysaccharidosis I: a randomized, double-blinded, placebo-controlled, multinational study of recombinant human alpha-L-iduronidase (laronidase) J. Pediatr. 2004;144(5):581–588. doi: 10.1016/j.jpeds.2004.01.046. [DOI] [PubMed] [Google Scholar]
- [87].Wraith JE. Enzyme replacement therapy in mucopolysaccharidosis type I: progress and emerging difficulties. J. Inherit. Metab Dis. 2001;24(2):245–250. doi: 10.1023/a:1010379320378. [DOI] [PubMed] [Google Scholar]
- [88].Clarke LA. Idursulfase for the treatment of mucopolysaccharidosis II. Expert. Opin. Pharmacother. 2008;9(2):311–317. doi: 10.1517/14656566.9.2.311. [DOI] [PubMed] [Google Scholar]
- [89].Harmatz P, Giugliani R, Schwartz IV, Guffon N, Teles EL, Miranda MC, Wraith JE, Beck M, Arash L, Scarpa M, Ketteridge D, Hopwood JJ, Plecko B, Steiner R, Whitley CB, Kaplan P, Yu ZF, Swiedler SJ, Decker C. Long-term follow-up of endurance and safety outcomes during enzyme replacement therapy for mucopolysaccharidosis VI: Final results of three clinical studies of recombinant human N-acetylgalactosamine 4-sulfatase. Mol. Genet. Metab. 2008;94(4):469–475. doi: 10.1016/j.ymgme.2008.04.001. [DOI] [PubMed] [Google Scholar]
- [90].Rohrbach M, Clarke JT. Treatment of lysosomal storage disorders : progress with enzyme replacement therapy. Drugs. 2007;67(18):2697–2716. doi: 10.2165/00003495-200767180-00005. [DOI] [PubMed] [Google Scholar]
- [91].Wang J, Lozier J, Johnson G, Kirshner S, Verthelyi D, Pariser A, Shores E, Rosenberg A. Neutralizing antibodies to therapeutic enzymes: considerations for testing, prevention and treatment. Nat. Biotechnol. 2008;26(8):901–908. doi: 10.1038/nbt.1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Chen YH, Chang M, Davidson BL. Molecular signatures of disease brain endothelia provide new sites for CNS-directed enzyme therapy. Nat. Med. 2009;15(10):1215–1218. doi: 10.1038/nm.2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Lin L, Lobel P. Production and characterization of recombinant human CLN2 protein for enzyme-replacement therapy in late infantile neuronal ceroid lipofuscinosis. Biochem. J. 2001;357(Pt 1):49–55. doi: 10.1042/0264-6021:3570049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Kakkis E, McEntee M, Vogler C, Le S, Levy B, Belichenko P, Mobley W, Dickson P, Hanson S, Passage M. Intrathecal enzyme replacement therapy reduces lysosomal storage in the brain and meninges of the canine model of MPS I. Mol. Genet. Metab. 2004;83(1-2):163–174. doi: 10.1016/j.ymgme.2004.07.003. [DOI] [PubMed] [Google Scholar]
- [95].Dickson P, McEntee M, Vogler C, Le S, Levy B, Peinovich M, Hanson S, Passage M, Kakkis E. Intrathecal enzyme replacement therapy: successful treatment of brain disease via the cerebrospinal fluid. Mol. Genet. Metab. 2007;91(1):61–68. doi: 10.1016/j.ymgme.2006.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Chang M, Cooper JD, Sleat DE, Cheng SH, Dodge JC, Passini MA, Lobel P, Davidson BL. Intraventricular enzyme replacement improves disease phenotypes in a mouse model of late infantile neuronal ceroid lipofuscinosis. Mol. Ther. 2008;16(4):649–656. doi: 10.1038/mt.2008.9. [DOI] [PubMed] [Google Scholar]
- [97].Sleat DE, El-Banna M, Sohar I, Kim KH, Dobrenis K, Walkley SU, Lobel P. Residual levels of tripeptidyl-peptidase I activity dramatically ameliorate disease in late-infantile neuronal ceroid lipofuscinosis. Mol. Genet. Metab. 2008;94(2):222–233. doi: 10.1016/j.ymgme.2008.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Lu JY, Hu J, Hofmann SL. Human recombinant palmitoyl-protein thioesterase-1 (PPT1) for preclinical evaluation of enzyme replacement therapy for infantile neuronal ceroid lipofuscinosis. Mol. Genet. Metab. 2010;99(4):374–378. doi: 10.1016/j.ymgme.2009.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Naldini L. Medicine. A comeback for gene therapy. Science. 2009;326(5954):805–806. doi: 10.1126/science.1181937. [DOI] [PubMed] [Google Scholar]
- [100].Cartier N, Hacein-Bey-Abina S, Bartholomae CC, Veres G, Schmidt M, Kutschera I, Vidaud M, Abel U, Dal-Cortivo L, Caccavelli L, Mahlaoui N, Kiermer V, Mittelstaedt D, Bellesme C, Lahlou N, Lefrere F, Blanche S, Audit M, Payen E, Leboulch P, l’Homme B, Bougneres P, Von KC, Fischer A, Cavazzana-Calvo M, Aubourg P. Hematopoietic stem cell gene therapy with a lentiviral vector in X-linked adrenoleukodystrophy. Science. 2009;326(5954):818–823. doi: 10.1126/science.1171242. [DOI] [PubMed] [Google Scholar]
- [101].Sondhi D, Hackett NR, Apblett RL, Kaminsky SM, Pergolizzi RG, Crystal RG. Feasibility of gene therapy for late neuronal ceroid lipofuscinosis. Arch. Neurol. 2001;58(11):1793–1798. doi: 10.1001/archneur.58.11.1793. [DOI] [PubMed] [Google Scholar]
- [102].Prasad VK, Kurtzberg J. Cord blood and bone marrow transplantation in inherited metabolic diseases: scientific basis, current status and future directions. Br. J. Haematol. 2010;148(3):356–372. doi: 10.1111/j.1365-2141.2009.07974.x. [DOI] [PubMed] [Google Scholar]
- [103].Muenzer J, Fisher A. Advances in the treatment of mucopolysac-charidosis type I. N. Engl. J. Med. 2004;350(19):1932–1934. doi: 10.1056/NEJMp048084. [DOI] [PubMed] [Google Scholar]
- [104].Griffey M, Macauley SL, Ogilvie JM, Sands MS. AAV2-mediated ocular gene therapy for infantile neuronal ceroid lipofuscinosis. Mol. Ther. 2005;12(3):413–421. doi: 10.1016/j.ymthe.2005.04.018. [DOI] [PubMed] [Google Scholar]
- [105].Griffey MA, Wozniak D, Wong M, Bible E, Johnson K, Rothman SM, Wentz AE, Cooper JD, Sands MS. CNS-directed AAV2-mediated gene therapy ameliorates functional deficits in a murine model of infantile neuronal ceroid lipofuscinosis. Mol. Ther. 2006;13(3):538–547. doi: 10.1016/j.ymthe.2005.11.008. [DOI] [PubMed] [Google Scholar]
- [106].Haskell RE, Hughes SM, Chiorini JA, Alisky JM, Davidson BL. Viral-mediated delivery of the late-infantile neuronal ceroid lipofuscinosis gene, TPP-I to the mouse central nervous system. Gene Ther. 2003;10(1):34–42. doi: 10.1038/sj.gt.3301843. [DOI] [PubMed] [Google Scholar]
- [107].Passini MA, Dodge JC, Bu J, Yang W, Zhao Q, Sondhi D, Hackett NR, Kaminsky SM, Mao Q, Shihabuddin LS, Cheng SH, Sleat DE, Stewart GR, Davidson BL, Lobel P, Crystal RG. Intracranial delivery of CLN2 reduces brain pathology in a mouse model of classical late infantile neuronal ceroid lipofuscinosis. J. Neurosci. 2006;26(5):1334–1342. doi: 10.1523/JNEUROSCI.2676-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Cabrera-Salazar MA, Roskelley EM, Bu J, Hodges BL, Yew N, Dodge JC, Shihabuddin LS, Sohar I, Sleat DE, Scheule RK, Davidson BL, Cheng SH, Lobel P, Passini MA. Timing of therapeutic intervention determines functional and survival outcomes in a mouse model of late infantile batten disease. Mol. Ther. 2007;15(10):1782–1788. doi: 10.1038/sj.mt.6300249. [DOI] [PubMed] [Google Scholar]
- [109].Sondhi D, Hackett NR, Peterson DA, Stratton J, Baad M, Travis KM, Wilson JM, Crystal RG. Enhanced survival of the LINCL mouse following CLN2 gene transfer using the rh.10 rhesus macaque-derived adeno-associated virus vector. Mol. Ther. 2007;15(3):481–491. doi: 10.1038/sj.mt.6300049. [DOI] [PubMed] [Google Scholar]
- [110].Mattson MP, Chan SL, Duan W. Modification of brain aging and neurodegenerative disorders by genes, diet, and behavior. Physiol. Rev. 2002;82(3):637–672. doi: 10.1152/physrev.00004.2002. [DOI] [PubMed] [Google Scholar]
- [111].Bennett MJ, Boriack RL, Boustany RM. Polyunsaturated fatty acids reverse the lysosomal storage and accumulation of subunit 9 of mitochondrial F1F0-ATP synthase in cultured lymphoblasts from patients with Batten disease. J. Inherit. Metab. Dis. 1997;20(3):457–460. doi: 10.1023/a:1005387608456. [DOI] [PubMed] [Google Scholar]
- [112].Boriack RL, Cortinas E, Bennett MJ. Mitochondrial damage results in a reversible increase in lysosomal storage material in lymphoblasts from patients with juvenile neuronal ceroidlipofuscinosis (Batten Disease) Am. J. Med. Genet. 1995;57(2):301–303. doi: 10.1002/ajmg.1320570238. [DOI] [PubMed] [Google Scholar]
- [113].Neal EG, Chaffe H, Schwartz RH, Lawson MS, Edwards N, Fitzsimmons G, Whitney A, Cross JH. The ketogenic diet for the treatment of childhood epilepsy: a randomised controlled trial. Lancet Neurol. 2008;7(6):500–506. doi: 10.1016/S1474-4422(08)70092-9. [DOI] [PubMed] [Google Scholar]
- [114].Kohan R, Noher de Halac I, Tapia Anzolini V, Cismondi IA, Oller Ramirez AM, Paschini Capra A, Dodelson de Kremer R. Palmitoyl Protein Thioesterase1 (PPT1) and Tripeptidyl Peptidase-I (TPP-I) are expressed in the human saliva. A reliable and non-invasive source for the diagnosis of infantile (CLN1) and late infantile (CLN2) neuronal ceroid lipofuscinoses. Clin. Biochem. 2005;38(5):492–494. doi: 10.1016/j.clinbiochem.2004.12.007. [DOI] [PubMed] [Google Scholar]
- [115].Zarate CA, Jr., Manji HK. The role of AMPA receptor modulation in the treatment of neuropsychiatric diseases. Exp. Neurol. 2008;211(1):7–10. doi: 10.1016/j.expneurol.2008.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Kovacs AD, Weimer JM, Pearce DA. Selectively increased sensitivity of cerebellar granule cells to AMPA receptor-mediated excitotoxicity in a mouse model of Batten disease. Neurobiol. Dis. 2006;22(3):575–585. doi: 10.1016/j.nbd.2005.12.018. [DOI] [PubMed] [Google Scholar]
- [117].Kovacs AD, Pearce DA. Attenuation of AMPA receptor activity improves motor skills in a mouse model of juvenile Batten disease. Exp. Neurol. 2008;209(1):288–291. doi: 10.1016/j.expneurol.2007.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Wang JY, Wen LL, Huang YN, Chen YT, Ku MC. Dual effects of antioxidants in neurodegeneration: direct neuroprotection against oxidative stress and indirect protection via suppression of glia-mediated inflammation. Curr. Pharm. Des. 2006;12(27):3521–3533. doi: 10.2174/138161206778343109. [DOI] [PubMed] [Google Scholar]
- [119].DeLegge MH, Smoke A. Neurodegeneration and inflammation. Nutr. Clin. Pract. 2008;23(1):35–41. doi: 10.1177/011542650802300135. [DOI] [PubMed] [Google Scholar]
- [120].Pupure J, Isajevs S, Skapare E, Rumaks J, Svirskis S, Svirina D, Kalvinsh I, Klusa V. Neuroprotective properties of mildronate, a mitochondria-targeted small molecule. Neurosci. Lett. 2010;470(2):100–105. doi: 10.1016/j.neulet.2009.12.055. [DOI] [PubMed] [Google Scholar]
- [121].Fan JQ. A counterintuitive approach to treat enzyme deficiencies: use of enzyme inhibitors for restoring mutant enzyme activity. Biol. Chem. 2008;389(1):1–11. doi: 10.1515/BC.2008.009. [DOI] [PubMed] [Google Scholar]
- [122].Loo TW, Clarke DM. Chemical and pharmacological chaperones as new therapeutic agents. Expert. Rev. Mol. Med. 2007;9(16):1–18. doi: 10.1017/S1462399407000361. [DOI] [PubMed] [Google Scholar]
- [123].Harding HP, Zhang Y, Zeng H, Novoa I, Lu PD, Calfon M, Sadri N, Yun C, Popko B, Paules R, Stojdl DF, Bell JC, Hettmann T, Leiden JM, Ron D. An integrated stress response regulates amino acid metabolism and resistance to oxidative stress. Mol. Cell. 2003;11(3):619–633. doi: 10.1016/s1097-2765(03)00105-9. [DOI] [PubMed] [Google Scholar]
- [124].Mori K. Tripartite management of unfolded proteins in the endoplasmic reticulum. Cell. 2000;101(5):451–454. doi: 10.1016/s0092-8674(00)80855-7. [DOI] [PubMed] [Google Scholar]
- [125].Tessitore A, del PM, Sano R, Ma Y, Mann L, Ingrassia A, Laywell ED, Steindler DA, Hendershot LM, d’Azzo A. GM1-ganglioside-mediated activation of the unfolded protein response causes neuronal death in a neurodegenerative gangliosidosis. Mol. Cell. 2004;15(5):753–766. doi: 10.1016/j.molcel.2004.08.029. [DOI] [PubMed] [Google Scholar]
- [126].Zhang Z, Lee YC, Kim SJ, Choi MS, Tsai PC, Xu Y, Xiao YJ, Zhang P, Heffer A, Mukherjee AB. Palmitoyl-protein thioesterase-1 deficiency mediates the activation of the unfolded protein response and neuronal apoptosis in INCL. Hum. Mol. Genet. 2006;15(2):337–346. doi: 10.1093/hmg/ddi451. [DOI] [PubMed] [Google Scholar]
- [127].Kim SJ, Zhang Z, Hitomi E, Lee YC, Mukherjee AB. Endoplasmic reticulum stress-induced caspase-4 activation mediates apoptosis and neurodegeneration in INCL. Hum. Mol. Genet. 2006;15(11):1826–1834. doi: 10.1093/hmg/ddl105. [DOI] [PubMed] [Google Scholar]
- [128].Kim SJ, Zhang Z, Lee YC, Mukherjee AB. Palmitoyl-protein thioesterase-1 deficiency leads to the activation of caspase-9 and contributes to rapid neurodegeneration in INCL. Hum. Mol. Genet. 2006;15(10):1580–1586. doi: 10.1093/hmg/ddl078. [DOI] [PubMed] [Google Scholar]
- [129].Farfel-Becker T, Vitner E, Dekel H, Leshem N, Enquist IB, Karlsson S, Futerman AH. No evidence for activation of the unfolded protein response in neuronopathic models of Gaucher disease. Hum. Mol. Genet. 2009;18(8):1482–1488. doi: 10.1093/hmg/ddp061. [DOI] [PubMed] [Google Scholar]
- [130].Lindholm D, Wootz H, Korhonen L. ER stress and neurodegenerative diseases. Cell Death. Differ. 2006;13(3):385–392. doi: 10.1038/sj.cdd.4401778. [DOI] [PubMed] [Google Scholar]
- [131].Pastores GM. Miglustat: substrate reduction therapy for lysosomal storage disorders associated with primary central nervous system involvement. Recent Pat. CNS. Drug Discov. 2006;1(1):77–82. doi: 10.2174/157488906775245282. [DOI] [PubMed] [Google Scholar]
- [132].Lachmann RH, te VD, Lloyd-Evans E, Reinkensmeier G, Sillence DJ, Fernandez-Guillen L, Dwek RA, Butters TD, Cox TM, Platt FM. Treatment with miglustat reverses the lipid-trafficking defect in Niemann-Pick disease type C. Neurobiol. Dis. 2004;16(3):654–658. doi: 10.1016/j.nbd.2004.05.002. [DOI] [PubMed] [Google Scholar]
- [133].Zervas M, Somers KL, Thrall MA, Walkley SU. Critical role for glycosphingolipids in Niemann-Pick disease type C. Curr. Biol. 2001;11(16):1283–1287. doi: 10.1016/s0960-9822(01)00396-7. [DOI] [PubMed] [Google Scholar]
- [134].Chien YH, Lee NC, Tsai LK, Huang AC, Peng SF, Chen SJ, Hwu WL. Treatment of Niemann-Pick disease type C in two children with miglustat: initial responses and maintenance of effects over 1 year. J. Inherit. Metab. Dis. 2007;30(5):826. doi: 10.1007/s10545-007-0630-y. [DOI] [PubMed] [Google Scholar]
- [135].Zeman RJ, Peng H, Etlinger JD. Clenbuterol retards loss of motor function in motor neuron degeneration mice. Exp. Neurol. 2004;187(2):460–467. doi: 10.1016/j.expneurol.2004.03.006. [DOI] [PubMed] [Google Scholar]
- [136].Culmsee C, Junker V, Thal S, Kremers W, Maier S, Schneider HJ, Plesnila N, Krieglstein J. Enantio-selective effects of clenbuterol in cultured neurons and astrocytes, and in a mouse model of cerebral ischemia. Eur. J. Pharmacol. 2007;575(1-3):57–65. doi: 10.1016/j.ejphar.2007.07.066. [DOI] [PubMed] [Google Scholar]
- [137].Linde L, Kerem B. Introducing sense into nonsense in treatments of human genetic diseases. Trends Genet. 2008;24(11):552–563. doi: 10.1016/j.tig.2008.08.010. [DOI] [PubMed] [Google Scholar]
- [138].Welch EM, Barton ER, Zhuo J, Tomizawa Y, Friesen WJ, Trifillis P, Paushkin S, Patel M, Trotta CR, Hwang S, Wilde RG, Karp G, Takasugi J, Chen G, Jones S, Ren H, Moon YC, Corson D, Turpoff AA, Campbell JA, Conn MM, Khan A, Almstead NG, Hedrick J, Mollin A, Risher N, Weetall M, Yeh S, Branstrom AA, Colacino JM, Babiak J, Ju WD, Hirawat S, Northcutt VJ, Miller LL, Spatrick P, He F, Kawana M, Feng H, Jacobson A, Peltz SW, Sweeney HL. PTC124 targets genetic disorders caused by nonsense mutations. Nature. 2007;447(7140):87–91. doi: 10.1038/nature05756. [DOI] [PubMed] [Google Scholar]
- [139].Schmitz A, Famulok M. Chemical biology: ignore the nonsense. Nature. 2007;447(7140):42–43. doi: 10.1038/nature05715. [DOI] [PubMed] [Google Scholar]
- [140].Byers PH. Killing the messenger: new insights into nonsense-mediated mRNA decay. J. Clin. Invest. 2002;109(1):3–6. doi: 10.1172/JCI14841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Sleat DE, Sohar I, Gin RM, Lobel P. Aminoglycoside-mediated suppression of nonsense mutations in late infantile neuronal ceroid lipofuscinosis. Eur. J. Paediatr. Neurol. 2001;5(Suppl A):57–62. doi: 10.1053/ejpn.2000.0436. [DOI] [PubMed] [Google Scholar]
- [142].Edelman A, Sermet-Gaudelus I, Rousset JP. Genetic testing to provide targeted treatment for cystic fibrosis patients. Pharmacogenomics. 2007;8(9):1101–1104. doi: 10.2217/14622416.8.9.1101. [DOI] [PubMed] [Google Scholar]
- [143].Kim KH, Sleat DE, Bernard O, Lobel P. Genetic modulation of apoptotic pathways fails to alter disease course in tripeptidylpeptidase 1 deficient mice. Neurosci. Lett. 2009;453(1):27–30. doi: 10.1016/j.neulet.2009.01.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Dhar S, Bitting RL, Rylova SN, Jansen PJ, Lockhart E, Koeberl DD, Amalfitano A, Boustany RM. Flupirtine blocks apoptosis in batten patient lymphoblasts and in human postmitotic. Ann. Neurol. 2002;51(4):448–466. doi: 10.1002/ana.10143. [DOI] [PubMed] [Google Scholar]
- [145].Klawe C, Maschke M. Flupirtine: pharmacology and clinical applications of a nonopioid analgesic and potentially neuroprotective compound. Expert. Opin. Pharmacother. 2009;10(9):1495–1500. doi: 10.1517/14656560902988528. [DOI] [PubMed] [Google Scholar]
- [146].DeWitt N. Regenerative medicine. Nature. 2008;453(7193):301. doi: 10.1038/453301a. [DOI] [PubMed] [Google Scholar]
- [147].Xu Y, Shi Y, Ding S. A chemical approach to stem-cell biology and regenerative medicine. Nature. 2008;453(7193):338–344. doi: 10.1038/nature07042. [DOI] [PubMed] [Google Scholar]
- [148].Badylak SF, Nerem RM. Progress in tissue engineering and regenerative medicine. Proc. Natl. Acad. Sci. U S A. 2010;107(8):3285–3286. doi: 10.1073/pnas.1000256107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Wang J, Lu J, Bond MC, Chen M, Ren XR, Lyerly HK, Barak LS, Chen W. Identification of select glucocorticoids as Smoothened agonists: potential utility for regenerative medicine. Proc. Natl. Acad. Sci. USA. 2010;107(20):9323–9328. doi: 10.1073/pnas.0910712107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Parker GC. Blood stem cells and non-hematological clinical practice: pragmatics before therapeutics. Curr. Pharm. Biotechnol. 2007;8(1):51–56. doi: 10.2174/138920107779941453. [DOI] [PubMed] [Google Scholar]
- [151].Boelens JJ, Prasad VK, Tolar J, Wynn RF, Peters C. Current international perspectives on hematopoietic stem cell transplantation for inherited metabolic disorders. Pediatr. Clin. North Am. 2010;57(1):123–145. doi: 10.1016/j.pcl.2009.11.004. [DOI] [PubMed] [Google Scholar]
- [152].Yuza Y, Yokoi K, Sakurai K, Ariga M, Yanagisawa T, Ohashi T, Hoshi Y, Eto Y. Allogenic bone marrow transplantation for late-infantile neuronal ceroid lipofuscinosis. Pediatr. Int. 2005;47(6):681–683. doi: 10.1111/j.1442-200x.2005.02126.x. [DOI] [PubMed] [Google Scholar]
- [153].Lonnqvist T, Vanhanen SL, Vettenranta K, Autti T, Rapola J, Santavuori P, Saarinen-Pihkala UM. Hematopoietic stem cell transplantation in infantile neuronal ceroid lipofuscinosis. Neurology. 2001;57(8):1411–1416. doi: 10.1212/wnl.57.8.1411. [DOI] [PubMed] [Google Scholar]
- [154].Lake BD, Steward CG, Oakhill A, Wilson J, Perham TG. Bone marrow transplantation in late infantile Batten disease and juvenile Batten disease. Neuropediatrics. 1997;28(1):80–81. doi: 10.1055/s-2007-973677. [DOI] [PubMed] [Google Scholar]
- [155].Wynn RF, Mercer J, Page J, Carr TF, Jones S, Wraith JE. Use of enzyme replacement therapy (Laronidase) before hematopoietic stem cell transplantation for mucopolysaccharidosis I: experience in 18 patients. J. Pediatr. 2009;154(1):135–139. doi: 10.1016/j.jpeds.2008.07.004. [DOI] [PubMed] [Google Scholar]
- [156].Deeg HJ, Shulman HM, Albrechtsen D, Graham TC, Storb R, Koppang N. Batten’s disease: failure of allogeneic bone marrow transplantation to arrest disease progression in a canine model. Clin. Genet. 1990;37(4):264–270. doi: 10.1111/j.1399-0004.1990.tb04188.x. [DOI] [PubMed] [Google Scholar]
- [157].Boelens JJ, Wynn RF, Bierings M. In: The EBMT-ESH Handbook. Apperley J, Carreras E, Gluckman E, Gratwohl A, Masszi T, editors. EBMT; Netherlands: 2008. pp. 545–553. [Google Scholar]
- [158].Escolar ML, Poe MD, Provenzale JM, Richards KC, Allison J, Wood S, Wenger DA, Pietryga D, Wall D, Champagne M, Morse R, Krivit W, Kurtzberg J. Transplantation of umbilical-cord blood in babies with infantile Krabbe’s disease. N. Engl. J. Med. 2005;352(20):2069–2081. doi: 10.1056/NEJMoa042604. [DOI] [PubMed] [Google Scholar]
- [159].Peters C, Charnas LR, Tan Y, Ziegler RS, Shapiro EG, DeFor T, Grewal SS, Orchard PJ, Abel SL, Goldman AI, Ramsay NK, Dusenbery KE, Loes DJ, Lockman LA, Kato S, Aubourg PR, Moser HW, Krivit W. Cerebral X-linked adrenoleukodystrophy: the international hematopoietic cell transplantation experience from 1982 to 1999. Blood. 2004;104(3):881–888. doi: 10.1182/blood-2003-10-3402. [DOI] [PubMed] [Google Scholar]
- [160].Nakafuku M, Nagao M, Grande A, Cancelliere A. Revisiting neural stem cell identity. Proc. Natl. Acad. Sci. USA. 2008;105(3):829–830. doi: 10.1073/pnas.0711637105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [161].Ge W, He F, Kim KJ, Blanchi B, Coskun V, Nguyen L, Wu X, Zhao J, Heng JI, Martinowich K, Tao J, Wu H, Castro D, Sobeih MM, Corfas G, Gleeson JG, Greenberg ME, Guillemot F, Sun YE. Coupling of cell migration with neurogenesis by proneural bHLH factors. Proc. Natl. Acad. Sci. USA. 2006;103(5):1319–1324. doi: 10.1073/pnas.0510419103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [162].Yadirgi G, Marino S. Adult neural stem cells and their role in brain pathology. J. Pathol. 2009;217(2):242–253. doi: 10.1002/path.2480. [DOI] [PubMed] [Google Scholar]
- [163].Cummings BJ, Uchida N, Tamaki SJ, Salazar DL, Hooshmand M, Summers R, Gage FH, Anderson AJ. Human neural stem cells differentiate and promote locomotor recovery in spinal cord-injured mice. Proc. Natl. Acad. Sci. USA. 2005;102(39):14069–14074. doi: 10.1073/pnas.0507063102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [164].Hooshmand MJ, Sontag CJ, Uchida N, Tamaki S, Anderson AJ, Cummings BJ. Analysis of host-mediated repair mechanisms after human CNS-stem cell transplantation for spinal cord injury: correlation of engraftment with recovery. PLoS. One. 2009;4(6):e5871. doi: 10.1371/journal.pone.0005871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [165].Tamaki SJ, Jacobs Y, Dohse M, Capela A, Cooper JD, Reitsma M, He D, Tushinski R, Belichenko PV, Salehi A, Mobley W, Gage FH, Huhn S, Tsukamoto AS, Weissman IL, Uchida N. Neuroprotection of host cells by human central nervous system stem cells in a mouse model of infantile neuronal ceroid lipofuscinosis. Cell Stem Cell. 2009;5(3):310–319. doi: 10.1016/j.stem.2009.05.022. [DOI] [PubMed] [Google Scholar]
- [166].Kielar C, Maddox L, Bible E, Pontikis CC, Macauley SL, Griffey MA, Wong M, Sands MS, Cooper JD. Successive neuron loss in the thalamus and cortex in a mouse model of infantile neuronal ceroid lipofuscinosis. Neurobiol. Dis. 2007;25(1):150–162. doi: 10.1016/j.nbd.2006.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [167].Pontikis CC, Cella CV, Parihar N, Lim MJ, Chakrabarti S, Mitchison HM, Mobley WC, Rezaie P, Pearce DA, Cooper JD. Late onset neurodegeneration in the Cln3−/− mouse model of juvenile neuronal ceroid lipofuscinosis is preceded by low level glial activation. Brain Res. 2004;1023(2):231–242. doi: 10.1016/j.brainres.2004.07.030. [DOI] [PubMed] [Google Scholar]
- [168].Bible E, Gupta P, Hofmann SL, Cooper JD. Regional and cellular neuropathology in the palmitoyl protein thioesterase-1 null mutant mouse model of infantile neuronal ceroid lipofuscinosis. Neurobiol. Dis. 2004;16(2):346–359. doi: 10.1016/j.nbd.2004.02.010. [DOI] [PubMed] [Google Scholar]
- [169].Kay GW, Palmer DN, Rezaie P, Cooper JD. Activation of non-neuronal cells within the prenatal developing brain of sheep with neuronal ceroid lipofuscinosis. Brain Pathol. 2006;16(2):110–116. doi: 10.1111/j.1750-3639.2006.00002.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [170].Danton GH, Dietrich WD. Inflammatory mechanisms after ischemia and stroke. J. Neuropathol. Exp. Neurol. 2003;62(2):127–136. doi: 10.1093/jnen/62.2.127. [DOI] [PubMed] [Google Scholar]
- [171].Carson MJ. Microglia as liaisons between the immune and central nervous systems: functional implications for multiple sclerosis. Glia. 2002;40(2):218–231. doi: 10.1002/glia.10145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [172].Eikelenboom P, Bate C, Van Gool WA, Hoozemans JJ, Rozemuller JM, Veerhuis R, Williams A. Neuroinflammation in Alzheimer’s disease and prion disease. Glia. 2002;40(2):232–239. doi: 10.1002/glia.10146. [DOI] [PubMed] [Google Scholar]
- [173].Saijo K, Winner B, Carson CT, Collier JG, Boyer L, Rosenfeld MG, Gage FH, Glass CK. A Nurr1/CoREST pathway in microglia and astrocytes protects dopaminergic neurons from inflammation-induced death. Cell. 2009;137(1):47–59. doi: 10.1016/j.cell.2009.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [174].Katz ML, Sanders DN, Mooney BP, Johnson GS. Accumulation of glial fibrillary acidic protein and histone H4 in brain storage bodies of Tibetan terriers with hereditary neuronal ceroid lipofuscinosis. J. Inherit. Metab. Dis. 2007;30(6):952–963. doi: 10.1007/s10545-007-0683-y. [DOI] [PubMed] [Google Scholar]
- [175].Xu S, Sleat DE, Jadot M, Lobel P. Glial fibrillary acidic protein is elevated in the lysosomal storage disease classical late-infantile neuronal ceroid lipofuscinosis but is not a component of the storage material. Biochem. J. 2010 doi: 10.1042/BJ20100128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [176].Cook DN, Beck MA, Coffman TM, Kirby SL, Sheridan JF, Pragnell IB, Smithies O. Requirement of MIP-1 alpha for an inflammatory response to viral infection. Science. 1995;269(5230):1583–1585. doi: 10.1126/science.7667639. [DOI] [PubMed] [Google Scholar]
- [177].Krathwohl MD, Kaiser JL. Chemokines promote quiescence and survival of human neural progenitor cells. Stem Cells. 2004;22(1):109–118. doi: 10.1634/stemcells.22-1-109. [DOI] [PubMed] [Google Scholar]
- [178].Wu YP, Proia RL. Deletion of macrophage-inflammatory protein 1 alpha retards neurodegeneration in Sandhoff disease mice. Proc. Natl. Acad. Sci. U S A. 2004;101(22):8425–8430. doi: 10.1073/pnas.0400625101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [179].Kielar C, Wishart TM, Palmer A, Dihanich S, Wong AM, Macauley SL, Chan CH, Sands MS, Pearce DA, Cooper JD, Gillingwater TH. Molecular correlates of axonal and synaptic pathology in mouse models of Batten disease. Hum. Mol. Genet. 2009;18(21):4066–4080. doi: 10.1093/hmg/ddp355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [180].Lim MJ, Beake J, Bible E, Curran TM, Ramirez-Montealegre D, Pearce DA, Cooper JD. Distinct patterns of serum immunoreactivity as evidence for multiple brain-directed autoantibodies in juvenile neuronal ceroid lipofuscinosis. Neuropathol. Appl. Neurobiol. 2006;32(5):469–482. doi: 10.1111/j.1365-2990.2006.00738.x. [DOI] [PubMed] [Google Scholar]
- [181].Lim MJ, Alexander N, Benedict JW, Chattopadhyay S, Shemilt SJ, Guerin CJ, Cooper JD, Pearce DA. IgG entry and deposition are components of the neuroimmune response in Batten disease. Neurobiol. Dis. 2007;25(2):239–251. doi: 10.1016/j.nbd.2006.09.005. [DOI] [PubMed] [Google Scholar]
- [182].de Winter BC, van GT, Sombogaard F, Shaw LM, van Hest RM, Mathot RA. Pharmacokinetic role of protein binding of mycophenolic acid and its glucuronide metabolite in renal transplant recipients. J. Pharmacokinet. Pharmacodyn. 2009;36(6):541–564. doi: 10.1007/s10928-009-9136-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [183].Aberg L, Talling M, Harkonen T, Lonnqvist T, Knip M, Alen R, Rantala H, Tyynela J. Intermittent prednisolone and autoantibodies to GAD65 in juvenile neuronal ceroid lipofuscinosis. Neurology. 2008;70(14):1218–1220. doi: 10.1212/01.wnl.0000307753.88839.29. [DOI] [PubMed] [Google Scholar]