SUMMARY
Marek’s disease virus (MDV) or Gallid herpesvirus 2 (GaHV-2) is a lymphotropic alphaherpesvirus and causes Marek’s disease. Former studies have demonstrated that MDV is spread from chicken to chicken about 2 wk postexposure as infectious dander shed from infected chickens. More recent reports, using highly sensitive quantitative PCR analyses of dander from infected chickens, suggested that MDV replicates and is shed from the chicken much earlier (5–7 days). However, detection of viral DNA in chicken dander does not indicate whether fully infectious virus is present. To determine if viral replication is present in the skin of infected chickens at these early times, expression of a late viral protein indicative of fully productive virus replication was evaluated using fluorescent microscopy. To do this, highly virulent and attenuated recombinant (r)MDV was generated that abundantly expresses the monomeric red fluorescent protein fused to the late UL47 (VP13/14) protein in feather follicle epithelial cells. Detection of viral DNA could be detected in the skin of infected chickens as early as 6 days postinfection (p.i.), consistent with previous reports detecting viral DNA in dander shed from infected chickens. Replication of virulent rMDV was evident in the feather follicles as early as 8 days p.i., while attenuated rMDV replication in the feather follicles was delayed 1–2 days. Former studies, using less sensitive techniques, suggested viral protein expression to occur about 10–12 days p.i. Undoubtedly differences in time of detection can partly be explained by multiple factors including the pathotype of virus, the route of infection, and the age and genetic line of the infected chickens used in different studies. In summary, though viral DNA can be detected as early as 6 days p.i., late viral protein expression, indicative of infectious virus production, occurs 2–3 days after DNA detection, but earlier than previously thought.
Keywords: Marek’s disease, herpesvirus, attenuation, chicken, feather follicle epithelium
Marek’s disease (MD) is caused by MD virus (MDV) or Gallid herpesvirus 2 (GaHV-2) in chickens. Clinical symptoms of MD include depression, neurological signs such as paralysis and ataxia, and the development of lymphoproliferative disease characterized by solid tumors in the viscera and other organs (4,21). Natural infection is believed to begin through inhalation of virus, after which primary cytolytic replication in B and then T lymphocytes ensue. Following lytic infection, latency is established in activated CD4+ T cells that can be transformed into highly proliferative T cell lymphomas. Irrespective of the transformation event, infected lymphocytesmigrating in the skin transfer virus to feather follicle epithelial (FFE) cells that leads to the production of infectious particles that are shed into the environment, providing a continuous source of infectious virus.
Much attention has been placed on the oncogenic aspect of MD and vaccinology since the identification of MDV as the etiologic agent (3,25). After the identification of the mode of transmission of MDV, that being infectious dander shed from infected chickens approximately 14 days postinfection (p.i.), information pertaining to this important aspect of the virus life cycle has been limited until recently. The identification of MDV encoded proteins that are essential for transmission has shed some light onto this area of research (16,19). Seminal studies in the late 1960s and early 1970s elucidated important aspects of MDV transmission. These reports suggested that infectious virus was present as early as 7 days p.i. in both nasal washings and feces (8,9,24,30,35); however, the data at that time were conflicting. Subsequently it was determined the main source of transmission for MDV was dust and dander shed from infected chickens (2,5,22). Since then, it has been accepted that MDV reaches the FFE and produces infectious virus around 10–12 days p.i., followed by shedding of infectious virus into the environment at an infective dose around 14 days p.i. (6,36). However, more recent studies, using highly sensitive PCR to detect viral genomes, found that virus was detectable as early as 7 days p.i. in feather tips (1) and as early as 5–7 days p.i. in dander shed from the chicken (10,13). The early detection of MDV DNA in these studies suggested transmission of MDV may occur earlier than originally thought, especially when more virulent viruses present today have earlier and more pronounced replicative rates (17,37).
Advances in molecular cloning and generation of recombinant (r)MDV allow us to revisit some of the long-standing questions that could not be addressed during the early studies on the elucidation of MDV pathogenesis. For example, fusing the enhanced green fluorescent protein (eGFP) to the C-terminus of the late UL47 (VP13/14) tegument protein of MDV (UL47-eGFP) led to the discovery that this protein is expressed at very low levels during in vitro MDV replication, while it is expressed abundantly in FFE cells in the skin (15). This finding circumstantially linked late protein expression levels to the production of infectious virus in FFE cells in vivo, and lack thereof, in the case of the strictly cell-associated nature of MDV during in vitro replication. Importantly, this study also showed that fusing the fluorescent protein to the viral UL47 protein resulted in no loss of pathogenicity, showing this is a powerful tool for studying MDV replication in the FFE cells of infected chickens. This approach was again utilized recently to generate additional fluorescent viruses using the monomeric red fluorescent protein (mRFP) fused to the UL47 protein (UL47-mRFP) in virulent and attenuated rMDV to examine dual infection of FFE cells with two viruses (14). These viruses were employed in the present study to address two important questions. First, when is the earliest time point at which expression of late MDV protein is detected in FFE cells, which would be indicative of a fully productive replicative cycle? Second, is there a difference between virulent and attenuated viruses with respect to when late protein expression can be detected and the relative number of infected follicles? Using qPCR for viral DNA and microscopy for viral antigens, the earliest time point at which viral DNA could be detected was at 6 days p.i. for both virulent and attenuated rMDV, consistent with earlier results, while expression of the late MDV protein, UL47-mRFP, could be detected at 8 days p.i during infection with virulent rMDV. Detection of late protein expression was delayed for attenuated viruses by 1–2 days.
MATERIALS AND METHODS
Construction and propagation of rMDV expressing UL47-mRFP
The generation of rMDVs expressing fluorescent UL47 protein has been recently described (14). Briefly, the mRFP protein was fused to the C-terminus of the MDV UL47 protein using standard two-step Red-mediated mutagenesis (33). Virulent rMDV expressing UL47-mRFP was generated using the infectious bacterial artificial chromosome (BAC) clone of the RB-1B strain (27) that had been restored for horizontal transmission (16), while two attenuated rMDV expressing UL47-mRFP were generated using previously characterized rMDV BAC clones. The first, ΔRLORF4, in which both copies of RLORF4 have been deleted, has been shown to be attenuated based on its increased replication rates in vitro and decreased replication and disease incidence in vivo (20). The second mutant, vTR AU5, has both copies of the viral telomerase RNA (vTR) template sequence mutated. This virus replicates like wild-type virus in vitro, but is attenuated in vivo exhibiting decreased replication levels and abrogation of tumorigenesis (23).
Following construction of rMDV BAC clones, rMDV was reconstituted in chicken embryo cell (CEC) cultures and propagated in chick kidney cell (CKC) cultures exactly as previously described (16). CEC and CKC cultures were prepared from 10 days-of-embryonation specific-pathogen-free (SPF) embryos or 14-day-old SPF chickens, respectively (29). Reconstituted viruses (v) were used at passage 4.
Fluorescent microscopy
Skin/feather tissues were collected from the ventral feather tracts of rMDV-infected chickens in 15 × 10 mm sections and snap-frozen in Tissue Tek®-optimal cutting temperature compound (Sankura® Finetek, Torrance, CA) and stored at −80 C until sectioned. Tissues were transversely cut through the feather follicle at 8 µm sections and affixed to Superfrost/Plus slides (Fisher Scientific, Pittsburgh, PA), then fixed with PFA buffer (2% paraformaldehyde, 0.1% Triton X-100) for 15 min. Cryosectioned tissues used for antigen detection were blocked in 10% neonatal calf serum and stained with monoclonal antibody O11 directed against the early lytic MDV protein pp38 (7). Goat anti-mouse IgG-Alexa Fluor® 488 (Molecular Probes, Eugene, OR) was used as secondary antibody. Hoechst 33342 (2 µg/ml, Molecular Probes) was used to visualize nuclei. The Axio Imager M1 system with AxioVision software (Carl Zeiss, Thornwood, NY) was used to analyze stained tissues. All images were compiled using Adobe® Photoshop® CS2 version 9.0.2.
DNA extraction from feather/skin tissues and qPCR assays
Feathers were cut off close to the skin, leaving the feather shaft tips embedded in the skin, and feather/skin tissues encompassing 8–10 feather follicles (~100 mm2) were dissected from ventral feather tracts of infected chickens at various times p.i. and frozen at −80 C until processed. DNA was extracted using the E.Z. 96® Tissue DNA Kit (Omega Bio-tek, Norcross, GA) as previously described (16). Quantification of MDV genomic copies using qPCR was also performed exactly as previously described (16) using primers and probe specific for the MDV-infected cell protein 4 and chicken inducible nitric oxide synthase as a normalizing control. All qPCR assays were performed in an ABI Prism 7300 Real-time PCR System and the results were analyzed using Sequence Detection Systems version 1.4.0 software supplied by Applied Biosystems (Foster City, CA).
In vivo experiments
SPF P2a (MHC: B19B19) chickens were obtained from flocks maintained at Cornell University, housed in isolation units, and water and food were provided ad libitum. Chickens were inoculated intra-abdominally with cell-associated inoculum of 2000 plaque-forming units for each rMDV at either 14 (Experiment 1) or 3 (Experiment 2) days of age. All experimental procedures were conducted in compliance with approved Cornell University Institutional Animal Care and Use Committee protocol number 2008-0018.
RESULTS
Generation of rMDV expressing UL47-mRFP
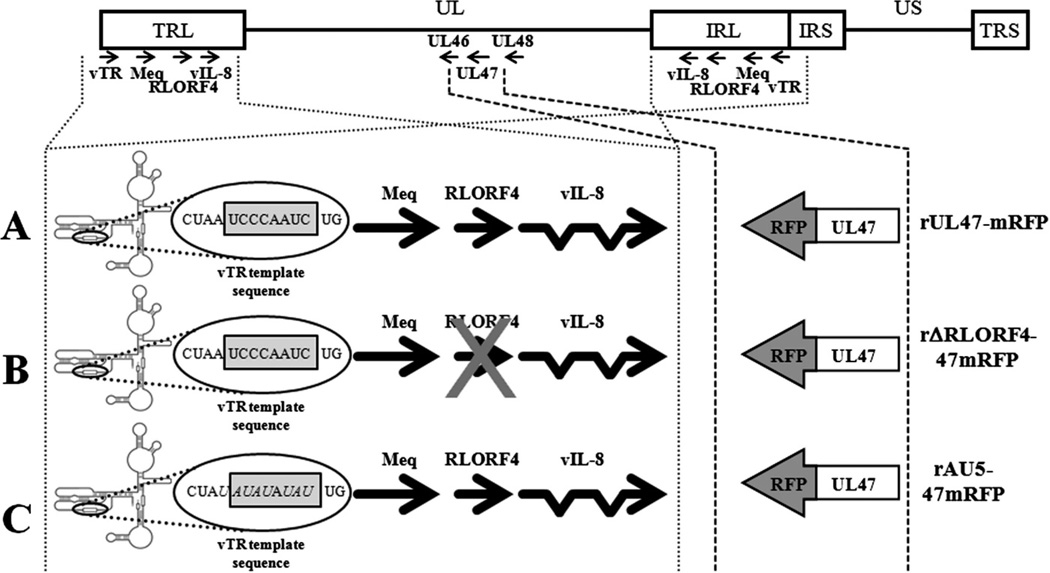
Three rMDVs were recently generated that express abundant UL47-mRFP in the FFE cells of infected chickens, while having no quantifiable effect on the pathogenicity of each virus (14). The generation and characterization of these viruses have recently been described (14) and were designated vUL47-mRFP (virulent), vΔRLORF4-47mRFP (attenuated), and vAU5-47mRFP (attenuated). Each rMDV showed no altered pathogenicity compared to their respective parental rMDV. Fig. 1 shows a schematic representation of the rMDV used in this report.
Fig. 1.
Schematic representation of rMDV expressing UL47-mRFP fusion proteins. Shown for each clone is the MDV genome depicting the locations of the terminal repeat long (TRL) and short (TRS), internal repeat long (IRL) and short (IRS), and unique long (UL) and short (US) regions. The position and orientation of the UL47 gene with respect to adjacent genes within the UL are shown. mRFP was fused to the C terminus of the UL47 tegument protein in three different BAC clones to generate rUL47-mRFP (A), rΔRLORF4-47mRFP (B), and rAU5-47mRFP (C) using the previously described BAC clones for rRB-1B (16), rRB-1B-ΔRLORF4 (20), and rAU5vTR (23). RLORF4 was deleted in both the TRL and IRL of the MDV genome in the rΔRLORF4-47mRFP clones, while both copies of the vTR template sequence (UCCCAAUC) was mutated to AUAUAUAU in rAU5-47mRFP. Only one copy within the TRL is shown for simplicity.
Late viral protein expression in FFE cells as early as 8 days p.i for virulent rMDV
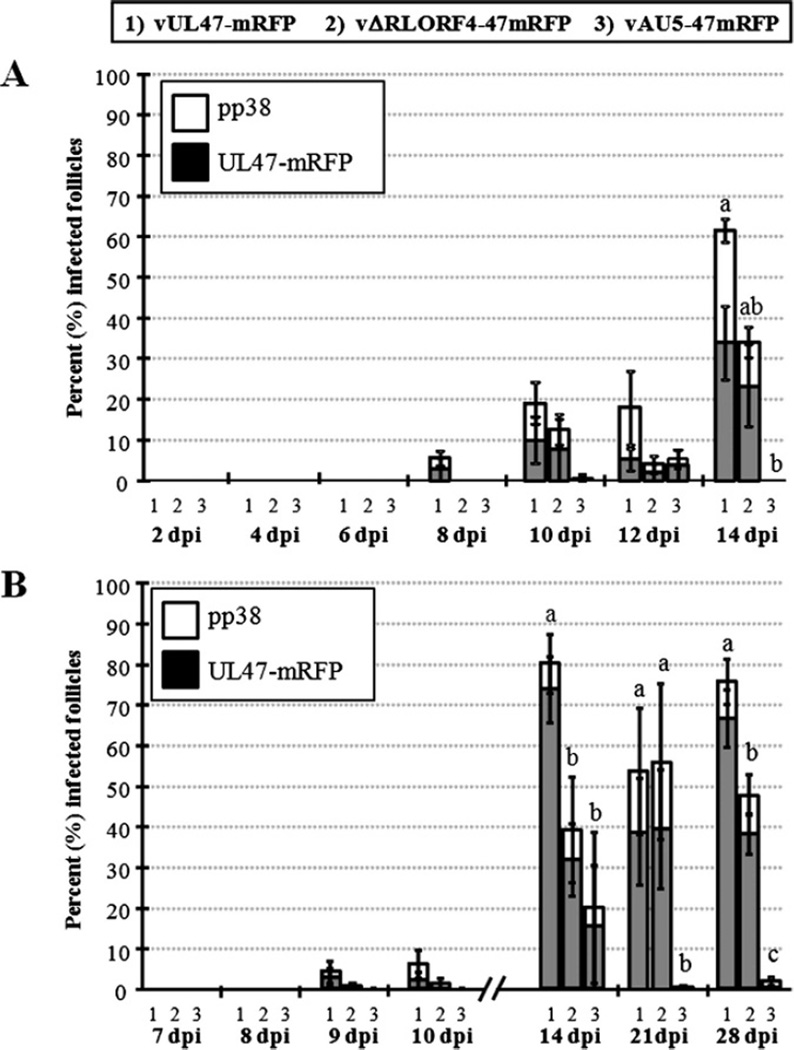
In the first experiment, chickens (n = 3) were infected with each virus at 14 days of age, and feather/skin tissues were collected at 2, 4, 6, 8, 10, 12, and 14 days p.i. Since it is well established that MDV is shed and transmitted after 14 days p.i. (6), analyses were not performed past this time point in this experiment. For each group, >100 feather follicles were examined at each time point for early lytic pp38 and late lytic UL47-mRFP expression by indirect IF assays or direct visual analysis, respectively. The percentage of follicles infected were averaged for each group, and the data are summarized in Fig. 2A. Both UL47-mRFP and pp38 were observed in FFE cells as early as 8 days p.i. with virulent rMDV (vUL47-mRFP). Fig. 3 shows microscopic images of two birds with detectable viral protein expression in FFE cells at 8 days p.i. The percentage of infected feather follicles remained approximately the same at 10 and 12 days p.i. (18%–19%) but increased significantly at 14 days p.i. (61%) in vUL47-mRFP-infected birds. Using qPCR assays on DNA collected from infected chicken feather/skin tissues, viral genomes could be detected at 6 days p.i. in 2/3 chickens infected with vUL47-mRFP indicating virus was present earlier (Table 1). All chickens infected with vUL47-mRFP remained positive for the remaining times tested.
Fig. 2.
Percent infected follicles in chickens infected with virulent and attenuated rMDVs. Chickens were infected with vUL47-mRFP (1), vΔRLORF4-47mRFP (2), or vAU5-47mRFP (3) at either 14 (A) or 3 (B) days of age, and skin/feather samples were collected at various times during the experiment from either three (A) or four (B) chickens per group. The number of feather follicles positive for pp38 or UL47-mRFP were determined at different days p.i. (dpi) and the data represented as the percent follicles infected (number of feather follicles positive for pp38 or UL47-mRFP/number of feather follicles examined) ± standard error of means. Groups were compared using a Tukey-Kramer comparison of means (P ≤ 0.05) for significance within a specific day p.i. Different letters above virus groups within a given day p.i. indicate that the groups are significantly different from each other.
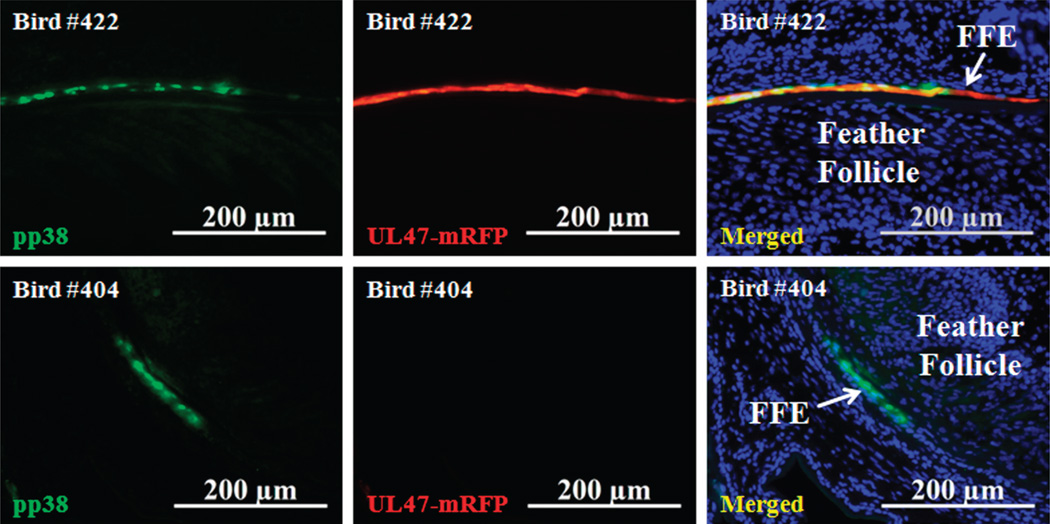
Fig. 3.
Expression of early and late MDV proteins at 8 days p.i. Skin/feather tissues were collected from vUL47-mRFP-infected chickens at 8 days p.i., and feather follicles (FFs) were stained for the pp38 early lytic MDV protein with anti-pp38 monoclonal antibody and nuclei (blue) with Hoechst 33342. The Axio Imager M1 system was used at 200× to detect early pp38 (green) and late UL47-mRFP (red) expression in two different birds.
Table 1.
Feather/skin tissues positive for Marek’s disease virus genomes in Experiment 1.
| Samples positive for MDV genomes/samples tested using qPCR assays |
|||
|---|---|---|---|
| Days p.i. | vUL47-mRFP | vΔRLORF4-47mRFP | vAU5-47mRFP |
| 2 | 0/3 | 0/3 | 0/3 |
| 4 | 0/3 | 0/3 | 0/3 |
| 6 | 2/3 | 1/3 | 0/3 |
| 8 | 3/3 | 3/3 | 3/3 |
| 10 | 3/3 | 2/3 | 3/3 |
| 12 | 3/3 | 3/3 | 2/3 |
| 14 | 3/3 | 3/3 | 1/3 |
Since the earliest point of lytic replication observed in Experiment 1 was at 8 days p.i. and no replication was observed at 6 days p.i., a second trial was performed in which chickens (n = 4) were infected with each virus at 3 days of age, and feather/skin tissues were collected 7, 8, 9, and 10 days p.i. to determine the earliest point MDV lytic replication could be observed. Additionally, later time points were included to evaluate the percentage of feather follicles infected later during infection. In this experiment, no chickens showed lytic replication at 7 or 8 days p.i.; however, lytic replication was evident with virulent vUL47-mRFP at 9 and 10 days p.i. (Fig. 2B). In this experiment, later time points indicated that a high percentage of feather follicles were infected with virulent vUL47-mRFP with more than 50% of the follicles examined being positive for late viral protein expression at 14, 21, and 28 days. There was a modest decrease in the average percent of infected follicles from 14 (85%) and 21 (60%) days p.i., but this was highly variable between birds indicated by large standard errors at 21 days p.i. At 28 days, the percent of infected follicles was >75% and with less variability between birds. qPCR results for MDV DNA in feather/skin samples in Experiment 2 were consistent with results in Experiment 1 with detection of viral genomes earlier than detection of viral protein expression. All chickens negative for MDV genomes in qPCR assays were also negative for viral protein expression using fluorescence microscopy. The data show that though viral genomes could be detected in the skin/feathers with highly sensitive qPCR assays as early as 6 days, viral protein expression was not evident until 8 days p.i. during infection with virulent rMDV.
Late viral protein expression in FFE cells is delayed for attenuated rMDV
In Experiment 1, late protein expression could be detected at 8 days p.i. with virulent rMDV, while this was delayed to 10 days p.i. for the attenuated rMDVs, vΔRLORF4-47mRFP, and vAU5-47mRFP (Fig. 2A). Viral genomes could be detected in one vΔRLORF4-infected chicken at 6 days p.i., and all chickens were positive in both attenuated virus groups at 8 days p.i. (Table 1), even though both early (pp38) and late (UL47-mRFP) protein could not be detected until 10 days p.i. (Fig. 2A). One chicken at 12 days p.i. and two chickens at 14 days p.i. were negative for viral genomes in the vAU5-47mRFP group (Table 1).
In the second experiment, few feather follicles were infected with vAU5-47mRFP (Fig. 2B) with the highest percentage (20%) detected at 14 days p.i., which dropped dramatically at 21 (0%) and 28 (4%) days p.i. In contrast to virulent vUL47-mRFP that had a drop in percent infected follicles at 21 days, the percentage of infected follicles in chickens infected with vΔRLORF4-47mRFP increased from 14 (40%) to 21 (54%) days p.i. Combined, these data show that detection of viral protein expression in feather follicles is delayed during infection with attenuated rMDVs compared to virulent rMDV.
DISCUSSION
Earlier studies showed that viral DNA could be detected, using highly sensitive qPCR assays, in either feather tips of infected chickens (1) or dander shed from infected chickens as early as 5–7 days p.i. (10,13); however, these studies do not discriminate between latently infected T cells circulating within the feather follicles and productive viral replication in FFE cells that would produce infectious virus. Interesting, in a follow-up study in which MDV DNA was detected in dander shed from infected chickens as early as 7 days p.i. (11,13), it was determined this material was noninfectious when administered via intratracheal insufflations (12). In this study, only material collected after 15 days p.i. could produce disease in chickens, suggesting either there was no infectious virus in the earlier samples or the levels were too low to produce disease.
Using new molecular tools to directly visualize late protein expression in feather follicles of rMDV infected chickens, two important questions were addressed in this report that previous studies could not answer. The first question was directed at determining whether late viral protein expression could be detected in feather follicles earlier than MDV is believed to be transmitted to naïve chickens (~14 days p.i.). Using qPCR assays, detection of viral DNA in feather/skin tissues could be detected as early as 6 days p.i. (Tables 1 and 2), consistent with earlier reports (1,11,13). Surprisingly, late protein expression could be detected as early as 8 days p.i. with virulent rMDV. It is not known whether infectious virus is being produced in these cells as transmission studies on this material were not performed; however, expression of the late viral protein UL47 is suggestive that fully productive infection is occurring. Given the low numbers of infected feather follicles producing UL47-mRFP at his time point, as well as at 10 and 12 days p.i. (Fig. 2), it would be expected that the time at which the numbers of infected follicles and level of infectious virus produced and shed into the environment that are infectious, is most likely what has been commonly considered around 12–14 days p.i. The exact day at which levels of infectious virus is shed and able to produce disease is most likely highly dependent on the pathotype of virus, the route of infection, and the age and genetic line of the infected chickens used in different studies; however, the data here support the information from earlier studies.
Table 2.
Feather/skin tissues positive for Marek’s disease virus genomes in Experiment 2.
| Samples positive for MDV genomes/samples tested using qPCR assays |
|||
|---|---|---|---|
| Days p.i. | vUL47-mRFP | vΔRLORF4-47mRFP | vAU5-47mRFP |
| 7 | 1/4 | 1/4 | 0/4 |
| 8 | 2/4 | 3/4 | 1/4 |
| 9 | 4/4 | 3/4 | 3/4 |
| 10 | 4/4 | 4/4 | 3/4 |
| 14 | 4/4 | 4/4 | 3/4 |
| 21 | 4/4 | 4/4 | 2/4 |
| 28 | 4/4 | 4/4 | 1/4 |
The second question addressed was to determine whether there was a difference in the time at which attenuated virus are detected in the feather/skin and when late viral protein expression can be seen compared to virulent rMDV. In two experiments, evidence of UL47-mRFP expression could be seen as early as 8 days p.i. with virulent rMDV, while replication was delayed by 1–2 days for attenuated vΔRLORF4-47mRFP and vAU5-47mRFP (Fig. 2). Viral DNA could be detected in one chicken at 6 days p.i. in the vΔRLORF4-47mRFP group and none for the vAU5-47mRFP group compared to two birds in the virulent vUL47-mRFP group (Table 1). By 8 days, all birds in each group were positive for viral DNA; however, no birds infected with the attenuated viruses showed late protein expression. These data indicate that the rate of entry into the feather/skin tissues appears to be relatively the same between virulent vUL47-mRFP and attenuated vΔRLORF4-47mRFP (6 days), but lytic protein expression is delayed during infection with attenuated viruses. This would be consistent with earlier reports on transmission of attenuated MDV (26). Expression of pp38 could be detected in some follicles with no UL47-mRFP expression, which would be consistent with pp38 being an early lytic protein and UL47 being a late protein (15), and therefore its detection expected to be prior to UL47-mRFP expression.
In the first experiment, expression of pp38 and pUL47-mRFP was clearly seen in vUL47-mRFP infected chickens at 8 days p.i.; however, in the second experiment, the earliest replication was not observed until 9 days p.i. This difference could be attributed to the difference in age of chickens used in each experiment. In the first experiment, chickens were inoculated at 14 days of age, while in the second experiment, 3-day-old birds were used. The stage of feather development between 3- or 14-day-old chickens is considerably different in that feathers are predominantly natal down or neossoptiles in the younger birds, whereas more feathers are fully developed pennaceous feathers or teleoptiles in the older birds (28). Thus, the number of mature feather follicles in the older birds is greater, and therefore, the chance of detecting replication of MDV in the FFE cells would be predictably much higher. It is possible, and likely, that replication of MDV in FFE cells is dependent on the maturity of the feather follicle as these cells have some, to date unknown, special trait able to facilitate fully productive MDV replication.
Comparing results in this report to previous studies, there was very little differences seen, other than an earlier detection of late protein expression shown in the current report. This difference could simply be due to increased sensitivity of detection of direct viral protein expression and a significant number of feather follicles examined used in this study. However, other differences may also contribute such as the virulence of the virus, the route of infection, and the age and genetic lines of chickens used in each report. For example, Carrozza et al. (6) used intratracheal inoculation of 1-day-old MD-susceptible Line 7 chickens with infectious dust obtained from chickens infected with the Conn-B MDV strain, while in the current study 1-day-old chickens were inoculated intra-abdominally with rMDV (RB-1B based) infected cell cultures. In their study, they used nonspecific fluorescent antibody staining to detect virus antigen in a small number of feather follicles (~3) per skin sample, whereas direct protein expression of a specific late viral protein in >100 feather follicles was examined here. The Conn B strain has not been pathotyped using the Witter pathotyping system (34), while RB-1B has been classified as a very virulent pathotype. Regardless of differences between experimental conditions, the data are consistent that very little viral protein is expressed prior to day 10.
When comparing virulent rMDV to the two attenuated rMDVs in this report, the virulent rMDV was observed in the FFE cells earlier and more abundantly than both attenuated rMDVs. As expected, vAU5-47mRFP was considerably more attenuated than vΔRLORF4-47mRFP with respect to infection of feather follicles in the skin. The mechanism for attenuation with vΔRLORF4 is not known, but it has been shown through multiple reports that truncation or deletion of RLORF4 leads to attenuation of MDV either through passing virulent MDV extensively in vitro (18,31,32) or by direct removal of the RLORF4 gene (20). Jarosinski et al. showed that deletion of RLORF4 in the highly virulent rMDV, the same parental virus used in this study (vΔRLORF4), led to attenuation of virus showing increased replication in vitro and decreased replication and disease induction in vivo (20). In contrast, a mechanism for attenuation of MDV through modification of the template region of vTR has been proposed. Kaufer et al. showed that modifying the template region, the sequence incorporated into the telomere, induces telomeric crisis and subsequently apoptosis in T cells transformed or latently infected with vAU5 (23). Thus, the constant circulation of infected T cells, which are presumed to be the cell transferring virus to the FFE cells, would be expected to be significantly diminished, subsequently reducing the level of transfer of virus to FFE cells. The data in Fig. 2 support this as the percentage of infected follicles in chickens infected with vAU5-47mRFP is significantly reduced compared to vUL47-mRFP. However, the delayed late MDV protein expression during infection with these two attenuated rMDV, with directed modifications that alter pathogenesis, may not necessarily reflect results of other attenuated MDV strains, as the mechanism by which natural attenuation occurs may be numerous.
It is interesting to note that in Experiment 2, when infection of feather follicles was examined at later time points, virulent vUL47-mRFP had significantly higher levels of infected feather follicles at 14 days, which were reduced at 21 days, only to increase again at 28 days. In contrast, the percentage of infected feather follicles increased from 14 to 21 days p.i. for the vΔRLORF4-47mRFP group, while the percentage of infected feather follicles was significantly diminished at 21 and 28 days in vAU5-47mRFP-infected chickens following a modest increase at 14 days p.i. These data would also be consistent with typical pathogenesis of virulent and attenuated viruses in which highly virulent viruses, once reactivated, typically replicate to high levels until ultimately the chicken succumbs to the disease, whereas attenuated virus often replicate at reduced levels following reactivation and typically maintain a low state of replication without causing significant disease.
In summary, the data presented here confirm that MDV DNA is present in feather follicles as early as 6 days p.i. and conclusively show that viral protein expression is evident at 8 days p.i. during infection with virulent rMDV. Additionally, during infection with attenuated rMDV, although viral DNA can also be detected as early as 6 days p.i., viral protein expression is delayed 1–2 days relative to infection with virulent rMDV.
ACKNOWLEDGMENTS
I would like to thank the Animal Care Staff in the Poultry Virus Isolation Unit of Cornell University for their help during the animal studies and Dr. Helen Valentine (Cornell University, Ithaca, NY) for technical help. Research reported in this publication was partially supported by the National Cancer Institute of the National Institutes of Health under Award No. RO1CA127238 and Agriculture and Food Research Initiative Competitive Grant No. 2010-65119-20493 from the USDA National Institute of Food and Agriculture.
Abbreviations
- BAC
bacterial artificial chromosome
- CEC
chicken embryo cell
- CKC
chick kidney cell
- eGFP
enhanced green fluorescent protein
- FFE
feather follicle epithelial
- GaHV-2
Gallid herpesvirus 2
- MD
Marek’s disease
- MDV
Marek’s disease virus
- mRFP
monomeric red fluorescent protein
- p.i.
postinfection
- rMDV
recombinant MDV
- SPF
specific-pathogen-free
- v
virus
- vTR
viral telomerase RNA
REFERENCES
- 1.Baigent SJ, Smith LP, Currie RJ, Nair VK. Replication kinetics of Marek’s disease vaccine virus in feathers and lymphoid tissues using PCR and virus isolation. J. Gen. Virol. 2005;86:2989–2998. doi: 10.1099/vir.0.81299-0. [DOI] [PubMed] [Google Scholar]
- 2.Beasley JN, Patterson LT, McWade DH. Transmission of Marek’s disease by poultry house dust and chicken dander. Am. J. Vet. Res. 1970;31:339–344. [PubMed] [Google Scholar]
- 3.Bublot M, Sharma J. Vaccination against Marek’s disease. In: Davison F, Nair V, editors. Marek’s disease: an evolving problem. London, UK: Elsevier Academic Press; 2004. pp. 168–185. [Google Scholar]
- 4.Calnek BW. Pathogenesis of Marek’s disease virus infection. Curr. Top. Microbiol. Immunol. 2001;255:25–55. doi: 10.1007/978-3-642-56863-3_2. [DOI] [PubMed] [Google Scholar]
- 5.Calnek BW, Adldinger HK, Kahn DE. Feather follicle epithelium: a source of enveloped and infectious cell-free herpesvirus from Marek’s disease. Avian Dis. 1970;14:219–233. [PubMed] [Google Scholar]
- 6.Carrozza JH, Fredrickson TN, Prince RP, Luginbuhl RE. Role of desquamated epithelial cells in transmission of Marek’s disease. Avian Dis. 1973;17:767–781. [PubMed] [Google Scholar]
- 7.Chbab N, Egerer A, Veiga I, Jarosinski KW, Osterrieder N. Viral control of vTR expression is critical for efficient formation and dissemination of lymphoma induced by Marek’s disease virus (MDV) Vet. Res. 2010;41:56. doi: 10.1051/vetres/2010026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Colwell WM, Schmittle SC. Studies on acute marek’s disease. VII. Airborne transmission of the GA isolate. Avian Dis. 1968;12:724–729. [PubMed] [Google Scholar]
- 9.Eidson CS, Schmittle SC. Studies on acute Marek’s disease. V. Attempted transmission of isolate GA with feces and nasal washings. Avian Dis. 1968;12:549–553. [PubMed] [Google Scholar]
- 10.Islam A, Walkden-Brown SW. Quantitative profiling of the shedding rate of the three Marek’s disease virus (MDV) serotypes reveals that challenge with virulent MDV markedly increases shedding of vaccinal viruses. J. Gen. Virol. 2007;88:2121–2128. doi: 10.1099/vir.0.82969-0. [DOI] [PubMed] [Google Scholar]
- 11.Islam AF, Walkden-Brown SW, Groves PJ, Underwood GJ. Effects of vaccine dose, virus challenge dose and interval from vaccination to challenge on protection of broiler chickens against Marek’s disease virus challenge. Aust. Vet. J. 2007;85:348–355. doi: 10.1111/j.1751-0813.2007.00195.x. [DOI] [PubMed] [Google Scholar]
- 12.Islam AFMF, Walkden-Brown SW. Infectivity of Marek’s disease virus shed in the early post-challenge period. In: Walkden-Brown SW, editor. The 8th International Marek’s Disease Symposium. Townsville, Queensland, Australia: James Cook University; 2008. [Google Scholar]
- 13.Islam AFMF, Walkden-Brown SW, Groves PJ, Underwood GJ. Kinetics of Marek’s disease virus (MDV) infection in broiler chickens 1: effect of varying vaccination to challenge interval on vaccinal protection and load of MDV and herpesvirus of turkey in the spleen and feather dander over time. Avian Pathol. 2008;37:225–235. doi: 10.1080/03079450701802230. [DOI] [PubMed] [Google Scholar]
- 14.Jarosinski KW. Dual infection and superinfection inhibition of epithelial skin cells by two alphaherpesviruses co-occur in the natural host. PLoS ONE. 2012;7:e37428. doi: 10.1371/journal.pone.0037428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jarosinski KW, Arndt S, Kaufer BB, Osterrieder N. Fluorescently tagged pUL47 of Marek’s disease virus reveals differential tissue expression of the tegument protein in vivo. J. Virol. 2012;86:2428–2436. doi: 10.1128/JVI.06719-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jarosinski KW, Margulis NG, Kamil JP, Spatz SJ, Nair VK, Osterrieder N. Horizontal transmission of Marek’s disease virus requires US2, the UL13 protein kinase, and gC. J. Virol. 2007;81:10575–10587. doi: 10.1128/JVI.01065-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jarosinski KW, Njaa BL, O’Connell PH, Schat KA. Proinflammatory responses in chicken spleen and brain tissues after infection with very virulent plus Marek’s disease virus. Viral Immunol. 2005;18:148–161. doi: 10.1089/vim.2005.18.148. [DOI] [PubMed] [Google Scholar]
- 18.Jarosinski KW, O’Connell PH, Schat KA. Impact of deletions within the Bam HI-L fragment of attenuated Marek’s disease virus on vIL-8 expression and the newly identified transcript of open reading frame LORF4. Virus Genes. 2003;26:255–269. doi: 10.1023/a:1024447230464. [DOI] [PubMed] [Google Scholar]
- 19.Jarosinski KW, Osterrieder N. Further analysis of Marek’s disease virus horizontal transmission confirms UL44 (gC) and UL13 protein kinase activity are essential, while US2 is non-essential. J. Virol. 2010;84:7911–7916. doi: 10.1128/JVI.00433-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jarosinski KW, Osterrieder N, Nair VK, Schat KA. Attenuation of Marek’s disease virus by deletion of open reading frame RLORF4 but not RLORF5a. J. Virol. 2005;79:11647–11659. doi: 10.1128/JVI.79.18.11647-11659.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jarosinski KW, Tischer BK, Trapp S, Osterrieder N. Marek’s disease virus: lytic replication, oncogenesis and control. Exp. Rev. Vaccines. 2006;5:761–772. doi: 10.1586/14760584.5.6.761. [DOI] [PubMed] [Google Scholar]
- 22.Johnson EA, Burke CN, Fredrickson TN, DiCapua RA. Morphogenesis of marek’s disease virus in feather follicle epithelium. J. Natl. Cancer Inst. 1975;55:89–99. doi: 10.1093/jnci/55.1.89. [DOI] [PubMed] [Google Scholar]
- 23.Kaufer BB, Arndt S, Trapp S, Osterrieder N, Jarosinski KW. Herpesvirus telomerase RNA (vTR) with a mutated template sequence abrogates herpesvirus-induced lymphomagenesis. PloS Pathogens. 2011;7:e1002333. doi: 10.1371/journal.ppat.1002333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kenzy SG, Biggs PM. Excretion of the Marek’s disease agent by infected chickens. Vet. Rec. 1967;80:565–568. [Google Scholar]
- 25.Nair VK, Kung HJ. Marek’s disease virus oncogenicity: molecular mechanisms. In: Davison TF, Nair VK, editors. Marek’s disease: an evolving problem. Compton, UK: Institute for Animal Health, Compton Laboratory; 2004. pp. 32–48. [Google Scholar]
- 26.Nazerian K, Witter RL. Cell-free transmission and in vivo replication of Marek’s disease virus. J. Virol. 1970;5:388–397. doi: 10.1128/jvi.5.3.388-397.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Petherbridge L, Brown AC, Baigent SJ, Howes K, Sacco MA, Osterrieder N, Nair VK. Oncogenicity of virulent Marek’s disease virus cloned as bacterial artificial chromosomes. J. Virol. 2004;78:13376–13380. doi: 10.1128/JVI.78.23.13376-13380.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Prum RO, Brush AH. The evolutionary origin and diversification of feathers. Q. Rev. Biol. 2002;77:261–295. doi: 10.1086/341993. [DOI] [PubMed] [Google Scholar]
- 29.Schat KA, Sellers HS. Cell-culture methods. In: Dufour-Zavala L, Swayne DE, Glisson JR, Pearson JE, Reed WM, Jackwood MW, Woolcock PR, editors. A laboratory manual for the identification and characterization of avian pathogens. 5th ed. Jacksonville, FL: American Association of Avian Pathologists; 2008. pp. 195–203. [Google Scholar]
- 30.Sevoian M, Chamberlain DM, Larose RN. Avian lymphomatosis. V. Air-borne transmission. Avian Dis. 1963;7:102–105. [PubMed] [Google Scholar]
- 31.Spatz SJ. Accumulation of attenuating mutations in varying proportions within a high passage very virulent plus strain of Gallid herpesvirus type 2. Virus Res. 2010;149:135–142. doi: 10.1016/j.virusres.2010.01.007. [DOI] [PubMed] [Google Scholar]
- 32.Spatz SJ, Rue C, Schumacher D, Osterrieder N. Clustering of mutations within the inverted repeat regions of a serially passaged attenuated gallid herpesvirus type 2 strain. Virus Genes. 2008;37:69–80. doi: 10.1007/s11262-008-0242-0. [DOI] [PubMed] [Google Scholar]
- 33.Tischer BK, Smith GA, Osterrieder N. En passant mutagenesis: a two step markerless red recombination system. Methods Mol. Biol. 2010;634:421–430. doi: 10.1007/978-1-60761-652-8_30. [DOI] [PubMed] [Google Scholar]
- 34.Witter RL. Increased virulence of Marek’s disease virus field isolates. Avian Dis. 1997;41:149–163. [PubMed] [Google Scholar]
- 35.Witter RL, Burmester BR. Transmission of Marek’s disease with oral washings and feces from infected chickens. Proc. Soc. Exp. Biol. Med. 1967;124:59–62. doi: 10.3181/00379727-124-31666. [DOI] [PubMed] [Google Scholar]
- 36.Witter RL, Solomon JJ, Champion LR, Nazerian K. Long-term studies of Marek’s disease infection in individual chickens. Avian Dis. 1971;15:346–365. [PubMed] [Google Scholar]
- 37.Yunis R, Jarosinski KW, Schat KA. Association between rate of viral genome replication and virulence of Marek’s disease herpesvirus strains. Virology. 2004;328:142–150. doi: 10.1016/j.virol.2004.07.017. [DOI] [PubMed] [Google Scholar]