Abstract
The evidence that antipsychotics improve brain function and reduce symptoms in schizophrenia is unmistakable, but how antipsychotics change brain function is poorly understood, especially within neuronal systems. In this review, we investigated the hypothesized normalization of the functional magnetic resonance imaging (fMRI) blood oxygen level dependent signal in the context of antipsychotic treatment. First, we conducted a systematic PubMed search to identify eight fMRI investigations that met the following inclusion criteria: case-control, longitudinal design; pre- and post-treatment contrasts with a healthy comparison group; and antipsychotic-free or antipsychotic-naïve patients with schizophrenia at the start of the investigation. We hypothesized that aberrant activation patterns or connectivity between patients with schizophrenia and healthy comparisons at the first imaging assessment would no longer be apparent or “normalize” at the second imaging assessment. The included studies differed by analysis method and fMRI task but demonstrated normalization of fMRI activation or connectivity during the treatment interval. Second, we reviewed putative mechanisms from animal studies that support normalization of the BOLD signal in schizophrenia. We provided several neuronal-based interpretations of these changes of the BOLD signal that may be attributable to long-term antipsychotic administration.
Keywords: antipsychotics, BOLD signal, drug effects, functional magnetic resonance imaging, schizophrenia
INTRODUCTION
Functional magnetic resonance imaging (fMRI), a widely available and non-invasive imaging modality, has advanced our understanding of neuropsychiatric diseases such as schizophrenia. Cross-sectional studies comparing medicated patients with schizophrenia (SP) and healthy comparison subjects (HC) often attribute group differences to neuronal dysfunction or disconnection related to the disease process despite the obvious confound of antipsychotic treatment. Schizophrenia investigations with first-degree relatives, unmedicated prodromal, or antipsychotic naïve, first-episode patients are immune to the effect of antipsychotics via the lack of exposure and are valuable at elucidating the pathophysiology of the disease process [1–3]. The costs of these investigations include the recruitment challenges, ethical considerations, and limited generalizability to clinical populations who are often medicated. Attempts to control for antipsychotic medications in cross-sectional studies with medicated SP are limited to correlations with dose equivalencies or type of antipsychotic (usually “typical” versus “atypical”) [4]. Most of these investigations will also include a section in the limitation paragraph acknowledging the possible contributions of group differences to antipsychotic medications in the SP group. Cross-sectional studies are unable to disentangle medication or treatment effects from the disease process in SP.
Antipsychotic Binding Affinities
The heterogeneity of different antipsychotics with different binding affinities and receptor profiles further clouds the effect of medications in fMRI investigations. D2 receptor antagonism appears to be critical for antipsychotic effect [5, 6]. D2 binding affinity (pharmacological context, as measured by Ki, equilibrium dissociation constant) varies by over three orders of magnitude in currently available antipsychotics [7]. Antipsychotics are “dirty” drugs and also have dopamine, serotonin, acetylcholine, norepinephrine, and histamine receptor affinities [8, 9]. Dose-equivalencies or an-tipsychotic classification strategies (typical versus atypical) run the risk of over-simplifying the heterogeneity of different antipsychotics. In order to elucidate the effect of different pharmacologic profiles, fMRI investigations that analyze SP by comparing different antipsychotic medications will be particularly useful. A recent review of fMRI investigations of medicated SP only included studies that had at least two groups of patients with different treatments (both cross-sectional or two different time points) [10]. Surprisingly, this review concluded that antipsychotics had “no general effect” on the BOLD signal, but antipsychotics that are potent D2 receptor antagonists may reduce the BOLD signal.
Background on the Blood Oxygen Level Dependent (BOLD) Signal
fMRI measures changes in the oxygenated blood flow with the blood oxygen level dependent (BOLD) contrast, a correlate of neuronal activity. The cascade of neural and vascular events challenges the biological interpretation of the BOLD signal. Neurovascular coupling defines the basic relationship between neuronal activity and increased blood flow. With intact neurovascular coupling, an orchestrated procession occurs from neuronal mass action to changes in cerebral blood flow and blood volume resulting in increased oxygenated hemoglobin and resultant increase in the BOLD signal [11, 12]. One weakness of fMRI is the inability to distinguish the pieces of the puzzle that compose the sum total of neuronal mass action: neuromodulatory, excitatory, and inhibitory inputs [12]. Astrocytes also have a role in neurovascular coupling [13]. In response to neuronal activity, astrocytes release potassium, prostaglandins and epoxyeicosatrienoic acids, all of which contribute to vasodilation of nearby arterioles [14, 15]. This systems-level process increases cerebral blood flow and oxygenated hemoglobin that exceeds the cerebral metabolic rate. This increased concentration of diamagnetic oxygenated hemoglobin and decreased field distortions from paramagnetic deoxyhemoglobin produces the increase in the BOLD signal contrast.
Antipsychotics and the BOLD Signal
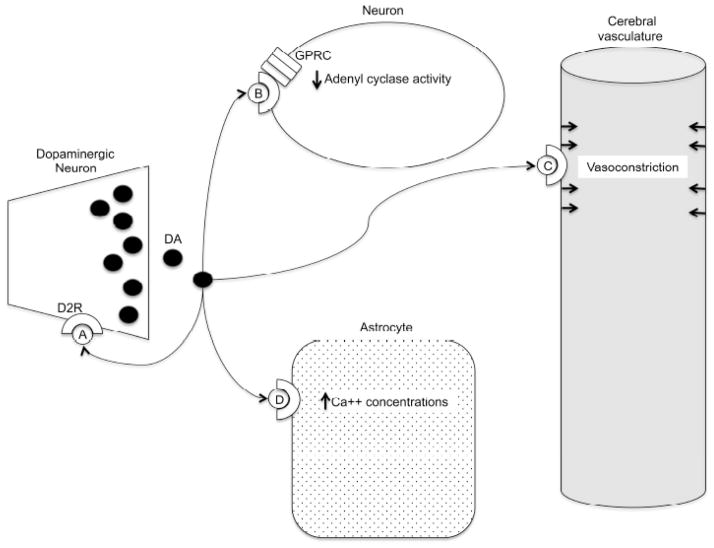
Changes in the BOLD signal observed in SP, with or without antipsychotic treatment, are typically interpreted to reflect alterations in neural activity. The possibility that such changes indicate alterations in the quantity or quality of the cerebral vasculature or neurovascular coupling, however, complicates this simple conclusion. Antipsychotics may affect the BOLD signal by direct modification of neurons, astrocytes, and cerebral blood flow and further compound the complexity of the biological interpretation of the BOLD signal [16, 17]. While recognizing the exceedingly complex receptor pharmacology of antipsychotic medications, we briefly review the variety of effects of D2 antagonism on neurovascular coupling, neuronal activity, and cerebral vascular function as summarized in (Fig. 1). D2 dopamine receptors are located in diffuse cortical and subcortical areas (striatum, nucleus accumbens, olfactory tubercle, ventral tegmental area, hypothalamus, amygdala and hippocampus) [18, 19]. Dopamine slowly modulates the fast GABA or glutamatergic neural transmission via G protein-coupled dopamine receptors (D1–D5) [19]. The D2 “class” of dopamine receptors (D2, D3, and D4) inhibit adenyl cyclase activity [20, 21]. D2 receptors are also expressed presynaptically on dopaminergic neurons and regulate dopamine synthesis and release via a negative feedback mechanism [22]. D2 receptors are located throughout the cerebral vasculature [23]. D2 antagonism reduces dopamine-induced vasoconstriction [23, 24]. The D2 blockade of vasoconstriction would increase cerebral blood flow and the BOLD signal. Lastly, D2 antagonists block a dopamine-induced calcium elevation within astrocytes [17]. The dopamine-mediated increase in astrocytic calcium may indirectly influence neuronal communication by modulating neuronal excitation and synaptic transmission.
Fig. 1.
Dopamine (DA), via the D2 receptor (D2R), A) provides negative feedback through presynaptic autoreceptors, B) inhibits adenyl cyclase activity through G protein coupled receptors (GPRC), C) increases calcium (Ca++) concentration in astrocytes, and D) constricts the cerebral vasculature. Antipsychotics, via the D2 receptor, modulate all of these dopaminergic activities, which may affect the blood oxygen level dependent signal.
Despite the results of Roder’s review, fMRI investigations examining the acute effects of antipsychotics in HC have demonstrated differences in the BOLD signal. fMRI investigations on visual-acoustic [25], reward [26–28], and motor [29] tasks all show reduced activation or diminished negative modulation with D2 antagonism. Dopaminergic antagonism also appears to alter the temporal coherence or functional connectivity in HC [30, 31]. The data suggests that, if D2 antagonists affect the BOLD signal via vascular effects, the BOLD signal would increase. The majority of acute antipsychotic investigations in HC show that D2 antagonism decreases the BOLD signal suggesting that changes may be may be attributable to neural factors. A complete understanding of the mechanistic bases of BOLD signal changes related to disease processes or pharmacotherapeutic interventions will require understanding both neural and neurovascular alterations and these are not likely to be mutually exclusive with respect to their contributions to BOLD signals.
Chronic antipsychotic administration affects dopamine and related signaling in a different ways than acute antipsychotic administration. First, chronic administration of haloperidol or fluphenazine may actually upregulate D2 receptor density and D2 agonist effects [32, 33]. However, this upregulation may be more related to loss of antipsychotic effect and may mediate motor side effects of neuroleptics such as tardive dyskinesia rather than the therapeutic mechanism of the antipsychotic in the treatment of schizophrenia [32, 34]. Second, acute antipsychotic administration increases firing of striatal and nucleus accumbens dopaminergic neurons, whereas chronic administration has been found to lead to decreased firing (depolarization inactivation) in the striatum [35]. The effects on dorsal striatal firing are believed to potentially mediate the motor side effects of antipsychotics like tardive dyskinesia. Preclinical studies indicate that the longer the exposure to D2 antagonism the more the striatal neurons are suppressed due to depolarization inactivation [36]. Third, chronic D2 antagonism protects against NMDA-mediated neurotoxicity, a potential mechanism of the antipsychotic effect [37].
The evidence that antipsychotics improve brain function and reduce symptoms in SP is unmistakable [38, 39], but how antipsychotics change brain function is poorly understood, especially within neuronal systems. Pharmacological magnetic resonance imaging (phMRI), the application of fMRI to drugs, has the potential to increase the translational impact of fMRI investigations in neuropsychiatric diseases. phMRI seeks to elucidate the mechanism of action of drug action and provide quantitative biological markers or “biomarkers” of the changing brain activity related to drug action [16]. Our goal in this review is to explore the potential impact of phMRI as applied to antipsychotics in schizophrenia. We hypothesize that chronic administration of antipsychotics will have a “normalizing” effect on brain function in SP. First, we review case-control, longitudinal fMRI investigations in schizophrenia. We aim to present the commonalities of the studies in the context of symptom improvement and changes in brain function in the context of antipsychotic treatment. Second, we review putative mechanisms from animal studies that support the normalization of the BOLD signal in schizophrenia. We aim to provide several neuronal-based interpretations of these changes of the BOLD signal that may be attributable to long-term antipsychotic administration.
METHODS
We searched PubMed with the following Medical Subject Headings (MeSH): “antipsychotic agents” OR “dopamine antagonists/pharmacology” AND “schizophrenia” AND “magnetic resonance imaging”. We further limited the results to “English” and “Humans”. We found a total of 319 studies up to May 30, 2012. We screened the abstracts to find investigations that met the following criteria: 1) functional magnetic resonance imaging 2) case-control, longitudinal design, 3) included both pre- and post-treatment contrasts with the HC group, and 4) antipsychotic-free or antipsychotic naïve SP at the start of the investigation (first time point or T1).
RESULTS
We identified eight longitudinal investigations published between 1999 and 2011 for a detailed review that met our inclusion criteria (presented in Table 1). The results of these investigations are often discussed in the context of “normalization”; that is, aberrant activation patterns or connectivity between SP and HC at the first imaging assessment (T1) that are no longer apparent at the second imaging assessment (T2). Many of the identified studies also discuss differences at T2 that were not apparent at T1. We operationally define the latter as “denormalization”. We discuss the longitudinal changes within the SP group, and we emphasize the following in the results: 1) within group longitudinal changes associated with treatment intervention; 2) the changes from the SP/HC contrasts at T2 relative to T1; and 3) correlations between changes in BOLD activation and behavioral correlates (symptoms or cognition).
Table 1.
Case-Control, Longitudinal Studies with Antipsychotic Naïve or Antipsychotic-Free SP at the First Imaging Assessment (T1).
| Study | Sample* | fMRI paradigm, analysis methods | Time interval; antipsychotics (mean dose, mg/day) | Results: normalization, denormalization, behavioral correlation# |
|---|---|---|---|---|
| 1. Stephan (2001) | 5 SP, 5 HC |
Task: finger-tapping Analysis: functional connectivity (seed voxel correlations) |
21 days; olanzapine (11.7 mg/day) |
Normalization: reduced right cerebellum functional connectivity Denormalizaiton: increased left cerebellum functional connectivity Behavioral Correlation: not performed |
| 2. Bertolino (2004)** | 17 SP, 17 HC |
Task: visually paced motor task. Analysis: general linear model (region of interest) |
Assessments after 28 and 56 days of treatment; olanzapine (20.3 mg/day) |
Normalization: increased activity in the contralateral primary sensory motor cortex Denormalization: not applicable Behavioral Correlation: no significant correlations between activation patterns and clinical measures |
| 3. Snitz (2005) | 11 SP, 16 HC |
Task: Preparing to Over-come Prepotency task Analysis: general linear model |
31 days: olanzapine (5–10 mg/day), quetiapine (300 mg/day), risperidone (1–4 mg/day) |
Normalization: increased activity in the anterior cingulate during the probe condition (conflict resolution) Denormalization: not applicable Behavioral correlation: disorganized symptoms correlated with dorsal lateral prefrontal cortex and anterior cingulate activity at baseline, but symptom changes and correlations after treatment were not significant |
| 4. Keedy (2009) | 9 SP, 9 HC |
Task: visually guided saccades Analysis: general linear model |
28 to 36 days; haloperidol (mean dose 4.5 mg/day) risperidone (4.2 mg/day), ziprasidone (200 mg/day) |
Normalization: increased activation in frontal eye fields, cerebellum; decreased activation in the superior temporal gyri, intraparietal sulci, ventral medial prefrontal cortex, subgenual anterior cingulate, and posterior right insula Denormalization: reduced activity in the cingulate motor area, caudate, supramarginal gyri, dorsal prefrontal cortex, presupplementary eye fields, and dorsal medial thalamus Behavioral correlation: not performed |
| 5. Blasi (2009)** | 12 SP, 12 HC |
Task: implicit and explicit emotional processing Analysis: general linear model |
Assessments after 28 and 56 days of treatment; olanzapine (16 mg/day) |
Normalization: decreased activation in the left amygdala during implicit and explicit emotional processing; increased activation in the right ventral lateral prefrontal cortex during implicit processing Denormalization: reduced activation in the right ventral lateral prefrontal cortex during explicit emotional processing Behavioral correlation: no significant correlations between neural changes and PANSS at any time points. |
| 6. Sambataro (2010)** | 17 SP, 19 HC |
Task: N-back working memory task. Analysis: Spatial ICA |
Assessments after 28 and 56 days of treatment; olanzapine (20 mg/day) |
Normalization: reduced negative modulation in the default mode network during the working memory task after 56 days of treatment Denormalization: increased default mode connectivity with the ventral medial prefrontal cortex Behavioral correlation: Symptom correlation was not performed. |
| 7. Lui (2010) | 34 SP, 34 HC |
Task: Resting state fMRI. Analysis: 1) ALFF; 2) functional connectivity (seed voxel correlations); and 3) functional network connectivity |
36 days (HC scanned at one time interval); aripiprazole (20 mg/day), clozapine (52.5 mg/day), olanzapine (16.9 mg/day), quetiapine (495 mg/day), risperidone (4.2 mg/day), and sulpiride (800 mg/day) |
Normalization: 1) SP had increased ALFF in the ventral medial cortex at T2; 2) SP had reduced functional network connectivity between fronto-pariental and temporal networks at T2 Denormalization: 1) the caudate and putamen had increased ALFF in SP relative to HC; 2) SP had decreased functional connectivity at T2 in multiple cortical and subcortical seeds; and 3) reduced functional connectivity between two pairs of cortical-subcortical and one cortical-cortical network Behavioral correlations: increased ALFF was associated with reduced positive symptoms (PANSS subscales) |
| 8. van Veelen (2011) | 23 SP, 33 HC |
Task: modified Sternberg working memory task Analysis: general linear model (region of interest) |
70 days; olanzapine (15 mg/day), quetiapine (733 mg/day), risperidone (4 mg/day), and ziprasidone (65 mg/day) |
Normalization and denormalization: no differences between T1 and T2 within SP Behavioral correlation: No symptom correlation performed for T1 and T2; however, SP dorsolateral prefrontal cortex practice effects at T1 were predictive of clinical outcome at T2 |
Sample size reports the number of subjects at the last imaging assessment.
SP were antipsychotic-free during study initiation, but the initial imaging assessment occurred after four weeks of treatment.
Behavioral correlation only includes cognitive or symptom ratings conducted outside of the scanning assessment
The first study to assess changes in functional connectivity related to antipsychotic medication used a finger-tapping task [40]. Four of the six SP were antipsychotic naïve. The remaining two SP had no antipsychotic medications for three weeks prior to the start of this investigation. After the first imaging assessment, SP started treatment with olanzapine with a titration to a mean dose of 11.7 mg/day by the second imaging assessment 21 days later. One SP and one HC had to be excluded for motion artifacts reducing the final sample size to five in each group. Seed-voxel correlations in the right and left cerebellum assessed functional connectivity, a measure of regional temporal synchrony. Longitudinal changes between un-medicated and medicated SP for the right and left cerebellar seeds were mixed but predominately displayed reduced functional connectivity between the cerebellum and the prefrontal cortex and medial dorsal thalamus. After treatment, SP and HC contrasts for the right cerebellum functional connectivity seed was reduced from 25 clusters (12027 voxels) to 18 clusters (4732 voxels). For prefrontal regions, medicated SP showed reduced cerebellar connectivity in 5/6 of the identified regions. In contrast, the left cerebellum functional connectivity showed the opposite pattern after treatment, which increased from 9 clusters (1870 voxels) to 29 clusters (13529 voxels). No clinical ratings or correlations between changes in cerebellum functional connectivity and symptomatology were reported.
Bertolino et al. (2004) assessed state versus trait abnormalities in the motor cortex by comparing 17 SP and 17 HC with a visually paced motor task [41]. Nine of the 17 SP were antipsychotic naïve while the remaining met the inclusion criteria of no oral antipsychotics for four weeks or missing two cycles of a depot antipsychotic. SP were having an acute exacerbation defined as PANSS subscales ≥ 4. SP were treated with olanzapine with titration during the first four weeks of the study (average dose 20.3 mg/day). The imaging assessments occurred after four and eight weeks of treatment. At the initial imaging assessment, SP had less activation in the contralateral motor cortex. After another four weeks of treatment, this reduction normalized and differences between SP and HC were no longer apparent. Paired t-tests confirmed that SP had more activation in the contralateral primary sensory motor cortex after eight weeks relative to four weeks of treatment. SP had significant improvements in PANSS during the course of the study, but clinical measures did not correlate with activation patterns. This investigation also assessed differences in a laterality quotient, which was defined as differences in left and right regions of interest (primary sensory and premotor regions). Unlike the activation patterns noted above, the laterality quotient did not change with treatment suggesting differences in state (activation patterns) and trait (laterality quotient) variables.
Snitz et al. (2005) assessed changes in the dorsolateral prefrontal cortex and anterior cingulate during control and conflict resolution [42]. The Preparing to Overcome Prepotency task requires subjects to respond with a button press after viewing an arrow (probe condition). A green square cue immediately prior to the arrow prompts the subjects to use their ipsilateral hand, and a red cue prompts the subjects to use their contralateral hand (cue condition). The cue condition requires subjects to overcome the prepotent response tendency and activates the dorsal lateral prefrontal cortex while the probe condition activates the anterior cingulate. Two of the eleven SP required a 72-hour antipsychotic wash out prior to the first imaging assessment (lifetime antipsychotic exposure less than two weeks). After the first imaging assessment, SP were then treated with olanzapine (n = 3), risperidone (n = 7), or quetiapine (n = 1). Treatment effects were significant with the anterior cingulate (cue condition) but not the dorsal lateral prefrontal cortex (probe condition). At baseline, conflict related modulation in the anterior cingulate was reduced in SP. After 31 days of antipsychotic treatment, anterior cingulate activity normalized (increased) in SP during the probe condition. At baseline, activity in the dorsal lateral prefrontal cortex was inversely correlated with disorganized symptoms, and activity in the anterior cingulate was inversely correlated with disorganized and poverty symptoms. Changes in symptoms over the 31-day treatment period and symptoms correlations after treatment were not significant.
Keedy et al. (2009) assessed the impact of 28 to 36 days of antipsychotic treatment in 9 SP and 9 matched HC with unpredictable, visually guided saccades that activated the saccade and spatial attention system [43]. One SP was antipsychotic naïve. The remaining SP had a minimum of 6 medication free-days prior to the initial image assessment. SP were treated with risperidone (n = 6), haloperidol (n = 2), or ziprasidone (n = 1). Cortical and cerebellar activity normalized after treatment in SP with increased activity in the bilateral supplementary eye fields, the left frontal eye fields, and cerebellum. Cortical activity also normalized in SP with reduced activity in the superior temporal gyri, intraparietal sulci, ventral medial prefrontal cortex, subgenual anterior cingulate, and posterior right insula. In contrast, cortical and subcortical areas denormalized after treatment with reduced activation in the dorsal lateral prefrontal cortex, presupplementary eye fields, dorsal anterior cingulate, and dorsal medial thalamus. The investigators concluded that antipsychotics may improve control of attention while the aberrant activation patterns evident in the post-treatment imaging assessment may be indicative of the deleterious effects on prefrontal systems associated with antipsychotic treatment.
Blasi et al. (2009) assessed changes in amygdala and prefrontal cortical activity during implicit and explicit emotional processing in SP treated with olanzapine [44]. Implicit (matching angry or afraid faces with a target image) and explicit (labeling a target face with the label of “angry” or “afraid”) emotional processing was assessed after 28 and 56 days of olanzapine treatment. Three of the 12-recruited SP were antipsychotic naïve while the remaining had an extensive medication wash-out (two weeks for oral antipsychotics or two cycles of depot antipsychotic treatment). Olanzapine titration occurred for the first two weeks of the investigation and was then held constant for the remainder of the study (mean dose 16 mg/day). The left amygdala in SP normalized after 56 days of treatment. The right prefrontal cortex also normalized with treatment during implicit processing, but denormalized with explicit processing with a significant reduction in activation. Correlations between symptom changes (PANSS) and changes in the left amygdala and right ventral lateral prefrontal activity were not significant.
In a related olanzapine treatment study, Sambataro and colleagues (2010) used spatial independent component analysis (ICA) to assess changes in functional connectivity in the default mode network during an N-back working memory task [45]. SP started olanzapine treatment during a hospitalization for an acute psychotic exacerbation (no imaging assessment at this time point). Thirteen of the 17-recruited SP were antipsychotic naïve while the remaining had an extensive medication wash-out as described in the Basli (2009) investigation [44]. The mean dose of olanzapine was 20mg/day. Imaging assessments occurred at 28 and 56 days of olanzapine treatment. ICA identifies spatially independent but widely distributed brain regions that share temporally synchronous activity. From 21 independent components, the default mode network was selected for spatial and time course analysis. The ICA spatial analysis compares subject-level spatial maps that reflect differences in functional within network connectivity of an average component time course. SP had increased connectivity within the default mode network at the ventral medial prefrontal cortex during the second imaging assessment (T2, 56 days) relative to the first imaging assessment (T1, 28 days). This increased connectivity for SP at 56 days was also greater than HC at the same time point and, therefore, reflects denormalization. In contrast, the ICA time course analysis compares stimulus-related responses of the default mode network time course. The time (T1 and T2) by diagnosis (SP and HC) interaction revealed smaller differences in negative modulation of the default mode network during the working memory task at T2. These changes were consistent with normalization of the default mode time course with treatment. Working memory performance was only correlated with ventral medial prefrontal cortex connectivity at 28 days of treatment for the SP. Symptom correlations were not performed.
The first resting state fMRI investigation tracked neural changes as 34 antipsychotic naïve, first episode SP were treated with antipsychotic medications over 36-day interval [46]. The antipsychotic medications were heterogeneous and included risperidone, olanzapine, clozapine, quetiapine, sulpiride, and aripiprazole titrated for the first 14 days of this investigation and then held constant for the next 28 days. This investigation used three complimentary analysis methods: amplitude of low frequency fluctuations (ALFF, measures voxel-wise temporal coherence), seed-voxel functional connectivity (measures regional temporal synchrony), and functional network connectivity (measures between network relationships). As SP responded to antipsychotic treatment with a significant reduction in the Positive and Negative Syndrome Scale (PANSS), the ALFF increased in nine cortical and subcortical regions. The changes in ALFF and symptoms were inversely correlated, with stronger relationships noted in cortical relative to subcortical areas. Relative to HC, ALFF normalized in the ventral medial cortex and denormalized in the caudate and putamen. These regions with increased ALFF were then used as seeds for functional connectivity assessment. Reduced functional connectivity correlated with increased ALFF in all seed areas from T1 to T2. The reduced functional connectivity in SP represented denormalization with treatment in the cortical (frontal, parietal, temporal cortices) and subcortical (caudate) regions. Functional network connectivity normalized between the frontal-parietal and temporal networks and denormalized between two pairs of cortical-subcortical networks (precuneus/basal ganglia, occipital/basal ganglia) and one pair cortical-cortical networks (parietal/temporal). The authors concluded that the denormalization of subcortical networks (both ALFF and functional network connectivity) might be related to antipsychotic side effects. The investigators performed a subsequent analysis with only the risperidone SP (n = 12) with similar increases in ALFF and reductions in functional connectivity.
The most recent investigation of antipsychotic-naïve SP examined the impact of antipsychotic treatment on prefrontal lobe dysfunction [47]. The investigators used a modified Sternberg working memory task to assess differences between novel and practice memory sets. The following regions of interest were identified from an independent sample that performed the same task: left fusiform gyrus, left dorsolateral prefrontal cortex, left and right superior parietal cortex, and anterior cingulate. A previous study from these investigators demonstrated that activation within the left dorsolateral prefrontal cortex did not attenuate with practice in antipsychotic naïve SP [3]. After 70 days of treatment with antipsychotic medications, SP continued to have more activation during the practice trails and did not change activation patterns in the dorsolateral prefrontal cortex. SP were divided into responders and non-responders based on 30% reduction in PANSS. Responders were no different from non-responders at each time point. The investigators did not compare symptom correlations between T1 and T2. However, practice effects during the T1 baseline scan were predictive of clinical response at T2. Eventual antipsychotic non-responders had a reduced practice effect (difference in activation between the novel and practiced memory sets) within the dorsolateral prefrontal cortex during the baseline scan.
DISCUSSION
This review summarizes the results of eight longitudinal fMRI investigations, which included antipsychotic-naïve or antipsychotic-free SP at the first imaging assessment. Seven of the eight investigations demonstrated normalization of the BOLD signal with antipsychotic treatment reflecting reversal of the aberrant fMRI signal evident at T1 and possible therapeutic benefit of antipsychotic treatment [40–47]. In contrast, five of the eight investigations had concurrent denormalization of different cortical and subcortical regions [40, 43–46]. Behavioral correlates (symptoms or cognition) were intermittently included among the eight investigations. Only one study found a relationship between symptom change and an fMRI correlate [46]. We review the patterns identified as a function of analysis method and discuss possible neural mechanisms that may lead to normalization of the fMRI signal as SP respond favorably to antipsychotic treatment.
The average length of the treatment interval between study initiation and the final imaging assessments (45 days, range: 21 to 70 days) was sufficient to detect treatment effects from antipsychotic medications. Previous research has shown that the majority of symptom improvement occurs within the first week of treatment with a more gradual improvement observed in subsequent weeks [48, 49]. Most of the investigations included SP that were in the midst of an acute psychotic exacerbation at T1 with an average total PANSS rating of 91.3 at study initiation (range: 73.5 to 105.4). To ensure comparable task performance between SP and HC, three investigations initiated standardized treatment with olanzapine prior to the first imaging assessment [41, 44, 45]. After 28 days of treatment, symptoms were reduced and the SP successfully completed the first fMRI assessment (visually paced motor task, implicit and explicit emotional processing, and an N-back working memory task). Subsequently, these investigations had a smaller change in PANSS ratings during the delayed scan interval (28 to 56 days after treatment was initiated) possibly obscuring any relationship between psychotic symptoms and fMRI imaging correlates. Resting state fMRI has the potential to expand the generalizability of imaging investigations to the most seriously impaired SP circumventing the need to delay the initial imaging assessment [50]. In the only resting state fMRI investigation included in this review, SP had a marked reduction in PANSS scores from 104.2 at T1 to 70.0 at T2 [46]. This was also the only investigation that had a significant relationship between fMRI signal (increased ALFF) and symptom reduction over the treatment interval.
SP were treated with a variety of different antipsychotics in the included studies. Four of the eight investigations used olanzapine monotherapy [40, 41, 44, 45]. The remaining studies used clinical judgment resulting in a mixture of different antipsychotics with markedly different D2 binding affinities from clozapine (Ki = 63) [46] to haloperidol (Ki = 0.55) [43]. D2 binding affinities may be associated with diminished BOLD signal activity in cross-sectional or longitudinal investigations comparing different types of antipsychotics [10]. These results, when applied to the included studies, suggest that normalization of the BOLD signal would only occur when antipsychotic-naïve or antipsychotic-free SP had increased BOLD activity at T1 relative to HC. Antipsychotic treatment would then decrease and normalize the BOLD signal. Although the included investigations do not permit comparisons of different types or binding affinities of antipsychotics, our results do not appear to entirely support this framework. Several investigations included risperidone (Ki = 1.1) and reported normalization of BOLD activity via increased activity in the anterior cingulate [42] as well as the frontal eye fields and cerebellum [43]. Furthermore, Lui et al. (2010) compared the results of subset of SP treated with risperidone monotherapy relative to the more heterogeneous sample included in the original study [46]. The results of the additional analysis were similar to the original investigation (increased ALFF, decreased functional connectivity) suggesting that these observed changes may be more consistent with general antipsychotic effects associated with treatment response as opposed to a specific type of antipsychotic. We conclude that the normalization of the BOLD signal (both increased and decreased BOLD signal) may be a general biological marker of antipsychotic efficacy and treatment response in SP. Future research comparing changes in BOLD signal to different types of D2 binding affinities are needed to disentangle the effect of D2 potency on the BOLD signal during treatment response.
Longitudinal changes in the BOLD within SP over the treatment interval may reflect plasticity of specific brain regions with respect to treatment effects [42]. The included studies have different tasks and analysis methods with dissimilar regions of response. Instead of anatomic regions, we focus on the generalized pattern of normalization with respect to analysis method. With respect to the general linear model, normalization resulted in both increased [42–44] and decreased activation [43, 44]. Seed-voxel correlations had mixed results with both increased and decreased connectivity with treatment [40, 46]. The larger of the two studies showed reduced functional connectivity with increased temporal coherence (ALFF) with seven of nine different seeds suggesting a general pattern of response to antipsychotic treatment [46]. ICA is a multivariate analysis technique that simultaneously analyzes information from multiple different regions or components. Furthermore, several different ICA-variables can be used to measure within network changes (spatial maps and component time courses) as well as between network relationship (functional network connectivity). The ICA default mode network time course during a working memory task normalized with treatment [45] and between network connectivity in the frontal/parietal networks normalized with treatment [46]. Each analysis method also identified striatal, motor and attention networks that denormalized with treatment. Investigators suggested that denormalization after antipsychotic treatment represents the adverse effect of antipsychotic medications leading to medication side effects [43, 46]. Therefore, the results of the included investigations may mirror clinical practice: antipsychotics ameliorate psychotic symptoms while normalizing the fMRI BOLD signal while potentially causing side effects (motor or cognitive) and de-normalizing the BOLD signal.
We are unable to completely exclude the possibility of changes in cerebral blood flow or cerebral vascular reactivity with normalization or denormalizaiton of the BOLD signal associated with antipsychotic treatment. Iannetti and Wise (2007) recommended several measures to limit potential confounds downstream of neuronal activation in phMRI investigations: concurrent inclusion of control tasks, recording physiological variables, and measuring baseline brain perfusion or vascular reactivity [51]. Other cross-sectional and longitudinal investigations that did not meet our inclusion criteria assessed the impact of dopamine on the BOLD signal included a separate region of interest not modulated by dopaminergic drugs [27, 52], breath-holding task as a hypercapnic challenge [28], and arterial spin labeling to measure cerebral blood flow [53]. None of the included investigations used these additional measurements thus limiting our conclusions about neuronal changes solely producing these BOLD signal changes.
Non-human animal investigations provide neural interpretation of antipsychotic-induced BOLD signal change
Putative neural mechanisms for changes in BOLD signals are difficult to disentangle from confounding variables in humans; however, the effects of chronic exposure to antipsychotic medications on neuron morphology and function have been extensively studied in non-human animals. By way of discussion of some constituents of this literature we hope to achieve two goals: 1) emphasize that there are conspicuous and reliable effects of chronic antipsychotic drugs on neural function and morphology at several levels, and 2) highlight the available data relevant to the issue of normalization or denormalization of neural function and morphology by antipsychotic exposure.
Although there are numerous levels of analysis upon which this discussion could focus, for the purposes of meeting the goals outlined above, we place emphasis on measures of neural activity, structural (dendritic morphology and spine density) and ultrastructural (synaptic density) measures. The rationale for the latter is based on the fact that neuron morphology has clear functional implications, represents a persistent change related to treatment and can be measured in SP post-mortem. For the present purposes, we focus discussion to only those studies that evaluated chronic exposures with established antipsychotic drugs but include acute effects for context where possible. In most of the studies cited here, animals were exposed to antipsychotic drugs or appropriate control substance for 14 to 30 days.
The effects of acute or chronic antipsychotic exposure on the firing rates and activity of individual neurons have been examined most thoroughly in the substantia nigra pars compacta (SNC) and the ventral tegmental area (VTA) (cell groups A9 and A10, respectively). Acute exposure to olanzapine or clozapine increases the number of spontaneously active dopaminergic neurons in A10 and reverses the acute inhibition of these neurons induced by d-amphetamine [54]. Todorova and Dimpfel [55] found that chronic haloperidol and clozapine exposure reduced the number of spontaneously active dopaminergic neurons in A10, while reductions in the number of spontaneously active dopaminergic neurons in A9 were observed only for haloperidol (see also ref. [56]). This basic pattern of reductions in the number of spontaneously active dopaminergic neurons in VTA, but not SNC, has been observed following chronic exposure to clozapine [55, 57], sertindole [57], and olanzapine [58], as well as other putative antipsychotic agents [59]. In cortical targets of VTA neurons (e.g., frontal cortex) chronic haloperidol increases excitatory signaling, while clozapine reduces basal levels of glutamate [60] and exposure to olanzapine reduces firing rates [61]. This can be contrasted with the increases in pre-frontal cortical activity associated with acute clozapine exposure [62]. Olanzapine’s acute effects on prefrontal neurons measured using ensemble recordings are, however, minimal and appear to be detectable only after chronic exposure [61].
In addition to measures of individual neurons and their firing properties following chronic antipsychotic exposure, the use of other markers of neural activity has been applied to identifying the regional distribution of alterations associated with blockade of D2 receptors. Immediate early genes (IEGs) are expressed when neurons are activated. Thus, measurement of IEG expression in specific neural populations can serve as a marker of neural activity at multiple spatial scales (from neurons to systems). Typically IEG expression protocols include an activation procedure (e.g., experience) to elicit IEG expression. The brain is extracted at the appropriate time-point after experience and processed via in situ hybridization, immunohistochemistry or polymerase chain reaction (PCR) for quantification of the IEG mRNA or protein products of interest. Measurement of experience-related IEG expression has proven useful in identifying brain regions recruited by various forms of experience [63–65] or exposure to drugs [66]. One feature of IEG measurement that is useful for this purpose is the distinct temporal profile of expression that different IEGs possess. This feature has been utilized to identify neural populations that are engaged immediately and/or persistently following experience or drug exposure.
Here, we briefly review studies that have quantified IEG mRNA or protein expression following chronic antipsychotic exposure for short-lived and long-lasting alterations in activity among candidate neural populations, primarily those comprising the mesolimbic, mesocortical and nigrostriatal DA systems. The expression of Fos and related gene products are among the most commonly measured markers used to examine neural populations recruited by experience or drug exposure. c-Fos (mRNA or protein) levels are increased in recently active neurons and it is often selected as the assay of choice for such measurement because of its sensitivity to a broad range of eliciting experiences compared to other IEGs. Measurement of FosB or c-Fos has been successfully employed to distinguish the effects of typical and atypical antipsychotics. Acute exposure to clozapine or olanzapine leads to increased expression of FosB or c-Fos in retrosplenial cortex [67], locus coeruleus [68, 69], PFC [69–73], and striatum [73–75] (but see refs. [72, 76]). In contrast, acute exposure to haloperidol leads to similar increases in expression in striatum [72, 74–76] but does not lead to increased Fos expression in neocortical regions [67, 70, 71]. Similar findings have been reported in striatum for other IEGs including activity regulated cytoskeletal protein (Arc) mRNA [77, 78], zif268 [76], and Homer1a [79]. One exception is the observation that olanzapine, but not haloperidol, results in decreased Arc expression in PFC and the related observation that olanzapine reduces the enhancement of Arc expression in PFC normally elicited by PCP [78]. Arc expression is critically involved in synaptic plasticity [80] and one possible explanation is that Arc expression might not provide a selective measure of reduced neural activity, but may be reduced due to other functional properties of this particular IEG. Further, local drug infusions may yield distinct patterns of IEG expression because behavioral states associated with systemic administration that would also influence IEG expression are not present [72]. For this reason, and because we wish to relate the current discussion to SP where drugs are administered systemically, we will focus on studies that utilized systemic administration.
Chronic exposure to antipsychotics leads to a similar pattern of Fos expression and related gene products as those observed with acute exposure. Chronic exposure to clozapine results in increased FosB expression in striatum [81], and chronic clozapine or olanzapine exposure increases c-Fos immunoreactivity in PFC [82]. In contrast, chronic haloperidol exposure decreased c-Fos immunore-activity in PFC [82]. Delta FosB is an IEG product whose expression remains elevated for a much longer duration (days to weeks) compared to other proteins, making its measurement particularly useful for identifying regions that show long-term alterations in relation to experience or drug exposure. Chronic exposure to clozapine increases Delta FosB expression in PFC and striatum, whereas chronic haloperidol exposure only resulted in increased expression in striatum [83].
The available data indicate that chronic exposure to clozapine or olanzapine reduces the activity of A10 and prefrontal neurons, while haloperidol increases prefrontal cortical activity and additionally alters activity in A9 neurons. The increased expression of Fos-related gene products in the same regions where reductions of neural activity are observed following chronic exposure to olanzapine or clozapine may reflect compensatory effects given the direction of the effects, however, it is important to emphasize two points: 1) the electrophysiological and IEG expression data consistently implicate the same brain regions; and 2) both sets of observations implicate alterations in neural activity associated with chronic exposure, which, because of the signals by which they are quantified, are not easily explained purely on the basis of vascular modifications. Future research will be required to directly address the relationship between the IEG expression and neural activation effects of antipsychotics.
The long-term functional consequences of disease processes and chronic drug exposure should be reflected in ultrastructural and structural modifications in neurons. Alterations in dendritic morphology and spine density are among the primary mechanisms by which experience alters the brain in the service of future behavior [84–86]. Such modifications reflect changes in total synaptic space and connections [86, 87]. Because dendritic spines are the primary cites of excitatory synapses, they also indicate possible changes in neural activity elicited via axodendritic connections. Exposure to drugs and other forms of experience induce persistent modifications in dendritic morphology and spine density [86, 88–91], that can be linked to specific behavioral, cognitive, and functional consequences [65, 86, 92]. Dendritic morphology and spine density are also thought to be related to functional consequences of neurological disorders [93]. For example, postmortem analyses of dendritic morphology and spine density in SP have consistently revealed decreases in cortical spine density, spine size, dendritic morphology and related measures [94–96], however, such effects are difficult to establish given the potential chronic effects of exposure to antipsychotic medications in SP on these measures.
Studies investigating the effects of chronic drug exposure on dendritic morphology and spine density in non-human animals are critical for distinguishing the long-term effects of drugs and disease processes. Chronic (1–12 months) exposure to the typical antipsychotic haloperidol in the adult rat has consistently been shown to reduce spine density but not spine size [97] in medium spiny neurons (MSNs) of the striatum [97–100]. These effects are persistent, but eventually subside following cessation [99]. A recent study by Frost and colleagues [101] found that chronic exposure to haloperidol or olanzapine during neonatal brain development in the rat primarily reduced measures of dendritic morphology and spine density in the NAc core, medial and orbital PFC and parietal cortex. DA depletion resulting from 6-OHDA lesions also reduces spine density in the medial PFC [102]. These findings can be contrasted with the increases in medial PFC and NAc spine density, but not parietal cortex, observed by following chronic stimulant [91, 103, 104] or PCP exposure [105], the latter of which represents one of the major approaches to modeling schizophrenia symptoms in rodents.
The numbers of asymmetric synapses (AS) and symmetric synapses (SS), which reflect classic excitatory and inhibitory connections, respectively, are also decreased in striatum following haloperidol [98, 99]. In contrast to the effects of haloperidol, chronic exposure to olanzapine does not affect striatal spine density, AS or SS [98], which may be related to reductions in extrapyramidal side effects. Vincent et al. [106] found that AS were decreased and SS were increased in the prefrontal cortex following chronic clozapine or haloperidol. Interestingly, PCP exposure has also been shown to reduce AS in the prefrontal cortex [107, 108], but despite the similarity to the effects of olanzapine, acute or chronic olanzapine exposure is effective at reversing the effect of PCP on AS [108]. Although dopamine depletion is used as a model of Parkinsonism rather than schizophrenia and yields similar effects on spine density in the prefrontal cortex to those observed following antipsychotic exposure, the effects of dopamine depletion are completely reversed by chronic olanzapine but not haloperidol exposure [102]. Collectively, these outcomes suggest that there are persistent effects of chronic antipsychotics on spine density and related ultrastructural measures, and the effects of alterations achieved by other pharmacological manipulations or treatments, such as those used to model disorders of the dopaminergic systems, can be normalized by olanzapine.
Overall, the available structural and ultrastructural consequences of chronic D2 blockade with antipsychotics are reliable and occur primarily in brain regions comprising the mesolimbic, mesocortical and nigrostriatal dopamine systems. As with the earlier treatment of basic activity measures, these changes are clear indicators of persistent alterations in neurons that should result in clear functional consequences not easily explained purely on the basis of vascular effects. The relative contributions of drug- and disease-related alterations on neural activity, vasculature and neurovascular coupling and their relationship to BOLD signal changes will require much research to elucidate. Studies that include measures in each domain will be most useful because drawing conclusions from independent observations of each variable using different exposure paradigms and drugs, or different approaches to modeling schizophrenia symptoms, will not be able to precisely establish the relationship between the variables of interest. For example, chronic exposure to olanzapine results in persistent increases in angiogenesis and vascular endothelial growth factor [109]. How such an observation can predict the patterns of activity or structural modifications outlined here and relate them to BOLD signal changes is not clear, however, studies that combine these measures could elucidate these relationships and examine the mechanistic bases of these relationships.
CONCLUSION
Antipsychotic medications clearly ameloriate the psychopathology associated with schizophrenia. Despite different analysis methods and diverse antipsychotics, the results of this review also suggest that specific neural regions normalize over the course of antipsychotic treatment. Although less frequently seen, this review also suggests that other regions denormalize with antipsychotics. Chronic antipsychotic administration may improve symptoms and affect BOLD signal by altering neuronal gene expression and neuronal function at the structural and ultrastructural level. Future studies could clarify the importance of D2 binding affinities in the effect of antipsychotics on BOLD signal, and could explore to what degree these changes (normalization and denormalization) mediate the therapeutic effects and psychiatric or neurocognitive side effect profiles of these medications. Normalization of the BOLD signal may prove to be an important biomarker of treatment response in schizophrenia.
Acknowledgments
NIH Centers of Biomedical Research Excellence. “Neural Mechanism of Schizophrenia: Use of Multiple Neuroimaging Tools to Examine Dysfunctions in Neural Integration” (1 P20 RR021938-01A1)
LIST OF ABBREVIATIONS
- fMRI
Functional magnetic resonance imaging
- phMRI
Pharmaceutical magnetic resonance imaging
- SP
Patients with schizophrenia
- HC
Healthy comparison subjects
- BOLD
Blood oxygen level dependent signal
- D2
Dopamine 2 receptor
- T1
First time point
- T2
Second time point
- ALFF
Amplitude of low frequency fluctuations
- ICA
Independent component analysis
- PANSS
Positive and Negative Syndrome Scale
- BA
Brodmann’s areas
- SNC
Substantia nigra pars compacta
- VTA
Ventral tegmental area
- IEG
Immediate early genes
- PCR
Polymerase chain reaction
- AS
Asymmetric synapses
- SS
Symmetric synapses
Footnotes
CONFLICT OF INTEREST
The author(s) confirm that this article content has no conflicts of interest.
References
- 1.Tamminga CA, Thomas BP, Chin R, Mihalakos P, Youens K, Wagner AD, Preston AR. Hippocampal novelty activations in schizophrenia: Disease and medication effects. Schizophrenia research. 2012;138(2–3):157–163. doi: 10.1016/j.schres.2012.03.019. [DOI] [PubMed] [Google Scholar]
- 2.Hofer A, Weiss EM, Golaszewski SM, Siedentopf CM, Brinkhoff C, Kremser C, Felber S, Fleischhacker WW. Neural correlates of episodic encoding and recognition of words in unmedicated patients during an acute episode of schizophrenia: a functional MRI study. The American journal of psychiatry. 2003;160(10):1802–1808. doi: 10.1176/appi.ajp.160.10.1802. [DOI] [PubMed] [Google Scholar]
- 3.van Veelen NM, Vink M, Ramsey NF, Kahn RS. Left dorsolateral prefrontal cortex dysfunction in medication-naive schizophrenia. Schizophrenia research. 2010;123(1):22–29. doi: 10.1016/j.schres.2010.07.004. [DOI] [PubMed] [Google Scholar]
- 4.Abbott C, Juarez M, White T, Gollub RL, Pearlson GD, Bustillo J, Lauriello J, Ho B, Bockholt HJ, Clark VP, Magnotta V, Calhoun VD. Antipsychotic dose and diminished neural modulation: A multi-site fMRI study. Progress in neuropsychopharmacology & biological psychiatry. 2010 doi: 10.1016/j.pnpbp.2010.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Strange PG. Antipsychotic drugs: importance of dopamine receptors for mechanisms of therapeutic actions and side effects. Pharmacological reviews. 2001;53(1):119–133. [PubMed] [Google Scholar]
- 6.Gardner DM, Baldessarini RJ, Waraich P. Modern antipsychotic drugs: a critical overview. CMAJ. 2005;172(13):1703–1711. doi: 10.1503/cmaj.1041064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Seeman P. Atypical antipsychotics: mechanism of action. Can J Psychiatry. 2002;47(1):27–38. [PubMed] [Google Scholar]
- 8.Richelson E. Receptor pharmacology of neuroleptics: relation to clinical effects. The Journal of clinical psychiatry. 1999;60(Suppl 10):5–14. [PubMed] [Google Scholar]
- 9.Roth BL, Sheffler DJ, Kroeze WK. Magic shotguns versus magic bullets: selectively non-selective drugs for mood disorders and schizophrenia. Nature reviews. Drug discovery. 2004;3(4):353–359. doi: 10.1038/nrd1346. [DOI] [PubMed] [Google Scholar]
- 10.Roder CH, Hoogendam JM, van der Veen FM. FMRI, antipsychotics and schizophrenia. Influence of different antipsychotics on BOLD-signal. Curr Pharm Des. 2010;16(18):2012–2025. doi: 10.2174/138161210791293088. [DOI] [PubMed] [Google Scholar]
- 11.Logothetis N, Pauls J, Augath M, Trinath T, Oeltermann A. Nature. 2001;412:150–157. doi: 10.1038/35084005. [DOI] [PubMed] [Google Scholar]
- 12.Logothetis NK. What we can do and what we cannot do with fMRI. Nature. 2008;453(7197):869–878. doi: 10.1038/nature06976. [DOI] [PubMed] [Google Scholar]
- 13.Attwell D, Buchan AM, Charpak S, Lauritzen M, Macvicar BA, Newman EA. Glial and neuronal control of brain blood flow. Nature. 2010;468(7321):232–243. doi: 10.1038/nature09613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Filosa JA, Bonev AD, Straub SV, Meredith AL, Wilkerson MK, Aldrich RW, Nelson MT. Local potassium signaling couples neuronal activity to vasodilation in the brain. Nature neuroscience. 2006;9(11):1397–1403. doi: 10.1038/nn1779. [DOI] [PubMed] [Google Scholar]
- 15.Gordon GR, Choi HB, Rungta RL, Ellis-Davies GC, MacVicar BA. Brain metabolism dictates the polarity of astrocyte control over arterioles. Nature. 2008;456(7223):745–749. doi: 10.1038/nature07525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wise RG, Tracey I. The role of fMRI in drug discovery. J Magn Reson Imaging. 2006;23(6):862–876. doi: 10.1002/jmri.20584. [DOI] [PubMed] [Google Scholar]
- 17.Khan ZU, Koulen P, Rubinstein M, Grandy DK, Goldman-Rakic PS. An astroglia-linked dopamine D2-receptor action in prefrontal cortex. Proceedings of the National Academy of Sciences of the United States of America. 2001;98(4):1964–1969. doi: 10.1073/pnas.98.4.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Missale C, Nash SR, Robinson SW, Jaber M, Caron MG. Dopamine receptors: from structure to function. Physiological reviews. 1998;78(1):189–225. doi: 10.1152/physrev.1998.78.1.189. [DOI] [PubMed] [Google Scholar]
- 19.Beaulieu JM, Gainetdinov RR. The physiology, signaling, and pharmacology of dopamine receptors. Pharmacological reviews. 2011;63(1):182–217. doi: 10.1124/pr.110.002642. [DOI] [PubMed] [Google Scholar]
- 20.Spano PF, Govoni S, Trabucchi M. Studies on the pharmacological properties of dopamine receptors in various areas of the central nervous system. Advances in biochemical psychopharmacology. 1978;19:155–165. [PubMed] [Google Scholar]
- 21.Kebabian JW, Calne DB. Multiple receptors for dopamine. Nature. 1979;277(5692):93–96. doi: 10.1038/277093a0. [DOI] [PubMed] [Google Scholar]
- 22.Wolf ME, Roth RH. Autoreceptor regulation of dopamine synthesis. Annals of the New York Academy of Sciences. 1990;604:323–343. doi: 10.1111/j.1749-6632.1990.tb32003.x. [DOI] [PubMed] [Google Scholar]
- 23.Amenta F, Ricci A, Vega JA. Autoradiographic localization of dopamine receptors in rat cerebral blood vessels. Eur J Pharmacol. 1991;192(1):123–132. doi: 10.1016/0014-2999(91)90078-5. [DOI] [PubMed] [Google Scholar]
- 24.Krimer LS, Muly EC, 3rd, Williams GV, Goldman-Rakic PS. Dopaminergic regulation of cerebral cortical microcirculation. Nature neuroscience. 1998;1(4):286–289. doi: 10.1038/1099. [DOI] [PubMed] [Google Scholar]
- 25.Brassen S, Tost H, Hoehn F, Weber-Fahr W, Klein S, Braus DF. Haloperidol challenge in healthy male humans: a functional magnetic resonance imaging study. Neurosci Lett. 2003;340(3):193–196. doi: 10.1016/s0304-3940(03)00104-6. [DOI] [PubMed] [Google Scholar]
- 26.Pessiglione M, Seymour B, Flandin G, Dolan RJ, Frith CD. Dopamine-dependent prediction errors underpin reward-seeking behaviour in humans. Nature. 2006;442(7106):1042–1045. doi: 10.1038/nature05051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Menon M, Jensen J, Vitcu I, Graff-Guerrero A, Crawley A, Smith MA, Kapur S. Temporal difference modeling of the blood-oxygen level dependent response during aversive conditioning in humans: effects of dopaminergic modulation. Biological psychiatry. 2007;62(7):765–772. doi: 10.1016/j.biopsych.2006.10.020. [DOI] [PubMed] [Google Scholar]
- 28.Abler B, Erk S, Walter H. Human reward system activation is modulated by a single dose of olanzapine in healthy subjects in an event-related, double-blind, placebo-controlled fMRI study. Psychopharmacology. 2007;191(3):823–833. doi: 10.1007/s00213-006-0690-y. [DOI] [PubMed] [Google Scholar]
- 29.Tost H, Meyer-Lindenberg A, Klein S, Schmitt A, Hohn F, Tenckhoff A, Ruf M, Ende G, Rietschel M, Henn FA, Braus DF. D2 antidopaminergic modulation of frontal lobe function in healthy human subjects. Biological psychiatry. 2006;60(11):1196–1205. doi: 10.1016/j.biopsych.2006.04.014. [DOI] [PubMed] [Google Scholar]
- 30.Tost H, Braus DF, Hakimi S, Ruf M, Vollmert C, Hohn F, Meyer-Lindenberg A. Acute D2 receptor blockade induces rapid, reversible remodeling in human cortical-striatal circuits. Nature neuroscience. 2010;13(8):920–922. doi: 10.1038/nn.2572. [DOI] [PubMed] [Google Scholar]
- 31.Honey GD, Suckling J, Zelaya F, Long C, Routledge C, Jackson S, Ng V, Fletcher PC, Williams SC, Brown J, Bullmore ET. Dopaminergic drug effects on physiological connectivity in a human cortico-striato-thalamic system. Brain. 2003;126(Pt 8):1767–1781. doi: 10.1093/brain/awg184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Boyson SJ, McGonigle P, Luthin GR, Wolfe BB, Molinoff PB. Effects of chronic administration of neuroleptic and anticholinergic agents on densities of D2 dopamine and muscarinic cholinergic receptors in rat striatum. J Pharmacol Exp Ther. 1988;244(3):987–993. [PubMed] [Google Scholar]
- 33.Wolffgramm J, Rommelspacher H, Buck E. Ethanol reduces tolerance, sensitization, and up-regulation of D2-receptors after subchronic haloperidol. Pharmacol Biochem Behav. 1990;36(4):907–914. doi: 10.1016/0091-3057(90)90099-4. [DOI] [PubMed] [Google Scholar]
- 34.Samaha AN, Seeman P, Stewart J, Rajabi H, Kapur S. “Breakthrough” dopamine supersensitivity during ongoing antipsychotic treatment leads to treatment failure over time. J Neurosci. 2007;27(11):2979–2986. doi: 10.1523/JNEUROSCI.5416-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chiodo LA, Bunney BS. Typical and atypical neuroleptics: differential effects of chronic administration on the activity of A9 and A10 midbrain dopaminergic neurons. J Neurosci. 1983;3(8):1607–1619. doi: 10.1523/JNEUROSCI.03-08-01607.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Valenti O, Cifelli P, Gill KM, Grace AA. Antipsychotic drugs rapidly induce dopamine neuron depolarization block in a developmental rat model of schizophrenia. J Neurosci. 2011;31(34):12330–12338. doi: 10.1523/JNEUROSCI.2808-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Farber NB, Nemmers B, Noguchi KK. Acute D2/D3 dopaminergic agonism but chronic D2/D3 antagonism prevents NMDA antagonist neurotoxicity. Biological psychiatry. 2006;60(6):630–638. doi: 10.1016/j.biopsych.2006.02.019. [DOI] [PubMed] [Google Scholar]
- 38.Tandon R. Antipsychotics in the treatment of schizophrenia: an overview. The Journal of clinical psychiatry. 2011;72(Suppl 1):4–8. doi: 10.4088/JCP.10075su1.01. [DOI] [PubMed] [Google Scholar]
- 39.Leucht S, Tardy M, Komossa K, Heres S, Kissling W, Davis JM. Maintenance treatment with antipsychotic drugs for schizophrenia. Cochrane database of systematic reviews (Online) 2012;5:CD008016. doi: 10.1002/14651858.CD008016.pub2. [DOI] [PubMed] [Google Scholar]
- 40.Stephan KE, Magnotta VA, White T, Arndt S, Flaum M, O’Leary DS, Andreasen NC. Effects of olanzapine on cerebellar functional connectivity in schizophrenia measured by fMRI during a simple motor task. Psychological medicine. 2001;31(6):1065–1078. doi: 10.1017/s0033291701004330. [DOI] [PubMed] [Google Scholar]
- 41.Bertolino A, Blasi G, Caforio G, Latorre V, De Candia M, Rubino V, Callicott JH, Mattay VS, Bellomo A, Scarabino T, Weinberger DR, Nardini M. Functional lateralization of the sensorimotor cortex in patients with schizophrenia: effects of treatment with olanzapine. Biological psychiatry. 2004;56(3):190–197. doi: 10.1016/j.biopsych.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 42.Snitz BE, MacDonald A, 3rd, Cohen JD, Cho RY, Becker T, Carter CS. Lateral and medial hypofrontality in first-episode schizophrenia: functional activity in a medication-naive state and effects of short-term atypical antipsychotic treatment. The American journal of psychiatry. 2005;162(12):2322–2329. doi: 10.1176/appi.ajp.162.12.2322. [DOI] [PubMed] [Google Scholar]
- 43.Keedy SK, Rosen C, Khine T, Rajarethinam R, Janicak PG, Sweeney JA. An fMRI study of visual attention and sensorimotor function before and after antipsychotic treatment in first-episode schizophrenia. Psychiatry research. 2009;172(1):16–23. doi: 10.1016/j.pscychresns.2008.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Blasi G, Popolizio T, Taurisano P, Caforio G, Romano R, Di Giorgio A, Sambataro F, Rubino V, Latorre V, Lo Bianco L, Fazio L, Nardini M, Weinberger DR, Bertolino A. Changes in prefrontal and amygdala activity during olanzapine treatment in schizophrenia. Psychiatry research. 2009;173(1):31–38. doi: 10.1016/j.pscychresns.2008.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sambataro F, Blasi G, Fazio L, Caforio G, Taurisano P, Romano R, Di Giorgio A, Gelao B, Lo Bianco L, Papazacharias A, Popolizio T, Nardini M, Bertolino A. Treatment with olanzapine is associated with modulation of the default mode network in patients with Schizophrenia. Neuropsychopharmacology. 2010;35(4):904–912. doi: 10.1038/npp.2009.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lui S, Li T, Deng W, Jiang L, Wu Q, Tang H, Yue Q, Huang X, Chan RC, Collier DA, Meda SA, Pearlson G, Mechelli A, Sweeney JA, Gong Q. Short-term effects of antipsychotic treatment on cerebral function in drug-naive first-episode schizophrenia revealed by “resting state” functional magnetic resonance imaging. Archives of general psychiatry. 2010;67(8):783–792. doi: 10.1001/archgenpsychiatry.2010.84. [DOI] [PubMed] [Google Scholar]
- 47.van Veelen NM, Vink M, Ramsey NF, van Buuren M, Hoogendam JM, Kahn RS. Prefrontal lobe dysfunction predicts treatment response in medication-naive first-episode schizophrenia. Schizophrenia research. 2011;129(2–3):156–162. doi: 10.1016/j.schres.2011.03.026. [DOI] [PubMed] [Google Scholar]
- 48.Agid O, Kapur S, Arenovich T, Zipursky RB. Delayed-onset hypothesis of antipsychotic action: a hypothesis tested and rejected. Archives of general psychiatry. 2003;60(12):1228–1235. doi: 10.1001/archpsyc.60.12.1228. [DOI] [PubMed] [Google Scholar]
- 49.Leucht S, Busch R, Hamann J, Kissling W, Kane JM. Early-onset hypothesis of antipsychotic drug action: a hypothesis tested, confirmed and extended. Biological psychiatry. 2005;57(12):1543–1549. doi: 10.1016/j.biopsych.2005.02.023. [DOI] [PubMed] [Google Scholar]
- 50.Greicius M. Resting-state functional connectivity in neuropsychiatric disorders. Current opinion in neurology. 2008;21(4):424–430. doi: 10.1097/WCO.0b013e328306f2c5. [DOI] [PubMed] [Google Scholar]
- 51.Iannetti GD, Wise RG. BOLD functional MRI in disease and pharmacological studies: room for improvement? Magn Reson Imaging. 2007;25(6):978–988. doi: 10.1016/j.mri.2007.03.018. [DOI] [PubMed] [Google Scholar]
- 52.Schlagenhauf F, Wustenberg T, Schmack K, Dinges M, Wrase J, Koslowski M, Kienast T, Bauer M, Gallinat J, Juckel G, Heinz A. Switching schizophrenia patients from typical neuroleptics to olanzapine: effects on BOLD response during attention and working memory. Eur Neuropsychopharmacol. 2008;18(8):589–599. doi: 10.1016/j.euroneuro.2008.04.013. [DOI] [PubMed] [Google Scholar]
- 53.Fernandez-Seara MA, Aznarez-Sanado M, Mengual E, Irigoyen J, Heukamp F, Pastor MA. Effects on resting cerebral blood flow and functional connectivity induced by metoclopramide: a perfusion MRI study in healthy volunteers. British journal of pharmacology. 2011;163(8):1639–1652. doi: 10.1111/j.1476-5381.2010.01161.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stockton ME, Rasmussen K. Olanzapine, a novel atypical antipsychotic, reverses d-amphetamine-induced inhibition of midbrain dopamine cells. Psychopharmacology. 1996;124(1–2):50–56. doi: 10.1007/BF02245605. [DOI] [PubMed] [Google Scholar]
- 55.Todorova A, Dimpfel W. Multiunit Activity From The A9 And A10 Areas In Rats Following Chronic Treatment With Different Neuroleptic Drugs. European Neuropsychopharmacology. 1994;4(4):491–501. doi: 10.1016/0924-977x(94)90298-4. [DOI] [PubMed] [Google Scholar]
- 56.Zhang J, Wang LP, Pitts DK. Prenatal haloperidol reduces the number of active midbrain dopamine neurons in rat offspring. Neurotoxicology and Teratology. 1996;18(1):49–57. doi: 10.1016/0892-0362(95)02023-3. [DOI] [PubMed] [Google Scholar]
- 57.Skarsfeldt T. Electrophysiological profile of the new atypical neuroleptic, sertindole, on midbrain dopamine neurons in rats - acute and repeated treatment. Synapse. 1992;10(1):25–33. doi: 10.1002/syn.890100105. [DOI] [PubMed] [Google Scholar]
- 58.Stockton ME, Rasmussen K. Electrophysiological effects of olanzapine, a novel atypical antipsychotic, on A9 and A10 dopamine neurons. Neuropsychopharmacology. 1996;14(2):97–104. doi: 10.1016/0893-133X(94)00130-R. [DOI] [PubMed] [Google Scholar]
- 59.Minabe Y, Hashimoto K, Shirayama Y, Ashby CR. Effect of the acute and chronic administration of the putative atypical antipsychotic drug Y-931 (8-fluoro-12(4-methylpiperazin-1-yl)-6H-l benzothieno 2,3b 1,5 benzodiazepine maleate) on spontaneously active rat midbrain dopamine neurons: Anin vivo electrophysiological study. Synapse. 2004;51(1):19–26. doi: 10.1002/syn.10280. [DOI] [PubMed] [Google Scholar]
- 60.Pietraszek M, Golembiowska K, Bijak M, Ossowska K, Wolfarth S. Differential effects of chronic haloperidol and clozapine administration on glutamatergic transmission in the fronto-parietal cortex in rats: microdialysis and electrophysiological studies. Naunyn-Schmiedebergs Archives of Pharmacology. 2002;366(5):417–424. doi: 10.1007/s00210-002-0619-x. [DOI] [PubMed] [Google Scholar]
- 61.Gronier BS, Rasmussen K. Electrophysiological effects of acute and chronic olanzapine and fluoxetine in the rat prefrontal cortex. Neurosci Lett. 2003;349(3):196–200. doi: 10.1016/s0304-3940(03)00851-6. [DOI] [PubMed] [Google Scholar]
- 62.Homayoun H, Moghaddam B. Fine-tuning of awake prefrontal cortex neurons by clozapine: Comparison with haloperidol and N-desmethylclozapine. Biological psychiatry. 2007;61(5):679–687. doi: 10.1016/j.biopsych.2006.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Guzowski JF, McNaughton BL, Barnes CA, Worley PF. Environment-specific expression of the immediate-early gene Arc in hippocampal neuronal ensembles. Nature neuroscience. 1999;2(12):1120–1124. doi: 10.1038/16046. [DOI] [PubMed] [Google Scholar]
- 64.Guzowski JF, Setlow B, Wagner EK, McGaugh JL. Experience-dependent gene expression in the rat hippocampus after spatial learning: A comparison of the immediate-early genes Arc, c-fos, and zif268. Journal of Neuroscience. 2001;21(14):5089–5098. doi: 10.1523/JNEUROSCI.21-14-05089.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hamilton DA, Candelaria-Cook FT, Akers KG, Rice JP, Maes LI, Rosenberg M, Valenzuela CF, Savage DD. Patterns of social-experience-related c-fos and Arc expression in the frontal cortices of rats exposed to saccharin or moderate levels of ethanol during prenatal brain development. Behavioural brain research. 2010;214(1):66–74. doi: 10.1016/j.bbr.2010.05.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Carrey N, Wilkinson M. A review of psychostimulant-induced neuroadaptation in developing animals. Neurosci Bull. 2011;27(3):197–214. doi: 10.1007/s12264-011-1004-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cochran SM, McKerchar CE, Morris BJ, Pratt JA. Induction of differential patterns of local cerebral glucose metabolism and immediate-early genes by acute clozapine and haloperidol. Neuropharmacology. 2002;43(3):394–407. doi: 10.1016/s0028-3908(02)00091-6. [DOI] [PubMed] [Google Scholar]
- 68.Dawe GS, Huff KD, Vandergriff JL, Sharp T, O’Neill MJ, Rasmussen K. Olanzapine activates the rat locus coeruleus: In vivo electrophysiology and c-fos immunoreactivity. Biological psychiatry. 2001;50(7):510–520. doi: 10.1016/s0006-3223(01)01171-4. [DOI] [PubMed] [Google Scholar]
- 69.Ohashi K, Hamamura T, Lee Y, Fujiwara Y, Suzuki H, Kuroda S. Clozapine- and olanzapine-induced Fos expression in the rat medial prefrontal cortex is mediated by beta-adrenoceptors. Neuropsychopharmacology. 2000;23(2):162–169. doi: 10.1016/S0893-133X(00)00105-6. [DOI] [PubMed] [Google Scholar]
- 70.Deutch AY, Duman RS. The effects of antipsychotic drugs on Fos protein expression in the prefrontal cortex: Cellular localization and pharmacological characterization. Neuroscience. 1996;70(2):377–389. doi: 10.1016/0306-4522(95)00357-6. [DOI] [PubMed] [Google Scholar]
- 71.Deutch AY, Lewis DA, Whitehead RE, Elsworth JD, Iadarola MJ, Redmond DE, Roth RH. Effects of D-2 dopamine receptor antagonists on fos protein expression in the striatal complex and entorhinal cortex of the nonhuman primate. Synapse. 1996;23(3):182–191. doi: 10.1002/(SICI)1098-2396(199607)23:3<182::AID-SYN7>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 72.Murphy CA, Feldon J. Interactions between environmental stimulation and antipsychotic drug effects on forebrain C-FOS activation. Neuroscience. 2001;104(3):717–730. doi: 10.1016/s0306-4522(01)00110-5. [DOI] [PubMed] [Google Scholar]
- 73.Robertson GS, Fibiger HC. Effects of olanzapine on regional c-Fos expression in rat forebrain. Neuropsychopharmacology. 1996;14(2):105–110. doi: 10.1016/0893-133X(95)00196-K. [DOI] [PubMed] [Google Scholar]
- 74.Macgibbon GA, Lawlor PA, Bravo R, Dragunow M. Clozapine and haloperidol produce a differential pattern of immediate-early gene-expression in rat caudate-putamen, nucleus-accumbens, lateral septum and islands-of-Calleja. Molecular Brain Research. 1994;23(1–2):21–32. doi: 10.1016/0169-328x(94)90207-0. [DOI] [PubMed] [Google Scholar]
- 75.Fujimura M, Hashimoto K, Yamagami K. The effect of the antipsychotic drug mosapramine on the expression of Fos protein in the rat brain - Comparison with haloperidol, clozapine and risperidone. Life Sciences. 2000;67(23):2865–2872. doi: 10.1016/s0024-3205(00)00872-9. [DOI] [PubMed] [Google Scholar]
- 76.Nguyen TV, Kosofsky BE, Birnbaum R, Cohen BM, Hyman SE. Differential expression of c-fos and zif268 in rat striatum after haloperidol, clozapine, and amphetamine. Proceedings of the National Academy of Sciences of the United States of America. 1992;89(10):4270–4274. doi: 10.1073/pnas.89.10.4270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Fumagalli F, Frasca A, Racagni G, Riva MA. Antipsychotic drugs modulate Arc expression in the rat brain. European Neuropsychopharmacology. 2009;19(2):109–115. doi: 10.1016/j.euroneuro.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 78.Nakahara T, Kuroki T, Hashimoto K, Hondo H, Tsutsumi T, Motomura K, Ueki H, Hirano M, Uchimura H. Effect of atypical antipsychotics on phencyclidine-induced expression of arc in rat brain. Neuroreport. 2000;11(3):551–555. doi: 10.1097/00001756-200002280-00025. [DOI] [PubMed] [Google Scholar]
- 79.Iasevoli F, Fiore G, Cicale M, Muscettola G, de Bartolomeis A. Haloperidol induces higher Homer1a expression than risperidone, olanzapine and sulpiride in striatal sub-regions. Psychiatry research. 2010;177(1–2):255–260. doi: 10.1016/j.psychres.2010.02.009. [DOI] [PubMed] [Google Scholar]
- 80.Guzowski JF, Lyford GL, Stevenson GD, Houston FP, McGaugh JL, Worley PF, Barnes CA. Inhibition of activity-dependent arc protein expression in the rat hippocampus impairs the maintenance of long-term potentiation and the consolidation of long-term memory. Journal of Neuroscience. 2000;20(11):3993–4001. doi: 10.1523/JNEUROSCI.20-11-03993.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rodriguez JJ, Garcia DR, Nakabeppu Y, Pickel VM. FosB in rat striatum: Normal regional distribution and enhanced expression after 6-month haloperidol administration. Synapse. 2001;39(2):122–132. doi: 10.1002/1098-2396(200102)39:2<122::AID-SYN3>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 82.Verma V, Lim EP, Han SP, Nagarajah R, Dawe GS. Chronic high-dose haloperidol has qualitatively similar effects to risperidone and clozapine on immediate-early gene and tyrosine hydroxylase expression in the rat locus coeruleus but not medial prefrontal cortex. Neuroscience Research. 2007;57(1):17–28. doi: 10.1016/j.neures.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 83.VahidAnsari F, Nakabeppu Y, Robertson GS. Contrasting effects of chronic clozapine, Seroquel(TM) (ICI 204,636) and haloperidol administration on Delta FosB-like immunoreactivity in the rodent forebrain. European Journal of Neuroscience. 1996;8(5):927–936. doi: 10.1111/j.1460-9568.1996.tb01579.x. [DOI] [PubMed] [Google Scholar]
- 84.Leuner B, Falduto J, Shors TJ. Associative memory formation increases the observation of dendritic spines in the hippocampus. Journal of Neuroscience. 2003;23(2):659–665. doi: 10.1523/JNEUROSCI.23-02-00659.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Moser MB, Trommald M, Andersen P. An Increase In Dendritic Spine Density On Hippocampal CA1 Pyramidal Cells Following Spatial-Learning In Adult-Rats Suggests The Formation Of New Synapses. Proceedings of the National Academy of Sciences of the United States of America. 1994;91(26):12673–12675. doi: 10.1073/pnas.91.26.12673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kolb B, Whishaw IQ. Brain plasticity and behavior. Annual Review of Psychology. 1998;49:43–64. doi: 10.1146/annurev.psych.49.1.43. [DOI] [PubMed] [Google Scholar]
- 87.Harris KM, Kater SB. Dendritic Spines - Cellular Specializations Imparting Both Stability And Flexibility To Synaptic Function. Annual Review of Neuroscience. 1994;17:341–371. doi: 10.1146/annurev.ne.17.030194.002013. [DOI] [PubMed] [Google Scholar]
- 88.Hamilton DA, Akers KG, Rice JP, Johnson TE, Candelaria-Cook FT, Maes LI, Rosenberg M, Valenzuela CF, Savage DD. Prenatal exposure to moderate levels of ethanol alters social behavior in adult rats: Relationship to structural plasticity and immediate early gene expression in frontal cortex. Behavioural brain research. 2010;207(2):290–304. doi: 10.1016/j.bbr.2009.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.van Praag H, Kempermann G, Gage FH. Neural consequences of environmental enrichment. Nature Reviews Neuroscience. 2000;1(3):191–198. doi: 10.1038/35044558. [DOI] [PubMed] [Google Scholar]
- 90.Kolb B, Gibb R, Gorny G. Experience-dependent changes in dendritic arbor and spine density in neocortex vary qualitatively with age and sex. Neurobiology of Learning and Memory. 2003;79(1):1–10. doi: 10.1016/s1074-7427(02)00021-7. [DOI] [PubMed] [Google Scholar]
- 91.Hamilton DA, Kolb B. Differential effects of nicotine and complex housing on subsequent experience-dependent structural plasticity in the nucleus accumbens. Behavioral Neuroscience. 2005;119(2):355–365. doi: 10.1037/0735-7044.119.2.355. [DOI] [PubMed] [Google Scholar]
- 92.Wosiski-Kuhn M, Stranahan AM. Transient increases in dendritic spine density contribute to dentate gyrus long-term potentiation. Synapse. 2012;66(7):661–664. doi: 10.1002/syn.21545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Purpura DP. Dendritic Spine Dysgenesis And Mental-Retardation. Science (New York, NY. 1974;186(4169):1126–1128. doi: 10.1126/science.186.4169.1126. [DOI] [PubMed] [Google Scholar]
- 94.Glantz LA, Lewis DA. Decreased dendritic spine density on prefrontal cortical pyramidal neurons in schizophrenia. Archives of general psychiatry. 2000;57(1):65–73. doi: 10.1001/archpsyc.57.1.65. [DOI] [PubMed] [Google Scholar]
- 95.Ide M, Lewis DA. Altered Cortical CDC42 Signaling Pathways in Schizophrenia: Implications for Dendritic Spine Deficits. Biological psychiatry. 2010;68(1):25–32. doi: 10.1016/j.biopsych.2010.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Pierri JN, Volk CLE, Auh S, Sampson A, Lewis DA. Decreased somal size of deep layer 3 pyramidal neurons in the prefrontal cortex of subjects with schizophrenia. Archives of general psychiatry. 2001;58(5):466–473. doi: 10.1001/archpsyc.58.5.466. [DOI] [PubMed] [Google Scholar]
- 97.Kelley JJ, Gao XM, Tamminga CA, Roberts RC. The effect of chronic haloperidol treatment on dendritic spines in the rat striatum. Experimental neurology. 1997;146(2):471–478. doi: 10.1006/exnr.1997.6552. [DOI] [PubMed] [Google Scholar]
- 98.Roberts RC. Effect of chronic olanzapine treatment on striatal synaptic organization. Synapse. 2001;39(1):8–15. doi: 10.1002/1098-2396(20010101)39:1<8::AID-SYN2>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 99.Roberts RC, Gaither LA, Gao XM, Kashyap SM, Tamminga CA. Ultrastructural Correlates Of Haloperidol-Induced Oral Dyskinesias In Rat Striatum. Synapse. 1995;20(3):234–243. doi: 10.1002/syn.890200307. [DOI] [PubMed] [Google Scholar]
- 100.Benes FM, Paskevich PA, Davidson J, Domesick VB. The Effects Of Haloperidol On Synaptic Patterns In The Rat Striatum. Brain Research. 1985;329(1–2):265–273. doi: 10.1016/0006-8993(85)90532-3. [DOI] [PubMed] [Google Scholar]
- 101.Frost DO, Page SC, Carroll C, Kolb B. Early Exposure to Haloperidol or Olanzapine Induces Long-Term Alterations of Dendritic Form. Synapse. 2010;64(3):191–199. doi: 10.1002/syn.20715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wang HD, Deutch AY. Dopamine depletion of the prefrontal cortex induces dendritic spine loss: Reversal by atypical antipsychotic drug treatment. Neuropsychopharmacology. 2008;33(6):1276–1286. doi: 10.1038/sj.npp.1301521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kolb B, Gorny G, Li YL, Samaha AN, Robinson TE. Amphetamine or cocaine limits the ability of later experience to promote structural plasticity in the neocortex and nucleus accumbens. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(18):10523–10528. doi: 10.1073/pnas.1834271100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Robinson TE, Kolb B. Structural plasticity associated with exposure to drugs of abuse. Neuropharmacology. 2004;47:33–46. doi: 10.1016/j.neuropharm.2004.06.025. [DOI] [PubMed] [Google Scholar]
- 105.Flores C, Wen XL, Labelle-Dumais C, Kolb B. Chronic phencyclidine treatment increases dendritic spine density in prefrontal cortex and nucleus accumbens neurons. Synapse. 2007;61(12):978–984. doi: 10.1002/syn.20452. [DOI] [PubMed] [Google Scholar]
- 106.Vincent SL, McSparren J, Wang RY, Benes FM. Edicience for ultrastructural-changes in cortical axodendritic synapses follwing long-term treatment with haloperidol or clozapine. Neuropsychopharmacology. 1991;5(3):147–155. [PubMed] [Google Scholar]
- 107.Elsworth JD, Hajszan T, Leranth C, Roth RH. Loss of asymmetric spine synapses in dorsolateral prefrontal cortex of cognitively impaired phencyclidine-treated monkeys. International Journal of Neuropsychopharmacology. 2011;14(10):1411–1415. doi: 10.1017/S1461145711000939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Elsworth JD, Morrow BA, Hajszan T, Leranth C, Roth RH. Phencyclidine-induced Loss of Asymmetric Spine Synapses in Rodent Prefrontal Cortex is Reversed by Acute and Chronic Treatment with Olanzapine. Neuropsychopharmacology. 2011;36(10):2054–2061. doi: 10.1038/npp.2011.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Pillai A, Mahadik SP. Differential effects of haloperidol and olanzapine on levels of vascular endothelial growth factor and angiogenesis in rat hippocampus. Schizophrenia research. 2006;87(1–3):48–59. doi: 10.1016/j.schres.2006.06.017. [DOI] [PubMed] [Google Scholar]