Abstract
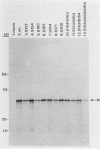
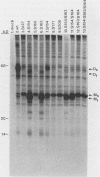
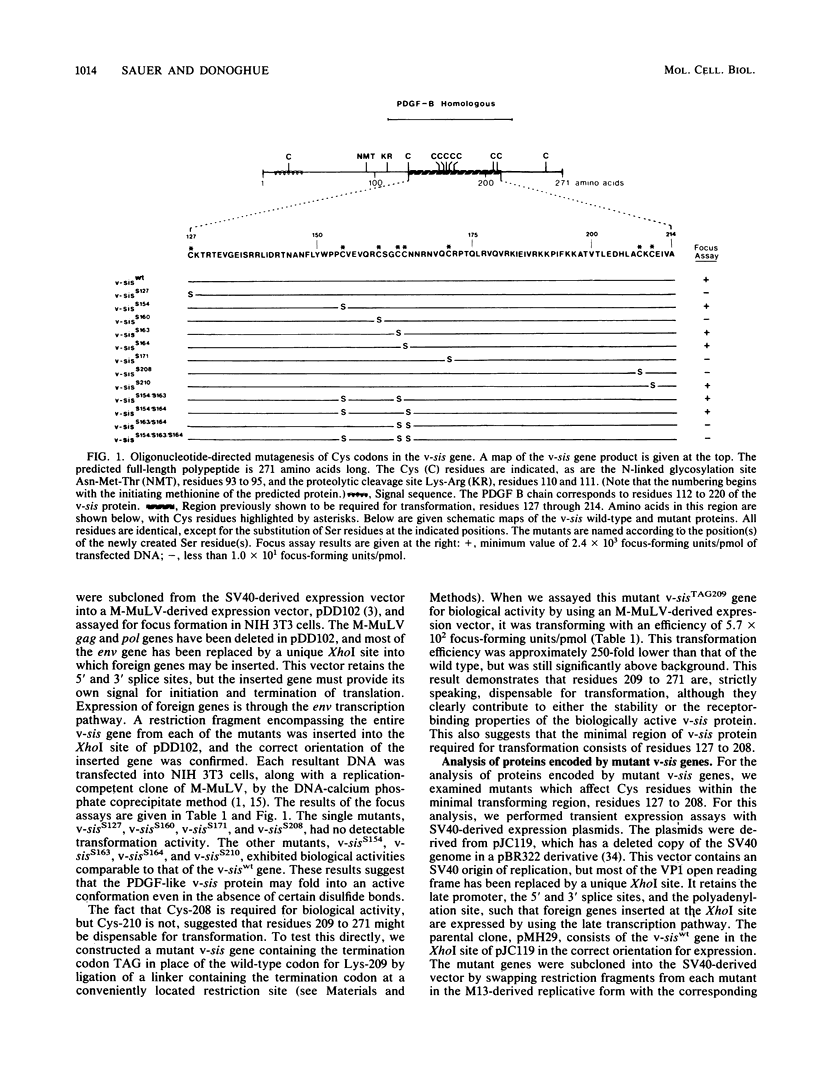
The protein encoded by v-sis, the oncogene of simian sarcoma virus, is homologous to the B chain of platelet-derived growth factor (PDGF). There are eight conserved Cys residues between PDGF-B and the v-sis protein. Both native PDGF and the v-sis protein occur as disulfide-bonded dimers, probably containing both intramolecular and intermolecular disulfide bonds. Oligonucleotide-directed mutagenesis was used to change the Cys codons to Ser codons in the v-sis gene. Four single mutants lacked detectable biological activity, indicating that Cys-127, Cys-160, Cys-171, and Cys-208 are required for formation of a biologically active v-sis protein. The other four single mutants retained biological activity as determined in transformation assays, indicating that Cys-154, Cys-163, Cys-164, and Cys-210 are dispensable for biological activity. Double and triple mutants containing three of these altered sites were constructed, some of which were transforming as well. The v-sis proteins encoded by biologically active mutants displayed significantly reduced levels of dimeric protein compared with the wild-type v-sis protein, which dimerized very efficiently. Furthermore, a mutant with a termination codon at residue 209 exhibited partial transforming activity. This study thus suggests that the minimal region required for transformation consists of residues 127 to 208. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis analysis indicated that the v-sis proteins encoded by some of the biologically active mutants exhibited an altered conformation when compared with the wild-type v-sis protein, and suggested that Cys-154 and Cys-163 participate in a nonessential disulfide bond.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersson P., Goldfarb M. P., Weinberg R. A. A defined subgenomic fragment of in vitro synthesized Moloney sarcoma virus DNA can induce cell transformation upon transfection. Cell. 1979 Jan;16(1):63–75. doi: 10.1016/0092-8674(79)90188-0. [DOI] [PubMed] [Google Scholar]
- Betsholtz C., Johnsson A., Heldin C. H., Westermark B., Lind P., Urdea M. S., Eddy R., Shows T. B., Philpott K., Mellor A. L. cDNA sequence and chromosomal localization of human platelet-derived growth factor A-chain and its expression in tumour cell lines. Nature. 1986 Apr 24;320(6064):695–699. doi: 10.1038/320695a0. [DOI] [PubMed] [Google Scholar]
- Bold R. J., Donoghue D. J. Biologically active mutants with deletions in the v-mos oncogene assayed with retroviral vectors. Mol Cell Biol. 1985 Nov;5(11):3131–3138. doi: 10.1128/mcb.5.11.3131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen E. Y., Seeburg P. H. Supercoil sequencing: a fast and simple method for sequencing plasmid DNA. DNA. 1985 Apr;4(2):165–170. doi: 10.1089/dna.1985.4.165. [DOI] [PubMed] [Google Scholar]
- Creighton T. E., Hillson D. A., Freedman R. B. Catalysis by protein-disulphide isomerase of the unfolding and refolding of proteins with disulphide bonds. J Mol Biol. 1980 Sep 5;142(1):43–62. doi: 10.1016/0022-2836(80)90205-3. [DOI] [PubMed] [Google Scholar]
- Creighton T. E. Renaturation of the reduced bovine pancreatic trypsin inhibitor. J Mol Biol. 1974 Aug 15;87(3):563–577. doi: 10.1016/0022-2836(74)90104-1. [DOI] [PubMed] [Google Scholar]
- Creighton T. E. The single-disulphide intermediates in the refolding of reduced pancreatic trypsin inhibitor. J Mol Biol. 1974 Aug 15;87(3):603–624. doi: 10.1016/0022-2836(74)90106-5. [DOI] [PubMed] [Google Scholar]
- Delbaere L. T., Hutcheon W. L., James M. N., Thiessen W. E. Tertiary structural differences between microbial serine proteases and pancreatic serine enzymes. Nature. 1975 Oct 30;257(5529):758–763. doi: 10.1038/257758a0. [DOI] [PubMed] [Google Scholar]
- Deuel T. F., Huang J. S., Huang S. S., Stroobant P., Waterfield M. D. Expression of a platelet-derived growth factor-like protein in simian sarcoma virus transformed cells. Science. 1983 Sep 30;221(4618):1348–1350. doi: 10.1126/science.6310754. [DOI] [PubMed] [Google Scholar]
- Deuel T. F., Huang J. S., Proffitt R. T., Baenziger J. U., Chang D., Kennedy B. B. Human platelet-derived growth factor. Purification and resolution into two active protein fractions. J Biol Chem. 1981 Sep 10;256(17):8896–8899. [PubMed] [Google Scholar]
- Devare S. G., Reddy E. P., Law J. D., Robbins K. C., Aaronson S. A. Nucleotide sequence of the simian sarcoma virus genome: demonstration that its acquired cellular sequences encode the transforming gene product p28sis. Proc Natl Acad Sci U S A. 1983 Feb;80(3):731–735. doi: 10.1073/pnas.80.3.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doolittle R. F., Hunkapiller M. W., Hood L. E., Devare S. G., Robbins K. C., Aaronson S. A., Antoniades H. N. Simian sarcoma virus onc gene, v-sis, is derived from the gene (or genes) encoding a platelet-derived growth factor. Science. 1983 Jul 15;221(4607):275–277. doi: 10.1126/science.6304883. [DOI] [PubMed] [Google Scholar]
- Garrett J. S., Coughlin S. R., Niman H. L., Tremble P. M., Giels G. M., Williams L. T. Blockade of autocrine stimulation in simian sarcoma virus-transformed cells reverses down-regulation of platelet-derived growth factor receptors. Proc Natl Acad Sci U S A. 1984 Dec;81(23):7466–7470. doi: 10.1073/pnas.81.23.7466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giese N. A., Robbins K. C., Aaronson S. A. The role of individual cysteine residues in the structure and function of the v-sis gene product. Science. 1987 Jun 5;236(4806):1315–1318. doi: 10.1126/science.3035718. [DOI] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Grunstein M., Hogness D. S. Colony hybridization: a method for the isolation of cloned DNAs that contain a specific gene. Proc Natl Acad Sci U S A. 1975 Oct;72(10):3961–3965. doi: 10.1073/pnas.72.10.3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannink M., Donoghue D. J. Requirement for a signal sequence in biological expression of the v-sis oncogene. Science. 1984 Dec 7;226(4679):1197–1199. doi: 10.1126/science.6095451. [DOI] [PubMed] [Google Scholar]
- Hannink M., Sauer M. K., Donoghue D. J. Deletions in the C-terminal coding region of the v-sis gene: dimerization is required for transformation. Mol Cell Biol. 1986 Apr;6(4):1304–1314. doi: 10.1128/mcb.6.4.1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann J. W., Steffen D., Gusella J., Tabin C., Bird S., Cowing D., Weinberg R. A. DNA methylation affecting the expression of murine leukemia proviruses. J Virol. 1982 Oct;44(1):144–157. doi: 10.1128/jvi.44.1.144-157.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J. S., Huang S. S., Deuel T. F. Transforming protein of simian sarcoma virus stimulates autocrine growth of SSV-transformed cells through PDGF cell-surface receptors. Cell. 1984 Nov;39(1):79–87. doi: 10.1016/0092-8674(84)90193-4. [DOI] [PubMed] [Google Scholar]
- Johnsson A., Heldin C. H., Wasteson A., Westermark B., Deuel T. F., Huang J. S., Seeburg P. H., Gray A., Ullrich A., Scrace G. The c-sis gene encodes a precursor of the B chain of platelet-derived growth factor. EMBO J. 1984 May;3(5):921–928. doi: 10.1002/j.1460-2075.1984.tb01908.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josephs S. F., Guo C., Ratner L., Wong-Staal F. Human-proto-oncogene nucleotide sequences corresponding to the transforming region of simian sarcoma virus. Science. 1984 Feb 3;223(4635):487–491. doi: 10.1126/science.6318322. [DOI] [PubMed] [Google Scholar]
- King C. R., Giese N. A., Robbins K. C., Aaronson S. A. In vitro mutagenesis of the v-sis transforming gene defines functional domains of its growth factor-related product. Proc Natl Acad Sci U S A. 1985 Aug;82(16):5295–5299. doi: 10.1073/pnas.82.16.5295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkel T. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Proc Natl Acad Sci U S A. 1985 Jan;82(2):488–492. doi: 10.1073/pnas.82.2.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Nishimura J., Huang J. S., Deuel T. F. Platelet-derived growth factor stimulates tyrosine-specific protein kinase activity in Swiss mouse 3T3 cell membranes. Proc Natl Acad Sci U S A. 1982 Jul;79(14):4303–4307. doi: 10.1073/pnas.79.14.4303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen A. J., Pantazis P., Antoniades H. N. Simian sarcoma virus--transformed cells secrete a mitogen identical to platelet-derived growth factor. Science. 1984 Jul 6;225(4657):54–56. doi: 10.1126/science.6328659. [DOI] [PubMed] [Google Scholar]
- Robbins K. C., Antoniades H. N., Devare S. G., Hunkapiller M. W., Aaronson S. A. Structural and immunological similarities between simian sarcoma virus gene product(s) and human platelet-derived growth factor. Nature. 1983 Oct 13;305(5935):605–608. doi: 10.1038/305605a0. [DOI] [PubMed] [Google Scholar]
- Robbins K. C., Leal F., Pierce J. H., Aaronson S. A. The v-sis/PDGF-2 transforming gene product localizes to cell membranes but is not a secretory protein. EMBO J. 1985 Jul;4(7):1783–1792. doi: 10.1002/j.1460-2075.1985.tb03851.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robson B., Platt E., Finn P. W., Millard P., Gibrat J. F., Garnier J. Prediction of the conformation and antigenic determinants of the V-sis viral oncogene product homologous with human platelet-derived growth factor. Int J Pept Protein Res. 1985 Jan;25(1):1–8. doi: 10.1111/j.1399-3011.1985.tb02140.x. [DOI] [PubMed] [Google Scholar]
- Ross R., Glomset J., Kariya B., Harker L. A platelet-dependent serum factor that stimulates the proliferation of arterial smooth muscle cells in vitro. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1207–1210. doi: 10.1073/pnas.71.4.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauer M. K., Hannink M., Donoghue D. J. Deletions in the N-terminal coding region of the v-sis gene: determination of the minimal transforming region. J Virol. 1986 Aug;59(2):292–300. doi: 10.1128/jvi.59.2.292-300.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprague J., Condra J. H., Arnheiter H., Lazzarini R. A. Expression of a recombinant DNA gene coding for the vesicular stomatitis virus nucleocapsid protein. J Virol. 1983 Feb;45(2):773–781. doi: 10.1128/jvi.45.2.773-781.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theilen G. H., Gould D., Fowler M., Dungworth D. L. C-type virus in tumor tissue of a woolly monkey (Lagothrix spp.) with fibrosarcoma. J Natl Cancer Inst. 1971 Oct;47(4):881–889. [PubMed] [Google Scholar]
- Waterfield M. D., Scrace G. T., Whittle N., Stroobant P., Johnsson A., Wasteson A., Westermark B., Heldin C. H., Huang J. S., Deuel T. F. Platelet-derived growth factor is structurally related to the putative transforming protein p28sis of simian sarcoma virus. Nature. 1983 Jul 7;304(5921):35–39. doi: 10.1038/304035a0. [DOI] [PubMed] [Google Scholar]
- Zoller M. J., Smith M. Oligonucleotide-directed mutagenesis of DNA fragments cloned into M13 vectors. Methods Enzymol. 1983;100:468–500. doi: 10.1016/0076-6879(83)00074-9. [DOI] [PubMed] [Google Scholar]