Abstract
A unified strategy for the syntheses of bi- and triantennary fully sialylated N-glycans is described. The synthesis capitalizes on a global glycosylation strategy that delivers the desired undeca- and tetradecasaccharide in excellent yields. Finally, conjugation of the glycan to PSMA oligopeptide is described.
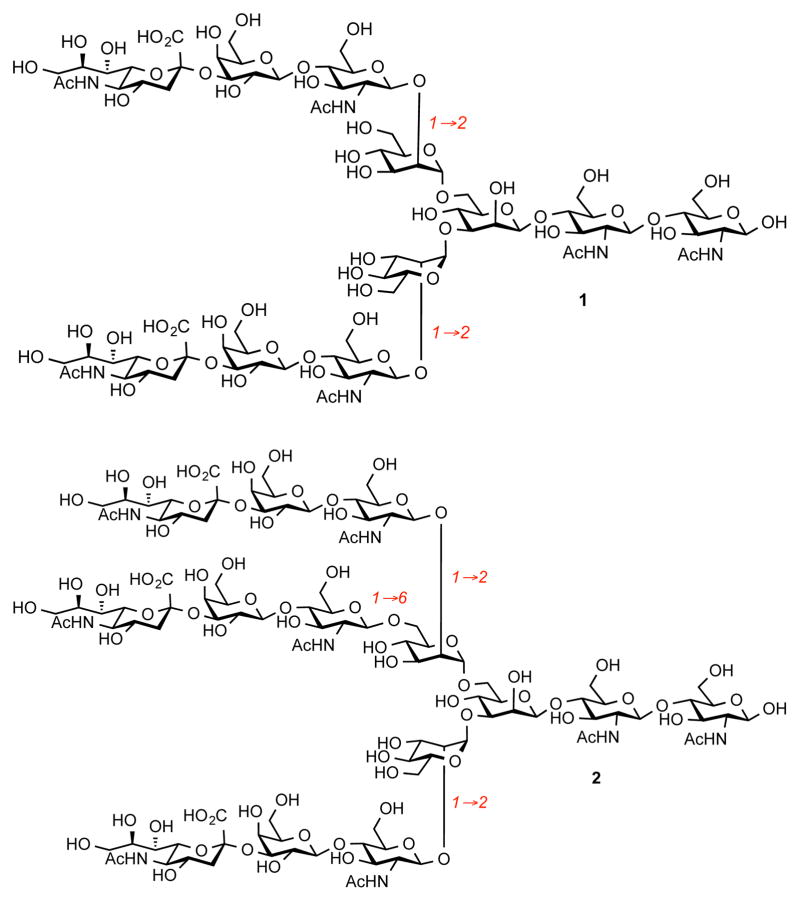
Remodeling of glycan composition in glycoproteins often results in critical changes in the (inter)cellular environment, and may signal metabolic imbalance.1 These changes can induce functional modifications of glycoproteins by altering their structure, stability, and site of localization.2 Given the importance of glycoproteins as potential therapeutic and diagnostic assets, their chemical synthesis could well prove to be of paramount importance in providing viable avenues for the investigations of covalent posttranslational modifications.3 A great deal of structural variability comes from N-linked glycans, which are linked to the peptidic backbone through a strategic asparagine residue. The parent sialylated glycan is believed to be a biantennary structure containing terminal N-acetylneuraminic acid residues connected via an α-2,6 and/or α-2,3-linkage (1, Figure 1). The nature of these glycosyl bonds depends on the origin of protein; many glycoproteins contain a mixture of both linkage regioisomers. The first chemical synthesis of an undecasaccharide containing an α-2,6-linked sialic acid was reported by Ogawa.4 However, it was found that this glycan can be easily obtained in substantial quantities from natural sources through isolation from egg yolk.5 Another typical regioisomer, containing α-2,3-linked bonds (1), was prepared via synthetic means by Ito.6 Not surprisingly, the chemical synthesis of this structure required lengthy sequences. Synthetic surrogates containing unnatural linkages have also been prepared.7 A higher order structure of N-linked glycan contains additional trisaccharide branching linked via the 6-OH position of a mannose residue (2). The structure 2 and its fucosylated analog have been identified in a panel of glycoproteins, including Erythropoietin (EPO),8 Follicle Stimulating Hormone (FSH),9 and human Chorionic Gonadotropin (hCG),10 rendering synthesis of these oligosaccharides a worthwhile pursuit. Here, we present a practical approach for the synthesis of dibranched (undecasaccharide) and tribranched (tetradecasaccharide) structures containing terminal Neu5Ac residues.
Figure 1.
As a part of our overall program directed towards the development of novel therapeutic agents and diagnostic strategies against cancer we investigated the role of glycoproteins in the emergence and progression of prostate cancer.11 We have shown that the extent of N-glycan branching in Prostate Specific Antigen (PSA) can be differentiated with synthetic glycoconjugates containing di-, tri-, and tetraantennary glycans. However, these systems did not contain terminal sialic acid residues, which are known to be critical components in cell signaling and promotion of immune response.12 Access to glycans 1 and 2 would allow for validation of the processing of the glycans in biological systems and analysis of the impact of various glycoforms on the structure and function of glycoproteins. Additionally, given the complexity of these systems, chemical synthesis would provide ultimate validation of the synthetic strategies for the preparation of branched oligosaccharides.
At the retrosynthetic level, we envisioned that each antenna could be installed in a single glycosylation event using a properly functionalized donor with Neu5Ac residues already in place.13 We were mindful of potential hurdles associated with this approach since incorporation of three sialylated antennae in a related system presented a challenging synthetic step requiring careful orchestration to achieve practical yields.14 However, attracted by the convergent nature of our synthetic plan, these critical steps seemed an attractive possibility to allow for a rapid assembly of the glycan core in a minimal number of synthetic operations.
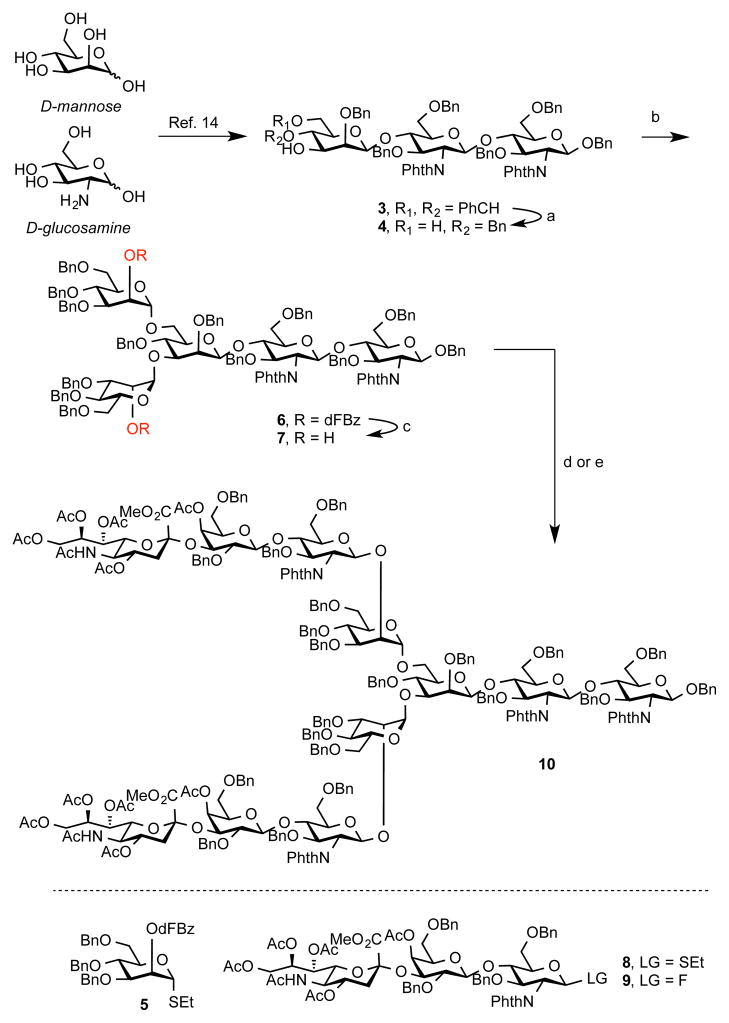
The synthesis began with trisaccharide 3,14 which was reductively opened to provide diol 4 in an excellent yield (Scheme 1). This intermediate was then coupled with mannose thioether 514 in 89% yield and the alcohol functionalities were liberated under basic conditions to afford diol 7. It is well to note the importance of the dFBz (2,5-difluorobenzoyl) group in this step. Attempts to remove unsubstituted benzoyl groups in an analogous pentasaccharide led only to 40% yield of 7, and a mixture of products arising from a partial cleavage of the phthalimide group was observed.
Scheme 1. Synthesis of Protected Undecasaccharide 10a.
aReagents and Conditions: (a) BH3·THF, n-Bu2BOTf, CH2Cl2, 0 °C, 3 h, 83%; (b) 5, NIS, AgOTf, CH2Cl2, −20 °C, 2 h, 89%; (c) NaOMe, MeOH, THF, rt, 1 h, 99%; (d) 8, NIS, AgOTf, CH2Cl2, −20 °C, 24 h, 75%; (e) 9, Cp2Zr(OTf)2·THF, CH2Cl2, 0 °C, 2 h, 74%.
At this point we were ready to evaluate the feasibility of conducting a double glycosylation.15 Accordingly, we investigated the utility of two trisaccharide donors in this transformation. Thioether 8,15 under NIS/TfO− conditions, did indeed provide the expected undecasaccharide 10 in 75% isolated yield. Similarly, glycosylation with anomeric fluoride proceeded very efficiently and reached completion in 1.5 h at 0 °C to deliver 10 in a similar yield (86% average yield per glycosyl acceptor).16 It is noteworthy that the reaction did not seem to produce, at least to the levels of our detection, transient monoglycosylated products. The stereochemistry of all of the glycosidic bonds was confirmed by analysis of 1JCH of anomeric carbon atoms.
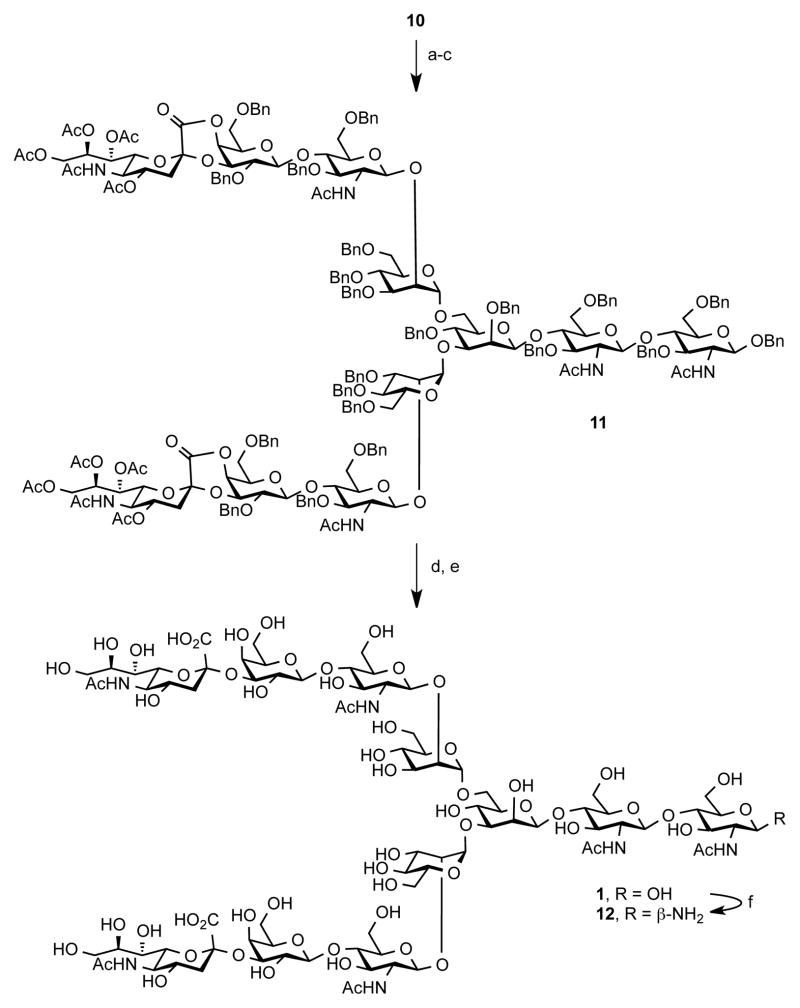
With sufficient quantities of undecasaccharide 10 in hand, we advanced to the deprotection sequence (Scheme 2). First, the acetate groups were removed with NaOMe/MeOH and the methyl ester functionalities were saponified with NaOH. The resultant diacid was then used in a crude state to remove four phthalimide groups with 1,2-ethylenediamine in refluxing ethanol. We found that this set of conditions proved to be a more robust protocol for the deprotection of nitrogen functionalities in comparison with the conditions reported previously,14 which required higher reaction temperatures. The resultant crude material was then subjected to acetylation conditions to provide bislactone 11 in 35% yield over three-step sequence. The peracetylation step allows for purification of the oligosaccharide material before the reductive removal of benzyl groups. To complete the synthesis of 1, saponification of 11 with NaOMe/NaOH furnished diacid, which was then subjected to Birch-type reduction to reveal undecasaccharide 1 in an appreciable yield of 77%. Occasionally, we found that some N-acetamide groups were cleaved under the reductive step, and an additional acetylation step (Ac2O, sat. NaHCO3(aq), 0 °C) was necessary to provide homogenous material. Unlike deprotection of fucosylated biantennary glycan, which was shown to lead to a partial cleavage of the oligosaccharide chain, we observed only clean removal of the benzyl groups without any truncated saccharide fragments and/or over-reduction products.15 Compared to the synthesis of 11 reported previously,6 our approach capitalizes on a more convergent assembly of the core undecasaccharide and it displays significantly improved yields. The analytical data for glycan 1 matches the previous reports.10, 17,6
Scheme 2. Deprotection Sequence for Glycan 1a.
aReagents and Conditions: (a) NaOMe/NaOH, MeOH/H2O, CH2Cl2, rt, 24 h; (b) 1,2-ethylenediamine, EtOH, 80 °C, 24 h; (c) Ac2O, pyridine, rt, 24 h, 35% (over three steps); (d) NaOMe, MeOH, CH2Cl2 then H2O, rt, 24 h; (e) Na, NH3, THF, −78 °C, 4 h,; (f) Ac2O, NaHCO3 (aq), 0 °C, 77% (over 3 steps); (g) NH4HCO3, H2O, 40 °C, 3 d, 99%.
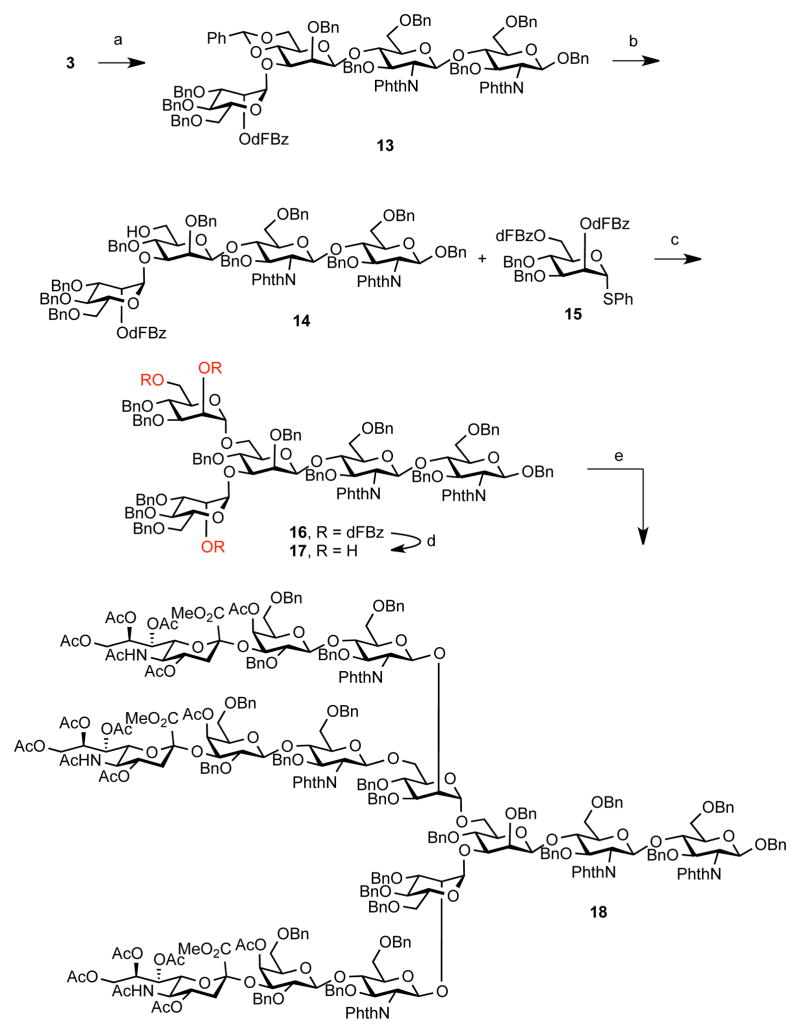
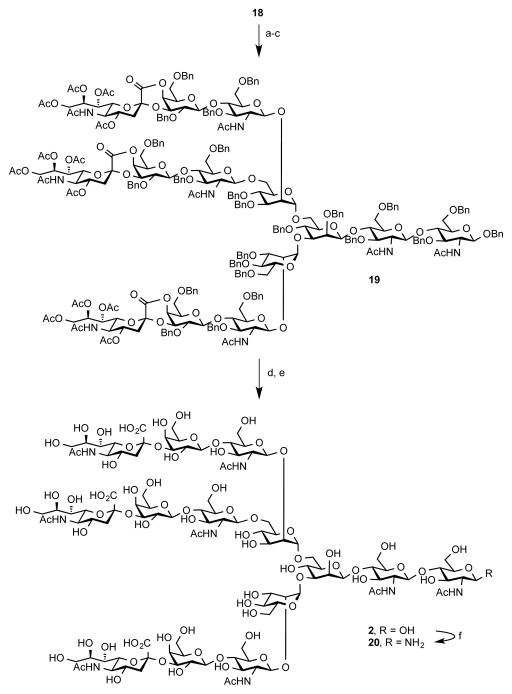
Having established a synthetic approach for the construction of the biantennary saccharide, we turned our attention to tetradecasaccharide 2 (Scheme 3). The synthesis started by coupling of 3 to a mannose thioether donor 5, which proceed in an excellent yield. Regioselective benzylidene opening revealed the desired 6-OH acceptor, as confirmed by 2D NMR analysis. The alcohol functionality served as the glycosyl acceptor site to the mannose donor 15 bearing the corresponding protective groups at positions 2 and 6. Finally, deprotection under basic conditions allowed for a rapid construction of triol 17 in excellent yield.
Scheme 3. Synthesis of Protected Tetradecasaccharide 18a.
aReagents and Conditions: (a) 5, NIS, AgOTf, CH2Cl2, 4Å MS, −50 to − 20 °C, 1.25 h, 91%; (b) BH3·THF, n-Bu2BOTf, CH2Cl2, 0 °C, 4 h, 99%; (c) 15, NIS, AgOTf, CH2Cl2, −10 °C, 2 h, 87%; (d) NaOMe, MeOH/THF, rt, 1.7 h, 93%; (e) 9, Cp2Zr(OTf)2·THF, CH2Cl2, 0 °C, 1.5 h, 71%.
Unlike the case in the synthesis of triantennary system described previously,14 it could be anticipated that the glycosylation reaction with the corresponding trisaccharide donors should be a rather more facile process. Indeed, coupling of 17 with anomeric fluoride donor 9 under the conditions optimized for the synthesis of biantennary glycan 1 provided the desired triantennary glycan 18 in a surprisingly high yield (89% per glycosyl acceptor). Notably, we were again unable to detect biantennary oligosaccharide intermediates upon completion of the reaction. However, thioether donor 8 afforded 18 in a significantly diminished yield (42%) and a significant amount of diglycosylated intermediate was observed. Completion of the synthesis of 2 is depicted in Scheme 4. A sequence of steps used in the preparation of 1 was used to provide tris-lactone 19. This compound was taken further to finish the deprotection sequence under the Birch conditions furnishing free oligosaccharide 2 in 71% yield.
Scheme 4. Deprotection Sequence for Glycan 2a.
aReagents and Conditions: (a) NaOMe/NaOH, MeOH/H2O, rt, 24 h; (b) 1,2-ethylenediamine, EtOH, 80 °C, 24 h; (c) Ac2O, pyridine 33% (over 3 steps); (d) NaOMe, MeOH/CH2Cl2 then H2O, rt, 24 h; (e) Na/NH3, THF, −78 °C, 71% (over 2 steps); (f) NH4HCO3, H2O, 40 °C, 3 d, 82%.
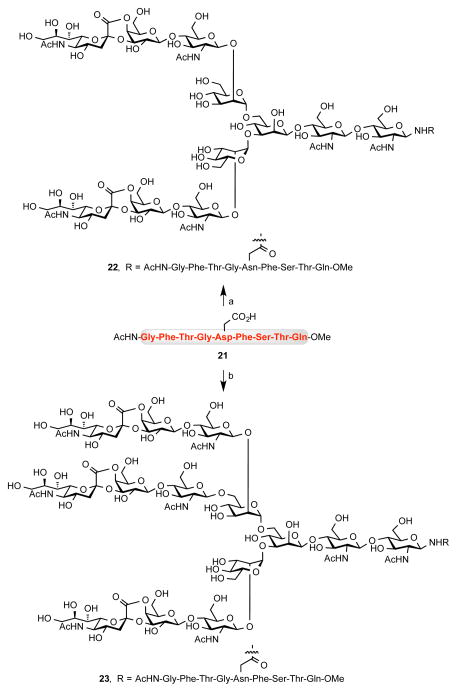
In order to evaluate the feasibility of chemical ligation of saccharides 1 and 2 to a peptide chain, a selected motif of Prostate-Specific Membrane Antigen (PSMA)18 21 was used. This peptide corresponds to Gly332-Gln340 native sequence of PSMA, and it was chosen based on the X-ray structure as the fragment containing one of the most exposed glycosylation sites in this glycoprotein.19 Thus, Kochetkov amination20 of 1 (Scheme 2) followed by repeated lyophilizations afforded anomeric amine 12 in an almost quantitative yield. The subsequent ligation to 21 was carried out under the Lansbury conditions21 to afford the PSMA glycoconjugate 22 in 54% (Scheme 5). Similarly, triantennary amine 20 was coupled with peptide 21 to furnish glycoconjugate 23 in 10% yield.
Scheme 5. Conjugation of Glycans 1 and 2 to PSMA Oligopeptidea.
aReagents and Conditions: (a) 12, HATU, DIPEA, DMSO, rt, 1.5 h, 54%; (b) 20, HATU, DIPEA, rt, 1 h, 10%.
In summary, we have described here a unified strategy for the synthesis of sialylated di- and tribranched N-linked glycans based on multiple glycosylations using very efficient anomeric fluoride donor. This approach allowed for preparation of fully sialylated glycans in excellent yields in a minimal number of steps. Finally, conjugation of the saccharides 1 and 2 to a native peptidic sequence of PSMA was demonstrated, supporting the feasibility of preparing diagnostic and therapeutic tools based on tumor-associated carbohydrate antigens related to prostate cancer.
Supplementary Material
Acknowledgments
Support for this research was provided by the National Institute of Health (GM102872 to S.J.D.). M.A.W. would like to acknowledge the Terrie Brodeur Breast Cancer Foundation for postdoctoral fellowship. We also thank Dr. George Sukenick, Hui Fang, and Sylvi Rusli of SKI’s NMR core facility for assistance.
Footnotes
Supporting Information. General experimental procedures, including spectroscopic and analytical data for new compounds. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Montreuil J, Vliegenthart JFG, HS, editors. Glycoproteins and Disease. Elsevier Science; 1996. [Google Scholar]
- 2.(a) Wittmann V. Glycopeptides and Glycoproteins. Synthesis, Structure, and Application Springer. 2010 [Google Scholar]; (b) Montreuil J. Glycoproteins. Elsevier Science; 1995. [Google Scholar]
- 3.(a) Davis BG. Chem Rev. 2002;102:579. doi: 10.1021/cr0004310. [DOI] [PubMed] [Google Scholar]; (b) Gamblin DP, Scanlan EM, Davis BG. Chem Rev. 2008;109:131. doi: 10.1021/cr078291i. [DOI] [PubMed] [Google Scholar]
- 4.Ogawa T, Sugimoto M, Kitajima T, Sadozai KK, Nukada T. Tetrahedron Lett. 1986;27:5739. [Google Scholar]
- 5.Kajihara Y, Suzuki Y, Yamamoto N, Sasaki K, Sakakibara T, Juneja LR. Chem Eur J. 2004;10:971. doi: 10.1002/chem.200305115. [DOI] [PubMed] [Google Scholar]
- 6.Seifert J, Lergenmüller M, Ito Y. Angew Chem Int Ed. 2000;39:531. doi: 10.1002/(sici)1521-3773(20000204)39:3<531::aid-anie531>3.3.co;2-6. [DOI] [PubMed] [Google Scholar]
- 7.Pratt MR, Bertozzi CR. J Am Chem Soc. 2003;125:6149. doi: 10.1021/ja029346v. [DOI] [PubMed] [Google Scholar]
- 8.Jensen PH, Karlsson NG, Kolarich D, Packer NH. Nature Protocols. 2012;7:1299. doi: 10.1038/nprot.2012.063. [DOI] [PubMed] [Google Scholar]
- 9.Gervais A, Hammel Y-a, Pelloux S, Lepage P, Baer G, Carte N, Sorokine O, Strub J-m, Koerner R, Leize E, Van Dorsselaer A. Glycobiology. 2003;13:179. doi: 10.1093/glycob/cwg020. [DOI] [PubMed] [Google Scholar]
- 10.Damm J, Kamerling J, Dedem G, Vliegenthart J. Glycoconjugate J. 1987;4:129. [Google Scholar]
- 11.(a) Dudkin VY, Miller JS, Danishefsky SJ. J Am Chem Soc. 2003;126:736. doi: 10.1021/ja037988s. [DOI] [PubMed] [Google Scholar]; (b) Dudkin VY, Miller JS, Dudkina AS, Antczak C, Scheinberg DA, Danishefsky SJ. J Am Chem Soc. 2008;130:13598. doi: 10.1021/ja8028137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schauer R. Curr Opin Struct Biol. 2009;19:507. doi: 10.1016/j.sbi.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu B, Hua Z, Warren JD, Ranganathan K, Wan Q, Chen G, Tan Z, Chen J, Endo A, Danishefsky SJ. Tetrahedron Lett. 2006;47:5577. doi: 10.1016/j.tetlet.2006.09.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Walczak MA, Danishefsky SJ. J Am Chem Soc. 2012;134:16430. doi: 10.1021/ja307628w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nagorny P, Fasching B, Li X, Chen G, Aussedat B, Danishefsky SJ. J Am Chem Soc. 2009;131:5792. doi: 10.1021/ja809554x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Allen JR, Allen JG, Zhang XF, Williams LJ, Zatorski A, Ragupathi G, Livingston PO, Danishefsky SJ. Chem Eur J. 2000;6:1366. doi: 10.1002/(sici)1521-3765(20000417)6:8<1366::aid-chem1366>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 17.Ishii K, Iwasaki M, Inoue S, Kenny PT, Komura H, Inoue Y. J Biol Chem. 1989;264:1623. [PubMed] [Google Scholar]
- 18.Barinka C, Šácha P, Sklenář J, Man P, Bezouška K, Slusher BS, Konvalinka J. Protein Sci. 2004;13:1627. doi: 10.1110/ps.04622104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Davis MI, Bennett MJ, Thomas LM, Bjorkman PJ. Proc Natl Acad Sci USA. 2005;102:5981. doi: 10.1073/pnas.0502101102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Likhosherstov LM, Novikova OS, Derevitskaja VA, Kochetkov NK. Carbohydr Res. 1986;146:C1. doi: 10.1016/0008-6215(90)84093-a. [DOI] [PubMed] [Google Scholar]
- 21.Cohen-Anisfeld ST, Lansbury PT. J Am Chem Soc. 1993;115:10531. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.