Abstract
Purpose
Fatigue is a highly prevalent and clinically significant symptom of advanced prostate cancer. To date, however, there are no published controlled trials of interventions for fatigue in men with prostate cancer.
Method
This six-week, randomized, double-blind, placebo-controlled design, evaluated the efficacy of methylphenidate to treat fatigue in prostate cancer patients. Inclusion criteria included men with advanced prostate cancer and the presence of moderate to severe fatigue. Patients with major depression, hypothyroidism, uncontrolled hypertension, arrhythmia or anemia were excluded. Fatigue levels, blood pressure, pulse and other safety concerns were monitored regularly.
Results
Thirty-two subjects were randomized to methylphenidate (N=16) or placebo (N=16). Brief Fatigue Inventory (BFI) total scores significantly decreased for both groups, however the methylphenidate group, as compared to placebo, reported greater decrease on BFI severity scores (p=.03) and a trend toward greater decrease on BFI total scores (p=.07). A significantly greater number of subjects in the methylphenidate group vs. the placebo group demonstrated clinically significant improvement in fatigue on total BFI scores (7/10 vs. 3/13) and BFI severity scores (8/10 vs. 3/13). Importantly, six subjects in the methylphenidate group discontinued due to increased blood pressure or tachycardia. There were no serious adverse events.
Conclusions
Methylphenidate is effective in treating fatigue in men with prostate cancer; however, oncologists need to monitor for possible pulse and blood pressure elevations.
Introduction
Fatigue is a common and distressing symptom of cancer and cancer treatments1–5. The impact of fatigue has been associated with considerable negative effects on mood, leading to or exacerbating anxiety or depressive symptoms, functional morbidity, poor quality of life, and has a significant negative impact on caregiver6–10.
Prostate cancer is the most common site of cancer in males in the United States11. Patients with advanced prostate cancer are amongst those most at risk for developing fatigue because they are likely to have widespread bony metastases and/or anemia and are likely to receive androgen deprivation agents or anti-androgens8, 12–14, radiation therapy15–17, 18–21, or chemotherapy. Reports estimate that fatigue is a distressing symptom in up to 67% of prostate cancer patients22, 23.
Psychostimulants have been used with some success in treating fatigue of various etiologies18–21, 24–27, 28–33, and are widely regarded to be safe34. While controlled studies examining non-pharmacological interventions35 as well as pharmacological interventions for fatigue related to cancer have being conducted,8, 18, 28, 29, 33, 36–38 pharmacological interventions for fatigue in prostate cancer patients have not been extensively studied39–42.
Bruera et al. recently published two double-blind, randomized controlled trials (RCT), where palliative cancer patients were prescribed either methylphenidate29 or donepezil28 against placebo for 7 days. The authors found that fatigue intensity improved in each of the three groups, yet there were no between group differences between the active medication groups and placebo groups, suggesting that these medications were not significantly superior to placebo. In an RCT of methylphenidate vs. pemoline vs placebo in HIV patients, Breitbart et al.6 also found a placebo effect; however, there was a significant but delayed improvement in fatigue in the two psychostimulant groups, with significant differences noted from the placebo group emerging at week three of the trial. Lower et al. recently reported on the impact of dexmethylphenidate in an RTC for fatigue following chemotherapy in cancer patients. In this heterogeneous population, the placebo effect was also strong, however the medication group reported lower levels of fatigue for all 8 weeks of the study43. Given the high levels of fatigue in prostate cancer, the lack of RCT studies testing pharmacological treatments for fatigue in prostate cancer, and the contradictory results of current RTC’s in cancer patients, it is important to test the potential benefit of psychostimulants with an appropriate time period in the management of fatigue in cancer patients in general and in prostate cancer patients in particular.
Methods/Study Design
This was a double-blind, placebo controlled, dose-titrated, six week intervention trial comparing the efficacy and monitoring safety of the psychostimulant methylphenidate against placebo in the treatment of fatigue in ambulatory men with advanced prostate cancer supported by an NIH RO1 grant # CA-85229-01A1. This study received Institutional Review Board approval at Memorial Sloan-Kettering Cancer Center (MSKCC).
Men with prostate cancer were identified and screened for fatigue in the outpatient waiting areas. To pass this initial one question screen, men had to rate their average level of fatigue over the previous two weeks as a 4 or greater on a 0–10 numeric rating scale (i.e., “moderate” to “severe” fatigue). Patients who qualified for the study under this initial screen were then asked secondary screening questions to rule out cognitive impairment (Mini Mental Status Exam), major depression (Structural Clinical Interview of the DSM-IV)), and medical conditions and medications that would contraindicate the use of a psychostimulant. Patients with anemia (i.e., severe anemia where the hemoglobin is less than 11.0) who had received six weeks of epoetin alfa therapy and still had significant fatigue were eligible for this stimulant trial. Patients with hypothyroidism who had received six weeks of thyroid supplementation therapy and still had significant fatigue were also eligible for this stimulant trial. Patients had to be able to give informed consent.
The patients were randomly assigned by the hospital pharmacy to receive either methylphenidate or placebo for a period of 6 weeks. The research staff remained blinded to this assignment. Methylphenidate was administered in capsules containing 5 mg each, with a starting dose of one capsule in the morning. Patients in the placebo group received identically appearing capsules. All other medications were held at their usual doses during the study period unless a change was mandated by the clinical situation. All medications taken during the study period were recorded by the patient on the medication diary.
The research nurse or physician was in contact with patients at least twice a week, and more often if necessary, in order to assess the need for titration of dosage and to ensure patients’ safety. The nurse monitored blood pressure, pulse and other vital signs weekly. The dose was increased by one capsule (5 mg) on day 3, added as a midday dose, if fatigue was not substantially reduced, there was no toxicity from the study treatment, and if the patient was willing to increase the dose. Dosage was titrated upwards (or down) every 2–3 days to a maximum of 6 capsules daily, divided into morning and midday doses (equivalent to a total maximum daily dose of 30 mg of methylphenidate).
One week’s supply of the study drug was dispensed at each weekly evaluation point. Baseline information related to fatigue etiology, severity, and treatment responsiveness of fatigue was collected at study entry and at the end of the study (6 weeks). Primary outcome measurements of fatigue were the Brief Fatigue Inventory(BFI)44 and the Fatigue Severity Scale(FSS)45. The BFI has two subscales: a 4-question fatigue severity subscale, and 5-question fatigue interference subscale. The BFI was chosen as the primary outcome because it has demonstrated good psychometrics, and it assesses both severity and interference related to fatigue44. The FSS questions focus primarily on interference questions about physical functioning, work, family, and social life. Secondary outcome measurements included depression and psychological distress (the Hospital Anxiety and Depression Scale46 and the Beck Depression Inventory47), quality of life (the Functional Assessment of Cancer Therapy Scale-Prostate Cancer48), and cognitive and neuropsychological test performance. Patients also completed a demographic questionnaire, the treatment emergent side effects (SAFTEE) questionnaire49, and the Extrapyramidal Side Effects Rating Scale (ESRS)50. The ESRS was chosen to closely monitor potential development of tics or involuntary movements that may accompany psychostimulant medication which were not assessed by SAFTEE. The patients completed the entire battery of questionnaires at baseline (before randomization) and then at the end of the study (the 6th week). Patients completed the BFI, SAFTEE, and the ESRS on a weekly basis when their vital signs were monitored. Patients were asked to complete a Medication Diary (MD) on a daily basis throughout the study.
Statistical Analysis
Descriptive statistics were used to report demographic and baseline data. Repeated measures t-tests were used to assess changes in study variables within each group to determine if there was a within-group medication or placebo effect. Change scores between baseline and the 6-week follow-up were calculated and between-subjects t-tests was used to determine if there were significant differences in these change scores for the medication group vs. the placebo group. In addition, Chi-square analysis was used to determine if a greater percentage of subjects in the medication group reported clinically significant change as compared to the placebo group.
Results
Screening and drop-out
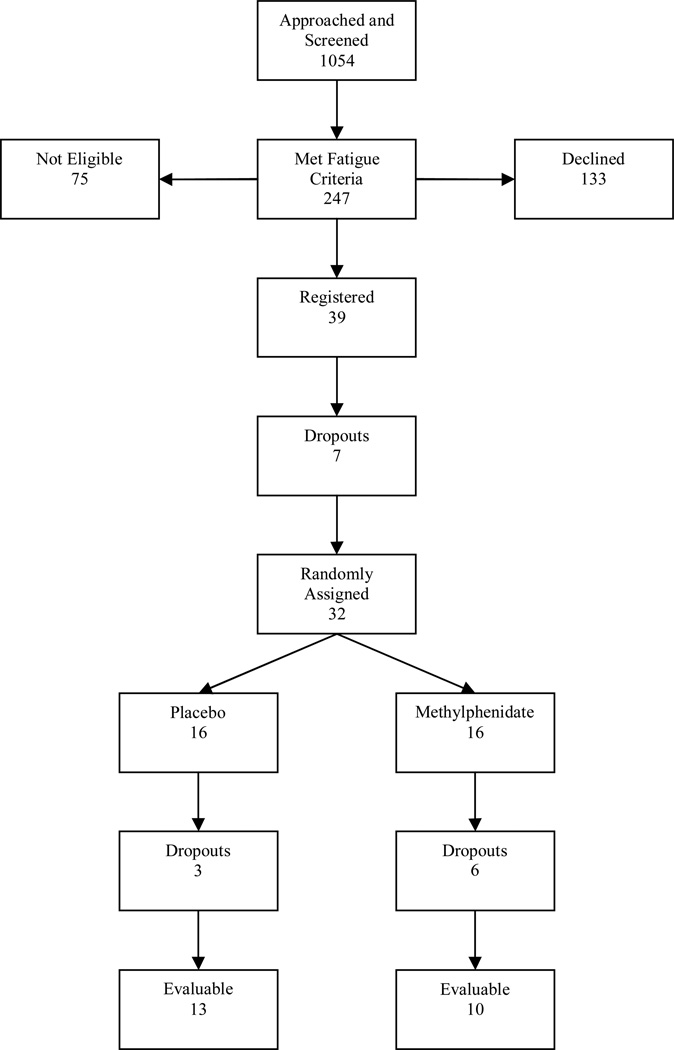
Our initial recruitment goal was to accrue 120 men to the study, with 60 subjects randomized to each treatment arm. A total of 1054 patients were screened for the study. Of those screened, 804 (77%) did not meet the fatigue eligibility criteria. Of the 247 subjects (23%) who met the initial fatigue eligibility criteria, only 39 (16%) agreed to participate in the study; 75 (30%) were ineligible because of a severe medical or psychiatric condition (i.e., uncontrolled hypertension, history of arrhythmia or other severe cardiac disease, severe renal or hepatic disease, severe anemia, major depressive disorder). 75 (30%) declined to participate in a research study, and 58 (24%) showed an interest in the study but eventually declined. Of the 39 subjects who registered for the study and completed baseline data, 7 subjects withdrew before being randomized to a treatment. Of the 32 remaining subjects, 16 were randomized to the methylphenidate group and 16 were randomized to the placebo group. The study was closed before our target enrollment goal was reached due to funding restrictions.
Of the 16 subjects assigned to the methylphenidate group, 4 (31%) men who started receiving methylphenidate had to be discontinued from the study due to increased blood pressure according to preset guidelines (equal or greater than 170/100 mmHg), and 2 (6%) man had to be discontinued from the study due to tachycardia. Of the 16 men in the placebo group, 3 (19%) dropped out of the study, citing personal reasons preventing continued study participation. There were no withdrawals from the placebo group due to reported adverse side effects. There were no abnormal movements or tics noted, and no serious adverse events related to study participation.
An intent-to-treat analysis (ITT) was considered when analyzing the decrease in fatigue and other study measures that used continuous variables. However, once a subject dropped out of the study these assessments were not collected and, as a result, there is missing data for these subjects that dropped out of the study. The last observation carried forward method would have to be used to conduct an ITT analysis in this situation, however that last observation carried forward method assumes the drop-out in the study is completely at random51, 52. As stated above, the drop-out in the medication group was in fact not random, but due to specific side effects of the medication. As a result, we will not use ITT analysis when assessing continuous variables. The final analysis of the continuous data will include 10 subjects in the methylphenidate group and 13 subjects in the placebo group (see Figure 1).
Figure 1.
Patient Flow Chart
In an attempt to comply with an ITT paradigm we conducted an adjunctive analysis where we developed a method to determine a clinically significant decrease in fatigue scores (see below)53–55.
Subject Characteristics
The average age of the subjects was 70±9 years old (range = 52 to 94). The vast majority was Caucasian (90%), married (71%), and had college educations (70%). There was no significant difference between the two study arms on baseline demographic variables or fatigue scores as measured by the BFI and the FSS (See Table 1).
Table 1.
Patient Characteristics
| Variable | Methylphenidate Group (n=10) |
Placebo Group (n = 13) |
Total (n=23) |
|---|---|---|---|
| Mean Age | 68±8 | 71±10 | 70±9 |
| Race | |||
| -Caucasian | 10 (100%) | 12 (92%) | 22 (96%) |
| -African-American | -- | 1 (8%) | 1 (4%) |
| Marital Status | |||
| -Married | 6 (60%) | 11 (85%) | 17 (74%) |
| -Single | 2 (20%) | 1 (8%) | 3 (13%) |
| -Divorced/Separated | 2 (20%) | 1 (8%) | 3 (13%) |
| Baseline BFI Score | |||
| -Total Scores | 5.13 (2.25) | 4.01 (2.00) | 4.54 (2.13) |
| -Severity Subscale | 6.50 (2.09) | 5.74 (2.05) | 6.07 (2.05) |
| -Interference Subscale | 4.45 (2.56) | 3.26 (2.30) | 3.78 (2.43) |
| Baseline FSS | |||
| -Mean Score | 4.27 (1.31) | 4.27 (1.37) | 4.27 (1.32) |
Note: No significant differences between the Methylphenidate group and the placebo group.
Change in Fatigue Scores
When analyzing the within-group results, the methylphenidate group showed a significant reduction in scores on the Brief Fatigue Inventory (BFI). The mean of the BFI total scores in the methylphenidate group decreased from 5.13 at baseline to 2.19 at end of study. This reduction was statistically significant (t (9) = 3.63, p = .01). The results for the methylphenidate group for the severity and interference subscales of the BFI were similar in magnitude and significance (See Table 2). There was also a placebo effect seen in this study. The placebo group showed a reduction in BFI total scores from baseline (4.09) to end of study (2.84), t (12) = 2.58, p = .02. The placebo group also reported a significant reduction in the interference subscale of the BFI, but not in the severity subscale. In terms of the second primary outcome, the FSS, the methylphenidate group showed a trend toward significant reduction (t (9) = 1.99, p = .08). There was not a significant reduction in FSS scores in the placebo group (See Table 2).
Table 2.
Improvement in fatigue from baseline to week 6
| Variable | Methylphenidate | Placebo |
|---|---|---|
| Brief Fatigue Inventory | ||
| -Total Scores | 2.9 (2.6) | 1.25 (1.7) |
| -Severity subscale | 3.5 (2.9) | 1.1 (2.1) |
| -Interference subscale | 2.7(3.0) | 1.3 (1.7) |
| Fatigue Severity Scale | .73 (1.2) | .59 (1.2) |
Note: Bold indicates p < .05
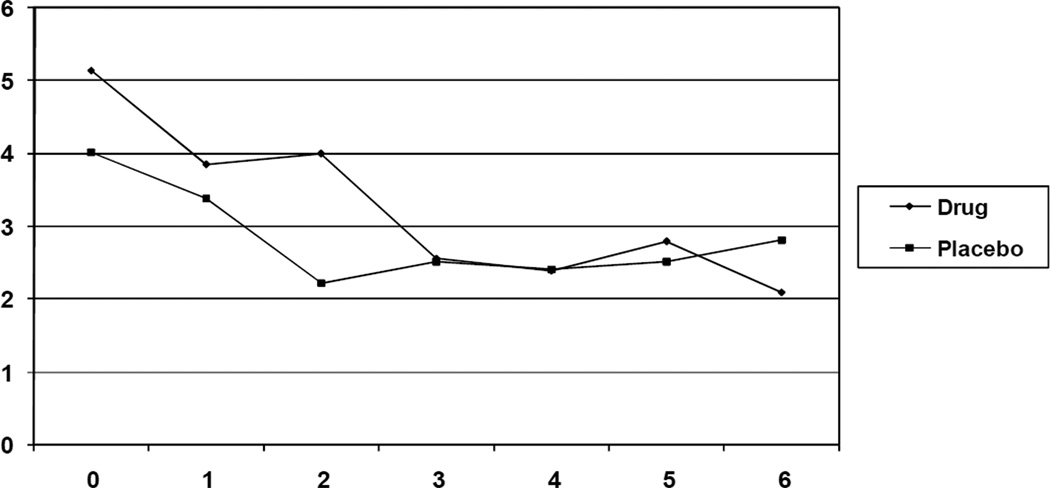
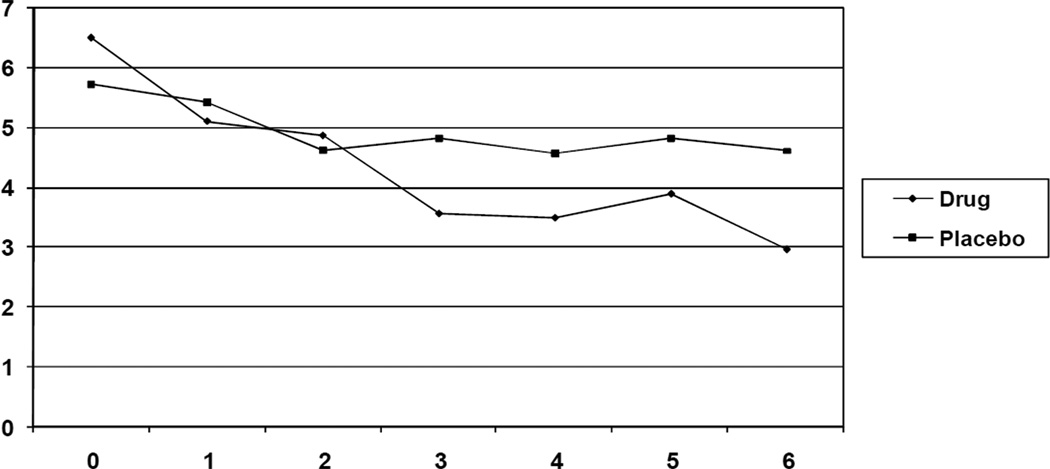
When comparing the change scores for the BFI total scores between the two groups, there was a trend toward a significant difference (t (21) = 1.89, p = .07, d = .80) indicating that the reduction in scores in the methylphenidate group was greater than the reduction in scores in the placebo group. When analyzing the subscales of the BFI, the methylphenidate group showed a significantly greater reduction in BFI severity scores (t (21) = 2.28, p = .03, d = .93) as compared to the placebo group. There was no significant difference in the change scores in the interference subscale of the BFI. All differences were associated with large effects sizes, indicating that if the sample size was larger, the differences between groups in change scores of the BFI total score and interference subscale would have been statistically significant (See Table 3). Figures 2 and 3 present the weekly BFI data for the total BFI scores and the fatigue severity scores, respectively. It appears the difference for the total BFI scores is not seen until week 6, while the separation for severity scores is seen at week 3. Additionally, there was no significant difference in change scores when analyzing the FSS scores (See Table 3).
Table 3.
Comparing fatigue change scores for Methylphenidate group vs. placebo
| Variable | Mean difference in change scores |
P | d |
|---|---|---|---|
| Brief Fatigue Inventory | |||
| - Total Scores | 1.7 | .07 | .80 |
| - Severity subscale | 2.4 | .03 | .93 |
| - Interference subscale | 1.3 | .19 | .57 |
| Fatigue Severity Scale | .14 | .78 | .12 |
Figure 2.
Mean BFI Total Scores
Figure 3.
Mean BFI Severity Scores
Clinically Significant Reduction in Fatigue
We classified subjects as having clinically significant reduction in fatigue scores on the BFI and FSS if their fatigue score decreased by 1 standard deviation of the baseline scores56. There are no formal methods for the BFI or the FSS to determine clinically meaningful changes in fatigue scores. The distribution method used here (i.e., 1 standard deviation) is consistent with the literature determining clinically meaningful differences56. One standard deviation of the baseline measures for the total BFI scores, the BFI severity subscale, and the BFI interference subscale were as follows: 2.1, 2.1, and 2.4 respectively. When using an ITT (i.e., retaining all those subjects who were randomized) those subjects who dropped out of the study were considered to not have reached a clinically significant decline in fatigue scores. When examining clinically meaningful change of total BFI scores, there was no difference between the number of subjects in the methylphenidate group (7/16) who reported a clinically significant reduction in BFI total scores as compared to the placebo group (3/16; X2 = 2.33, p = 0.13). However, there was a trend toward significant results on the BFI severity scale (8/16 in the methylphenidate group vs. 3/16 in the placebo group, p=0.06). There were no differences seen in the BFI interference scale or the FSS scores (1 standard deviation = 1.3).
Since the reasons for drop-out in the methylphenidate group are detailed, and ITT analysis has been criticized because it may miss potentially important findings57 when including drop-outs washes out significant results, this analysis was also run using only those subjects who completed the study. When examining clinically meaningful change of total BFI scores, significantly more subjects in the methylphenidate group (7/10) reported a clinically significant reduction in BFI total scores as compared to the placebo group (3/13; X2 = 5.06, p = 0.02; RR = 3.03 CI: 1.04 to 8.83). Similar results in significance and magnitude were also reported for the BFI severity subscale; and similar results in magnitude were reported for the interference subscale although not statistically significant (p = 0.07; see Table 4). There was no difference in clinically significant change in FSS scores (1 standard deviation) between groups.
Table 4.
Clinically significant decrease in fatigue scores
| Variables | Methylphenidate | Placebo | P | RR | CI |
|---|---|---|---|---|---|
| BFI | |||||
| -Total Scores | 7/10 | 3/13 | 0.02 | 3.04 | 1.04 to 8.86 |
| -Severity | 8/10 | 3/13 | 0.01 | 3.47 | 1.23 to 9.81 |
| -Interference | 5/10 | 2/13 | 0.07 | 3.25 | 0.79 to 13.41 |
| FSS | 2/10 | 5/13 | 0.34 | 0.52 | 0.15 to 2.15 |
When assessing the impact of the interventions on depression, anxiety, QOL, and measures of cognitive functioning, there were no significant differences seen between the two groups. However, the differences in the change in depression scores did produce potentially important effects sizes. The Cohen’s d effect size for the HADS Depression subscale and the Beck Depression inventory were 0.54 and 0.38, respectively.
Discussion
Our results suggest there is a decline in fatigue levels with the use of methylphenidate in men with advanced prostate cancer. Not unlike other psychostimulant trials for fatigue6, 29 there was a large placebo effect in our study; and our results indicate a separation of placebo and study drug effects are seen at week 3 (severity scores) and week 6 (total scores). It is important to consider this placebo effect and time to detect difference between groups when comparing these results to previous studies designed to test the efficacy of stimulants in cancer patients. Bruera et al. reported negative results in palliative cancer patients, however the study period was only 7 days28, 29, and may not have been long enough to see separation from the methylphenidate arm. The negative results may also have been related to the severity of disease in the palliative population. To contrast the Bruera et al. results, two recent reports show efficacy of stimulants for fatigue in cancer patients. Blackhall et al. reported a decline in fatigue in 19 cancer patients using Modafinil, and significant results were seen at week 4 of the study58. These results should be considered preliminary as there was no control group in this study. In a well designed and powered RCT testing dexmethylphenidate for chemotherapy related fatigue, Lower et al. reported a significant reduction in fatigue in the study group compared to placebo43. This study also showed a large placebo effect, however the separation between groups occurred the first week post initiation of treatment and continued for the remaining 7 weeks of the study. The patients in the Lower et al. study were physically healthier (none were palliative) than the patients studied by Bruera et al. When compared to the study group in this current manuscript, the Lower et al. subjects were considerably younger (mean age 53 vs. 70) and the study medication used was D-isomer of methylphenidate whose clinical efficacy is found a half the dose. Lastly, the placebo effect in all these studies is rather remarkable. For example, in both the data reported here and also in the Lower et al. study, the placebo group demonstrated a 30% to 50% decrease in fatigue scores, and in both studies it appears the placebo remained effective through the entire study period. This underscores the importance that future studies that focus on fatigue should have a placebo control.
There was noteworthy difficulty accruing patients to the study which highlights some interesting issues. With over 1000 men screened for fatigue from the medical genitourinary (GU) oncology clinic at MSKCC, only 23% met criteria for moderate to severe fatigue. This is a much lower level of fatigue than noted in the literature. Of those men who were eligible for the study, 84% declined entry into the study, often stating that they did not want to be on yet another medication, or they did not want to participate in a research study. Older cancer patients are not easily recruited to clinical trials59. Many who would consider participation in a clinical trial are hesitant to accept taking a medication that does not specifically target treatment of the cancer. Older patients are also fearful about potential side effects and drug interactions that may hamper an already compromised quality of life.
Many physicians display some hesitancy in prescribing controlled substances to older cancer patients because of concerns about the ability to tolerate these medications. Although our study did have some men reporting side effects from the medication, with careful observation, there were no severe adverse reactions. There are now additional studies that use stimulants to safely treat cancer related fatigue. Of the men who tolerated methylphenidate, many showed significant improvement in their fatigue and most wanted to continue the medicine at the end of the trial, In fact many called it a “wonder drug.” Despite this, we did have 6 men who had to come off of the study because of medication side effects.: 4 subjects experienced a rise in blood pressure beyond study parameters, and 2 patients had tachycardia. This is a high rate compared to other studies testing methylphenidate. We have reviewed the study charts of these men and have attempted to find some predictors of these cardiovascular side effects, however no consistent predictors emerged. One reason for this may be that the study was designed to test efficacy and maintain safety as opposed to explicitly examining the etiology of those men who experience cardiovascular side effects from the medication. As a result, the approved IRB protocol was not designed to keep a detailed medical history for each subject once the extensive eligibility requirements were satisfied. We can report that all men were either on hormone ablation therapy and/or chemotherapy, however to the best of our knowledge methylphenidate does not interact with these medications in a way that would raise blood pressure. The range of medication dose for men who experienced cardiovascular side effects was 10mg to 30mg, and the rise in blood pressure or pulse rate was experienced between week 2 and week 5. The medication escalation in this study was similar to other studies that investigated methylphenidate6. In fact, the Breitbart study, started subjects at a higher dose (7.5mg/day) and titrated the study drug to higher end dosages (60mg/day). In terms of medication escalation, it is difficult to compare this current study with the Lower et al study, since the Lower et al. study used a different isomer of methylphenidate and therefore had a different dosing regimen. All of the men who experienced these side effects were on multiple medications, but all medications with known interactions with methylphenidate were exclusionary criteria. Despite the fact that the average age of the study population was high (mean age of 70 years old), this also does not appear to be a predictors since the average age of those men who experienced these side effects was 64. Considering these results, it is important to conduct good clinical practice with all men who are prescribed these stimulants and appropriately monitor blood pressure and heart rate.
The limitations of the study have been alluded to previously. First, the total number of patients accrued to the trial as well as the numbers in each study group was extremely small, especially compared to our original target numbers. As such, these results should be considered preliminary and suggest that continued effort should be extended to conducting a larger trial. It is possible that the population of older prostate cancer patients at our tertiary care cancer center has different fatigue levels than at other treatment locations. It is also possible that our one question screening measure of ‘fatigue in the last 2 weeks’ was not sensitive enough to identify the actual prevalence of fatigue in our clinic patients.
Our study points to future research that will help improve the quality of life of men with prostate cancer. Consideration of a psychostimulant that might have fewer cardiovascular side effects than methylphenidate, such as modafinil which is a wakefulness-promoting agent that is less potently sympathomimetic than conventional stimulants such as methylphenidate and dextroamphetamine, may lead to better tolerance of the study medication and therefore fewer dropouts. A feasibility trial of longer acting psychostimulants which have become available in the last few years may decrease the burden of patients who have to take multiple dosing of medications, often leading to decreased compliance.
In conclusion, fatigue is an important quality of life parameter in cancer patients, though it was not as prevalent in our population of men with prostate cancer as the literature suggests. This is the first randomized placebo controlled trial of a psychostimulant for fatigue in a prostate cancer population. Data from this study suggests that methylphenidate is effective in treating fatigue in men with prostate cancer; however, oncologists need to monitor these men regularly for possible pulse and blood pressure elevations.
Acknowledgments
This study was funded by a grant from the NIH (Grant #5R01- CA-85229) and ongoing support from the PepsiCo Foundation.
References
- 1.Reddy S, Bruera E, Pace E, Zhang K, Reyes-Gibby CC. Clinically important improvement in the intensity of fatigue in patients with advanced cancer. J Palliat Med. 2007;10(5):1068–1075. doi: 10.1089/jpm.2007.0007. [DOI] [PubMed] [Google Scholar]
- 2.Forlenza M, Hall P, Lichtenstein P, Evengard B, Sullivan PF. Epidemiology of cancer-related fatigue in the Swedish twin registry. Cancer. 2005;104(9):2022–2031. doi: 10.1002/cncr.21373. [DOI] [PubMed] [Google Scholar]
- 3.Wagner L, Cella D. Fatigue and cancer: causes, prevalence and treatment approaches. Br J Cancer. 2004;91(5):822–828. doi: 10.1038/sj.bjc.6602012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ahlberg K, Ekman T, Gaston-Johansson F, Mock V. Assessment and management of cancer-related fatigue in adults. Lancet. 2003;362(9384):640–650. doi: 10.1016/S0140-6736(03)14186-4. [DOI] [PubMed] [Google Scholar]
- 5.Stone P, Richards M, A'Hern R, Hardy J. A study to investigate the prevalence, severity and correlates of fatigue among patients with cancer in comparison with a control group of volunteers without cancer. Ann Oncol. 2000;11(5):561–567. doi: 10.1023/a:1008331230608. [DOI] [PubMed] [Google Scholar]
- 6.Breitbart W, Rosenfeld B, Kaim M, Funesti-Esch J. A randomized, double blind placebo-controlled trial of psychostimulants for the treatment of fatigue in ambulatory patients with HIV disease. Arch Internal Medicine. 2001;161(3):411–420. doi: 10.1001/archinte.161.3.411. [DOI] [PubMed] [Google Scholar]
- 7.Smets E, Garssen B, Schuster-Ultterhoeve ALJ, de Haes JCJM. Fatigue in cancer patients. British Journal of Cancer. 1993;68:220–224. doi: 10.1038/bjc.1993.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stone P, Hardy J, Huddart R, A’Hern R, Richards M. Fatigue in patients with prostate cancer receiving hormone therapy. Eur. J Cancer. 2000;36:1134–1141. doi: 10.1016/s0959-8049(00)00084-8. [DOI] [PubMed] [Google Scholar]
- 9.Barnes E, Bruera E. Fatigue in patients with advanced cancer: a review. Int J Gynecol Cancer. 2002;12(5):424–428. doi: 10.1046/j.1525-1438.2002.t01-1-01147.x. [DOI] [PubMed] [Google Scholar]
- 10.Butt Z, Rosenbloom SK, Abernethy AP, Beaumont JL, Paul D, Hampton D, Jacobsen PB, Syrjala KL, Von Roenn JH, Cella D. Fatigue is the most important symptom for advanced cancer patients who have chemotherapy. J Natl Compr Canc Netw. 2008;6(5):448–455. doi: 10.6004/jnccn.2008.0036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cancer Facts & Figures. American Cancer Society. 2008 [Google Scholar]
- 12.Joly F, Alibhai SM, Galica J, Park A, Yi QL, Wagner L, Tannock IF. Impact of androgen deprivation therapy on physical and cognitive function, as well as quality of life of patients with nonmetastatic prostate cancer. J Urol. 2006;176(Pt 1):2443–2447. doi: 10.1016/j.juro.2006.07.151. [DOI] [PubMed] [Google Scholar]
- 13.Nakabayashi M, Xie W, Regan MM, Jackman DM, Kantoff PW, Oh WK. Repsonse to low-dose ketoconazole and subsequent dose escalation to high-doe ketoconazole in patients with androgen-independent prostate cancer. Cancer. 107(5):975–981. doi: 10.1002/cncr.22085. 206. [DOI] [PubMed] [Google Scholar]
- 14.Bok R, Small EJ. The treatment of advanced prostate cancer with ketoconazole: safety issues. Drug Saf. 1999;20:451–458. doi: 10.2165/00002018-199920050-00005. [DOI] [PubMed] [Google Scholar]
- 15.Kunkel E, Bakker JMR, Myers RE, Oyesanmi O, Gomella LG. Biopsychosocial aspects of prostate cancer. Psychosomatics. 2000;41:85–94. doi: 10.1176/appi.psy.41.2.85. [DOI] [PubMed] [Google Scholar]
- 16.Zelefsky M, Kelly WK, Scher HI, Lee H, Smart T, Metz E, Schwartz L, Fuks Z, Leibel SA. Results of a phase II study using estramustine phosphate and vinblastine in combination with high-dose three-dimensional conformal radiotherapy for patients with locally advanced prostate cancer. Journal of Clinical Oncology. 2000;18:1936–1941. doi: 10.1200/JCO.2000.18.9.1936. [DOI] [PubMed] [Google Scholar]
- 17.Monga U, Kerrigan AJ, Thornby J, Monga TN. Prospective study of fatigue in localized prostate cancer patients undergoing radiotherapy. Radia. Oncol. Investig. 1999;7:178–185. doi: 10.1002/(SICI)1520-6823(1999)7:3<178::AID-ROI7>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 18.Bruera E, Chadwick S, Brennels C, Hanson J, MacDonald RN. Methylphenidate associated with narcotics for the treatment of cancer pain. Cancer Treatment Reports. 1987;71:67–70. [PubMed] [Google Scholar]
- 19.Breitbart W, Mermelstein C. An alternative psychostimulant for the management of depressive disorders in cancer patients. Psychosomatics. 1992;33:353–356. doi: 10.1016/s0033-3182(92)71979-3. [DOI] [PubMed] [Google Scholar]
- 20.Sarhill N, Walsh D, Nelson KA, Homsi J, LeGrand S, Davis MP. Methylphenidate for fatigue in advanced cancer: a prospective open-label pilot study. Am J Hosp Palliat Care. 2001;18(3):187–192. doi: 10.1177/104990910101800310. [DOI] [PubMed] [Google Scholar]
- 21.Iop A, Manfredi AM, Bonura S. Fatigue in cancer patients receiving chemotherapy: an analysis of published studies. Ann Oncol. 2004;15(5):712–720. doi: 10.1093/annonc/mdh102. [DOI] [PubMed] [Google Scholar]
- 22.Kornblith A, Herr HW, Ofman US, Scher HI, Holland JC. Quality of life of patients with prostate cancer and their spouses: The value of a database in clinical care. Cancer. 1994;73:2791–2802. doi: 10.1002/1097-0142(19940601)73:11<2791::aid-cncr2820731123>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 23.Portenoy R. The Memorial Symptom Assessment Scale: an instrument for the evaluation of symptom prevalence, characteristics and distress. European Journal of Cancer. 1994;30A(9):1326–1336. doi: 10.1016/0959-8049(94)90182-1. [DOI] [PubMed] [Google Scholar]
- 24.Katon W, Raskind M. Treatment of depression in the medically ill elderly with methylphenidate. Am J Psychiatry. 1980;137:963–965. doi: 10.1176/ajp.137.8.963. [DOI] [PubMed] [Google Scholar]
- 25.Kaufmann M, Murray GB, Cassem NH. Use of psychostimulants in medically ill depressed patients. Psychosomatics. 1982;23:817–819. doi: 10.1016/S0033-3182(82)73080-4. [DOI] [PubMed] [Google Scholar]
- 26.Fernandez F, Levy J, Galizzi H. Response of HIV-related depression to psychostimulants: case reports. Hospital and Community Psychiatry. 1988b;39:628–631. doi: 10.1176/ps.39.6.628. [DOI] [PubMed] [Google Scholar]
- 27.Holmes V, Fernandez F, Levy JK. Psychostimulant response in AIDS-related complex patients. J Clin Psychiatry. 1989;50:5–8. [PubMed] [Google Scholar]
- 28.Bruera E, El Osta B, Valero V, Driver LC, Pei BL, Shen L, Poulter VA, Palmer JL. Donepezil for cancer fatigue: a double-blind, randomized, placebo-controlled trial. J Clin Oncol. 2007;25(23):475–481. doi: 10.1200/JCO.2007.10.9231. [DOI] [PubMed] [Google Scholar]
- 29.Bruera E, Valero V, Driver L, Shen L, Willey J, Zhang T, Palmer JL. Patient-controlled methylphenidate for cancer fatigue: a double-blind, randomized, placebo-controlled trial. J Clin Oncol. 2006;24(13):2073–2078. doi: 10.1200/JCO.2005.02.8506. [DOI] [PubMed] [Google Scholar]
- 30.Sood A, Barton DL, Loprinzi CL. Use of methylphenidate in patients with cancer. Am J Hosp Palliat Care. 2006;23(1):35–40. doi: 10.1177/104990910602300106. [DOI] [PubMed] [Google Scholar]
- 31.Lower E, Fleishman S, Cooper A, Zeldis J, Faleck H, Manning D. A phase III, randomized placebo-controlled trial of the safety and efficacy of d-MPH as new treatment of fatigue and “chemobrain” in adult cancer patients. JCO. 2005;(Supplement 23)(16S):8000. [Google Scholar]
- 32.Mock V. Evidence-based treatment for cancer-related fatigue. J Natl Cancer Inst Monogr. 2004;(32):112–118. doi: 10.1093/jncimonographs/lgh025. [DOI] [PubMed] [Google Scholar]
- 33.Cullum J, Wojciechowski AE, Pelletier G, Simpson JS. Bupropion sustained release treatment reduces fatigue in cancer patients. Can J Psychiatry. 2004;49(2):139–144. doi: 10.1177/070674370404900209. [DOI] [PubMed] [Google Scholar]
- 34.Bruera E, Brenneis C, Paterson AH, MacDonald RN. Use of methylphenidate as an adjuvant to narcotic analgesics in patients with advanced cancer. J Pain Symptom Manage. 1989;4:3–6. doi: 10.1016/0885-3924(89)90057-2. [DOI] [PubMed] [Google Scholar]
- 35.Schmitz K, Holtzman J, Courneya KS, Mâsse LC, Duval S, Kane R. Controlled physical activity trials in cancer survivors: a systematic review and meta-analysis. Cancer Epidemiol Biomarkers Prev. 2005;14(7):1588–1595. doi: 10.1158/1055-9965.EPI-04-0703. [DOI] [PubMed] [Google Scholar]
- 36.Monga U, Garber SL, Thornby J, Vallbona C, Kerrigan AJ, Monga TN, Zimmerman KP. Exercise prevents fatigue and improves quality of life in prostate cancer patients undergoing radiotherapy. Arch Phys Med Rehabil. 2007;88(11):1416–1422. doi: 10.1016/j.apmr.2007.08.110. [DOI] [PubMed] [Google Scholar]
- 37.Atkinson A, Barsevick A, Cella D, Cimprich B, Cleeland C, Donnelly J, Eisenberger MA, Escalante C, Hinds P, Jacobsen PB, Kaldor P, Knight SJ, Peterman A, Piper BF, Rugo H, Sabbatini P, Stahl C. NCCN practice Guidelines for cancer-related fatigue. Oncology. 2000;14(11A):151–161. [PubMed] [Google Scholar]
- 38.Bruera E, MacDonald RN. Asthenia in patients with advanced cancer. J Pain Sympt Manag. 1988;3:9–14. doi: 10.1016/0885-3924(88)90132-7. [DOI] [PubMed] [Google Scholar]
- 39.Segal R, Reid RD, Courneya KS, Sigal RJ, Kenny GP, Prud'homme DG, Malone SC, Wells GA, Scott CG, Slovinec D'Angelo ME. Randomized Controlled Trial of Resistance or Aerobic Exercise in Men Receiving Radiation Therapy for Prostate Cancer. J Clin Oncol. 2008 Dec 8; doi: 10.1200/JCO.2007.15.4963. [DOI] [PubMed] [Google Scholar]
- 40.Culos-Reed S, Robinson JL, Lau H, O'Connor K, Keats MR. Benefits of a physical activity intervention for men with prostate cancer. J Sport Exerc Psychol. 2007;29(1):118–127. doi: 10.1123/jsep.29.1.118. [DOI] [PubMed] [Google Scholar]
- 41.Windsor P, Nicol KF, Potter J. A randomized, controlled trial of aerobic exercise for treatment-related fatigue in men receiing radical external beam radiotherapy for localized prostate carcinoma. Cancer. 2004;101(3):550–557. doi: 10.1002/cncr.20378. [DOI] [PubMed] [Google Scholar]
- 42.Segal R, Reid RD, Courneya KS, Malone SC, Parliament MB, Scott CG, Venner PM, Quinney HA, Jones LW, D'Angelo ME, Wells GA. Resistance exercise in men receiving androgen deprivation therapy for prostate cancer. J Clin Oncol. 2003;21(9):1653–1659. doi: 10.1200/JCO.2003.09.534. [DOI] [PubMed] [Google Scholar]
- 43.Lower EE, Fleishman S, Cooper A, et al. Efficacy of dexmethylphenidate for the treatment of fatigue after cancer chemotherapy: a randomized clinical trial. J Pain Symptom Manage. 2009 Nov;38(5):650–662. doi: 10.1016/j.jpainsymman.2009.03.011. [DOI] [PubMed] [Google Scholar]
- 44.Mendoza TRWX, Cleeland CS, Morrissey M, Johnson BA, Wendt JK, Huber SL. The rapid assessment of fatigue severity in cancer patients: use of the Brief Fatigue Inventory. Cancer. 1999;85(5):1186–1196. doi: 10.1002/(sici)1097-0142(19990301)85:5<1186::aid-cncr24>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 45.Krupp LBLN, Muir-Nash J, Steinberg AD. The fatigue severity scale. Application to patients with multiple sclerosis and systemic lupus erythematosus. Arch Neurol. 1989;46(10):1121–1123. doi: 10.1001/archneur.1989.00520460115022. [DOI] [PubMed] [Google Scholar]
- 46.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta psychiatrica Scandinavica. 1983;67(6):361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 47.Beck AT. Psychiatry: Cognitive Therapy for Depression and Panic Disorder. West J Med. 1989 Sep;151(3):311. [PMC free article] [PubMed] [Google Scholar]
- 48.Cella DF, Tulsky DS, Gray G, et al. The Functional Assessment of Cancer Therapy scale: development and validation of the general measure. Journal of clinical oncology. 1993;11(3):570–579. doi: 10.1200/JCO.1993.11.3.570. [DOI] [PubMed] [Google Scholar]
- 49.Rabkin JG, Markowitz JS, Ocepek-Welikson K, Wager SS. General versus systematic inquiry about emergent clinical events with SAFTEE: implications for clinical research. J Clin Psychopharmacol. 1992 Feb;12(1):3–10. doi: 10.1097/00001573-199202000-00002. [DOI] [PubMed] [Google Scholar]
- 50.Chouinard G, Chouinard AR, Aunable L, Jones BD. Extrapyramidal symptoms rating scale. The Canadian Journal of Neurological Sciences. 1980;3:233–238. [Google Scholar]
- 51.Gadbury GL, Coffey CS, Allison DB. Modern statistical methods for handling missing repeated measurements in obesity trial data: beyond LOCF. Obes Rev. 2003 Aug;4(3):175–184. doi: 10.1046/j.1467-789x.2003.00109.x. [DOI] [PubMed] [Google Scholar]
- 52.Mallinckrodt CH, Clark SW, Carroll RJ, Molenbergh G. Assessing response profiles from incomplete longitudinal clinical trial data under regulatory considerations. J Biopharm Stat. 2003 May;13(2):179–190. doi: 10.1081/BIP-120019265. [DOI] [PubMed] [Google Scholar]
- 53.Babyak MA. What you see may not be what you get: a brief, nontechnical introduction to overfitting in regression-type models. Psychosom Med. 2004 May-Jun;66(3):411–421. doi: 10.1097/01.psy.0000127692.23278.a9. [DOI] [PubMed] [Google Scholar]
- 54.MacCallum RC, Zhang S, Preacher KJ, Rucker DD. On the practice of dichotomization of quantitative variables. Psychol Methods. 2002 Mar;7(1):19–40. doi: 10.1037/1082-989x.7.1.19. [DOI] [PubMed] [Google Scholar]
- 55.Royston P, Altman DG, Sauerbrei W. Dichotomizing continuous predictors in multiple regression: a bad idea. Stat Med. 2006 Jan 15;25(1):127–141. doi: 10.1002/sim.2331. [DOI] [PubMed] [Google Scholar]
- 56.Guyatt G, Osaba D, Wu AW, Wyrwich KW, Norman GR. Methods to Explain the Clinical Significance of Health Status Measures (Symposium on Quality of Life in Cancer Patients); Paper presented at: Mayo Clinic Proceedings; 2002. [DOI] [PubMed] [Google Scholar]
- 57.Gross D, Fogg L. A critical analysis of the intend-to-treat principle in preventin research. The Jouranl of Primary Prevention. 2004;25:475–489. [Google Scholar]
- 58.Blackhall L, Petroni G, Shu J, Baum L, Farace E. A pilot study evaluating the safety and efficacy of modafinal for cancer-related fatigue. J Palliat Med. 2009 May;12(5):433–439. doi: 10.1089/jpm.2008.0230. [DOI] [PubMed] [Google Scholar]
- 59.Townsley C, Selby R, Siu LL. Systematic barriers to the recruitment of older patients with cancer onto clinical trials. J Clin Oncol. 2005;23(13):3112–3124. doi: 10.1200/JCO.2005.00.141. [DOI] [PubMed] [Google Scholar]