Abstract
Localized scleroderma (LS) is a disfiguring autoimmune disease of the skin and underlying tissue that mainly affects the pediatric population. Inflammation of the tissue leads to fibrosis and atrophy, causing physical and psychological disability that can continue throughout childhood into adulthood. Available therapies for LS have had variable effects and are associated with morbidity themselves. A better understanding of the pathophysiology of LS, especially during the active inflammatory phase, would lead to more directed and efficacious therapies.
As in systemic sclerosis (SSc), the other form of scleroderma, T-helper (Th) cells and their associated cytokines have been suggested to contribute significantly to the pathophysiology of LS supported by the presence of cytokines from these lineages in the sera and tissue of LS patients. It is postulated that the imbalance between Th1/Th2/Th17 cell subsets drives inflammation in the early stages of disease (Th1 and Th17 predominant) and fibrosis in the later stages of scleroderma (Th2 predominant). We review the available experimental data regarding cytokines in LS and compare them to available clinical disease severity and activity features. This provides the platform to launch further investigations into the role of select cytokines in the pathogenesis of LS and to provide directed therapeutic options in the future.
Keywords: Localized scleroderma, Cytokines, T-lymphocytes
1. Introduction
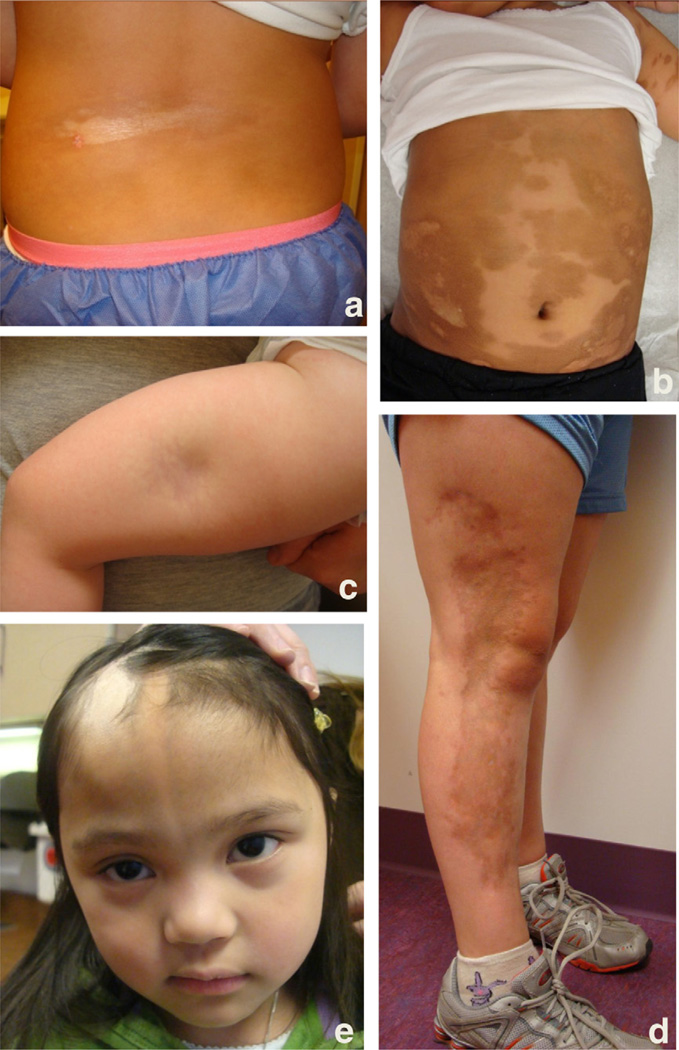
Localized scleroderma (LS) is a disfiguring fibrotic autoimmune disease of the skin and underlying tissue that is more common in children than adults, with a prevalence of 50 per 100,000 children [1]. It manifests clinically as discolored fibrotic patches or bands of skin and is divided into clinical subtypes: plaque morphea, generalized morphea, deep morphea, and linear scleroderma (of extremities and/or head) [2]. Plaque morphea (Fig. 1a), or circumscribed morphea, is characterized by well defined oval-shaped lesions, and typically does not extend beyond the dermis. Generalized morphea (Fig. 1b) is termed when there are several plaques, which are often confluent and cover a large total surface area of the patient’s skin. Deep morphea (Fig. 1c) is defined as lesions that affect deeper layer of the dermis, extending into the subcutaneous tissue and often muscle. Linear scleroderma (Fig. 1d and e), the most common subtype in children, is characterized by one or more linear streaks down the extremities or head, and causes the most significant deformities and morbidity, including joint contractures, leg length discrepancy, optic neuritis, vasculitic strokes and seizures. Of the subtypes of LS, linear scleroderma and generalized morphea are felt to be more severe, while plaque morphea is considered more benign.
Fig. 1.
Subtypes of LS. (a) Plaque morphea of the back, (b) confluent lesions of the abdomen characteristic of generalized morphea, (c) deep morphea lesion of the left thigh marked by subcutaneous atrophy, (d) linear scleroderma of the right leg, (e) linear scleroderma of the face extending from the scalp to forehead with associated hair loss.
The lesions of LS initially undergo an ‘active phase’ which is clinically recognized by erythema or violaceous color, warmth, expansion of lesions, and/or appearance of new lesions. Histologically, a dense dermal and subcutaneous lymphocytic infiltrate is identified during this stage. This is followed by a ‘damage’ phase characterized by closely packed homogenous dense collagen deposition with minimal inflammation corresponding to physical examination findings of fibrotic patches or linear bands of skin that are thick, hard and discolored on the face, trunk, and extremities [3,4]. The fibrosis and resultant atrophy of the skin and underlying connective tissue, including subcutaneous fat, muscle, tendons and bone, can cause significant deformity and severe functional impairment in actively growing children and psychological disability that often continues into adulthood [5]. Many therapies have been used to treat this condition but have had variable effects and are associated with morbidity themselves. A better understanding of the pathophysiology of LS would lead toward more directed and efficacious therapies.
Although the pathogenesis of localized scleroderma (LS) remains unknown, several lines of evidence support CD4+ T-helper (Th) cells and the cytokines they produce as playing an important role, as demonstrated in systemic sclerosis (SSc). Despite the different clinical features of LS and SSc regarding organ involvement and skin lesion distribution, the histopathologic features of the skin lesions are indistinguishable and both demonstrate a predominant CD4+ T-helper (Th) lymphocytic infiltrate [6,7]. In addition to findings in the tissue, Th cells and their associated cytokines have also been identified in the circulating sera and stimulated PBMCs of LS and SSc patients.
T-helper cells consist of three main effector lineages, Th1, Th2 and Th17, each identified by the cytokines they produce. The process of Th cell differentiation is dynamic, incorporating epigenetic factors with antagonistic and self-perpetuating signals from mature T cell effectors to create a transient cell lineage. The plasticity of Th effector cells has caused some debate concerning proper cytokine lineage identification. Classically, Th1 cells have been known to secrete IL-2, IFN-γ, and TNF-α, and are stimulated by IL-2 and IL-12. Th2 cells have been shown to be activated by IL-4 and produce IL-4, IL-5, IL-10 and IL-13. Th17 cells, a more recently identified Th cell subset that has altered the classic Th1/Th2 paradigm, produce IL-17 A/F, IL-21, and IL-22. IL-1, IL-6, IL-23, and TGF-β are now known to play important roles in the differentiation and propagation of the Th17 cell lineage.
Many autoimmune diseases, including scleroderma, are thought to be propagated by an imbalance of Th cell effector lineages and their associated cytokines. Evaluation of the literature reveals a Th2 predominant cytokine profile in the biological specimens (sera, PBMCs, and tissue) of those with SSc [8–10]. However, the literature available from studies in LS show that Th1, Th2, and Th17 cytokines may contribute equally to the pathogenesis of the disease. The data in LS consists mostly of cytokine analysis of serologic specimens and are outlined below. These studies examined sera IL-2, IL-4, IL-6, IL-8, IL-13, TNF-α, and TGF-β as well as the soluble IL-2 and IL-6 receptors in LS cohorts. Studies with substantial clinical data to compare cytokine presence or level in LS patients with disease characteristics are included in Table 1. A review of the literature for each cytokine or receptor as well as its associated clinical findings in LS is described.
Table 1.
Cytokines and their soluble receptors evaluated in localized scleroderma.a
| Reference | No. LS patients | Subgroups | Cytokines | Disease features associated with cytokine presence or quantity |
|---|---|---|---|---|
| Ihn et al. [14]b | 48 | Linear (22) Generalized (16) Plaque (10) |
IL-2 IL-4 IL-6 |
Presence of IL-2, IL-4, and IL-6 correlated to one another and had higher frequency of detection in generalized morphea IL-2 correlated with RF positivity IL-4 and IL-6 correlated with AHA positivity |
| Ihn et al. [51]b | 48 | Linear (22) Generalized (16) Plaque (10) |
IL-8 | Detected in all subgroups, but higher detection frequency in generalized morphea and linear scleroderma |
| Hasegawa et al. [19] | 45 | Linear (22) Generalized (12) Plaque (11) |
IL-13 | Correlation with number of plaque lesions and total number of lesions Higher detection of frequency in generalized and plaque morphea |
| TNF-α | Correlation with IL-6, AHA, and anti-ssDNA presence, number of linear lesions, and frequency of muscle involvement Higher levels correlated with shorter disease duration Higher detection frequency in generalized morphea and linear scleroderma |
|||
| Uziel et al. [47]c,d | 55 | Linear (29) Generalized (16) Plaque (10) |
TGF-β | None |
| Ihn et al. [57]b | 48 | Linear (22) Generalized (16) Plaque (10) |
sIL-2r | Correlation with anti-ssDNA, AHA, IL-2, and IL-4 levels, and number of sclerotic lesions and body areas involved Higher levels and detection frequency in generalized morphea |
| Uziel et al. [58]c | 17 | Active (5) Inactive (8) Indeterminate (5) |
sIL-2r | Correlation with disease activity (clinician judgment of active disease) |
| Nagaoka et al. [65]b | 45 | Linear (22) Generalized (12) Plaque (11) |
sIL-6r | Correlation with AHA levels, RF presence, number of linear lesions and body areas involved Higher detection frequency in generalized morphea and linear scleroderma |
All cytokines were evaluated in the sera of adult localized scleroderma cohort using ELISA and were found to be elevated compared to controls, unless otherwise specified.
Studied in comparison to healthy and systemic sclerosis (SSc) controls.
Evaluated in a pediatric localized scleroderma cohort.
Also grouped into active (21), inactive (23) and indeterminate (11) groups.
2. Th1 associated cytokines
Effectors of the Th1 lineage are involved in the cellular immune response and are central to the host inflammatory response. Th1 cells function to eliminate intracellular pathogens via macrophage activation. A large body of evidence also supports the involvement of Th1-released cytokines in autoimmune inflammation. IL-2 and IL-12 work through specific signal transduction pathways to promote the differentiation of naive T-cell precursors into this lineage. IL-2 and IL-12, in turn, are secreted from Th1 cells, perpetuating the development of Th-1 effectors. IL-12 also demonstrates antagonistic effects on Th2 cells, thus shifting the balance back towards Th1. Additionally, IFN-γ and TNF-α have been shown to be secreted by Th1 cells. IL-2 and TNF-α have been evaluated in the sera of LS patients, are reviewed below.
2.1. Interleukin-2
IL-2 is known to be secreted in response to the mitogenic or antigenic stimulation of T-cells, which then promote the proliferation and maturation of activated B and T cells. Studies of SSc support the presence and elevation of IL-2 in the serum in comparison to healthy controls [11]. A correlation between IL-2 and early rapidly progressive disease was noted in SSc by Lis et al. [12], suggesting a role of this pro-inflammatory cytokine in disease activity. A correlation between serum IL-2 levels and disease progression, as evaluated by skin score and disease duration, was also noted in patients with SSc [11].
Although no direct correlation between the presence of IL-2 and disease activity has been described for LS, studies support IL-2’s presence, elevation, and correlation to disease severity in LS [13,14]. In a serum ELISA analysis of 88 patients (48 LS, 20 SSc, 20 healthy), Ihn et al. [14] demonstrated a significant presence of IL-2 in the serum of LS patients in comparison to both healthy controls and SSc patients. The mean disease duration for the LS patients was 3 years (range 5 months to 10 years), and the disease duration of SSc patients was not reported. LS patients were analyzed by subtype, and elevated IL-2 levels associated with the more severe subtypes, linear scleroderma and generalized morphea (Table 1) [14]. Results showed higher levels of IL-2 (263.5 ± 371.6 pg/mL) in 63% of patients with generalized morphea (10/16), in 14% (3/22) of the patients with linear scleroderma (level 73.59 ± 53.9 pg/mL) and in none of the patients with plaque morphea (0/10) using a minimum detection level of 31.3 pg/mL for the assay [14]. The presence of IL-2 in LS patients also correlated with a positive Rheumatoid Factor (RF) and increased concentrations of IL-4 and IL-6. When evaluated longitudinally, IL-2 sera levels decreased over time as the skin softened, signifying cutaneous improvement [14], possibly reflecting IL-2 as a marker of disease activity in LS.
2.2. Tumor necrosis factor-alpha (TNF-α)
TNF-α is a commonly studied pro-inflammatory and immunoregulatory cytokine that is synthesized by activated monocytes and plays a varying role in tissue fibrosis. Studies conducted by Thornton et al. have found lower concentrations of TNF-α to stimulate fibroblast synthesis and higher levels to be inhibitory [15]. SSc analyses conducted in vitro have implicated TNF-α as a potential contributor to fibrosis, demonstrating SSc dermal fibroblasts to be hyperresponsive to TNF-α [16]. In vivo studies have illustrated elevated serum levels of TNF-α to be significantly correlated with the presence of pulmonary fibrosis [17]. TNF-α also induces production of the profibrotic cytokine IL-6, further implicating TNFα in the development of fibrosis [18]. Recent studies have shown a higher serological TNF-α presence in SSc patients when compared to the sera of healthy controls [17].
In a serum ELISA analysis of 45 Japanese LS patients (33 female, 12 male) with a mean age of 27 (range 5–67) grouped according to disease subtype with 20 age and gender matched healthy controls, Hasegawa et al. [19] showed detectable levels of TNF-α in 24% of LS patients (11/45) and no significant detection of TNF-α in controls (Table 1). Levels of detectable serum TNF-α were similar among the three subtypes of LS evaluated, with a median value of 20 pg/mL when analyzed using an ELISA with a minimum detection limit of 4.4 pg/mL. However, TNF-α was more frequently detected in the generalized morphea (3/12) and linear scleroderma subtypes (6/22) when compared with the detection frequencies of plaque morphea (2/11) and healthy controls (0/20) [19].
The presence of TNF-α in the serum correlated positively with the serological presence of anti-histone antibodies (AHA), anti-single-stranded-DNA (ss-DNA) antibodies, and IL-6. Moreover, the number of linear lesions and the frequency of muscle involvement in LS patients correlated positively with the presence of serum TNF-α. The propensity for the more severe subtypes of LS, association with auto-antibodies, and relationship to disease burden (muscle involvement and number of lesions) support TNF-α as a marker of more severe LS disease. In addition, TNF-α may contribute to pathogenesis in early disease as disease duration was demonstrated to be shorter in patients with elevated serum TNF-α (2.5 ± 2.7 years) than in those without elevated levels of the cytokine (6.0 ± 7.0 years) [19].
3. Th2 associated cytokines
Th2 cells are known to produce IL-4, IL-5, IL-10, and IL-13, and function to eliminate extracellular pathogens through the upregulation of antibody synthesis by B-cells. Cytokines of Th2 lineage have been characterized as pro-fibrotic and anti-inflammatory due to their respective actions as initiators of extracellular matrix production and inhibitors of Th1 cell function. Development of the Th2 cell lineage is induced by IL-4 and propagated by a positive feedback loop involving this effector cytokine. IL-4 and IL-13 have been evaluated in LS, as described below. However, findings regarding IL-5 and IL-10 have not been published in LS.
3.1. Interleukin-4
IL-4 is a glycoprotein produced in response to repeated antigenic stimulation of CD4+ and CD8+ Th2 cells as well as stimulation of mast cells and basophilic neutrophils [10]. IL-4 functions to stimulate the production and proliferation of B-cells, and has been shown to increase immunoglobulin and adhesion molecule synthesis in vivo [20]. In fibroblasts, IL-4 has been shown to regulate TGF-β levels yielding in vitro fibrosis [21] and to stimulate fibroblast proliferation. IL-4 also promotes extracellular matrix production by increasing collagen [22], fibronectin [23] and proteoglycan synthesis [24] and inhibiting the synthesis of specific collagenases [25] further supporting its involvement in tissue fibrosis. Patients with SSc exhibit elevated levels of IL-4 in serum and in the dermis of skin biopsies [8–10].
IL-4 has been demonstrated in the serum of LS patients by Ihn et al. The same set of patient sera used to evaluate IL-2 by Ihn described above [14], was examined for IL-4, including LS, SSc and healthy patients. Those with LS exhibited detectable levels of serum IL-4 (>31.3 pg/mL) in comparison to healthy patients who lacked serum IL-4 (Table 1). A correlation of IL-4 and AHA positivity was also demonstrated [14]. The presence of AHA in patients with LS is thought to signify disease severity by correlating to the number of plaque lesions and number of involved areas of the body (Section 7) [26]. Therefore, it was not surprising to find that IL-4 was most commonly present in patients with generalized morphea (6/16) with a median level of 110 pg/mL when LS patients were divided into subtypes. Two out of 22 patients with linear scleroderma exhibited statistically significant levels of serum IL-4 (median level of 50 pg/mL) while none of the 10 patients with plaque morphea and only one of 20 SSc patients (100 pg/mL) exhibited significant levels of the cytokine [14].
3.2. Interleukin-13
IL-13 is a well known pro-fibrotic cytokine. There is a large functional overlap between IL-13 and IL-4, which is most likely explained by the proximity of the genes for these cytokines on chromosome 5q. Both IL-4 and IL-13 share a common receptor, IL-4Rα1, and are pro-fibrotic through enhanced fibroblast proliferation [20], increased production of collagen, and inhibition of collagenase synthesis [27]. IL-13 is produced by activated Th2 cells and, like IL-4, functions to inhibit the production of pro-inflammatory cytokines, specifically Th1 associated cytokines. Hasegawa et al. [9] demonstrated the presence of IL-13 in the sera of SSc patients in comparison to healthy controls.
Using the same patient cohort as that from their TNF-α studies reported above (25 LS patients and 20 healthy controls), Hasegawa et al. [19] also investigated serum IL-13 levels in LS using a serum ELISA assay with a sensitivity of 34 pg/mL and demonstrated a significantly greater frequency of detection in LS patients (13/45) when compared to healthy controls (0/20) (Table 1).
Similar to IL-2, IL-4, and IL-6, IL-13 was most commonly detected in generalized morphea (7/12), with a median concentration of 77 pg/mL. Interestingly, IL-13 was also found to be frequently elevated in patients with plaque morphea (4/11 detected at a median level of 70 pg/mL). Although IL-13 was detected in 2 out of 22 patients with linear scleroderma (with a median level of 90 pg/mL) it was determined that the frequency of detection in patients with this subtype did not differ significantly from the frequency of IL-13 detection in controls (0/20). Elevated levels were associated with number of plaque lesions and the total number of sclerotic lesions, supporting a correlation with disease severity. IL-13 did not correlate with the serological presence of IL-6 or TNF-α [19].
4. Th17 associated cytokines
Th17 cells and effectors are part of a recently defined subset that plays an integral role in the pathogenesis of many inflammatory and autoimmune diseases including rheumatoid arthritis, systemic lupus erythematosus, psoriasis, inflammatory bowel disease, and multiple sclerosis [28–30]. Th17 effectors include IL-17A/F, IL-21, and IL-22. Interplay among IL-1, IL-6, IL-23, and TGF-β is required for the development and propagation of the Th17 lineage. Th17 differentiation is inhibited by IFN-γ and IL-4, Th1 and Th2 cell-derived effectors, respectively [31,32]. The literature available for Th17 function proposes that effectors of this lineage encompass both pro-inflammatory and pro-fibrotic characteristics, suggesting that this cell type may act as an intermediate between the Th1 and Th2 lineages. The other Th17 associated cytokines have not been reported in reference to LS in the literature at this time. Our preliminary results in 71 pediatric LS patients have supported the possible role of Th17 cells in early active disease, with elevated serum levels of the Th17 inducers, IL-1 and IL-6, in those with disease onset of less than 24 months and elevated levels of Th-17 effectors, IL-17F and IL-22, in the serum between 24 and 48 months of disease onset [33]. IL-6 and TGF-β have been previously characterized in LS patients and are reviewed here.
4.1. Interleukin-17
IL-17 is a more recently identified cytokine that is secreted by CD4+ cells and has been shown to enhance the secretion of the pro-inflammatory and pro-fibrotic cytokines IL-6 and IL-8 from fibroblasts [34,35]. Studies comparing SSc patients to healthy controls have demonstrated an increase in IL-17 levels in SSc serum that correlated significantly with the early stage disease. IL-17 was also shown to enhance the proliferation of fibroblasts, further indicating a possible role in fibrosis [36]. To date, a serological presence of IL-17 has yet to be formally established in LS patients. However, our preliminary results have shown IL-17F and IL-22 to be elevated in serum samples obtained from pediatric LS patients compared to healthy age-matched controls. Of interest was that these two cytokines were particularly elevated in patients with disease duration between 24 and 48 month compared to those with <24 months duration and those with >48 months duration, suggesting that Th17 associated cytokines may play an intermediate role between the active and damage phases [33]. IL-17F and IL-22 positivity also correlated with ANA, anti-histone, and ss-DNA Ab positivity, reflecting more severe and perhaps immunogenically active group of LS patients [33].
4.2. Interleukin-6
IL-6 is a commonly involved cytokine of inflammatory and immune responses, and is produced by multiple cell types including T cells, B cells, monocytes, and fibroblasts. In association with autoimmune disease, IL-6 promotes the proliferation and maturation of B-cells, and is known to regulate fibroblast activity [37]. IL-6 also favors the development of fibrosis through stimulation of collagen production and inhibition of the synthesis of collagenases [25]. Recent studies have shown that IL-6 is required for the differentiation of Th17 cells, suggesting its role in the pro-inflammatory and fibrotic pathways.
IL-6 has been implicated in the pathogenesis of SSc, as it has been shown to be present and increased in the sera [8] and dermis of SSc patients when compared to healthy controls [38]. An increase in the production of IL-6 by dermal fibroblasts of SSc patients has also been demonstrated, while production cannot be detected in normal fibroblasts without external cytokine stimulation [39]. Furthermore, studies have demonstrated the presence of IL-6 in the sera of patients with early or severe systemic disease and have suggested the cytokine as a marker for disease activity [12,40].
IL-6 has been shown to be present in the serum of LS patients when compared to healthy controls [14] and was detected at higher levels in LS patients when compared to SSc patients using the same patient population as that described by Ihn et al. for IL-2 and IL-4 assays [14]. With an ELISA assay sensitivity of 3.0 pg/mL, serum IL-6 was detectable in 23 out of 48 LS patients with a mean of 16.4 ± 15.3 pg/mL, whereas 7 out of 20 SSc patients exhibited significant levels of IL-6 with a mean concentration of 9.0 ± 3.7 pg/mL. With regards to LS subtypes, trends with IL-6 levels were similar to those observed with IL-2 and IL-4, as IL-6 was more frequently detected in patients with the generalized morphea (12/16) and linear scleroderma (9/22) subtypes of LS (Table 1), with median serological levels of 17 pg/mL and 10 pg/mL, respectively. Statistically significant levels of IL-6 were detected in only 2 out of 10 patients with plaque morphea, with a median level of 4 pg/mL. Longitudinal studies undertaken by Ihn et al. also demonstrated a decrease in circulating levels of IL-6 that paralleled disease improvement over time, possibly supporting IL-6 as a marker of disease activity. Serum IL-6 levels also correlated positively with elevated IL-2 and IL-4 levels, as well as with the presence of AHA, reflecting disease severity [14].
4.3. Transforming growth factor-beta (TGF-β)
TGF-β has multiple well known functions in anti-inflammatory and immunoregulatory pathways. Recently, TGF-β has been defined as a T-regulatory molecule involved in the differentiation of naive T-cells into the Th17 lineage. TGF-β also plays an important and well characterized role tissue fibrosis by stimulating fibroblasts to increase the production of extracellular matrix proteins including collagen and fibronectin [41]. TGF-β has also been described as a potent chemoattractant for fibroblasts and an inhibitor of extracellular matrix breakdown [42]. Higley et al. [43] found an increase in serum TGF-β levels in LS patients, but did not see elevated levels in SSc patients. However, recent findings in SSc patients showed an increase in the levels of circulating TGF-β when compared to that of controls [44]. Analysis of SSc patient skin biopsy specimens demonstrated levels of TGF-β signaling to parallel disease severity as measured by the modified Rodnan Skin Score (mRSS) [45].
Skin biopsies from LS patients have demonstrated increased TGF-β expression and co-localization with activated fibroblasts and other inflammatory molecules implicating its involvement in the pathogenesis of LS [43]. Immunohistochemical studies performed by Querfeld et al. [46] further showed TGF-β to be present in inflammatory LS skin samples. In examining a population of 55 pediatric LS patients with a mean age of onset of 9.2 years and an average disease duration of 4.9 years [47], Uziel et al. showed a significant increase in the mean TGF-β serum levels in LS patients (51,393 ± 33,953 pg/mL) when compared to average levels in healthy age-matched control subjects (9825 ± 5287 pg/mL). The LS patient population was also grouped according to disease subtype; generalized morphea, linear scleroderma, and plaque morphea, and by clinical activity status as active, inactive, or indeterminate. However, there was no difference noted in serum TGF-β levels among LS subtypes or disease activity status groups (Table 1) [47].
5. Other cytokines
5.1. Interleukin-8
IL-8 has been described as chemotactic pro-inflammatory cytokine produced by several cell types including monocytes, endothelial cells, and fibroblasts [48,49]. Similar to IL-2, IL-4, and IL-6, IL-8 has been detected in the serum of SSc patients, yet is absent from that of healthy controls [50]. As seen with IL-6, IL-8 production by dermal fibroblasts in SSc patients is increased while production by normal, unstimulated fibroblasts is undetectable, suggesting that the molecule also plays a role in fibrotic pathways [39].
Although the data of Reitamo et al. did not support the presence of IL-8 in LS patient serum (0 out of 3), ELISA analyses by Ihn et al. using a larger population of LS patients demonstrated the detection of IL-8 in 75% (36/48) of LS patients compared to 25% (5/20) in SSc patients and 5% (1/20) of healthy controls [51]. Sera IL-8 was detected in 13 out of 16 patients with generalized morphea (median 55 pg/mL), 20 out of 22 patients with linear scleroderma (median 60 pg/mL), and 3 out of 10 patients with plaque morphea (median 65 pg/mL) using a detection limit of 30 pg/mL. Though more prevalent in generalized morphea and linear scleroderma, IL-8 was present in the serum of all LS subtypes in contrast to IL-2, IL-4, and IL-6 which did not have a signal in plaque morphea (Table 1). Thus, IL-8 did not appear to reflect disease severity as strongly as observed with the previously mentioned cytokines. There was also a lack of correlation between IL-8 and antibody positivity, including anti-nuclear antibody (ANA), AHA, and antiss-DNA antibodies and to disease burden, documented by the number of sclerotic lesions and affected areas of the body [51].
6. Soluble interleukin receptors
In addition to an increased cytokine presence in serum and tissue of LS and SSc patients, an increase in levels of serum soluble interleukin-2 receptor (sIL-2r) and interleukin-6 receptor (sIL-6r) has been identified in the sera of SSc and LS patients, suggesting that the receptors may play a role in the pathogenesis of both diseases. Cytokine receptor expression indicates an activation of T-lymphocytes, further supporting the involvement and imbalance of Th1/Th2 cells in SSc and LS.
6.1. Soluble interleukin-2 receptor (sIL-2r)
sIL-2r has previously been shown to be a marker of immune activity, and increased levels of sIL-2r can be indicative of autoimmune or inflammatory disease [52]. Expression of sIL-2r on T-lymphocytes indicates that the molecule most likely regulates immune function [53], and it has been suggested that sIL-2r plays a role in IL-2 inhibition. The receptor has been characterized in many autoimmune diseases including systemic lupus erythematosus, rheumatoid arthritis, and SSc. sIL-2r has been demonstrated to be present at higher levels in SSc patient serum compared to healthy subject serum [54], and a correlation between sIL-2r levels and SSc disease progression and activity has been proposed [55]. Steen et al. [56] demonstrated a significant association between sIL-2r serum levels and mortality, extent of skin thickness, early disease and disease activity of SSc patients. Longitudinal analysis of sIL-2r in SSc demonstrated a decrease in the receptor over time, paralleling cutaneous improvement [56].
Serum levels of sIL-2r were also studied in LS by Ihn et al. using a cohort of 48 LS patients (16 generalized morphea, 22 linear scleroderma, 10 plaque morphea), 20 SSc patients, and 20 healthy controls (Table 1) [57]. An ELISA with a sensitivity of 5 pmol/L was used to determine serum levels of sIL-2r. Higher concentrations were observed in those with LS (104.8 ± 123.7 pmol/L) compared to healthy individuals (53.8 ± 28.9 pmol/L). Serum IL-2r levels were most commonly elevated in general morphea (6/16, level 172.6 ± 199.2 pmol/L) and linear scleroderma (4/22, level 84.8 ± 43.4 pmol/L) patients, while no patients with plaque morphea exhibited a statistically significant increase in sIL-2r levels (level 40.4 ± 16.6 pmol/L). Eight out of 20 patients with SSc exhibited elevated sIL-2r serum levels, with a mean value of 153.8 pmol/L [58].
Uziel et al. [58] showed a significant increase in levels of sIL-2r in pediatric patients with active LS, as judged by physician, when compared to those with inactive disease and healthy controls (Table 1). Low levels of sIL-2r were seen in the inactive phase of LS which corresponded to lower anti-ssDNA levels and paralleled skin softening. Patients with statistically elevated levels of sIL-2r were also found to have a greater number of sclerotic lesions (4.88 ± 4.19 lesions) when compared to those without elevated sIL-2r levels (2.342 ± 2.23 lesions). Correlations were also demonstrated between the presence of elevated serum sIL-2r levels and AHA levels, and the number of involved areas of skin, suggesting that sIL-2r may reflect disease severity in LS. However, no associations between receptor levels and disease duration were observed in this study [58]. Ihn et al. [57] confirmed a higher frequency of sIL-2r detection in generalized morphea patients (Table 1).
6.2. Soluble interleukin-6 receptor (sIL-6r)
sIL-6r is secreted from T-cells and macrophages [59] and has been shown to bind and inhibit the activity of IL-6, which has well characterized functions in autoimmune disease [60]. sIL-6r levels demonstrate an increased presence in many clinical conditions, such as interstitial lung disease and rheumatoid arthritis [61,62]. When complexed, sIL-6r and IL-6 have been shown to inhibit fibroblast proliferation, suggesting a possible anti-fibrotic function for the paired molecules [63]. Studies have shown a significant increase in serum sIL-6r levels in SSc patients over levels of healthy controls, including both early and late SSc [64,65]. Interestingly, no correlation between sIL-6r and IL-6 levels in SSc patients was noted [64].
sIL-6r levels have been studied in the sera of LS patients. Nagaoka et al. [65] used ELISA analysis (sensitivity 140 pg/mL) to determine serum sIL-6r levels in a group of 45 Japanese, untreated LS patients (33 female, 12 male, mean age 27, age range of 5–67), 20 SSc patients (15 female, 5 male, mean age 27, age range 6–69 years), and 20 age and sex-matched healthy controls. The group found sIL-6r levels to be significantly higher in LS and SSc patients when compared to levels in healthy control subjects as 24% of LS (11/45) and 35% of SSc (7/20) patients exhibited serum levels that were two standard deviations above the mean sIL-6r level in healthy controls. Of the subsets of LS, general morphea and linear scleroderma patients exhibited a higher frequency of sIL-6r elevation (5/12 and 5/22, respectively) in comparison with plaque morphea (1/11). sIL-6r levels were demonstrated to correlate with levels of AHA and the presence of RF (Table 1). The number of linear lesions and involved body areas of LS patients also correlated positively with the elevated sIL-6r levels [65], supporting the receptor as a marker of disease severity.
7. Autoantibody association
Many of described studies analyzing serum cytokines in LS compare cytokine presence and level to more well established serologic autoimmune markers of disease, such as anti-nuclear antibody (ANA), anti-histone antibody (AHA) and single-stranded DNA antibody (ss-DNA Ab). ANA is a classic marker of autoimmune disease in general and higher titers have been associated with early onset LS [66] as well as increased risk for extracutaneous manifestations of LS [67]. The presence of ss-DNA Ab in LS is associated with linear scleroderma, deep muscle involvement and more extensive skin disease, all reflecting increased disease severity [66,68]. In a sub-group of LS patients, ss-DNA Ab has been shown to reflect disease activity status with titers decreasing as the skin lesions clinically improve [66,68]. As mentioned previously, AHA correlates with surface area of body involved and number of lesions [66], it also correlates with linear disease and joint contractures [68], both of which support its reflection of disease severity.
8. Conclusion
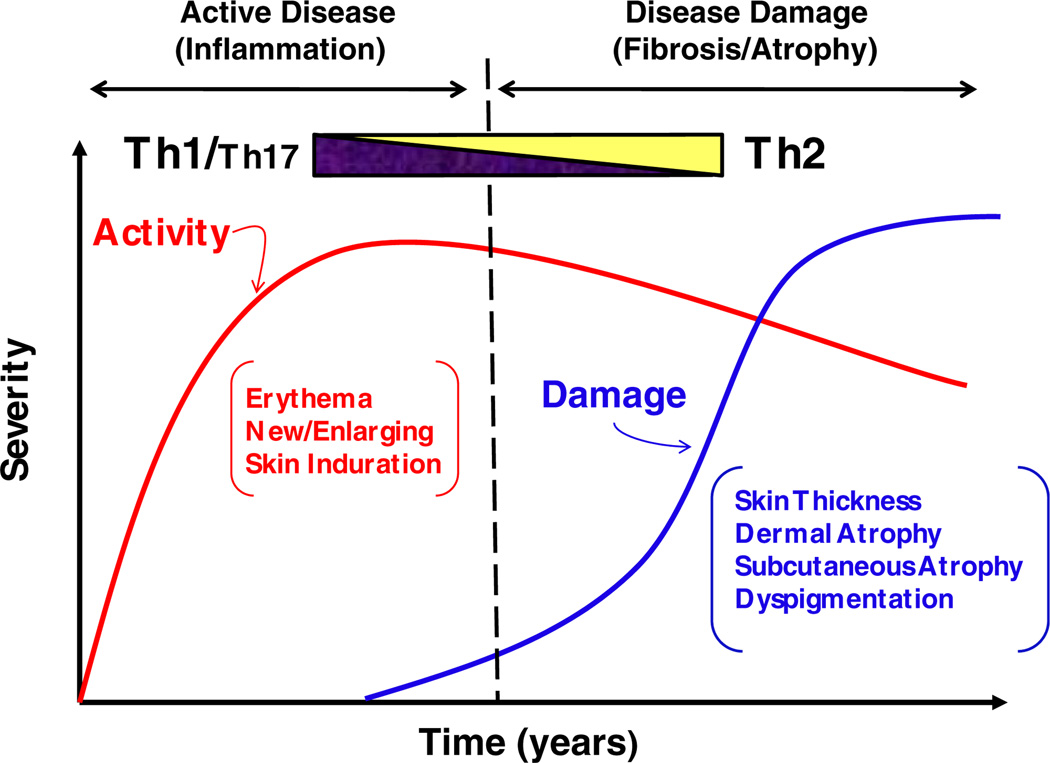
In conclusion, the literature suggests that cytokines of Th1, Th2, and Th17 cell lineages contribute to the pathogenesis of both forms of scleroderma, systemic sclerosis (SSc) and localized scleroderma (LS). Prior studies support the theory of Th1/Th2 imbalance in SSc propagating disease in a Th2 manner, leading to skin fibrosis and damage. Although there are discrepancies within results of various published studies, there is an overall notion that pro-inflammatory Th1 and Th17 associated cytokines are elevated during the early stages of scleroderma, whereas Th2 cytokines mainly correlate with disease damage and fibrosis extent. This holds true in SSc but has only been partially investigated in LS, as reviewed above. Longitudinal studies have shown an early presence of the proinflammatory cytokine IL-2 and the Th17 inducer, IL-6, suggesting that an early influx of Th1 cytokines mediate the active, inflammatory phase of the disease, and the subsequent propagation of inflammation and transition to the fibrotic damage phase is achieved through the differentiation and development of Th17 effectors (Fig. 2). This is further supported by preliminary results showing the elevated Th17 effector cytokines two to four years after the initial onset of disease activity. It can be postulated that the Th2 effector cytokines shown to be present at elevated levels in LS patient serum promote the development of tissue damage and fibrosis later in the course of the disease (Fig. 2).
Fig. 2.
Proposed conceptual model of localized scleroderma: transition from Th1/Th17 to Th2 response.
The literature available, however, does not examine the presence or elevation of Th effector subsets in reference to early or late disease. Future studies using serum ELISA or Luminex assays as well as studies in tissue and PBMCs should be used to compare levels of cytokine subsets, including Th17 effectors, longitudinally and to well defined disease activity parameters in order to elucidate their true role in the pathogenesis of LS. Despite a limited understanding of the relationship between the serological presence of Th1/Th2 cytokines and the development and propagation of LS, studies have demonstrated positive correlations between the detection of certain Th cytokines with established markers of disease, including AHA and anti-ss-DNA presence. Future investigation of the functions of Th1 and Th2 derived cytokines as well as Th17 and Treg cells in the serum, tissue, and PBMCs of LS patients could provide further insight into the mechanisms mediating the progression of localized scleroderma and provide novel therapies for this chronic and disfiguring disease.
Acknowledgement
This work was supported by a grant from the Nancy Taylor Foundation for Chronic Diseases, Inc.
Footnotes
Source of support: The Nancy Taylor Foundation for Chronic Diseases. No financial support or other benefits from commercial sources were used for the work reported on in the manuscript. The authors have no financial interests which could create a potential conflict of interest or the appearance of a conflict of interest with regard to the work.
Contributor Information
Katherine Kurzinski, Email: katherine.kurzinski@chp.edu.
Kathryn S. Torok, Email: kathryn.torok@chp.edu.
References
- 1.Peterson LS, Nelson AM, Su WP, Mason T, O’Fallon WM, Gabriel SE. The epidemiology of morphea (localized scleroderma) in Olmsted County 1960–1993. J Rheumatol. 1997;24(1):73–80. [PubMed] [Google Scholar]
- 2.Peterson LS, Nelson AM, Su WP. Classification of morphea (localized scleroderma) Mayo Clin Proc. 1995;70(11):1068–1076. doi: 10.4065/70.11.1068. [DOI] [PubMed] [Google Scholar]
- 3.Lever WF, Elder DE, Elenitsas R, Jaworsky C, Johnson BL. Lever’s histopathology of the skin. 8th ed. Philadelphia: Lippincott-Raven; 1997. [Google Scholar]
- 4.McKee PH. Pathology of the skin: with clinical correlations. 2nd ed. London: Mosby-Wolfe; 1996. [Google Scholar]
- 5.Zulian F. New developments in localized scleroderma. Curr Opin Rheumatol. 2008;20(5):601–607. doi: 10.1097/BOR.0b013e328309a5eb. [DOI] [PubMed] [Google Scholar]
- 6.Roumm AD, Whiteside TL, Medsger TA, Jr, Rodnan GP. Lymphocytes in the skin of patients with progressive systemic sclerosis. Quantification, subtyping, and clinical correlations. Arthritis Rheum. 1984;27(6):645–653. doi: 10.1002/art.1780270607. [DOI] [PubMed] [Google Scholar]
- 7.Torres JE, Sanchez JL. Histopathologic differentiation between localized and systemic scleroderma. Am J Dermatopathol. 1998;20(3):242–245. doi: 10.1097/00000372-199806000-00003. [DOI] [PubMed] [Google Scholar]
- 8.Needleman BW, Wigley FM, Stair RW. Interleukin-1, interleukin-2, interleukin-4, interleukin-6, tumor necrosis factor alpha, and interferon-gamma levels in sera from patients with scleroderma. Arthritis Rheum. 1992;35(1):67–72. doi: 10.1002/art.1780350111. [DOI] [PubMed] [Google Scholar]
- 9.Hasegawa M, Fujimoto M, Kikuchi K, Takehara K. Elevated serum levels of interleukin-4 (IL-4), IL-10, and IL-13 in patients with systemic sclerosis. J Rheumatol. 1997;24(2):328–332. [PubMed] [Google Scholar]
- 10.Salmon-Ehr V, Serpier H, Nawrocki B, Gillery P, Clavel C, Kalis B, et al. Expression of interleukin-4 in scleroderma skin specimens and scleroderma fibroblast cultures. Potential role in fibrosis. Arch Dermatol. 1996;132(7):802–806. [PubMed] [Google Scholar]
- 11.Kahaleh MB, LeRoy EC. Interleukin-2 in scleroderma: correlation of serum level with extent of skin involvement and disease duration. Ann Intern Med. 1989;110(6):446–450. doi: 10.7326/0003-4819-110-6-446. [DOI] [PubMed] [Google Scholar]
- 12.Lis A, Brzezinska-Wcislo L. Interleukin-2 and interleukin-6 in serum as markers of disease progression in systemic sclerosis. Pol Merkuriusz Lek. 2001;11(63):206–209. [PubMed] [Google Scholar]
- 13.Alecu M, Geleriu L, Coman G, Galatescu L. The interleukin-1, interleukin-2, interleukin-6 and tumour necrosis factor alpha serological levels in localised and systemic sclerosis. Rom J Intern Med. 1998;36(3–4):251–259. [PubMed] [Google Scholar]
- 14.Ihn H, Sato S, Fujimoto M, Kikuchi K, Takehara K. Demonstration of interleukin-2, interleukin-4 and interleukin-6 in sera from patients with localized scleroderma. Arch Dermatol Res. 1995;287(2):193–197. doi: 10.1007/BF01262331. [DOI] [PubMed] [Google Scholar]
- 15.Thornton SC, Por SB, Walsh BJ, Penny R, Breit SN. Interaction of immune and connective tissue cells: I. The effect of lymphokines and monokines on fibroblast growth. J Leukoc Biol. 1990;47(4):312–320. doi: 10.1002/jlb.47.4.312. [DOI] [PubMed] [Google Scholar]
- 16.Cho MM, Jimenez SA, Johnson BA, Harlow LA, Burrows JC, Koch AE. In vitro cytokine modulation of intercellular adhesion molecule-1 expression on systemic sclerosis dermal fibroblasts. Pathobiology. 1994;62(2):73–81. doi: 10.1159/000163881. [DOI] [PubMed] [Google Scholar]
- 17.Hasegawa M, Fujimoto M, Kikuchi K, Takehara K. Elevated serum tumor necrosis factor-alpha levels in patients with systemic sclerosis: association with pulmonary fibrosis. J Rheumatol. 1997;24(4):663–665. [PubMed] [Google Scholar]
- 18.Jablons DM, Mule JJ, McIntosh JK, Sehgal PB, May LT, Huang CM, et al. IL-6/IFN-beta-2 as a circulating hormone. Induction by cytokine administration in humans. J Immunol. 1989;142(5):1542–1547. [PubMed] [Google Scholar]
- 19.Hasegawa M, Sato S, Nagaoka T, Fujimoto M, Takehara K. Serum levels of tumor necrosis factor and interleukin-13 are elevated in patients with localized scleroderma. Dermatology. 2003;207(2):141–147. doi: 10.1159/000071783. [DOI] [PubMed] [Google Scholar]
- 20.Doucet C, Brouty-Boye D, Pottin-Clemenceau C, Jasmin C, Canonica GW, Azzarone B. IL-4 and IL-13 specifically increase adhesion molecule and inflammatory cytokine expression in human lung fibroblasts. Int Immunol. 1998;10(10):1421–1433. doi: 10.1093/intimm/10.10.1421. [DOI] [PubMed] [Google Scholar]
- 21.Kodera T, McGaha TL, Phelps R, Paul WE, Bona CA. Disrupting the IL-4 gene rescues mice homozygous for the tight-skin mutation from embryonic death and diminishes TGF-beta production by fibroblasts. Proc Natl Acad Sci USA. 2002;99(6):3800–3805. doi: 10.1073/pnas.052709999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fertin C, Nicolas JF, Gillery P, Kalis B, Banchereau J, Maquart FX. Interleukin-4 stimulates collagen synthesis by normal and scleroderma fibroblasts in dermal equivalents. Cell Mol Biol. 1991;37(8):823–829. [PubMed] [Google Scholar]
- 23.Peyron E. Interleukin-4 structure, function and clinical aspects. Eur J Dermatol. 1994;4:181–188. [Google Scholar]
- 24.Wegrowski Y, Paltot V, Gillery P, Kalis B, Randoux A, Maquart FX. Stimulation of sulphated glycosaminoglycan and decorin production in adult dermal fibroblasts by recombinant human interleukin-4. Biochem J. 1995;307(Pt 3):673–678. doi: 10.1042/bj3070673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shingu M, Miyauchi S, Nagai Y, Yasutake C, Horie K. The role of IL-4 and IL-6 in IL-1-dependent cartilage matrix degradation. Br J Rheumatol. 1995;34(2):101–106. doi: 10.1093/rheumatology/34.2.101. [DOI] [PubMed] [Google Scholar]
- 26.Sato S, Fujimoto M, Ihn H, Kikuchi K, Takehara K. Clinical characteristics associated with antihistone antibodies in patients with localized scleroderma. J Am Acad Dermatol. 1994;31(4):567–571. doi: 10.1016/s0190-9622(94)70217-9. [DOI] [PubMed] [Google Scholar]
- 27.Oriente A, Fedarko NS, Pacocha SE, Huang SK, Lichtenstein LM, Essayan DM. Interleukin-13 modulates collagen homeostasis in human skin and keloid fibroblasts. J Pharmacol Exp Ther. 2000;292(3):988–994. [PubMed] [Google Scholar]
- 28.Garrett-Sinha LA, John S, Gaffen SL. IL-17 and the Th17 lineage in systemic lupus erythematosus. Curr Opin Rheumatol. 2008;20(5):519–525. doi: 10.1097/BOR.0b013e328304b6b5. [DOI] [PubMed] [Google Scholar]
- 29.Stockinger B, Veldhoen M. Differentiation and function of Th17 T cells. Curr Opin Immunol. 2007;19(3):281–286. doi: 10.1016/j.coi.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 30.Fitch E, Harper E, Skorcheva I, Kurtz SE, Blauvelt A. Pathophysiology of psoriasis: recent advances on IL-23 and Th17 cytokines. Curr Rheumatol Rep. 2007;9(6):461–467. doi: 10.1007/s11926-007-0075-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Park H, Li Z, Yang XO, Chang SH, Nurieva R, Wang YH, et al. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol. 2005;6(11):1133–1141. doi: 10.1038/ni1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Harrington LE, Hatton RD, Mangan PR, Turner H, Murphy TL, Murphy KM, et al. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol. 2005;6(11):1123–1132. doi: 10.1038/ni1254. [DOI] [PubMed] [Google Scholar]
- 33.Torok K, Arkachaisri T, Medsger TA, Jr, Feghali-Bostwick CA. Th1 and Th17 cytokine signatures in early pediatric localized scleroderma. Arthritis Rheum. 2010;62(10):S839. [Google Scholar]
- 34.Yao Z, Painter SL, Fanslow WC, Ulrich D, Macduff BM, Spriggs MK, et al. Human IL-17: a novel cytokine derived from T cells. J Immunol. 1995;155(12):5483–5486. [PubMed] [Google Scholar]
- 35.Fossiez F, Djossou O, Chomarat P, Flores-Romo L, Ait-Yahia S, Maat C, et al. T cell interleukin-17 induces stromal cells to produce proinflammatory and hematopoietic cytokines. J Exp Med. 1996;183(6):2593–2603. doi: 10.1084/jem.183.6.2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kurasawa K, Hirose K, Sano H, Endo H, Shinkai H, Nawata Y, et al. Increased interleukin-17 production in patients with systemic sclerosis. Arthritis Rheum. 2000;43(11):2455–2463. doi: 10.1002/1529-0131(200011)43:11<2455::AID-ANR12>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 37.Naka T, Nishimoto N, Kishimoto T. The paradigm of IL-6: from basic science to medicine. Arthritis Res. 2002;4(Suppl. 3):S233–S242. doi: 10.1186/ar565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Romero LI, Pincus SH. In situ localization of interleukin-6 in normal skin and atrophic cutaneous disease. Int Arch Allergy Immunol. 1992;99(1):44–49. doi: 10.1159/000236334. [DOI] [PubMed] [Google Scholar]
- 39.Kadono T, Kikuchi K, Ihn H, Takehara K, Tamaki K. Increased production of interleukin 6 and interleukin 8 in scleroderma fibroblasts. J Rheumatol. 1998;25(2):296–301. [PubMed] [Google Scholar]
- 40.Gourh P, Arnett FC, Assassi S, Tan FK, Huang M, Diekman L, et al. Plasma cytokine profiles in systemic sclerosis: associations with autoantibody subsets and clinical manifestations. Arthritis Res Ther. 2009;11(5):R147. doi: 10.1186/ar2821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Varga J, Rosenbloom J, Jimenez SA. Transforming growth factor beta (TGF beta) causes a persistent increase in steady-state amounts of type I and type III collagen and fibronectin mRNAs in normal human dermal fibroblasts. Biochem J. 1987;247(3):597–604. doi: 10.1042/bj2470597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Edwards DR, Murphy G, Reynolds JJ, Whitham SE, Docherty AJ, Angel P, et al. Transforming growth factor beta modulates the expression of collagenase and metalloproteinase inhibitor. EMBO J. 1987;6(7):1899–1904. doi: 10.1002/j.1460-2075.1987.tb02449.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Higley H, Persichitte K, Chu S, Waegell W, Vancheeswaran R, Black C. Immunocytochemical localization and serologic detection of transforming growth factor beta 1. Association with type I procollagen and inflammatory cell markers in diffuse and limited systemic sclerosis, morphea, and Raynaud’s phenomenon. Arthritis Rheum. 1994;37(2):278–288. doi: 10.1002/art.1780370218. [DOI] [PubMed] [Google Scholar]
- 44.Radstake TR, van Bon L, Broen J, Hussiani A, Hesselstrand R, Wuttge DM, et al. The pronounced Th17 profile in systemic sclerosis (SSc) together with intracellular expression of TGFbeta and IFNgamma distinguishes SSc phenotypes. PLoS One. 2009;4(6):e5903. doi: 10.1371/journal.pone.0005903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Verrecchia F, Laboureau J, Verola O, Roos N, Porcher R, Bruneval P, et al. Skin involvement in scleroderma – where histological and clinical scores meet. Rheumatology (Oxford) 2007;46(5):833–841. doi: 10.1093/rheumatology/kel451. [DOI] [PubMed] [Google Scholar]
- 46.Querfeld C, Eckes B, Huerkamp C, Krieg T, Sollberg S. Expression of TGF-beta 1, -beta 2 and -beta 3 in localized and systemic scleroderma. J Dermatol Sci. 1999;21(1):13–22. doi: 10.1016/s0923-1811(99)00008-0. [DOI] [PubMed] [Google Scholar]
- 47.Uziel Y, Feldman BM, Krafchik BR, Laxer RM, Yeung RS. Increased serum levels of TGFbeta1 in children with localized scleroderma. Pediatr Rheumatol Online J. 2007;5:22. doi: 10.1186/1546-0096-5-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Matsushima K, Oppenheim JJ. Interleukin 8 and MCAF: novel inflammatory cytokines inducible by IL 1 and TNF. Cytokine. 1989;1(1):2–13. doi: 10.1016/1043-4666(89)91043-0. [DOI] [PubMed] [Google Scholar]
- 49.Larsen CG, Anderson AO, Oppenheim JJ, Matsushima K. Production of interleukin-8 by human dermal fibroblasts and keratinocytes in response to interleukin-1 or tumour necrosis factor. Immunology. 1989;68(1):31–36. [PMC free article] [PubMed] [Google Scholar]
- 50.Reitamo S, Remitz A, Varga J, Ceska M, Effenberger F, Jimenez S, et al. Demonstration of interleukin 8 and autoantibodies to interleukin 8 in the serum of patients with systemic sclerosis and related disorders. Arch Dermatol. 1993;129(2):189–193. [PubMed] [Google Scholar]
- 51.Ihn H, Sato S, Fujimoto M, Kikuchi K, Takehara K. Demonstration of interleukin 8 in serum samples of patients with localized scleroderma. Arch Dermatol. 1994;130(10):1327–1328. [PubMed] [Google Scholar]
- 52.Zerler B. The soluble interleukin-2 receptor as a marker for human neoplasia and immune status. Cancer Cells. 1991;3(12):471–479. [PubMed] [Google Scholar]
- 53.Sondergaard K, Stengaard-Pedersen K, Zachariae H, Heickendorff L, Deleuran M, Deleuran B. Soluble intercellular adhesion molecule-1 (sICAM-1) and soluble interleukin-2 receptors (sIL-2R) in scleroderma skin. Br J Rheumatol. 1998;37(3):304–310. doi: 10.1093/rheumatology/37.3.304. [DOI] [PubMed] [Google Scholar]
- 54.Degiannis D, Seibold JR, Czarnecki M, Raskova J, Raska K., Jr Soluble and cellular markers of immune activation in patients with systemic sclerosis. Clin Immunol Immunopathol. 1990;56(2):259–270. doi: 10.1016/0090-1229(90)90147-i. [DOI] [PubMed] [Google Scholar]
- 55.Lis AD, Brzezinska-Wcislo LA. Soluble receptors of cytokines in sera of patients with systemic sclerosis–clinical correlation. Wiad Lek. 2003;56(11–12):532–536. [PubMed] [Google Scholar]
- 56.Steen VD, Engel EE, Charley MR, Medsger TA., Jr Soluble serum interleukin 2 receptors in patients with systemic sclerosis. J Rheumatol. 1996;23(4):646–649. [PubMed] [Google Scholar]
- 57.Ihn H, Sato S, Fujimoto M, Kikuchi K, Takehara K. Clinical significance of serum levels of soluble interleukin-2 receptor in patients with localized scleroderma. Br J Dermatol. 1996;134(5):843–847. [PubMed] [Google Scholar]
- 58.Uziel Y, Krafchik BR, Feldman B, Silverman ED, Rubin LA, Laxer RM. Serum levels of soluble interleukin-2 receptor. A marker of disease activity in localized scleroderma. Arthritis Rheum. 1994;37(6):898–901. doi: 10.1002/art.1780370618. [DOI] [PubMed] [Google Scholar]
- 59.Honda M, Yamamoto S, Cheng M, Yasukawa K, Suzuki H, Saito T, et al. Human soluble IL-6 receptor: its detection and enhanced release by HIV infection. J Immunol. 1992;148(7):2175–2180. [PubMed] [Google Scholar]
- 60.Narazaki M, Yasukawa K, Saito T, Ohsugi Y, Fukui H, Koishihara Y, et al. Soluble forms of the interleukin-6 signal-transducing receptor component gp130 in human serum possessing a potential to inhibit signals through membraneanchored gp130. Blood. 1993;82(4):1120–1126. [PubMed] [Google Scholar]
- 61.Yokoyama A, Kohno N, Hirasawa Y, Kondo K, Abe M, Inoue Y, et al. Evaluation of soluble IL-6 receptor concentration in serum and epithelial lining fluid from patients with interstitial lung diseases. Clin Exp Immunol. 1995;100(2):325–329. doi: 10.1111/j.1365-2249.1995.tb03672.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Uson J, Balsa A, Pascual-Salcedo D, Cabezas JA, Gonzalez-Tarrio JM, Martin-Mola E, et al. Soluble interleukin 6 (IL-6) receptor and IL-6 levels in serum and synovial fluid of patients with different arthropathies. J Rheumatol. 1997;24(11):2069–2075. [PubMed] [Google Scholar]
- 63.Mihara M, Moriya Y, Ohsugi Y. IL-6-soluble IL-6 receptor complex inhibits the proliferation of dermal fibroblasts. Int J Immunopharmacol. 1996;18(1):89–94. doi: 10.1016/0192-0561(95)00106-9. [DOI] [PubMed] [Google Scholar]
- 64.Hasegawa M, Sato S, Fujimoto M, Ihn H, Kikuchi K, Takehara K. Serum levels of interleukin 6 (IL-6), oncostatin M, soluble IL-6 receptor, and soluble gp130 in patients with systemic sclerosis. J Rheumatol. 1998;25(2):308–313. [PubMed] [Google Scholar]
- 65.Nagaoka T, Sato S, Hasegawa M, Ihn H, Takehara K. Serum levels of soluble interleukin 6 receptor and soluble gp130 are elevated in patients with localized scleroderma. J Rheumatol. 2000;27(8):1917–1921. [PubMed] [Google Scholar]
- 66.Takehara K, Sato S. Localized scleroderma is an autoimmune disorder. Rheumatology (Oxford) 2005;44(3):274–279. doi: 10.1093/rheumatology/keh487. [DOI] [PubMed] [Google Scholar]
- 67.Zulian F, Vallongo C, Woo P, Russo R, Ruperto N, Harper J, et al. Localized scleroderma in childhood is not just a skin disease. Arthritis Rheum. 2005;52(9):2873–2881. doi: 10.1002/art.21264. [DOI] [PubMed] [Google Scholar]
- 68.Arkachaisri T, Fertig N, Pino S, Medsger TA., Jr Serum autoantibodies and their clinical associations in patients with childhood- and adult-onset linear scleroderma. A single-center study. J Rheumatol. 2008;35(12):2439–2444. doi: 10.3899/jrheum.080098. [DOI] [PubMed] [Google Scholar]