Abstract
Objective
The incidence of low back pain is extremely high and is often linked to intervertebral disc (IVD) degeneration. The mechanism of this disease is currently unknown. In this study, we have investigated the role of β-catenin signaling in IVD tissue function.
Methods
β-catenin protein levels were measured in disc samples derived from patients with disc degeneration and normal subjects by immunohistochemistry (IHC). To generate β-catenin conditional activation (cAct) mice, Col2a1-CreERT2 transgenic mice were bred with β-cateninfx(Ex3)/fx(Ex3) mice. Changes in disc tissue morphology and function were analyzed by micro-CT, histology and real-time PCR assays.
Results
We found that β-catenin protein was up-regulated in disc tissues from patients with disc degeneration. To assess the effects of increased β-catenin on disc tissue we generated β-catenin cAct mice. Overexpression of β-catenin in disc cells led to extensive osteophyte formation in 3- and 6-month-old β-catenin cAct mice which were associated with significant changes in the cells and extracellular matrix of disc tissues and growth plate. Gene expression analysis demonstrated that activation of β-catenin could enhance Runx2-dependent Mmp13 and Adamts5 expression. Moreover, genetic ablation of the Mmp13 or Adamts5 under β-catenin cAct background, or treatment of β-catenin cAct mice with a specific MMP13 inhibitor, ameliorated the mutant phenotype.
Conclusions
β-catenin signaling pathway plays a critical role in disc tissue function.
Introduction
Low back pain has been considered to be linked to degenerative changes in the intervertebral disc (IVD) (1). The incidence of low back pain is extremely high. This degenerative disc disease can occur in any of the twenty-three IVDs that span the cervical, thoracic and lumbar regions of the spine. Spine diseases and low back pain are the leading cause of disability in people under 45 years-of-age, and result in US national economic losses of over 90 billion dollars per year (2). Approximately 1% of the US population is chronically disabled because of back pain and an additional 1% experiences temporary disability. Numerous surgical approaches have been developed in recent years to deal with damaged or traumatized discs. These procedures are aimed at symptom relief, and none of the current therapies can restore the normal function of a degenerated IVD.
The major cartilaginous joint of the vertebral column is the IVD. Each disc consists of an inner zone, the nucleus pulposus, surrounded by a peripheral outer region, the annulus fibrosus. On the inner border of the annulus, the fibers form a tissue composed predominantly of type II collagen. The inner annulus fibrosus encloses the nucleus pulposus, a highly viscous gel-like tissue that serves to generate aggrecan and other proteoglycans, that provide the disc with its critical water-retaining characteristics. The superior and inferior boundaries of the IVD are formed by the growth plate and cartilage endplate. It has been suggested that mechanical factors produce growth plate and endplate damage, the antecedent to disc degeneration (3). The metabolism of the nucleus puplosus is dependent on diffusion of fluid either from the marrow of the vertebral bodies across the subchondral bone, growth plate and cartilage endplate or through the annulus fibrosus from the surrounding blood vessels. Morphological changes in the vertebral bone, growth plate and cartilage endplate, which occur with advancing age or trauma-related degeneration, can interfere with normal disc nutrition and further the degenerative process. These changes could eventually lead to abnormal disc cell metabolism and alter the integrity of the proteoglycans and water concentration, reducing the number of viable cells with subsequent alteration in the movement of solutes into and out of the disc (4).
Wnt proteins play critical roles in bone and cartilage development (5). Wnts form a dual-receptor complex with Frizzled and low-density-lipoprotein (LDL)-receptor like protein 5 or 6 (LRP-5/6) on cell surfaces. This triggers signaling through a large protein complex in the Wnt canonical pathway, including glycogen synthase kinase 3β (GSK-3β), casein kinase I, and the scaffolding proteins, adenomatous polyposis coli (APC), disheveled and Axins. This complex has multiple effects on β-catenin: it promotes β-catenin phosphorylation by GSK-3β at specific amino terminal residues and creates docking sites for F-box protein/E3 ligase complexes (5, 6, 7) and enables β-catenin to be detected and destroyed by the 26S proteasome in the absence of Wnt signaling (8). The activation of Wnt signaling inhibits the stimulatory effect of Axins on β-catenin phosphorylation and allows β-catenin to move to the nucleus (9). Nuclear β-catenin combines with the transcription factors TCFs and LEF1 to activate expression of target genes. β-catenin is a key molecule in the canonical Wnt signaling pathway and plays a critical role in multiple steps during osteoblast and chondrocyte differentiation. However, the role of β-catenin signaling in IVD tissue has not been fully investigated.
In the present studies, we have analyzed β-catenin expression in disc tissues derived from patients with disc degeneration by IHC. We found that β-catenin protein was significantly up-regulated in the disc tissues of these samples. To create a mouse model which mimics the β-catenin up-regulation condition observed in patients with disc degeneration, we have generated β-catenin cAct mice. Severe defects in disc tissue were found in these mice, including up-regulation of expression of Mmp13 and Adamts4 and Adamts5 genes, significant loss of growth plate cartilage, and severe osteophyte formation. Deletion of the Mmp13 or Adamts5 gene under β-catenin cAct background or treatment of β-catenin cAct mice with MMP13 inhibitor significantly reversed the defective phenotype observed in disc tissues of β-catenin cAct mice. These findings demonstrate that β-catenin signaling plays a critical role in disc tissue function and may be involved in the development of disc degeneration.
Methods
Immunohistochemistry
3-μm paraffin sections were heated at 95°C in citrate buffer (pH 6.0) for 2 hours, and then treated with a dual endogenous enzyme blocking reagent for 10 minutes (Dako S2003). After blocked with 1/20 normal goat serum (Vector S-1000) for 30 minutes, sections were treated with 1/30 rabbit anti-β-catenin antibody (Cell Signaling, 9562) overnight at 4°C and incubated with 1/200 secondary biotinylated goat anti-rabbit antibody (Vector BA-1000) for 30 minutes, followed by 1/250 streptavidine-peroxidase (Pierce 21130) for 30 minutes at room temperature. Peroxidase activity was revealed by Romulin AEC Chromogen (Biocare Medical RAEC810L) staining.
Animals
We obtained Rosa26 reporter mice and Mmp13fx/fx mice from Jackson Laboratories (Bar Harbor, ME) (10, 11). We used Col2a1-CreERT2 transgenic mice as previously described (12, 13). β-cateninfx(Ex3)/fx(Ex3) mice were originally reported by Harada et al. (14) and were used in our previous studies (13). Tamoxifen (Sigma, St. Louis, MO) was administered to 2-week-old mice by i.p. injection (1mg/10g body weight for 5 days). All protocols were approved by the University Committee on Animal Resources of the University of Rochester.
Micro-Computed Tomography (μCT)
Prior to histological processing, we evaluated formalin-fixed spines by μCT. We used a Scanco vivaCT40 cone-beam scanner (SCANCO Medical, Switzerland) with 55 kVp source and 142 μAmp current. We scanned the spines at a resolution of 10.5 μm. The scanned images from each group were evaluated at the same thresholds to allow 3-dimensional structural rendering of each sample.
Histology and histomorphometry
We dissected lumbar spines from Col2a1-CreERT2;R26R mice, Col2a1-CreERT2;β-cateninfx(Ex3)/wt mice, Col2a1-CreERT2;Mmp13fx/fxmice, Col2a1-CreERT2;β-cateninfx(Ex3)/wt;Mmp13fx/fx mice and their corresponding Cre-negative control mice. Samples were fixed in 10% formalin, decalcified, and embedded in paraffin. Serial sections were taken at 3 levels spaced 15μm apart within the midsagittal region of the intervetebral bodies. Sections were cut at 3μm thickness. The sections were stained with Alcian Blue/H&E (AB/H&E) and Safranin O/Fast green (SO/FG). We quantified growth plate cartilage area using ImagePro 4.5 (Leeds Precision Instruments, Minneapolis, MN, USA) by tracing the alcian blue-positive area. To quantify lumbar spine length, we measured C1 to S4 vertebra distance from μCT images using ImagePro 4.5. To quantify disc space, we measured the distance between L4 and L5 from μCT images by ImagePro 4.5. Statistical analysis was conducted using one-way ANOVA followed by Dunnett's test or unpaired Student t-test.
Treatment with MMP13 inhibitor
A MMP13 inhibitor CL82198 (Tocris Bioscience) was used for the in vivo experiment. The effect of CL82198 on inhibition of MMP13 enzymatic activity was confirmed by MMP-13 fluorimetric drug discovery kit (Enzo Life Sciences International). Both β-catenin cAct mice and Cre-negative control mice were injected intraperitoneally with tamoxifen (1mg /10g body weight for 5 days) at 2 weeks of age and then injected intraperitoneally with MMP13 inhibitor (5mg/kg body weight per day) or vehicle (PBS) every other day. At 3 months of age, mice were sacrificed and the whole lumbar vertebras were harvested for micro-CT and histological analyses.
Results
Our recent studies demonstrate that β-catenin expression is highly up-regulated in knee joint samples from patients with osteoarthritis (OA) (13). Since disc generation is often correlated with OA development in patients (15, 16), in the present studies, we examined β-catenin expression in patients with disc degeneration by IHC. Expression of β-catenin protein in human disc samples was semi-quantified. Each sample was scored twice for the percentage of labeled disc cells (0, absence of labeling over disc cells; 1, <30% of disc cells are labeled; 2, 30-60%; 3, >60%); and for the intensity of the IHC (0, no staining; 1, weak; 2, mild; 3, strong staining). Multiplication of both scores allowed the final quotation ranging from 0-9. A double-blind analysis was performed by three independent individuals. The final scores of 1-4 were considered as weak positive and the final scores of 6-9 were considered as strong positive. We found that β-catenin protein was up-regulated in most samples derived from patients with disc degeneration. We analyzed 30 patient samples and found 6 of them to be strong positive and 22 of them were weak positive with β-catenin protein expression. In contrast, β-catenin was only detected in 1 sample with weak expression in disc tissue derived from normal subjects (n=10) (Figure 1). In most cases, β-catenin expression was detected in the annular fibrosus and cartilage endplate cells. Formation of chondrocyte clusters was found in degenerative disc samples where β-catenin is highly up-regulated (Figure 1A and F). These results suggest that β-catenin expression may be activated in different types of IVD cells through different mechanisms during the development of disc degeneration.
Figure 1.
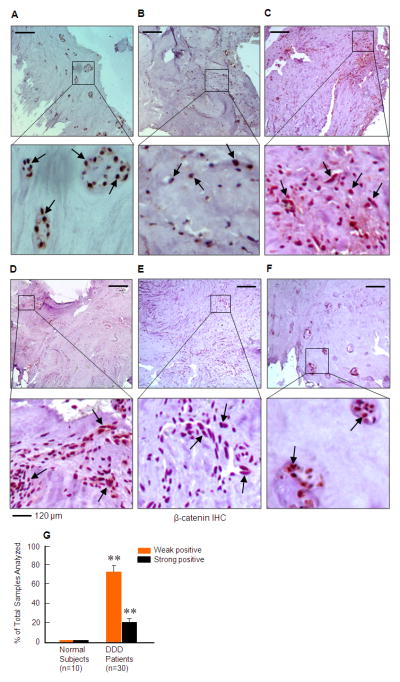
β-catenin protein expression is up-regulated in disc tissues of patients with disc degeneration. The expression of β-catenin protein was examined by IHC in samples derived from normal subjects (n=10) and patients with disc degeneration as determined by MRI and histology. β-catenin protein is highly expressed in 6 samples and weakly expressed in 22 samples derived from patients with disc degeneration (black arrows) (n=30) but was weakly expressed in 1 sample from normal subjects. In some samples, disc cells form clusters where β-catenin is highly up-regulated (A and F). In most samples, β-catenin expression was found in localized areas of disc tissues. Expression of β-catenin protein was semi-quantified (G). Each sample was scored twice for the percentage of labeled disc cells (0, absence of labeling over disc cells; 1, <30% of disc cells are labeled; 2, 30-60%; 3, >60%); and for the intensity of the IHC (0, no staining; 1, weak; 2, mild; 3, strong staining). Multiplication of both scores allowed the final quotation ranging from 0-9. The final scores of 1-4 were considered as weak positive and the final scores of 6-9 were considered as strong positive. The percentage of weak and strong positive β-catenin staining was much higher in patient samples with disc degeneration compared to those from normal subjects. **p<0.01, unpaired Student t-test.
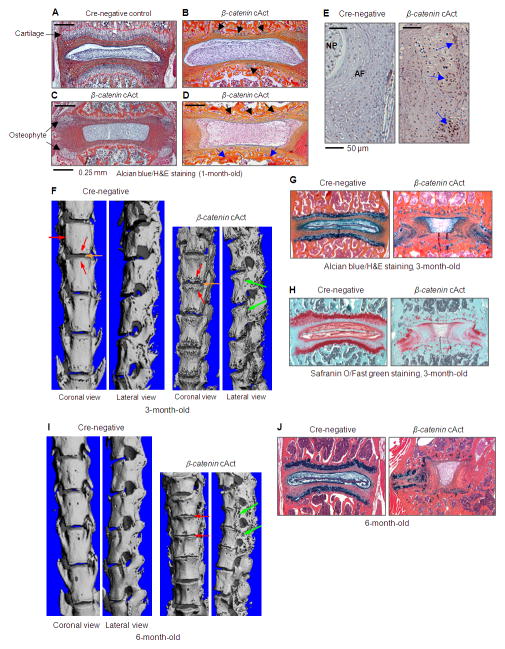
To develop a mouse model to mimic human disc degeneration, we generated inducible β-catenin conditional activation (cAct) mice by breeding β-cateninfx(E x3)/fx(Ex3) mice with Col2a1-CreERT2 transgenic mice. The advantage of this approach is that it overcomes the embryonic lethality of chondrocyte-specific β-catenin activation (17, 18), and it permits normal spine development up to the time of Cre recombination. Tamoxifen (1 mg/10 g body weight, i.p. injection for 5 consecutive days) was administered into 2-week-old Col2a1-CreERT2;β-cateninfx(Ex3)/wt (β-catenin cAct) and Cre-negative control mice. These mice were sacrificed at 3 and 6 months of age to analyze changes in the structure and morphology of IVD tissues. To determine Cre-mediated recombination efficiency, we have bred Col2a1-CreERT2 transgenic mice with Rosa26 reporter mice. Tamoxifen was administered into 2-week-old mice and X-Gal staining was performed at 2 months of age. High Cre-recombination efficiency in growth plate chondrocytes (75%) and inner annulus fibrosus cells (81%) was observed in Col2a1-CreERT2;R26R mice (data not shown) (19). This is consistent with the expression pattern of endogenous type II collagen in the disc tissue (Figure 3J). We analyzed β-catenin expression in 4-week-old β-catenin cAct mice which received tamoxifen via IHC, and found that β-catenin protein was overexpressed in disc cells of β-catenin cAct mice (Figure 2A). Due to the loss of growth plate chondrocytes in 4-week-old β-catenin cAct mice, β-catenin expression was mainly detected in annulus fibrosus cells in β-catenin cAct mice (Figure 2A).
Figure 3.
Changes in expression of disc tissue-specific genes and proteins. (A-H) Total RNA was extracted from primary disc cells isolated from 3-week-old β-catenin cAct mice or Cre-negative control mice for real-time PCR analysis. The results showed that expression of Adamts4 (2-fold), Adamts5 (4-fold), Mmp13 (4-fold), colX (2.5-fold), Alp (2-fold) and osteocalcin (2-fold) were significantly increased (A-F), while Col2a1 and Col9a1 mRNA levels were significantly reduced in disc cells derived from β-catenin cAct mice (G and H) (*p<0.05, unpaired Student t-test). (I-K) IHC data demonstrated that MMP13 protein expression was markedly increased (I) (green arrows), type II collagen (Col2a1) protein levels were slightly reduced (J) (black arrow) and type IX collagen (Col9a1) protein levels were significantly reduced (K) (blue arrow) in 1-month-old β-catenin cAct mice.
Figure 2.
Characterization of β-catenin cAct mice. β-catenin cAct mice were generated by breeding β-cateninfx(Ex3)/fx(Ex3) mice with Col2a1-CreERT2 mice and treating them with tamoxifen. (A-D) Loss of growth plate cartilage (The growth plate cartilage in Cre-negative control mice was outlined by the red dotted line, A), formation of chondrocyte clusters (B, black arrows), formation of osteophyte (C, black arrows) and formation of new blood vessels (D, black arrows) and new woven bone (D, blue arrows) were observed in 1-month-old β-catenin cAct mice, demonstrated by Alcian blue/H&E staining. (E) Overexpression of β-catenin protein was detected by IHC in β-catenin cAct mice (blue arrows). (F and I) Micro-CT analysis showed that the length of spine was reduced18% and 25% in 3- and 6-month-old β-catenin cAct mice, respectively. Additonal pathologic features in the mutant mice included extensive osteophyte formation (green arrows) and disk space narrowing (red arrows). (G, H and J) Histologic analysis of Alcian blue/H&E and Safranin O/Fast green stained spine sections showed that endplate cartilage and growth plate cartilage were markedly lost, together with a dramatic IVD tissue disorganization in 3- (G and H) and 6-month-old (J)β-catenin cAct mice.
The loss of growth plate chondrocytes was detected as early as two weeks after tamoxifen induction in 1-month-old β-catenin cAct mice. The formation of chondrocyte clusters was found in 1-month-old β-catenin cAct mice (Figure 2B), and similar chondrocyte clusters were also observed in disc samples from patients with disc degeneration (Figure 1A and F). Early osteophyte formation can be detected in 1-month-old β-catenin cAct mice (Figure 2C). New blood vessels and new woven bone formation were also observed at the original location of the growth plate (Figure 2D). We then analyzed phenotypic changes in disc tissues of 3- and 6-month-old β-catenin cAct mice using micro-CT and histology. Our results showed that the spine length of 3-month-old β-catenin cAct mice were reduced 18% compared to Cre-negative control mice (Figure 2F). The lengths of C1-S4 of Cre-negative control mice were 60±0.58 mm (n=3) while the lengths of C1-S4 of β-catenin cAct mice were 49±2.19 mm (n=6) (Figure 2F). The average body weight of β-catenin cAct mice was reduced 23% (Cre-negative: 19.7±2.18 g, n=3; β-catenin cAct: 15.0±1.24 g, n=6). At 6 months old, the spine length of β-catenin cAct mice were reduced 25% compared to Cre-negative control mice (Figure 2I). The lengths of C1-S4 of Cre-negative control mice were 62.5±1.08 mm (n=7) while the lengths of C1-S4 of β-catenin cAct mice were 46.5±1.26 mm (n=4) (Figure 2I). The average body weight of β-catenin cAct mice was reduced 49% (Cre-negative: 26.3±1.95 g, n=7; β-catenin cAct: 16±.0.71 g, n=4). Extensive osteophyte formation, disc space narrowing and fusion of adjacent vertebra were evident in the entire spine of β-catenin cAct mice, as determined by micro-CT analysis (Figure 2F and 2I). The disc space narrowing could be related to the loss of growth plate cartilage and severe osteophyte formation which blocks the disc space. Histology was performed on paraffin-embedded coronal sections and, samples were stained with Alcian blue/H&E (AB/H&E) and Safranin O/Fast green. Histologic results demonstrated a severe loss of endplate and growth plate cartilage, a reduced number of growth plate chondrocytes and disorganized annulus fibrosus and nucleus pulposus tissues in 3-month-old β-catenin cAct mice (Figure 2G and 2H). The defects of disc tissues were worse in 6-month-old mice. The AB/H&E staining data showed that the end plate and growth plate cartilage almost completely disappeared, there were much fewer chondrocytes left, and the annulus fibrosus and nucleus pulposus structures were completely damaged (Figure 2J).
To investigate the gene expression changes in β-catenin cAct mice, we isolated primary disc cells from 3-week-old β-catenin cAct and Cre-negative control mice for real-time PCR assay. 2- and 4-fold increases in expression of Adamts4 and Adamts5 were detected in disc cells derived from β-catenin cAct mice (Figure 3A and B). Mmp13 expression was also significantly increased (4-fold) (Figure 3C) and Mmp2 expression was not changed (data not shown) in β-catenin cAct mice. ColX expression was significantly increased (Figure 3D). Alp and osteocalcin expression was also up-regulated (2-fold increases) in β-catenin cAct mice (Figure 3E and F). In contrast, Col2a1 and Col9a1 expression was significantly reduced in disc cells of β-catenin cAct mice (Figure 3G and H). Consistent with the finding on increased Mmp13 mRNA expression, MMP13 protein levels were also significantly increased in disc tissues of 4-week-old β-catenin cAct mice (Figure 3I). To further determine if activation of β-catenin signaling will lead to the up-regulation of Mmp13 in human chondrocytes, we treated human articular chondrocytes with BIO for 48 hours and found that BIO also stimulated Mmp13 expression in human articular chondrocytes (data not shown). To determine changes in protein levels of type II and type IX collagen, we performed IHC using mouse monoclonal antibodies against Col2a1 and Col9a1 proteins (Developmental Studies Hybridoma Bank, DSHB, University of Iowa, Iowa City) and found that protein levels of type II collagen were slightly reduced but protein levels of type IX collagen were markedly reduced in β-catenin cAct mice (Figure 3J and K).
To determine the signaling mechanism by which β-catenin regulates Mmp13 expression, and to provide an initial insight into the mechanism underlying the disc phenotype seen in β-catenin cAct mice, in vitro studies were performed using the RCS chondrogenic cell line. Treatment of RCS cells with BIO (a GSK-3β inhibitor which induces β-catenin activation) had no effect on Mmp13 expression within 12 hours, but significantly up-regulated Mmp13 mRNA expression at 24 and 48 hour time points (Figure 4A). Interestingly, treatment with BIO (48 hours) also significantly up-regulated Runx2 protein expression in RCS cells (Figure 4B) which is consistent with the up-regulation of Runx2 mRNA in disc cells of β-catenin cAct mice (Figure 4C). The regulation of the Mmp13 gene by Runx2 has been reported in recent years (20, 21, 22). To directly assess the effect of Runx2 on Mmp13 gene transcription and to determine if it might mediate the overall effect of β-catenin on Mmp13, we cloned the 3.4 kb human Mmp13 promoter into the pGL3 luciferase reporter plasmid. DNA sequencing of this Mmp13 promoter identified a Runx2 binding site in the proximal region (-138/-132, AACCACA), which is conserved among human, mouse and rat. We found that transfection of Runx2 as well as treatment with BIO significantly stimulated Mmp13 promoter activity (Figure 4D). Mutation of the Runx2 binding site (mutant sequence: ACTAACA) completely abolished the stimulatory effect of Runx2 as well as BIO-induced Mmp13 promoter activity (Figure 4D), suggesting that BIO (or activation of β-catenin signaling) may stimulate Mmp13 gene transcription through up-regulation of transcription factor Runx2. To further determine the role of Runx2 in BIO-induced Mmp13 promoter activity, we treated RCS cells with BIO with or without transfection of Runx2 siRNA and found that transfection of Runx2 siRNA completely inhibited BIO-induced Mmp13 promoter activity (Figure 4E). Results from chromatin immunoprecipitation assay further demonstrated that Runx2 specifically bound to the proximal region of the Mmp13 promoter, which contains the Runx2 binding site but not the upstream or downstream distant region of the Mmp13 gene (Figure 4F). These results demonstrate that activation of β-catenin signaling could stimulate Runx2 expression which subsequently promotes Mmp13 gene transcription.
Figure 4.
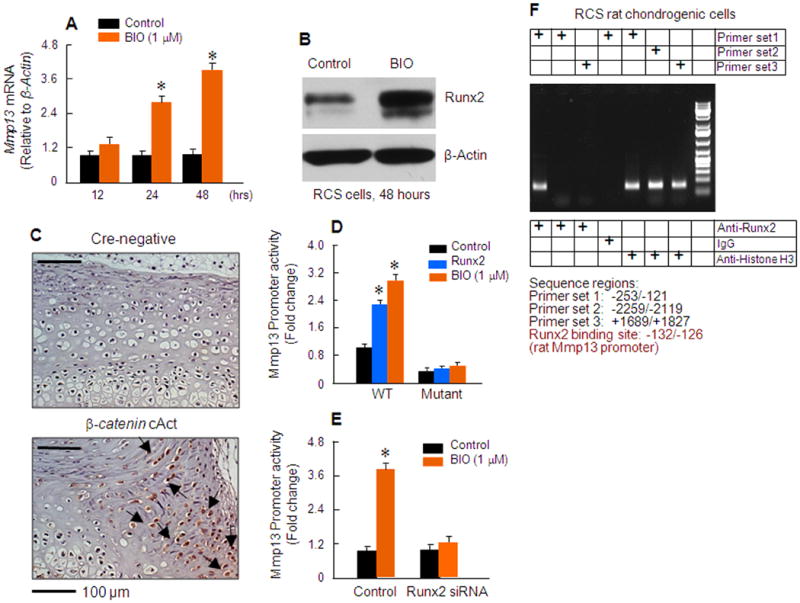
Activation of β-catenin signaling stimulates Mmp13 gene transcription in a Runx2-dependent manner. (A) Effects of BIO, a GSK-3β inhibitor, on Mmp13 mRNA expression were examined in RCS chondrogenic cells. BIO (1 μM) significantly up-regulated Mmp13 expression after RCS cells were incubated with BIO for 24 and 48 hours. *p<0.05, one-way ANOVA followed by Dunnett's test, n=4, compared to control group. (B) Runx2 protein expression was also significantly up-regulated by BIO in RCS cells (48 hour incubation). (C) Runx2 protein expression was examined by IHC, showing a dramatic up-regulation of Runx2 protein levels in disc cells of 3-week-old β-catenin cAct mice (black arrows). (D) The 3.4 kb human Mmp13 promoter was cloned into pGL3 vector and a conserved Runx2 binding site was found in the proximal region of the human Mmp13 promoter (-138/-132). Transfection of Runx2 or treatment with BIO (1 μM) significantly enhanced luciferase activity of the Mmp13 promoter. Mutation of the Runx2 binding site completely abolished the stimulatory effect of Runx2 as well as BIO on Mmp13 promoter activity in RCS cells (*p<0.05, one-way ANOVA followed by Dunnett's test, n=4). (E) Transfection of Runx2 siRNA also significantly inhibited BIO-induced Mmp13 promoter activity (*p<0.05, one-way ANOVA followed by Dunnett's test, n=4). (F) Chromatin immunoprecipitation (ChIP) assay showed Runx2 specifically bound to the proximal promoter region of the Mmp13 promoter, but not the upstream or downstream distant region of the Mmp13 gene.
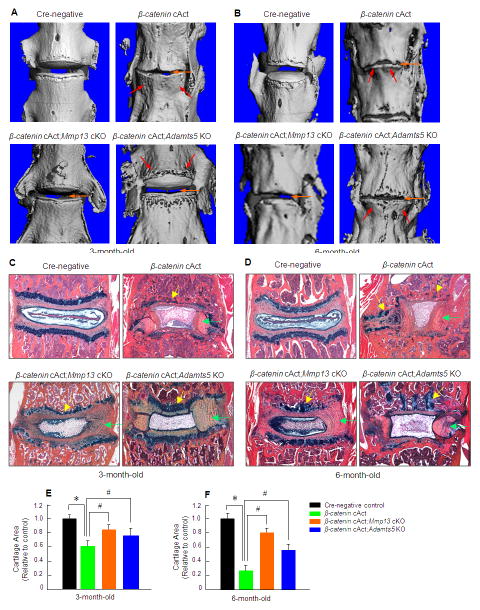
MMP13 is a collagenase which degrades type II and type IX collagens, and ADAMTS5 is an aggrecanase which degrades aggrecan. Type II and type IX collagens and aggrecan are the principal components present in disc tissue. We observed significant up-regulation of Mmp13 and Adamts5 expression in β-catenin cAct mice. Because both MMP13 and ADAMTS5 play critical roles in the development of osteoarthritis (23, 24, 25), we reasoned that Mmp13 and Adamts5 may be the key downstream target genes of β-catenin signaling in disc cells during the development of disc defective phenotype. To test this hypothesis, we bred β-catenin cAct mice with Mmp13fx/fx mice (11) or Adamts5−/− mice (26) to produce Col2a1-CreERT2;β-cateninfx(Ex3)/wt;Mmp13fx/fx or Col2a1-CreERT2;β-cateninfx(Ex3)/wt;Adamts5−/− double mutant mice. Deletion of the Mmp13 gene in β-catenin-induced disc cells was confirmed by IHC (data not shown). Micro-CT data showed that deletion of the Mmp13 or the Adamts5 gene in β-catenin cAct mice significantly ameliorated the phenotype of disc defects in 3-month-old mice, including the loss of growth plate cartilage and disc space narrowing (Figure 5A). At 6-month-old, deletion of the Mmp13 gene in β-catenin cAct mice significantly decelerated the disc defects (Figure 5B). Though deletion of the Adamts5 gene in β-catenin cAct mice ameliorated disc defects to a certain degree, it is less potent than deletion of the Mmp13 gene (Figure 5A and B). Results from histology and histomorphometry further demonstrated maintenance of normal disc tissue morphology with a significant increase in proteoglycan levels and restoration of the normal growth plate cartilage when the Mmp13 or the Adamts5 gene was deleted under β-catenin cAct background in 3-month-old double mutant mice (Figure 5C). In 6-month-old mice, deletion of the Mmp13 gene is more effective to rescue the disc defects in β-catenin cAct mice compared to deletion of the Adamts5 gene (Figure 5D). These results indicate that Mmp13 and Adamts5 are the critical downstream target genes of β-catenin signaling in disc cells and may play key roles in mediating β-catenin-induced disc defects. Since Mmp13 conditional knockout (cKO) mice and Adamts5 KO mice had relatively normal disc phenotype, the results observed in β-catenin cAct;Mmp13 cKO and β-catenin cAct;Adamts5−/− double mutant mice are mainly caused by rescuing the β-catenin activation phenotype in disc cells.
Figure 5.
Deletion of the Mmp13 or Adamts5 gene in β-catenin cAct mice ameliorates the disc defect phenotype. (A and B) Micro-CT analysis (L3/L4 disc) was performed in 4 groups of mice: 1) Cre-negative, 2) β-catenin cAct, 3) β-catenin cAct;Mmp13 cKO, and 4) β-catenin cAct;Adamts5 KO. Deletion of the Mmp13 or Adamts5 gene significantly ameliorated the phenotype of disc space narrowing (orange arrows) and osteophyte formation (red arrows) observed in 3- and 6-month-old β-catenin cAct mice. In β-catenin cAct;Adamts5 KO group, osteophyte formation was reduced compared to β-catenin cAct group. (C and D) Histologic results of Alcian blue/H&E staining showed dramatic loss of growth plate cartilage in 3- and 6-month-old β-catenin cAct mice (yellow arrows). The structures of annulus fibrosus and nucleus pulposus tissues were severely disorganized in β-catenin cAct mice (green arrows). Deletion of the Mmp13 or Adamts5 gene significantly prevents the loss of growth plate cartilage tissues (yellow arrows) and preserves the structure disorganization of the annulus fibrosus and nucleus pulposus tissues (green arrows) observed in β-catenin cAct mice. The growth plate cartilage areas were measured by histomorphometry. *p<0.05, one-way ANOVA followed by Dunnett's test, n=5, compared to Cre-negative group; #p<0.05, one-way ANOVA followed by Dunnett's test, n=5, compared to β-catenin cAct group.
With β-catenin-dependent up-regulation of Mmp13 as a potential mechanism underlying disc defects, we investigated the efficacy of the MMP13 inhibitor CL82198 in reversing the disc phenotype in β-catenin cAct mice. CL82198 is a specific MMP13 inhibitor that binds to the S1' pocket of MMP13 rather than via metal chelation and has no effect on MMP1, MMP9, or TACE (27, 28, 29) so it is relatively specific on inhibition of MMP13 activity. CL82198 was injected i.p. every other day (5 mg/kg) into β-catenin cAct mice right after tamoxifen induction. Mice were sacrificed at 3 months of age and effects of CL82198 on the disc phenotype were analyzed by micro-CT and histology. The disc space narrowing found by micro-CT and loss of proteoglycan and disorganized annulus fibrosus and nucleus pulposus tissues found by histology and histomorphometry were remarkably ameliorated by MMP13 inhibition (Figure 6A-D). These results suggest that the use of an MMP13 inhibitor may represent a potential therapy for the treatment or diseases such as disc degeneration.
Figure 6.
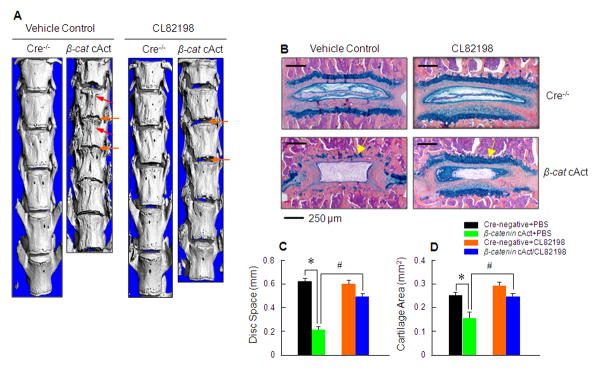
Inhibition of the MMP13 enzyme activity in β-catenin cAct mice ameliorates the disc defect phenotype. (A) Micro-CT analysis (L1 to L6 disc) was performed in 4 groups of mice: 1) Cre-negative+PBS, 2) β-catenin cAct+PBS, 3) Cre-negative+CL82198, and 4) β-catenin cAct+CL82198. Treatment with the MMP13 inhibitor CL82198 significantly ameliorated the disc space narrowing (orange arrows) and osteophyte formation (red arrows) observed in β-catenin cAct mice. (B and C) Histologic results of Alcian blue/H&E staining showed dramatic loss of the growth plate cartilage and proteoglycan (yellow arrows) in β-catenin cAct mice. Treatment with MMP13 inhibitor CL82198 significantly reverses the cartilage loss and disc structure disorganization phenotype observed in β-catenin cAct mice. The changes in growth plate cartilage area were measured by histomorphometry. *p<0.05, one-way ANOVA followed by Dunnett's test, n=5, compared to Cre-negative+PBS group; #p<0.05, one-way ANOVA followed by Dunnett's test, n=5, compared to β-catenin cAct+PBS group.
Discussion
The present studies show for the first time that β-catenin protein is up-regulated and activated in disc tissues from patients with disc degeneration. We created a β-catenin cAct mouse model and the phenotype of these mice resembles some of features of human disc degeneration. Similar findings were also reported in recent studies when β-catenin gene is overexpressed in transgenic mice (30). β-catenin cAct mice have reduced spine length. This is likely related to the general growth retardation seen in β-catenin cAct mice. We also observed severe loss of proteoglycan and growth plate cartilage, severely disorganized annulus fibrosus and nucleus pulposus tissues and prevalence of osteophyte formation in β-catenin cAct mice. Although β-catenin cAct mice have multiple features that resemble human disc degeneration, this mouse model may only partially mimic the human disc diseases. One reason is that nucleus pulposus cells were not targeted by Col2a1-CreERT2 transgenic mice. The change in the shape of nucleus pulposus could be caused by the osteophyte formation and structural changes in annulus fibrosus tissues. It is also possible that the defect seen in nucleus pulposus tissues is related to the disruption of nutrient and solute supplies to this region after the loss of the growth plate cartilage in β-catenin cAct mice.
We found that Mmp13 mRNA and protein expression was significantly up-regulated in β-catenin cAct mice and our in vitro studies demonstrated that activation of β-catenin signaling stimulates Mmp13 gene transcription in a Runx2-dependent manner. To determine the role of Runx2 in β-catenin-induced disc defects in vivo, it will require genetically targeting the Runx2 gene and this important aspect needs to be further investigated. Deletion of the Mmp13 or Adamts5 gene under the β-catenin cAct background significantly reversed the disc destruction phenotype observed in β-catenin cAct mice. It seems that deletion of the Mmp13 gene is more efficient in protecting the loss of growth plate cartilage and preserves the disc tissue structure than the deletion of the Adamts5 gene. These findings suggest that Mmp13 may serve as a major downstream target of β-catenin signaling in disc cells although both Mmp13 and Adamts5 were up-regulated in disc cells in β-catenin cAct mice. Strikingly, treatment of β-catenin cAct mice with MMP13 inhibitor also significantly inhibited disc defects. Our studies demonstrate that β-catenin is a key mediator causing defects in disc tissues. A recent study demonstrated that transcription factor ED-rich tail 2 (CITED2) is a negative regulator of MMP1 in articular chondrocytes and has chondroprotective effect (31). Taken together these findings suggest that either inhibition of MMP13 or activation of CITED2 could serve as potential treatments for disc degeneration in patients.
Findings from histology and histomorphometry showed that treatment with MMP13 inhibitor is more effective than deletion of the Mmp13 gene in protecting against the disc defects observed in β-catenin cAct mice. There are two possible reasons to account for this. Although the MMP13 inhibitor used in this study is relatively specific for MMP13, it does have some additional non-specific effects on inhibition of other MMPs which may contribute to the protection of the disc defects observed in β-catenin cAct mice. Alternatively, the Cre-recombination efficiency mediated by Col2a1-CreERT2 is about 80% in inner annulus fibrosus cells and about 75% in growth plate chondrocytes (19); thus, MMP13 activity in the other 20-25% of inner annulus fibrosus cells and growth plate chondrocytes as well as the entire population of nucleus pulposus cells remainsnormal in β-catenin cAct;Mmp13 cKO double mutant mice.
Although deletion of the Mmp13 gene significantly reversed the disc degeneration phenotype observed in β-catenin cAct mice, the phenotype was not completely protected by deletion of the Mmp13 gene. There is still significant loss of proteoglycans and growth plate cartilage tissues in these double mutant mice compared to Cre-negative controls. It is known that deletion of Adamts5 protects against the development of OA (25, 32, 33). Since the mRNA expression for both of these genes was significantly up-regulated in the disc tissue in β-catenin cAct mice, the impact of aggrecanase enzymatic activity likely contributes to the development of disc defects observed in β-catenin cAct mice. We found that deletion of the Adamts5 gene under the β-catenin cAct background reversed the disc destruction phenotype in β-catenin cAct mice as well, although it is less effective than deletion of the Mmp13 gene under the β-catenin cAct background. These results indicate that Mmp13 may play a major role in β-catenin-mediated disc destruction observed in β-catenin cAct mice.
The current study shows that β-catenin plays a key role in activating downstream target genes such as Mmp13 and Adamts5 in disc cells leading to severe defects in disc tissues. However, how β-catenin signaling is activated during disc degeneration remains unknown. Potential causes of β-catenin activation in disc cells include 1) an activation mutation of the β-catenin gene or mutations of other genes that regulate and inhibit β-catenin signaling, such as Axin1 and APC, 2) injury or mechanical loading-induced β-catenin activation, and 3) inflammation-induced β-catenin activation. Activating mutations of the β-catenin gene has been linked to the development of multiple cancers including colorectal cancer, acute and chronic myeloid leukemia and multiple myeloma (34, 35, 36, 37). Several lines of evidence have demonstrated that mechanical loading leads to activation of β-catenin signaling in bone cells (38, 39, 40). Finally, TNFα and IL-1β are two important inflammatory cytokines involved in the development of osteoarthritis. Recent findings demonstrate that these cytokines could activate β-catenin signaling in other cell types (41, 42). Thus, they may also play a role in the activation of β-catenin signaling in disc cells and contribute to the development of disc diseases.
Acknowledgments
This work was supported by Grants R01-AR055915, R01-AR054465 and R01-AR051189 to D.C. from National Institute of Health and by Grant N08G-070 to D.C. from New York State Department of Health and Empire State Stem Cell Board. This work was also partially supported by the National Basic Research Program of China (973 program, 2010cb530400) to Y.W.
Footnotes
Conflict of interest: The authors declare that no competing interests exist.
Financial disclosure: The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Luoma K, Riihimaki H, Luukkonen R, Raininko R, Viikari-Juntura E, Lamminen A. Low back pain in relation to lumbar disc degeneration. Spine. 2000;25:487–492. doi: 10.1097/00007632-200002150-00016. [DOI] [PubMed] [Google Scholar]
- 2.Phillips K, Ch'ien AP, Norwood BR, Smith C. Chronic low back pain management in primary care. Nurse Pract. 2003;28:26–31. doi: 10.1097/00006205-200308000-00008. [DOI] [PubMed] [Google Scholar]
- 3.Adams MA, Freeman BJ, Morrison HP, Nelson IW, Dolan P. Mechanical initiation of intervertebral disc degeneration. Spine. 2000;25:1625–36. doi: 10.1097/00007632-200007010-00005. [DOI] [PubMed] [Google Scholar]
- 4.Hurri H, Karppinen J. Discogenic pain. Pain. 2004;112:225–8. doi: 10.1016/j.pain.2004.08.016. [DOI] [PubMed] [Google Scholar]
- 5.Westendorf JJ, Kahler RA, Schroeder TM. Wnt signaling in osteoblasts and bone diseases. Gene. 2004;341:19–39. doi: 10.1016/j.gene.2004.06.044. [DOI] [PubMed] [Google Scholar]
- 6.Behrens J, Jerchow BA, Wurtele M, Grimm J, Asbrand C, Wirtz R, et al. Functional interaction of an axin homolog, conductin, with beta-catenin, APC, and GSK3beta. Science. 1998;280:596–9. doi: 10.1126/science.280.5363.596. [DOI] [PubMed] [Google Scholar]
- 7.Jiang J, Struhl G. Regulation of the Hedgehog and Wingless signaling pathways by the F-box/WD40-repeat protein Slimb. Nature. 1998;391:493–6. doi: 10.1038/35154. [DOI] [PubMed] [Google Scholar]
- 8.Aberle H, Bauer A, Stappert J, Kispert A, Kemler R. beta-catenin is a target for the ubiquitinproteasome pathway. EMBO J. 1997;16:3797–804. doi: 10.1093/emboj/16.13.3797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mao J, Wang J, Liu B, Pan W, Farr GH, 3rd, Flynn C, et al. Low-density lipoprotein receptor-related protein-5 binds to Axin and regulates the canonical Wnt signaling pathway. Mol Cell. 2001;7:801–9. doi: 10.1016/s1097-2765(01)00224-6. [DOI] [PubMed] [Google Scholar]
- 10.Soriano P. Generalized lacZ expression with the Rosa26 Cre reporter strain. Nat Genet. 1999;21:70–1. doi: 10.1038/5007. [DOI] [PubMed] [Google Scholar]
- 11.Stickens D, Behonick DJ, Ortega N, Heyer B, Hartenstein B, Yu Y, et al. Altered endochondral bone development in matrix metalloproteinase 13-deficient mice. Development. 2004;131:5883–95. doi: 10.1242/dev.01461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen M, Lichtler AC, Sheu T, Xie C, Zhang X, O'Keefe RJ, et al. Generation of a transgenic mouse model with chondrocyte-specific and tamoxifen-inducible expression of Cre recombinase. Genesis. 2007;45:44–50. doi: 10.1002/dvg.20261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhu M, Tang D, Wu Q, Hao S, Chen M, Xie C, et al. Activation of β-catenin signaling in articular chondrocytes leads to osteoarthritis-like phenotype in adult β-catenin conditional activation mice. J Bone Miner Res. 2009;24:12–21. doi: 10.1359/JBMR.080901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harada N, Tamai Y, Ishikawa T, Sauer B, Takaku K, Oshima M, et al. Intestinal polyposis in mice with a dominant stable mutation of the beta-catenin gene. EMBO J. 1999;18:5931–5942. doi: 10.1093/emboj/18.21.5931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lane NE, Nevitt MC, Genant HK, Hochberg MC. Reliability of new indices of radiographic osteoarthritis of the hand and hip and lumbar disc degeneration. J Rheumatol. 1993;20:1911–8. [PubMed] [Google Scholar]
- 16.Goldring MB. The link between structural damage and pain in a genetic model of osteoarthritis and intervertebral disc degeneration: a joint misadventure. Arthritis Rheum. 2009;60:2550–2. doi: 10.1002/art.24771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Akiyama H, Lyons JP, Mori-Akiyama Y, Yang X, Zhang R, Zhang Z, et al. Interactions between Sox9 and beta-catenin control chondrocyte differentiation. Genes Dev. 2004;18:1072–87. doi: 10.1101/gad.1171104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Day TF, Guo X, Garrett-Beal L, Yang Y. Wnt/beta-catenin signaling in mesenchymal progenitors controls osteoblast and chondrocyte differentiation during vertebrate skeletogenesis. Dev Cell. 2005;8:739–750. doi: 10.1016/j.devcel.2005.03.016. [DOI] [PubMed] [Google Scholar]
- 19.Jin H, Shen J, Wang B, Wang M, Shu B, Chen D. TGF-β signaling plays an essential role in the growth and maintenance of intervertebral disc tissue. FEBS Lett. 2011;585:1209–1215. doi: 10.1016/j.febslet.2011.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Selvamurugan N, Jefcoat SC, Kwok S, Kowalewski R, Tamasi JA, Partridge NC. Overexpression of Runx2 directed by the matrix metalloproteinase-13 promoter containing the AP-1 and Runx/RD/Cbfa sites alters bone remodeling in vivo. J Cell Biochem. 2006;99:545–57. doi: 10.1002/jcb.20878. [DOI] [PubMed] [Google Scholar]
- 21.Akech J, Wixted JJ, Bedard K, van der Deen M, Hussain S, Guise TA, et al. Runx2 association with progression of prostate cancer in patients: mechanisms mediating bone osteolysis and osteoblastic metastatic lesions. Oncogene. 2010;29:811–21. doi: 10.1038/onc.2009.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee M, Partridge NC. Parathyroid hormone activation of matrix metalloproteinase-13 transcription requires the histone acetyltransferase activity of p300 and PCAF and p300-dependent acetylation of PCAF. J Biol Chem. 2010;285:38014–22. doi: 10.1074/jbc.M110.142141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mitchell PG, Magna HA, Reeves LM, Lopresti-Morrow LL, Yocum SA, Rosner PJ, et al. Cloning, expression, and type II collagenolytic activity of matrix metalloproteinase-13 from human osteoarthritic cartilage. J Clin Invest. 1996;97:761–768. doi: 10.1172/JCI118475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Neuhold LA, Killar L, Zhao W, Sung ML, Warner L, Kulik J, et al. Postnatal expression in hyaline cartilage of constitutively active human collagenase-3 (MMP-13) induces osteoarthritis in mice. J Clin Invest. 2001;107:35–44. doi: 10.1172/JCI10564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Glasson SS, Askew R, Sheppard B, Carito B, Blanchet T, Ma HL, et al. Deletion of active ADAMTS5 prevents cartilage degradation in a murine model of osteoarthritis. Nature. 2005;434:644–8. doi: 10.1038/nature03369. [DOI] [PubMed] [Google Scholar]
- 26.McCulloch DR, Le Goff C, Bhatt S, Dixon LJ, Sandy JD, Apte SS. Adamts5, the gene encoding a proteoglycan-degrading metalloprotease, is expressed by specific cell lineages during mouse embryonic development and in adult tissues. Gene Expr Patterns. 2009;9(5):314–23. doi: 10.1016/j.gep.2009.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen JM, Nelson FC, Levin JI, Mobilio D, Moy FJ, Nilakantan R, et al. Structure-based design of a novel, potent, and selective inhibitor for MMP-13 utilizing NMR spectroscopy and computer-aided molecular design. J Am Chem Soc. 2000;122:9648–9654. [Google Scholar]
- 28.Borg SA, Royds JA, Jones TH. IL1 alpha, IL6 and MMP13 are required for invasion by the human pituitary cell line HP75. Endocrine Abstr. 2003;5:122. [Google Scholar]
- 29.Hernández Ríos M, Sorsa T, Obregón F, Tervahartiala T, Valenzuela MA, Pozo P, et al. Proteolytic roles of matrix metalloproteinase (MMP)-13 during progression of chronic periodontitis: initial evidence for MMP-13/MMP-9 activation cascade. J Clin Periodontol. 2009;36:1011–7. doi: 10.1111/j.1600-051X.2009.01488.x. [DOI] [PubMed] [Google Scholar]
- 30.Kondo N, Yuasa T, Shimono K, Tung W, Okabe T, Yasuhara R, Pacifici M, Zhang Y, Iwamoto M, Enomoto-Iwamoto M. Intervertebral disc development is regulated by Wnt/β-catenin signaling. Spine. 2011;36:E513–8. doi: 10.1097/BRS.0b013e3181f52cb5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Leong DJ, Li YH, Gu XI, Sun L, Zhou Z, Nasser P, Laudier DM, Iqbal J, Majeska RJ, Schaffler MB, Goldring MB, Cardoso L, Zaidi M, Sun HB. Physiological loading of joints prevents cartilage degradation through CITED2. FASEB J. 2011;25:182–91. doi: 10.1096/fj.10-164277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stanton H, Rogerson FM, East CJ, Golub SB, Lawlor KE, Meeker CT, et al. ADAMTS5 is the major aggrecanase in mouse cartilage in vivo and in vitro. Nature. 2005;434:648–52. doi: 10.1038/nature03417. [DOI] [PubMed] [Google Scholar]
- 33.Majumdar MK, Askew R, Schelling S, Stedman N, Blanchet T, Hopkins B, et al. Double-knockout of ADAMTS-4 and ADAMTS-5 in mice results in physiologically normal animals and prevents the progression of osteoarthritis. Arthritis Rheum. 2007;56:3670–4. doi: 10.1002/art.23027. [DOI] [PubMed] [Google Scholar]
- 34.Morin PJ, Sparks AB, Korinek V, Barker N, Clevers H, Vogelstein B, et al. Activation of beta-catenin-Tcf signaling in colon cancer by mutations in beta-catenin or APC. Science. 1997;275:1787–90. doi: 10.1126/science.275.5307.1787. [DOI] [PubMed] [Google Scholar]
- 35.Simon M, Grandage VL, Linch DC, Khwaja A. Constitutive activation of the Wnt/beta-catenin signalling pathway in acute myeloid leukaemia. Oncogene. 2005;24:2410–20. doi: 10.1038/sj.onc.1208431. [DOI] [PubMed] [Google Scholar]
- 36.Jamieson CH, Ailles LE, Dylla SJ, Muijtjens M, Jones C, Zehnder JL, et al. Granulocyte-macrophage progenitors as candidate leukemic stem cells in blast-crisis CML. N Engl J Med. 2004;351:657–67. doi: 10.1056/NEJMoa040258. [DOI] [PubMed] [Google Scholar]
- 37.Derksen PW, Tjin E, Meijer HP, Klok MD, MacGillavry HD, van Oers MH, et al. Illegitimate WNT signaling promotes proliferation of multiple myeloma cells. Proc Natl Acad Sci USA. 2004;101:6122–7. doi: 10.1073/pnas.0305855101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hens JR, Wilson KM, Dann P, Chen X, Horowitz MC, Wysolmerski JJ. TOPGAL mice show that the canonical Wnt signaling pathway is active during bone development and growth and is activated by mechanical loading in vitro. J Bone Miner Res. 2005;20:1103–13. doi: 10.1359/JBMR.050210. [DOI] [PubMed] [Google Scholar]
- 39.Robinson JA, Chatterjee-Kishore M, Yaworsky PJ, Cullen DM, Zhao W, Li C, et al. Wnt/beta-catenin signaling is a normal physiological response to mechanical loading in bone. J Biol Chem. 2006;281:31720–8. doi: 10.1074/jbc.M602308200. [DOI] [PubMed] [Google Scholar]
- 40.Sen B, Styner M, Xie Z, Case N, Rubin CT, Rubin J. Mechanical loading regulates NFATc1 and beta-catenin signaling through a GSK3beta control node. J Biol Chem. 2009;284:34607–17. doi: 10.1074/jbc.M109.039453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Johnson A. TNF-induced activation of pulmonary microvessel endothelial cells: a role for GSK3beta. Am J Physiol Lung Cell Mol Physiol. 2009;296:L700–9. doi: 10.1152/ajplung.90566.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kaler P, Augenlicht L, Klampfer L. Macrophage-derived IL-1beta stimulates Wnt signaling and growth of colon cancer cells: a crosstalk interrupted by vitamin D3. Oncogene. 2009;28:3892–902. doi: 10.1038/onc.2009.247. [DOI] [PMC free article] [PubMed] [Google Scholar]