Abstract
Humans are able to continuously monitor environmental situations and adjust their behavioral strategies to optimize performance. Here we investigate the behavioral and brain adjustments that occur when conflicting stimulus elements are, or are not, temporally predictable. Event-related potentials (ERPs) were collected while manual-response variants of the Stroop task were performed in which the stimulus onset asynchronies (SOAs) between the relevant-color and irrelevant-word stimulus components were either randomly intermixed, or held constant, within each experimental run. Results indicated that the size of both the neural and behavioral effects of stimulus incongruency varied with the temporal arrangement of the stimulus components, such that the random-SOA arrangements produced the greatest incongruency effects at the earliest irrelevant-first SOA (−200 ms) and the constant-SOA arrangements produced the greatest effects with simultaneous presentation. These differences in conflict processing were accompanied by rapid (~150 ms) modulations of the sensory ERPs to the irrelevant distracter components when they occurred consistently first. These effects suggest that individuals are able to strategically allocate attention in time to mitigate the influence of a temporally predictable distracter. As these adjustments are instantiated by the subjects without instruction, they reveal a form of rapid strategic learning for dealing with temporally predictable stimulus incongruency.
Keywords: Stroop task, conflict processing, event-related potentials (ERPs), incongruency, Stimulus Onset Asynchrony (SOA)
Activities in everyday life require us to attend selectively to certain features in the environment while excluding other irrelevant or distracting information that may lead us to the wrong, or less appropriate, action. A fundamental and widely studied aspect of human psychology deals with the allocation of attentional resources and cognitive control faculties to resolve distracting or conflicting environmental stimuli. Experimental paradigms that pit competition between target stimuli and irrelevant distracters, such as the Stroop (Stroop, 1935), Simon (Simon, 1990), and Flanker (Eriksen & Eriksen, 1974) tasks, have proved extremely fruitful in elucidating the behavioral (MacLeod, 1991; Miller, 1991) and brain (Egner, 2008; MacLeod & MacDonald, 2000; Roberts & Hall, 2008) mechanisms that are invoked in response to situations that elicit conflicting response tendencies. While numerous studies have successfully invoked some form of conflict effect, the presence and degree of interference evoked by competing stimuli have been shown to depend heavily on the arrangement of incompatible elements in the environment (Reynolds, Kwan, & Smilek, 2010) the strategies used by observers to focus on and select relevant information (Egner, 2007; Scerif, Worden, Davidson, Seiger, & Casey, 2006; Tzelgov, Henik, & Berger, 1992), and the manner in which stimuli need to be translated into specific responses (e.g., Coderre, van Heuven, & Conklin, 2010; Sugg & McDonald, 1994)
The strategic allocation of attention is a key aspect of behavioral control that allows individuals to optimize performance in the face of conflicting stimulus input by generating, maintaining and adjusting sets of goal-directed processing tactics. The determination and reinforcement of such task sets reflects the operation of multiple, complementary processes that are determined by top-down objectives and ongoing regulatory control processes as they interact with the dynamically changing array of stimulus input. Critical aspects of the allocation of attentional resources for the purpose of resolving conflict have been demonstrated to operate both over space and over time. While spatial attention has been the subject of substantial research efforts, the orienting of attention in time (e.g. Nobre, 2001) has received considerably less focus. One notable exception to this has been in a family of experiments that have manipulated the stimulus onset asynchrony (SOA) between component parts of the stimuli used in the color-naming Stroop task to study the time course of the incongruency effects in this well-known paradigm (Appelbaum, Meyerhoff, & Woldorff, 2009; Dyer, 1971; M. O. Glaser & Glaser, 1982; W. R. Glaser & Dungelhoff, 1984; W. R. Glaser & Glaser, 1989; Long & Lyman, 1987; Lu & Proctor, 2001; Rayner & Springer, 1986; Roelofs, 2003, 2005, 2006, 2010; Starreveld & La Heij, 1996; Sugg & McDonald, 1994),(see also Flowers, 1990; and Mattler, 2003 for related Flanker-SOA tasks).
In these Stroop-SOA experiments, the onset of a task-irrelevant distracter feature (the word in the Stroop task) is separated in time from the onset of the task-relevant target element (the color patch), with temporal separations typically ranging from −400 to +400 ms. In general, Stroop behavioral effects in these tasks, such as the relative slowing of response times for incongruent versus congruent stimuli, has been found to be maximal when the color patch and word were presented close to each other in time (i.e., with a zero SOA), with the size of this incongruency effect falling off monotonically in both directions as the irrelevant stimulus was presented earlier or later than the target stimulus (i.e., an inverted u-shape function). While these studies have provided a useful depiction of the behavioral time course of facilitation and interference effects, and have served as the basis of important computational models of human cognition (Cohen, Dunbar, & McClelland, 1990; Phaf, Van der Heijden, & Hudson, 1990; Roelofs, 2003; Stafford & Gurney, 2007; Zhang, Zhang, & Kornblum, 1999), they have typically only used trial arrangements where the SOA between the target and distracter elements was held constant in a given experimental run. Accordingly, the typical inverted u-shaped interference function obtained in these ‘constant-SOA’ tasks could be partly explained by assuming that subjects were able to deduce the predictable temporal structure of trials within a block. This simple inference would then allow subjects to implement an attentional filter that selectively modulates the visual input over time, opening and closing dynamically to attenuate the influence of irrelevant distracters not temporally aligned with the target (Broadbent, 1970; Yu & Choe, 2006).
In previous work by our group (Appelbaum, Meyerhoff, et al., 2009) behavioral and event-related potential (ERP) measures in variants of the Stroop SOA tasks showed a distinctly different pattern of effects. In these studies, the SOAs were randomly intermixed from trial-to-trial within an experimental run, which led to maximal incongruency effects being observed at the earliest tested pre-exposure SOAs (i.e., when the word information was presented before the color), with the magnitude of these effects decreasing monotonically with later SOAs. These differences suggest that subjects may be exploiting different strategies to more effectively filter irrelevant information based on the composition of SOA trials within an experimental run (although see Roelofs, 2010 and discussion of below, for a contrary view). Moreover, in that conflicting stimulus elements in the world very frequently do not occur in a temporally predictable fashion, a fundamental open question is to what degree conflict resolution mechanisms can flexibly adjust to differing environmental demands with differing levels of temporal predictability.
In the present study, we directly tested for such possible strategic adjustments by having the same set of subjects perform separate Stroop-SOA tasks in which temporal separations between the color and word elements were either random or constant within an experimental run. We focused on task and SOA effects on two well-established Stroop-related ERP components: the negative-polarity ERP wave associated with incongruency1 (NINC), thought to reflect conflict-induced interference processes in the brain (Hanslmayr et al., 2008; Larson, Kaufman, & Perlstein, 2009; Liotti, Woldorff, Perez, & Mayberg, 2000; Perlstein, Larson, Dotson, & Kelly, 2006; Van Veen & Carter, 2002; West, 2003; West & Alain, 1999; West, Jakubek, Wymbs, Perry, & Moore, 2005); and the late positivity component2 (LPC), proposed to reflect semantic re-evaluation or post-conflict processes (Hanslmayr, et al., 2008; Liotti, et al., 2000). For this purpose we wished to specifically assess the amplitudes and latencies of these components to determine what, if any, stage of the cortical processing showed dependence on the temporal predictability of stimulus conflict.
In addition to expected effects of SOA-arrangement on the conflict-related NINC and LPC components, we also anticipated that the temporal predictability of stimulus elements would lead to context-specific modulation of the sensory processing of the stimulus input. Accordingly, we planned to analyze the sensory evoked potentials to the task-irrelevant stimulus component when it preceded the target to assess if such arrangements revealed differential processing of the irrelevant distracter input when it came predictably first, versus when the temporal arrangement was random. Such modulations of early visual ERP responses to these task-irrelevant stimulus inputs would provide direct evidence for early sensory modulation being a key part of the neural adjustments that accompany the strategic allocation of attention in time for reducing the effects of temporally predictable conflicting stimulus input.
Methods
Participants
Thirteen neurologically intact subjects with normal, or corrected-to-normal, visual acuity participated in the present experiments. All participants were screened for colorblindness, and informed consent was obtained prior to experimentation under a protocol approved by the Duke University Institutional Review Board. Participants were instructed on the task and given practice experimental runs prior to the start of each experimental session. All participants were paid $15/hour for their participation and participated in two session on different days spaced no more than two weeks apart. The data from three participants were excluded from the final behavioral and ERP analyses due to problematically high levels of EEG artifact (e.g., eye blinks) or failure to complete both sessions, leaving 10 subjects in the final analyses (mean age = 25 yrs, 5 female, 8 right handed).
Experimental Design and Procedure
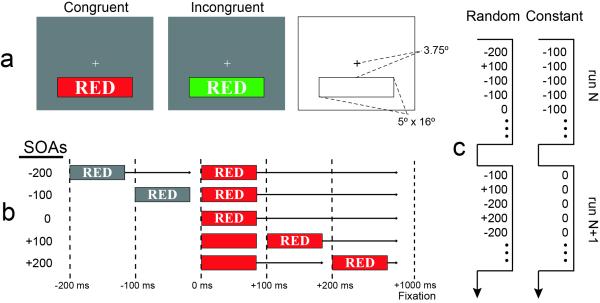
Example experimental stimuli and task parameters are illustrated in Figure 1. Stimuli consisted of red, green, blue, or yellow colored horizontal rectangular patches overlaid with English color-word text strings ‘RED’, ‘GREEN’, ‘BLUE’, or ‘YELLOW’, written in white font with black borders and positioned in the center of the rectangular patch. These stimuli were presented on a gray screen with a white fixation cross at the center. Colored bars subtended 5° × 16° and were centered 3.75° below fixation.
Figure 1. Schematic illustration of stimuli and tasks.
a) Example congruent and incongruent stimuli with stimulus dimensions. b) Schematic illustration of the timing sequence for the two stimulus components at each SOA condition. Each temporal separation (−200, −100, 0, +100, and +200 ms) is shown in a separate row with vertical, dotted lines indicating times at which stimuli components were presented. Once both stimulus components were presented, they remained on the screen for an additional 1000 ms until the fixation screen reappeared. The participant’s task was always to report the color of the bar, which was defined as 0 ms in this schematic. c) Schematic of SOA trial-type arrangements over successive ~3 minute runs for the constant-SOA and random-SOA tasks.
The current experimental design consisted of three independent variables that were varied for each subject. The first independent variable was ‘Stroop congruency’, which was defined by the correspondence between the color-bar physical color and the written-word meaning on each trial (Figure 1a). In all experimental sessions congruent pairings appeared on half of the trials (red-RED, green-GREEN, blue-BLUE, yellow-YELLOW), while the other half of the trials were split evenly between the twelve possible non-corresponding, incongruent pairings (red-GREEN, red-BLUE, red-YELLOW, green-RED, green-BLUE, green-YELLOW, blue-RED, blue-GREEN, blue-YELLOW, yellow-RED, yellow-GREEN, yellow-BLUE).
The second independent variable was the Stimulus Onset Asynchrony between the presentation of the task-irrelevant distracter word and the target color bar (Figure 1b). There were five levels of SOA; −200, −100, 0, +100, and +200 ms, so that the task-irrelevant word stimulus could precede the target color bar (−200 and −100 ms conditions), occur simultaneously with it, (0 ms, or “no-delay”), or follow it (+100 and +200 ms).
The third independent variable was ‘SOA arrangement’ (Figure 1c). In separate experimental sessions administered on separate days, participants were either presented with a ‘random-SOA’ arrangement in which all five SOA conditions were intermixed and appeared randomly within each experimental run, or they were presented with a ‘constant-SOA’ arrangement in which the same SOA was presented on every trial in an experimental run. Constant- and random-SOA session orders were counterbalanced over subjects. For half of the participants the constant-SOA runs progressed −200, −100, 0, 100, +200, with the same SOA type being maintained throughout three successive runs. In the other half of the participants this order was reversed. As no difference was observed in the RT or error rates based on the order of constant-SOA runs, the data was collapsed over this factor for all subsequent analyses (see Supplementary Figure 1).
In all cases the participants’ task was to report the physical color of the color-bar stimulus as quickly possible, while ignoring the semantic meaning of the task-irrelevant word. In the random-SOA and constant-SOA task variants participants were instructed to respond manually by pressing one of four keys on the keyboard corresponding to four possible colors, indicated with colored stickers attached to the letter keys (see Sugg and MacDonald (1994), for a discussion of feature translation issues related to the mapping of stimulus-response associations). Red and green responses were mapped to the ‘D’ and ‘F’ keys of the left hand, and blue and yellow were mapped to the ‘J’ and ‘K’ keys on the right hand.
For all tasks, participants were instructed to maintain central fixation and to minimize eye blinks during the experimental run. Reaction times and error rates were monitored while 64-channel EEG was recorded. On every trial, the color bar and the color word remained on the screen together for 1000 ms after the onset of the later of the two stimulus components. Individual trials were separated by a trial onset asynchrony that varied randomly between 1300 and 1700 ms, with only the fixation cross remaining on the screen in between trials. Each run consisted of 48 trials and lasted approximately three minutes. Before recording began, participants were given one or two training runs to learn the mapping of the four-color response buttons. A total of 15 runs were collected for each participant in each of two experimental sessions, and participants were given the opportunity to rest between the runs. Each of the two tasks (random-SOA and constant-SOA) had the same number of trials, and the order was counterbalanced such that half of the participants performed one of the tasks during the first session, and the other half performed the other task first.
Data Acquisition and Analysis
Behavioral analysis
Behavioral responses were monitored and recorded while participants performed the color-naming task. Trials were counted as correct if the subject responded correctly between 200 and 1000 ms following the presentation of the target color bar. As no systematic behavioral differences were observed for the four different colors of the target color bars, data were collapsed over the different colors to arrive at within-participant mean response times (RTs; correct trials only) and error rates for the congruent and incongruent instances of the five SOA conditions. RT and error rates were submitted to repeated measures analyses of variance (rANOVA), with factors congruency (2 levels), SOA (5 levels), and SOA arrangement (2 levels). Additional two-tailed, paired t-tests were performed on the incongruent minus congruent RT and error-rate differences between the two tasks (constant and random) for each SOA. The significance thresholds were set to a p-value of 0.05 and, when applicable, adjusted using the Greenhouse-Geisser correction for non-sphericity. Partial eta-squared values () are reported as a metric of effect size for each ANOVA contrast.
ERP recording and analysis
The electroencephalogram (EEG) was recorded continuously from 64 channels mounted in a customized elastic cap (Electro-Cap International, https://www.electro-cap.com) using a bandpass filter of 0.01 – 100 Hz at a sampling rate of 500 Hz (SynAmps, Neuroscan). All channels were referenced to the right mastoid during recording. The positions of all 64 channels were equally spaced across the customized cap and covered the whole head from slightly above the eyebrows to below the inion (Woldorff et al., 2002). Impedances of all channels were kept below 5kΩ, and visual fixation was monitored with both electrooculogram (EOG) recordings and a zoom-lens camera. Recordings took place in an electrically shielded, sound-attenuated, dimly lit, experimental chamber.
For each participant, ERPs to the onset of the colored bars were selectively averaged for each condition and SOA. ERP processing included the re-referencing of all channels to the algebraic mean of the two mastoid electrodes. A digital, non-causal, nine-point running-average filter was applied to the ERP averages, which greatly reduces signal at frequencies of 56 Hz and above at our sampling frequency of 500 Hz. Artifact rejection was performed off-line before averaging by excluding epochs of the EEG that exceeded a specifiable threshold in the window from −200 to 900 ms around a given event type. The artifact rejection thresholds were set individually for each subject. An average of 13% of the trials was rejected, per subject, leaving an average of 628 total trials per condition in the grand average.
Separate ERPs were computed for correctly reported congruent and correctly reported incongruent presentations at each of the five SOA conditions (−200, −100, 0, +100, +200) by time-locking to the onset of the target color bar stimulus. As with the behavioral performance, since no differences were observed in the ERP responses for the different colors of the target color bars, responses were collapsed over the different colors, yielding 10 (5 SOA × 2 congruency conditions) evoked response types for each experimental task. To isolate brain potentials related to the Stroop incongruency effect under the different conditions, difference waves were computed by subtracting the ERPs for congruent trials from the ERPs for incongruent trials, separately for each SOA in each SOA arrangement condition. We explicitly focus our ERP analyses here on the incongruency difference waves, because the SOA manipulation utilized in these experiments introduces differential amounts of overlap in the ERP record depending on the temporal separation between stimulus components (Woldorff, 1993). As this overlap is equivalent for the congruent and incongruent stimuli within each SOA condition, the difference wave isolates processes related to the Stroop stimulus incongruency and serves as a principled ERP marker for assessing interactions between the SOA and the neural processing related to the conflict processing interactions.
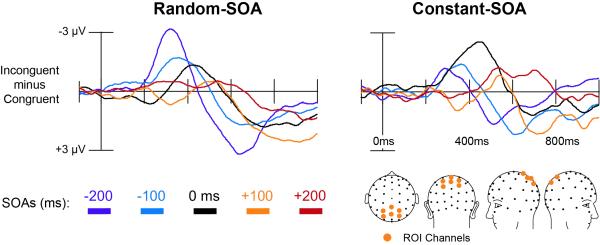
Statistical analyses of the SOA-incongruency effects were carried out using four regions-of-interest to separately test for effects of stimulus congruency (1) and sensory biasing (3). First, in order to test for effects of congruency, a six-channel ROI was derived from our previous Stroop experiments that also roughly corresponded to the peaks of the incongruency effects for the current random-SOA and constant-SOA tasks (see orange dots in Figure 3). This ROI consisted of same set of posterior-parietal left- (P01, P1) and right- (P02, P2) channels as was used in our previous paper (Appelbaum, Meyerhoff, et al., 2009). However, since lateralization was not considered as a factor here, as it was in our previous paper, the midline channels CPz and Pz were also included in the ROI for the present study.
Figure 3. ERP incongruency difference waves for the random-SOA and constant-SOA variants of the Stroop task.
Group average difference waves (incongruent minus congruent) computed over the 6-channel ROI (depicted in the lower right) are shown for the 5 SOAs in each task. The pattern of ERP incongruency effects, as a function of the SOA, differed between the random- and constant-SOA conditions, indicating that Stroop incongruency depended on the contextual arrangement of SOA trials over the course of an experimental run.
To test for main effects and interactions of SOA and SOA-arrangement, peak amplitude values of the negative (NINC) and positive (LPC) incongruency difference waves (incongruent minus congruent) were submitted to repeated-measures ANOVAs. Local peak amplitudes were extracted by first identifying the peak latency of the NINC and LPC components for each SOA. Next, the peak voltage falling within +/−150 ms of this time point was extracted for each subject and at each channel. These values were then averaged and subjected to rANOVA with factors SOA and SOA-arrangement. In addition, the peak latency values were submitted to rANOVAs to infer the presence of significant linear trends in the data. Task interactions were statistically explored further through paired t-tests.
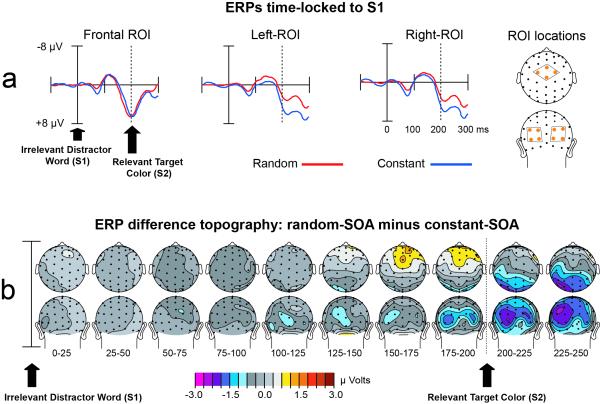
In order to test for evidence for differential sensory processing of the stimuli between the random- and constant-SOA task blocks, we probed for task differences in the −200 ms SOA condition. This condition provides an uninterrupted window for 200 ms from the onset of stimulus component 1 (S1) until the onset of stimulus component 2 (S2), during which no other overlapping stimulus response activity would be present. Based on previous reports of cortical biasing due to attentional selection (Anllo-Vento & Hillyard, 1996; Hillyard & Anllo-Vento, 1998), we probed the response amplitudes over ROIs comprised of frontal-central sensors Cz, FCz, C1a, and C2a, left posterior sensors (O1i, O1′, TO1, P3i), and right posterior sensors (O2i, O2’, TO2, P4i). These ROIs also corresponded roughly to the peaks of the random- minus constant-SOA subtraction for the −200 ms SOA condition and are depicted by the orange dots in Figure 5. Mean amplitude values computed over 25 ms windows extending from 0 to 250ms following the S1 stimulus were derived for each four-channel ROI and were submitted to rANOVA where they were compared to the 100 ms baseline preceding the presentation of the S1 stimulus.
Figure 5. Distracter predictability modulates sensory stimulus processing.
In order to visualize task differences due to the arrangement of SOAs, random- and constant-SOA waveforms, along with topographic distributions of the difference between these two conditions, are shown for the −200ms SOA (i.e., when the distracter word occurred 200 ms before the target color bar to be named). a) ERPs time-locked to the distracter-word (S1) are shown for the random-SOA (red) and constant-SOA (blue) task variants. Each of these waveforms is collapsed over congruent and incongruent trial types as congruency has no meaning prior to the presentation of the S2 stimulus. b) Topographic distribution of the random-SOA minus constant-SOA difference for the S1-elicited ERP in the −200ms SOA condition. This subtraction produces a frontal-positive/occipital-negative difference that initiates prior to the S2 stimulus presentation, indicating modulations in visual processing of the distracter word stimulus due to the SOA arrangement of the task.
Results
Behavioral Performance
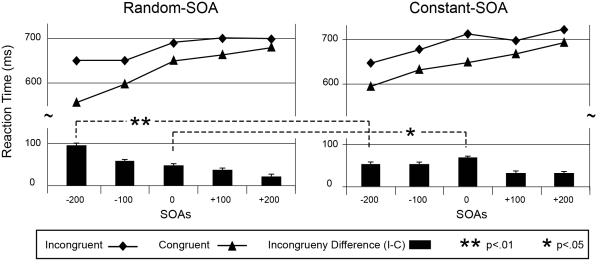
Robust and statistically significant behavioral effects of stimulus incompatibility were observed in both the random-SOA and constant-SOA variants of these tasks. For both tasks, response times were faster for congruent trials than for incongruent trials. Similarly, error rates were generally lower for congruent trials than incongruent trials. Mean reaction times for the two tasks are shown graphically in Figure 2 and are presented along with paired t-test results in Table 1.
Figure 2. SOA arrangement alters the pattern of incongruency effects.
Reaction times for the random-SOA, and constant-SOA variants of the Stroop task produced different profiles of SOA-incongruency interactions. While statistically significant behavioral incongruency effects were observed across all SOA conditions (see Table 1), incongruency effects were maximal at the earliest distracter-exposure condition for the random-SOA arrangement (−200 ms) but was largest at the simultaneous (0 ms) condition for the constant-SOA arrangement. Error bars indicate 1 standard error of the mean (SEM).
Table 1.
Summary of incongruency differences for the two SOA arrangements. Group mean RT and error rate differences and paired t-test results for all within-SOA contrasts of incongruent minus congruent trials.
| a) Random-SOA (incongruent versus congruent) | ||||||
|---|---|---|---|---|---|---|
| SOA | RT difference (ms) | t | sig (2 tail) | Error-rate diffs (%) | t | sig (2 tail) |
| −200 | 94.57 | t(9)=9.07 | p<.001** | 2.96 | t(9)=2.23 | p=.053 |
| −100 | 58.86 | t(9)=5.78 | p<.001** | 1.91 | t(9)=2.19 | p=.056 |
| 0 | 47.94 | t(9)=5.81 | p<.001** | 2.58 | t(9)=3.52 | p=.006** |
| 100 | 37.73 | t(9)=4.41 | p=.002** | 0.50 | t(9)=0.44 | p=.667 |
| 200 | 23.28 | t(9)=2.53 | p=.032* | −0.85 | t(9)=−1.01 | p=.338 |
| b) Constant-SOA (incongruent versus congruent) | ||||||
|---|---|---|---|---|---|---|
| SOA | RT difference (ms) | t | sig (2 tail) | Error-rate diffs (%) | t | sig (2 tail) |
| −200 | 51.60 | t(9)=7.91 | p<.001** | 1.77 | t(9)=1.37 | p=.205 |
| −100 | 48.84 | t(9)=5.80 | p<.001** | 2.31 | t(9)=2.71 | p=.024* |
| 0 | 67.24 | t(9)=7.69 | p<.001** | 2.64 | t(9)=2.69 | p=.025* |
| 100 | 30.76 | t(9)=4.25 | p=.002** | 1.48 | t(9)=1.50 | p=.169 |
| 200 | 30.19 | t(9)=2.94 | p=.017* | 1.39 | t(9)=1.94 | p=.085 |
| c) Task Differences (random-SOA minus constant-SOA) | ||||||
|---|---|---|---|---|---|---|
| SOA | RT diff (ms) | t | sig (2 tail) | Error-rate diff (%) | t | sig (2 tail) |
| −200 | 42.96 | t(9)=3.36 | p=.008** | 1.18 | t(9)=0.61 | p=.554 |
| −100 | 10.01 | t(9)=0.83 | p=.427 | −0.41 | t(9)=−0.34 | p=.744 |
| 0 | −19.31 | t(9)=−2.89 | p=.018* | −0.06 | t(9)=−0.45 | p=.965 |
| 100 | 6.97 | t(9)=0.68 | p=.521 | −0.97 | t(9)=−0.56 | p=.589 |
| 200 | −6.91 | t(9)=−0.45 | p=.661 | −2.24 | t(9)=−1.93 | p=.086 |
For general statistical evaluation of these data, 2 × 5 × 2 (congruency by SOA by SOA-arrangement) repeated-measures analyses of variance (rANOVA) were performed separately on the RT and percent-error data. The rANOVA for the RTs demonstrated a significant main effect of both congruency [F(1,9)=128.70, p<.001, =0.93] and SOA [F(4,36)=25.98, p<.001, =0.74], and a significant congruency by SOA interaction [F(4,36)=11.1, p<.001, =0.55]. Critically, there was also a significant congruency by SOA by SOA-arrangement interaction [F(4,36)=3.69, p=.03, =0.29], indicating that the effect of SOA on Stroop incongruency differed depending on the arrangement of SOA conditions within a run. More specifically, this effect appeared to be due to the incongruency effect being largest at the 0 SOA for the constant-SOA arrangement, whereas it was largest at the −200 SOA in the random-SOA case. For the error rates (shown graphically in Supplementary Figure 2), the three-way ANOVA showed only a significant main effect of congruency [F(1,9)=17.01, p=.003, =0.65] and a significant congruency by SOA interaction [F(4,36)=3.18, p=.047, =0.26].
In order to further explore interactions between experimental variables, paired t-tests were performed between the incongruent and congruent RTs and error rates at each SOA (Table 1a & b) and for the incongruent minus congruent differences for the two SOA arrangements (Table 1c). These planned comparisons revealed that behavioral incongruency effects were present at all SOAs and for both tasks. As depicted by the black bars in Figure 2 showing the incongruency differences for each SOA, however, the greatest behavioral incongruency effects occurred at different SOAs for the two tasks. Between-task, paired t-tests reveal that this pattern is driven by a significant reduction in the amount of incongruency effect for the constant-SOA arrangement at the −200 SOA (42.96 ms; t=3.36, p=0.008), while the behavioral incongruency effect was significantly larger at the 0 ms SOA for the constant-SOA arrangement (−19.31 ms; t=−2.89, p=0.018). Additional, direct comparisons between the congruent and incongruent trial types indicate that the effect at the −200ms SOA is largely driven by a reduction in RTs for the congruent trial types (p=.038), whereas the difference for incongruent trials was not significant (p=.19). The task differences were not significant at the 0 ms SOA for either trial type alone (congruent, p=.3; incongruent, p=.23). No significant congruency differences were observed between the RT or error rates at any other SOAs.
Electrophysiological incongruency effects as a function of SOA arrangement
Having established at the behavioral level both that Stroop incongruency was robustly evoked over a range of SOA separations and that the pattern of SOA-incongruency interaction depended on the contextual arrangement of SOA trials over the course of an experimental run, we examined how these effects are manifested in the brain. For this purpose we compared the peak amplitude profiles of the negative and positive incongruency difference waves over central-parietal cortex using ROIs derived from our previous experiments (Appelbaum, Meyerhoff, et al., 2009). As before, this ROI roughly corresponded to the peak of the incongruency difference distributions for both the random-SOA and constant-SOA tasks (see Supplementary Figure 3 for full spatio-temporal profiles of the effects).
Figure 3 shows the ROI incongruency difference wave for each of the five SOAs in the two versions of the task. Two primary observations are easily visible in these waveforms. First, for both task variants, the grand-average difference waves contained both earlier-latency negative-polarity effects (NINC) and the longer-latency positive-polarity effects (LPC) that followed roughly monotonic patterns in which the later SOAs (later occurrence of the distracter word information) yielded longer-latency effects. Secondly, as was observed in the RT data, the profile of the incongruency effect amplitudes across the SOAs depended heavily on the within-run arrangement of the SOA trial types.
In order to quantitatively assess the influence of SOA-arrangement on the response profiles of these components, we first considered the local peak latencies. Using the local peak latencies of the grand-average incongruency difference waveform as a starting point (listed as “Grand Average Latency” in Table 2), the individual-subject local peak latencies occurring within +/−150ms of the grand average peak were extracted (Figure 4a). Repeated-measures ANOVAs on these values demonstrated a significant main effect of SOA [F(4,36)=60.13, p=<.001, =0.87] on the latencies of the NINC and a nearly significant main effect of SOA on the LPC latencies [F(4,36)=2.47, p=.06, =0.22], but no main effect of SOA-arrangement (NINC: [F(1,36)=1.84, p=0.21, =0.17]; LPC: [F(1,36)=1.01, p=0.34, =0.10]), or a SOA by SOA-arrangement interaction (NINC: [F(4,36)=0.77, p=0.55, =0.08]; LPC: [F(4,36)=0.46, p=0.77, =0.05]), on the latency of either component. These main effects of SOA were in turn driven by a significant linear trend [F(1,9)=104.77, p<.001] for the NINC, and marginally significant linear trend [F(1,9)=3.67, p=.089] for the LPC component. In agreement with the absence of main effects of SOA-arrangement, paired t-tests confirmed that the peak latencies did not differ between the two SOA-arrangement variants at any of the SOAs, for either the NINC or LPC components. In order to further investigate the linear trends in these responses, post-hoc paired comparisons were performed between the latencies at each SOA for each component collapsed over the two tasks. These analyses revealed that for the NINC, each successive SOA evoked a longer latency response than the previous SOA (all p<.01). In contrast, LPC latencies increased monotonically between the −200, −100, and 0 ms 3 SOAs (p<.05), but were not statistically different for the 0 and +100 SOA (p=0.22) or between the +100 and +200 ms SOAs (p=0.18).
Table 2.
Grand average peak NINC and LPC amplitudes (μV) and latencies (ms) for the random-SOA and constant-SOA tasks across the five SOAs. Significant paired t-test results are indicated with asterisks.
| −200 SOA | −100 SOA | 0 SOA | +100 SOA | +200 SOA | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Grand Average Latency | Random 320 ms |
Constant 286 ms |
Random 348 ms |
Constant 362 ms |
Random 424 ms |
Constant 446ms |
Random 518 ms |
Constant 544 ms |
Random 536 ms |
Constant 538 ms |
| NINC Amplitude (uV) | −3.68 | −1.66 | −2.24 | −1.83 | −1.79 | −3.35 | −1.06 | −1.39 | −1.28 | −2.05 |
| p=0.01* | p=0.21 | p=0.02* | p=0.26 | p=0.08 | ||||||
| NINC Latency (ms) | 325 | 320 | 368 | 363 | 393 | 432 | 523 | 544 | 589 | 607 |
| p=0.40 | p=0.36 | p=0.1 | p=0.15 | p=0.24 | ||||||
| Grand Average Latency | 654 ms | 512 ms | 693 ms | 610 ms | 888 ms | 926 ms | 912 ms | 932 ms | 936 | 904 ms |
|---|---|---|---|---|---|---|---|---|---|---|
| LPC Amplitude (uV) | 3.74 | 2.34 | 2.67 | 3.40 | 2.59 | 2.97 | 3.22 | 2.99 | 2.35 | 1.66 |
| p=0.04* | p=0.44 | p= 0.60 | p=0.69 | p=0.50 | ||||||
| LPC Latency (ms) | 648 | 663 | 741 | 708 | 781 | 755 | 824 | 761 | 760 | 727 |
| p=0.63 | 0.29 | p=0.66 | p=0.16 | p=0.60 | ||||||
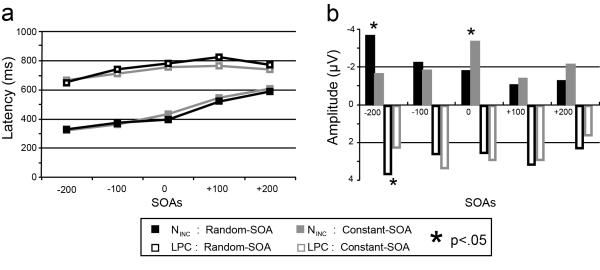
Figure 4. Quantitative summary of ERP measures.
a) Peak latencies and b) peak amplitudes and for the random-SOA and constant-SOA task variants across the five SOAs. Average NINC (filled symbols) and LPC (open symbols) latencies did not differ for the two tasks at any SOA. In close agreement with the behavioral effects, however (see Figure 2), the incongruency-effect amplitudes produced differing SOA by SOA-arrangement profiles. In particular, at the −200 ms SOA, the random-SOA arrangements produced greater activity for both the NINC and the LPC, while at the 0 ms SOA NINC activity was larger for the constant-SOA task. Significant paired t-test results are indicated with asterisks.
In contrast, as can be seen in Figure 4b, the amplitude patterns of the incongruency effects differed substantially between the two SOA-arrangement variants of the task, with the effect amplitudes being largest at the −200 ms SOA for the random arrangement and at 0 ms (simultaneous) for the blocked one. ANOVAs performed on the NINC peak amplitudes of these effects indeed confirmed both a main effect of SOA [F(4,36)=5.26, p=.011, =0.36] and a significant SOA by SOA-arrangement interaction [F(4,36)=5.21, p=.009, =0.37]. Subsequent paired t-test comparisons between the two task variants revealed significant differences for the −200 ms SOA condition (p=0.01) and for the 0 ms SOA condition (p=0.02). Peak LPC amplitudes showed neither main effects, nor significant SOA by SOA-arrangement interactions, although planned paired t-tests indicated a significant reduction in the LPC amplitude of the −200 SOA condition for the constant-SOA task.
Sensory biasing due to SOA arrangement
Having established that the arrangement of SOAs within a task evoked different patterns of SOA-incongruency interaction, both behaviorally and for the NINC ERP effect, we probed the data further to determine whether there were alterations in the stimulus processing that may have accompanied these conflict-dependent modulations. In particular, we analyzed the sensory evoked activity to the task-irrelevant word stimulus in the −200 ms SOA condition, which provides an uninterrupted window of at least 200 ms following the first stimulus component (S1) during which no other overlapping stimulus response is present. Based on previous reports of cortical biasing due to attentional selection (Anllo-Vento & Hillyard, 1996; Hillyard & Anllo-Vento, 1998), we probed the response amplitudes over fronto-central and lateral-occipital ROIs in order to test the hypothesis that the SOA-arrangement might be accompanied by differential processing of the evoked response to the irrelevant distracter when it always comes predictably first, versus when the SOAs are random across trials.
ERPs time-locked to the S1 stimulus in the −200 ms SOA condition (i.e., when the task-irrelevant distracter-word came 200 ms before the color bar) showed no difference between the congruent and incongruent trial types over the first 300 ms. This result was expected since the influence of compatibility between the S1 and S2 stimulus component has no meaning prior to the occurrence, and processing, of the second stimulus (S2). Subsequent analyses of the time-range before and around the presentation of S2 were therefore collapsed over the congruent and incongruent trial types and are shown visually in Figure 5.
ANOVAs performed over 25 ms windows for the anterior and posterior ROIs (orange dots in Fig. 5A) revealed a significant (p=.028) difference over fronto-central electrodes that was more positive from 150-175 ms post S1 for random than constant trial types. This difference onset at around the same time as a bilateral posterior difference that was more negative for random than constant trial types, which was significant through the four 25 ms windows beginning at 150 ms (150-175ms, p=.039; 175-200ms, p=.007; 200-225ms, p=.004, 225-250ms, p=.014). These posterior effects were significantly larger in the left than in the right ROI from 200-225ms (p=.011) and 225-250ms (p=.014). At latencies beyond ~250 ms the cortical ERP to the S2 bar stimulus would necessarily begin and would overlap and distort the ERP activity evoked in response to the S1, and thus activity in these longer latencies are not considered here.
Discussion
Goal-oriented behavior requires flexible mechanisms that are able to select correct actions in the presence of many competing external stimuli, especially when some of those stimuli may be associated with incorrect responses. Strategic processes that govern these abilities vary as a function of the context under which choices are made and represent a critical adaptive mechanism by which behavior is optimized. In the present work we used variants of the Stroop task in which temporal separations between the task-relevant color elements and the task-irrelevant word elements of each of the stimuli were varied, either randomly intermixed or held constant within an experimental run. By comparing behavioral and neural-activity differences that accompanied Stroop conflict under these arrangements, we were able to infer how the temporal predictability of stimuli in the environment may lead to strategic cognitive adjustments.
The influence of temporal predictability on stimulus incongruency
Behavioral results from SOA variants of the Stroop task are generally regarded as key, robust findings that must be accounted for in successful models of conflict processing in the Stroop and related tasks (MacLeod, 1991; Roelofs, 2003). It is generally appreciated from numerous SOA variants of the Stroop task that the irrelevant stimulus element is able to cause interference if it appears at any point before target processing is finished. A number of previous behavioral Stroop-SOA tasks have typically reported that incongruency effects are greatest when the SOA between the two dimensions is close to 0 ms, falling off for either positive or negative SOAs (Dyer, 1971; M. O. Glaser & Glaser, 1982; W. R. Glaser & Dungelhoff, 1984; Lu & Proctor, 2001; Sugg & McDonald, 1994; Taylor, 1977). In addition to this inverted u-shaped incongruency function, these studies have tended to show that the pre-exposure of irrelevant stimuli elicits somewhat greater RT slowing than comparable post-exposure of the distracter (e.g. ~75 ms for −200 SOA compared to ~25 ms for +200 as measured by Glasser and Glasser (1982)), although with both less than simultaneous occurrence.
In our previous paper (Appelbaum, Meyerhoff, et al., 2009) we recorded both behavioral and EEG measures of stimulus conflict processing in a Stroop-SOA task variant in which the task-irrelevant color words could appear at one of five SOAs relative to the task-relevant bar-color occurrence: 100 or 200 ms before, 100 or 200 ms after, or simultaneously. In this design, however, the SOAs were randomly intermixed from trial-to-trial within each experimental run. This arrangement produced the greatest behavioral and electrophysiological effects when irrelevant stimulus information preceded the task-relevant target and reduced effects when the irrelevant information followed the relevant target. We interpreted this pattern of effects as reflecting two separate processes: (1) a ‘priming influence’ that enhanced the magnitude of the conflict-related incongruency effect when a task-relevant target was preceded by an irrelevant distracter; and (2) a reduced ‘backward influence’ of stimulus conflict when the irrelevant distracter information followed the task-relevant target. As this pattern of incongruency by SOA effects differed from the commonly reported inverted-U incongruency pattern for behavioral effects that had typically been reported with blocked SOA designs, we wished in the present experiment to specifically test the influence of SOA-arrangement on Stroop behavioral and neural incongruency effects.
Direct comparisons of the incongruency effects collected under conditions of constant- and random-SOA arrangements in the present study indicate that the increased pre-exposure priming of the incongruency effects observed in our original experiment may indeed be due to the temporal uncertainty introduced by the random-SOA arrangements. Consistent with our previous results, random-SOA arrangements produced RT and electrophysiological effects that were robustly larger (nearly twice) at the −200ms SOA relative to the simultaneous presentation. Importantly, this effect was reversed for the constant-SOA arrangement (significantly larger for simultaneous than with 200ms SOA). Moreover, this condition showed differential early-latency processing differences for the S1 stimulus relative to in the random-SOA arrangement. This combination of results suggests that with temporal predictability of potentially conflicting input, subjects are better able to impose temporally selective filters that reduce the influence of the irrelevant stimulus dimension. In the absence of such temporal predictability, the processing of the irrelevant distracter would necessarily be relatively unconstrained and could thereby result in greater pre-target priming for the random-SOA condition. It is of importance to note that the random-SOA arrangement seems likely to have important ecological validity given that conflicting or distracting stimuli in the natural world frequently do not occur with temporal regularities, and therefore the distinction between stimulus conflict processing under randomized and predictable temporal arrangements provides an important new contribution to the Stroop-conflict literature.
In the current constant-SOA task, incongruency effects were greatest at the 0 ms SOA, in line with the behavioral results pattern reported by MacLeod (1991) and Roelofs (2003)3 using this SOA configuration, and with recent computational simulations of the Stroop-SOA effect (Yu & Choe, 2006). A further observation of interest in the present study is that according to paired comparisons, both the behavioral and ERP effect sizes were larger at the 0 ms SOA for the constant-SOA arrangements than for the random-SOA one. Since in either SOA arrangement, the simultaneous presentation of distracters and targets would not be expected to result in any differential amount of priming, it can be inferred that other mechanisms beyond a strict temporal filter may be contributing to the overall pattern of incongruency effects observed here. In fact, this result resembles findings reported by Mattler (2003) who observed larger effects of time uncertainty at short SOAs for spatially-induced conflict resulting from Flanker incongruency. Given such observations, it may be the case that in the constant-SOA arrangement the synchronous presentation of the color and word stimuli may induce relatively more binding of the two elements, greater processing of the distracter, and therefore larger overall incongruency effects in relation to the random-SOA condition. Nonetheless, future research will be needed to assess such hypotheses.
The present results may also bear a close resemblance to the cued temporal orienting literature (Coull & Nobre, 1998; Griffin, Miniussi, & Nobre, 2001; Miniussi, Wilding, Coull, & Nobre, 1999; Nobre, 2001). Although there have not been a great many of such studies, they have utilized interval manipulations in a variety of cue-to-target tasks to demonstrate that time interval expectation can guide the attentional selection of stimuli to improve behavior. It is worth noting, however, that in the present tasks subjects were not explicitly informed about the temporal arrangement of the stimuli, as they have been in the cued temporal orienting studies. The contextual effects observed here must therefore be a quickly emergent property that is derived from the recent history of trials in the experimental runs, rather than any pre-specified cue meaning. This notwithstanding, the present results indicate that the processing of stimulus conflict can be influenced by strategic attentional orienting in time.
The temporal dynamics of Stroop incongruency as revealed by ERPs
Measurement of scalp-recorded ERPs have provided an important means by which to explore the time course of brain processes underlying Stroop conflict (e.g. Atkinson, Drysdale, & Fulham, 2003; Hesse, Moller, Arnold, & Schack, 2003; Markela-Lerenc et al., 2004; Rebai, Bernard, & Lannou, 1997; West & Alain, 1999; West, et al., 2005). As with the present findings, typical ERP studies of the Stroop task report that responses to incongruent stimuli, as compared to congruent stimuli, tend to elicit a negative voltage deflection that is centrally distributed over the head and peaks at around 450 ms post stimulus (Badzakova-Trajkov, Barnett, Waldie, & Kirk, 2009; Bruchmann, Herper, Konrad, Pantev, & Huster, 2010; Hanslmayr, et al., 2008; Lansbergen, van Hell, & Kenemans, 2007; Larson, et al., 2009; Liotti, et al., 2000; Perlstein, et al., 2006; Rebai, et al., 1997; Van Veen & Carter, 2002; West, 2003; West & Alain, 1999; West, et al., 2005). This component, often referred to as the N450, here termed the NINC, is thought to index incongruency-related interactions that occur at a rather late point in the cortical processing hierarchy, following initial stimulus evaluation. In fact, several ERP studies have shown that Stroop incongruency has little effect on the amplitude or latency of the common P300 component, which is sensitive to the probability and task relevance of an eliciting stimulus (Duncan-Johnson & Kopell, 1981; Ilan & Polich, 1999; Rosenfeld & Skogsberg, 2006). Similarly, modulations akin to the P300 do not appear to be present in the current tasks.
Studies investigating the neural sources of the NINC/N450 effect have typically modeled this component as arising from generators in the dorsal ACC (Badzakova-Trajkov, et al., 2009; Hanslmayr, et al., 2008; Liotti, et al., 2000; Markela-Lerenc, et al., 2004; West, 2003; West, Bowry, & McConville, 2004). However, more recent source localization efforts have suggested additional contributions from generators in the lateral prefrontal and more superior medial frontal regions, as well as possibly the motor cortices bilaterally (Bruchmann, et al., 2010). As in our previous study (Appelbaum et al 2009), the response distribution of the present incongruency negativity produced a broad central-parietal deflection. This somewhat posterior distribution is largely consistent with that reported by Liotti et al. (2000) and West and Alain (1999) for a manual-response Stroop task and is potentially consistent with a source in the more posterior regions of the ACC (Liotti, et al., 2000; Rebai, et al., 1997; West & Alain, 1999).
The present incongruency subtraction also produced a more sustained, parietal positive/lateral frontal negative wave occurring between 500 and 900 ms post-stimulus that is largely consistent with similar components reported at later latencies in other Stroop conflict tasks (Bailey, West, & Anderson, 2009; Larson, et al., 2009; Liotti, et al., 2000; Perlstein, et al., 2006; West, 2003; West & Alain, 2000; West, et al., 2005). This tonic, conflict-sensitive potential, typically termed either the conflict sustained potential (conflict-SP) or the late positive component (LPC) by different researchers, is more positive for incongruent relative to congruent trials over parietal electrode sites and tends to be somewhat larger over the left than the right hemisphere. As the amplitude of the LPC is positively correlated with both accuracy and reaction time with incongruent stimuli, it has been suggested that this component may be associated with response selection on the current trial (West, et al., 2005). Alternately, based on the timing and distribution, others have suggested that the LPC may be related to the processing of semantic meaning of the Stroop stimulus words (Liotti, et al., 2000).
Microscopic and macroscopic cognitive control influences on the ERP
In recent years, researchers have begun to study how strategic properties, such as the degree of impulsivity or cautiousness influence the flexible regulation of behavior (Botvinick, Braver, Barch, Carter, & Cohen, 2001; Egner, 2007) and whether such regulation is implemented at the local or global level (Blais & Bunge, 2010). In general, the application of strategic processes has been subdivided into two broad classes; micro-adjustments that act from trial-to-trial in order to alter performance based on the recent history of stimuli and/or the commission of incidental errors, and macro-adjustments that involve long-term modifications in response to the context under which different types of stimulus events occur (Ridderinkhof, 2002).
Event-related potentials possess millisecond temporal resolution and are therefore particularly well suited for investigating the dynamic micro adjustments that are made in response to specific factors that change rapidly over time. Recent ERP research has begun to focus on how the sequence of different trial types manifests in differential effects on conflict-induced ERPs components such as the NINC (N450) and LPC (conflict-SP). One such type of first-order sequence effects is the so-called conflict adaptation effect in which trials containing a relatively high (incongruent) versus low (congruent) amount of conflict induce corresponding reductions, or enhancements, in the influence of the irrelevant stimulus dimension during the subsequent trial. In one recent experiment, Larson and colleagues (2009) investigated the influence of conflict adaptation on the NINC and LPC components. They reported that while the LPC monotonically differentiated current trial congruency on the basis of previous-trial context, no such conflict adaptation effects were observed in the amplitude of the NINC component.
Macro-scale conflict-related adjustments are those that occur in response to factors that are likely to remain constant for some time. One experimental manipulation that has been extensively used to study the behavioral ramifications and neural mechanisms that correspond to macro adjustments has been to alter the level of conflict potency by varying the relative proportion of congruent versus incongruent trials presented in an experimental run. Such ‘trial-type frequency’ manipulations have demonstrated that increasing the probability of incongruent trials reduces not only the magnitude of the behavioral incongruency effects (Botvinick, et al., 2001; Crump, Gong, & Milliken, 2006; M. O. Glaser & Glaser, 1982; W. R. Glaser & Dungelhoff, 1984; Larson, et al., 2009; Logan & Zbrodoff, 1979; Rosenfeld & Skogsberg, 2006; Schmidt & Besner, 2008; Taylor, 1977; Tzelgov, et al., 1992), but also the amplitude of ERP responses to these stimuli (West & Alain, 2000), suggesting that macroscopic control can be experimentally manipulated and measured in the brain.
Results from the present experiment demonstrate a pattern of macro-scale adjustments that result from trial-type context differences that are in place over a relatively long time frame (i.e. blocks, not trial-to-trial). We observed that the conflict-related NINC component produced a pattern of amplitude effects that closely matched the incongruency-related RT effects over the two SOA arrangements. For both arrangements, greater behavioral RT effects corresponded to larger NINC amplitudes and therefore it can be concluded that the arrangement of SOA trials serves as an effective means by which to adjust the level of conflict potency on a macroscopic scale.
Evidence that temporal predictability modulates stimulus-conflict processing
Previous studies have suggested that conflict resolution can be accompanied by neural strategies that act to bias stimulus processing in sensory pathways in order to facilitate behavioral performance (Cohen, et al., 1990; Egner, Delano, & Hirsch, 2007). Direct evidence for such conflict-related stimulus-biasing indicates that the cortical representations of task-relevant stimulus features are amplified relative to task-irrelevant ones during sequential-trial conflict adaptation (Egner & Hirsch, 2005). ERP studies, in particular, have reported that the context under which stimulus-response conflict occurs modulates stimulus processing as early as the P1 (Scerif, et al., 2006) and N2 (Folstein & Van Petten, 2008) components. One possibility that has been widely postulated is that these modulations are achieved through sensory amplification or ‘gain control’ effects which would be reflected in ERPs by larger P1 and N1 sensory-evoked responses being elicited by attended input relative to ignored input (Hillyard, Vogel, & Luck, 1998; Posner & Driver, 1992). Alternately, such contextually driven adjustments act to modulate feature-specific mechanisms, which would be revealed by later-latency ERP effects such as the selection negativity and selection positivity (SN/SP) (Anllo-Vento & Hillyard, 1996; Hillyard & Anllo-Vento, 1998).
In order to evaluate if context-driven modulations in stimulus evoked processing were present for the current tasks, we carried out analyses of the sensory evoked component to the irrelevant distracter stimulus component (S1) for the −200 ms SOA under the two SOA arrangements. In this analysis we observed clear differences in the ERP to the S1 stimulus as a function of the temporal predictability due to the SOA arrangement. This difference initiated at ~150 ms post-S1 as a bilateral occipital deflection that was more negative for the random-SOA than for the constant-SOA arrangements, concurrently with a fronto-central positive deflection difference. T he posterior negative difference was more pronounced in the left than right hemisphere and increased in amplitude till well after the presentation of the S2 stimulus.
Overall, these patterns of ERP modulations would suggest that the task differences captured here reflect operations more akin to the selection of relevant stimulus features associated with feature-selection ERP effects, rather than early P1/N1 modulations that accompany sensory amplification or ‘gain control’ that tends to occur with spatial attention. In particular, both the latencies and distributions of the present effects bear a close resemblance to the selection-negativity (SN) ERP effect that has been linked the selection of relevant features including color (Harter, Aine, & Schroeder, 1982; Hillyard & Munte, 1984) and other non-spatial features (Kenemans, Kok, & Smulders, 1993). This effect has consisted of a bilateral, posterior negativity (SN) that typically occurs between 150 and 300 ms, which is sometimes accompanied by and a frontal positivity (sometimes called “SP”). The onset of the SN/SP waveform provides a high-resolution measure of the time at which a particular feature, or feature conjunction, is discriminated and selectively processed according to its task relevance (Hillyard & Anllo-Vento, 1998). Accordingly, it may serve as a plausible mechanism by which stimulus conflict from a task-irrelevant feature can be mitigated in the current Stroop-SOA tasks.
The occipital negative effects derived from this task subtraction also demonstrate a degree of left lateralization. One possible explanation for this lateralization comes from the N170 ERP literature. The N170 component is a left-lateralized ERP wave that has been reported as showing prelexical sensitivity to orthographic versus non-orthographic strings (Appelbaum, Liotti, Perez, Fox, & Woldorff, 2009; Bentin, Mouchetant-Rostaing, Giard, Echallier, & Pernier, 1999; Ruz & Nobre, 2008). As the sensory response considered here is that of the task-irrelevant word component, it is reasonable that the task modulations reported here may also contain some degree of left-lateralization. An alternate account of this lateralization may come from the temporal framework literature. In the account of their temporal orienting effects Coull and colleagues (1998) reported a pronounced “left- sided bias in activations during temporal orienting [that] was reminiscent of the laterality in tasks involving fine temporal discriminations”. It may therefore be the case that the present task differences are also tapping into mechanisms that are invoked during the temporal discrimination of rapidly occurring stimulus events (Fiez, Raichle, Petersen, Tallal, & Katz, 1995; Platel et al., 1997).
Comparison with recent study of temporal predictability effects by Roelofs (2010)
While SOA manipulations have been applied to Stroop tasks since as early as 1971 (Dyer, 1971), the first direct empirical test of the temporal predictability hypothesis applied to the Stroop task has only recently been attempted. In this study, Roelofs (2010) used constant- and random-SOA arrangements in different runs to test how temporal predictability influenced incongruency effects in verbal naming variants of the Stroop-SOA task. In direct contrast to the present results, Roelofs reported seeing no evidence of strategic orienting due to the temporal arrangement of the SOAs, reporting that behavioral interference was greatest at the 0 ms SOA for both intermixed and blocked SOA conditions.
As discussed by MacLeod in his extensive review of the Stroop literature (1991), there are a considerable number of factors that can influence the type and degree of congruency-related interactions elicited in the Stroop task. One such factor is the behavioral response modality used. Previous literature has reported behavioral performance differences depending on response mode (Barch et al., 2001; Weekes & Zaidel, 1996), such that manual responses elicit generally faster overall RTs than vocal responses. Specifically as it relates to Stroop-SOA tasks, it has recently been demonstrated that changes in response modality can alter the pattern of incongruency effects in a Stroop-SOA task. As reported by Coderre and colleagues (Coderre, et al., 2010) the peak of Stroop interference experiences a negative shift in a manual task such that it occurs at the −200 ms SOA rather than the 0 ms SOA as observed for verbal responses. Accordingly, this response-mode difference would be related to the earlier shift in incongruency effects in the present findings, relative to those of Roelofs. Thus, it is possible that the different response modes and the degree to which they are susceptible to response-translation effects may have at least partially contributed to differences reported between these two studies (e.g., see Sugg & McDonald, 1994), an open possibility for future studies to disentangle.
Conclusions
In the present study, we investigated the behavioral and brain adjustments that accompany stimulus conflict in the color-naming Stroop task when the relative timing of the conflicting stimulus elements were predictable versus when they were not. The results demonstrated that the pattern of behavioral and neural incongruency effects vary as a function of the temporal predictability, producing the greatest incongruency effects when the conflicting irrelevant stimulus is presented earlier than the target for unpredictable arrangements, but with simultaneous onset for the temporally predictable ones. Moreover, additional analysis of the sensory responses to the irrelevant distracters when they occurred prior to the target revealed a marked modulation of the early-latency stimulus processing between the two SOA arrangements, providing important evidence for a possible mechanism contributing to the differential incongruency effects. Thus, the current results indicate that, at least under some circumstances, subjects are able to rapidly deduce temporally predictable contingencies between regularly occurring stimulus elements and invoke contextually driven cognitive control mechanisms to modulate interference effects that can be induced when these elements conflict.
Supplementary Material
Acknowledgments
Funding: This work was supported by grants from the National Institutes of Heath [grants R01-MH60415 and R01-NS051048 to M.G.W.].
Footnotes
The negative-polarity ERP wave associated with incongruency (NINC) -- This component is analogous to the previously reported N450 component observed in many 0 ms SOA versions of the Stroop task, but since the latency of this component varies with SOA in the present design, we will use the label NINC.
This component has also been termed the conflict SP, representing the “conflict slow potential” (Larson et al., 2009) or “conflict sustained potential” (West, 2003; West, et al., 2005).
These correspond to points #4 and #11 in the respective text’s “Critical Factors for Stroop Interference” tables.
References
- Anllo-Vento L, Hillyard SA. Selective attention to the color and direction of moving stimuli: electrophysiological correlates of hierarchical feature selection. Percept Psychophys. 1996;58(2):191–206. doi: 10.3758/bf03211875. [DOI] [PubMed] [Google Scholar]
- Appelbaum LG, Liotti M, Perez R, Fox SP, Woldorff MG. The temporal dynamics of implicit processing of non-letter, letter, and word-forms in the human visual cortex. Front Hum Neurosci. 2009;3:56. doi: 10.3389/neuro.09.056.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appelbaum LG, Meyerhoff KL, Woldorff MG. Priming and backward influences in the human brain: processing interactions during the stroop interference effect. Cereb Cortex. 2009;19(11):2508–2521. doi: 10.1093/cercor/bhp036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkinson CM, Drysdale KA, Fulham WR. Event-related potentials to Stroop and reverse Stroop stimuli. Int J Psychophysiol. 2003;47(1):1–21. doi: 10.1016/s0167-8760(02)00038-7. [DOI] [PubMed] [Google Scholar]
- Badzakova-Trajkov G, Barnett KJ, Waldie KE, Kirk IJ. An ERP investigation of the Stroop task: the role of the cingulate in attentional allocation and conflict resolution. Brain Res. 2009;1253:139–148. doi: 10.1016/j.brainres.2008.11.069. [DOI] [PubMed] [Google Scholar]
- Bailey K, West R, Anderson CA. A negative association between video game experience and proactive cognitive control. Psychophysiology. 2009;47(1):34–42. doi: 10.1111/j.1469-8986.2009.00925.x. [DOI] [PubMed] [Google Scholar]
- Barch DM, Braver TS, Akbudak E, Conturo T, Ollinger J, Snyder A. Anterior cingulate cortex and response conflict: effects of response modality and processing domain. Cereb Cortex. 2001;11(9):837–848. doi: 10.1093/cercor/11.9.837. [DOI] [PubMed] [Google Scholar]
- Bentin S, Mouchetant-Rostaing Y, Giard MH, Echallier JF, Pernier J. ERP manifestations of processing printed words at different psycholinguistic levels: time course and scalp distribution. J Cogn Neurosci. 1999;11(3):235–260. doi: 10.1162/089892999563373. [DOI] [PubMed] [Google Scholar]
- Blais C, Bunge S. Behavioral and neural evidence for item-specific performance monitoring. J Cogn Neurosci. 2010;22(12):2758–2767. doi: 10.1162/jocn.2009.21365. [DOI] [PubMed] [Google Scholar]
- Botvinick MM, Braver TS, Barch DM, Carter CS, Cohen JD. Conflict monitoring and cognitive control. Psychol Rev. 2001;108(3):624–652. doi: 10.1037/0033-295x.108.3.624. [DOI] [PubMed] [Google Scholar]
- Broadbent DE. Stimulus set and response set: Two kinds of selective attention. In: Mostofsky DI, editor. Attention: Contemporary theory and analysis. Appleton-Century-Crofts; New York: 1970. pp. 51–60. [Google Scholar]
- Bruchmann M, Herper K, Konrad C, Pantev C, Huster RJ. Individualized EEG source reconstruction of Stroop interference with masked color words. Neuroimage. 2010;49(2):1800–1809. doi: 10.1016/j.neuroimage.2009.09.032. [DOI] [PubMed] [Google Scholar]
- Coderre E, van Heuven W, Conklin K. Lexical Access and Executive Control in Monolinguals and Bilinguals; Paper presented at the Architectures and Mechanisms for Language Processing; York, UK. 2010. [Google Scholar]
- Cohen JD, Dunbar K, McClelland JL. On the control of automatic processes: a parallel distributed processing account of the Stroop effect. Psychol Rev. 1990;97(3):332–361. doi: 10.1037/0033-295x.97.3.332. [DOI] [PubMed] [Google Scholar]
- Coull JT, Nobre AC. Where and when to pay attention: the neural systems for directing attention to spatial locations and to time intervals as revealed by both PET and fMRI. J Neurosci. 1998;18(18):7426–7435. doi: 10.1523/JNEUROSCI.18-18-07426.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crump MJ, Gong Z, Milliken B. The context-specific proportion congruent Stroop effect: location as a contextual cue. Psychon Bull Rev. 2006;13(2):316–321. doi: 10.3758/bf03193850. [DOI] [PubMed] [Google Scholar]
- Duncan-Johnson CC, Kopell BS. The Stroop effect: brain potentials localize the source of interference. Science. 1981;214(4523):938–940. doi: 10.1126/science.7302571. [DOI] [PubMed] [Google Scholar]
- Dyer FN. The effect of word meaning response: Stroop interference for different preexposures of the word. Psychonomic Science. 1971;25:229–231. [Google Scholar]
- Egner T. Congruency sequence effects and cognitive control. Cogn Affect Behav Neurosci. 2007;7(4):380–390. doi: 10.3758/cabn.7.4.380. [DOI] [PubMed] [Google Scholar]
- Egner T. Multiple conflict-driven control mechanisms in the human brain. Trends Cogn Sci. 2008;12(10):374–380. doi: 10.1016/j.tics.2008.07.001. [DOI] [PubMed] [Google Scholar]
- Egner T, Delano M, Hirsch J. Separate conflict-specific cognitive control mechanisms in the human brain. Neuroimage. 2007;35(2):940–948. doi: 10.1016/j.neuroimage.2006.11.061. [DOI] [PubMed] [Google Scholar]
- Egner T, Hirsch J. Cognitive control mechanisms resolve conflict through cortical amplification of task-relevant information. Nat Neurosci. 2005;8(12):1784–1790. doi: 10.1038/nn1594. [DOI] [PubMed] [Google Scholar]
- Eriksen BA, Eriksen CW. Effects of noise letters upon the identification of a target letter in a nonsearch task. Percept Psychophys. 1974;16:143–149. [Google Scholar]
- Fiez JA, Raichle ME, Petersen PE, Tallal P, Katz WF. PET studies of auditory and phonological processing effects of stimulus characteristics and task demands. J Cogn Neurosci. 1995;7:357–375. doi: 10.1162/jocn.1995.7.3.357. [DOI] [PubMed] [Google Scholar]
- Flowers JH. Priming effects in perceptual classification. Percept Psychophys. 1990;47(2):135–148. doi: 10.3758/bf03205978. [DOI] [PubMed] [Google Scholar]
- Folstein JR, Van Petten C. Influence of cognitive control and mismatch on the N2 component of the ERP: a review. Psychophysiology. 2008;45(1):152–170. doi: 10.1111/j.1469-8986.2007.00602.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaser MO, Glaser WR. Time course analysis of the Stroop phenomenon. J Exp Psychol Hum Percept Perform. 1982;8(6):875–894. doi: 10.1037//0096-1523.8.6.875. [DOI] [PubMed] [Google Scholar]
- Glaser WR, Dungelhoff FJ. The time course of picture-word interference. J Exp Psychol Hum Percept Perform. 1984;10(5):640–654. doi: 10.1037//0096-1523.10.5.640. [DOI] [PubMed] [Google Scholar]
- Glaser WR, Glaser MO. Context effects in stroop-like word and picture processing. J Exp Psychol Gen. 1989;118(1):13–42. doi: 10.1037//0096-3445.118.1.13. [DOI] [PubMed] [Google Scholar]
- Griffin IC, Miniussi C, Nobre AC. Orienting attention in time. Front Biosci. 2001;6:D660–671. doi: 10.2741/griffin. [DOI] [PubMed] [Google Scholar]
- Hanslmayr S, Pastotter B, Bauml KH, Gruber S, Wimber M, Klimesch W. The electrophysiological dynamics of interference during the Stroop task. J Cogn Neurosci. 2008;20(2):215–225. doi: 10.1162/jocn.2008.20020. [DOI] [PubMed] [Google Scholar]
- Harter MR, Aine C, Schroeder C. Hemispheric differences in the neural processing of stimulus location and type: effects of selective attention on visual evoked potentials. Neuropsychologia. 1982;20(4):421–438. doi: 10.1016/0028-3932(82)90041-0. [DOI] [PubMed] [Google Scholar]
- Hesse W, Moller E, Arnold M, Schack B. The use of time-variant EEG Granger causality for inspecting directed interdependencies of neural assemblies. J Neurosci Methods. 2003;124(1):27–44. doi: 10.1016/s0165-0270(02)00366-7. [DOI] [PubMed] [Google Scholar]
- Hillyard SA, Anllo-Vento L. Event-related brain potentials in the study of visual selective attention. Proc Natl Acad Sci U S A. 1998;95(3):781–787. doi: 10.1073/pnas.95.3.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillyard SA, Munte TF. Selective attention to color and location: an analysis with event-related brain potentials. Percept Psychophys. 1984;36(2):185–198. doi: 10.3758/bf03202679. [DOI] [PubMed] [Google Scholar]
- Hillyard SA, Vogel EK, Luck SJ. Sensory gain control (amplification) as a mechanism of selective attention: electrophysiological and neuroimaging evidence. Philos Trans R Soc Lond B Biol Sci. 1998;353(1373):1257–1270. doi: 10.1098/rstb.1998.0281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilan AB, Polich J. P300 and response time from a manual Stroop task. Clin Neurophysiol. 1999;110(2):367–373. doi: 10.1016/s0168-5597(98)00053-7. [DOI] [PubMed] [Google Scholar]
- Kenemans JL, Kok A, Smulders FT. Event-related potentials to conjunctions of spatial frequency and orientation as a function of stimulus parameters and response requirements. Electroencephalogr Clin Neurophysiol. 1993;88(1):51–63. doi: 10.1016/0168-5597(93)90028-n. [DOI] [PubMed] [Google Scholar]
- Lansbergen MM, van Hell E, Kenemans JL. Impulsivity and Conflict in the Stroop Task An ERP Study. Journal of Psychophysiology. 2007;21(1):33059. [Google Scholar]
- Larson MJ, Kaufman DA, Perlstein WM. Neural time course of conflict adaptation effects on the Stroop task. Neuropsychologia. 2009;47(3):663–670. doi: 10.1016/j.neuropsychologia.2008.11.013. [DOI] [PubMed] [Google Scholar]
- Liotti M, Woldorff MG, Perez R, Mayberg HS. An ERP study of the temporal course of the Stroop color-word interference effect. Neuropsychologia. 2000;38(5):701–711. doi: 10.1016/s0028-3932(99)00106-2. [DOI] [PubMed] [Google Scholar]
- Logan GD, Zbrodoff NJ. When it helps to be misled: Facilitative effects of increasing of increasing the frequency of conflicting stimuli in a Stroop task. Memory and Cognition. 1979;7:166–174. [Google Scholar]
- Long GM, Lyman BJ. Foveal and parafoveal processing of asynchronous Stroop stimuli. Br J Psychol. 1987;78(Pt 2):151–162. doi: 10.1111/j.2044-8295.1987.tb02236.x. [DOI] [PubMed] [Google Scholar]
- Lu CH, Proctor RW. Influence of irrelevant information on human performance: effects of S-R association strength and relative timing. Q J Exp Psychol A. 2001;54(1):95–136. doi: 10.1080/02724980042000048. [DOI] [PubMed] [Google Scholar]
- MacLeod CM. Half a century of research on the Stroop effect: an integrative review. Psychol Bull. 1991;109(2):163–203. doi: 10.1037/0033-2909.109.2.163. [DOI] [PubMed] [Google Scholar]
- MacLeod CM, MacDonald PA. Interdimensional interference in the Stroop effect: uncovering the cognitive and neural anatomy of attention. Trends Cogn Sci. 2000;4(10):383–391. doi: 10.1016/s1364-6613(00)01530-8. [DOI] [PubMed] [Google Scholar]
- Markela-Lerenc J, Ille N, Kaiser S, Fiedler P, Mundt C, Weisbrod M. Prefrontal-cingulate activation during executive control: which comes first? Brain Res Cogn Brain Res. 2004;18(3):278–287. doi: 10.1016/j.cogbrainres.2003.10.013. [DOI] [PubMed] [Google Scholar]
- Mattler U. Delayed flanker effects on lateralized readiness potentials. [Clinical Trial Research Support, Non-U.S. Gov’t] Experimental brain research. Experimentelle Hirnforschung. Experimentation cerebrale. 2003;151(2):272–288. doi: 10.1007/s00221-003-1486-5. doi:10.1007/s00221-003-1486-5. [DOI] [PubMed] [Google Scholar]
- Miller J. The flanker compatibility effect as a function of visual angle, attentional focus, visual transients, and perceptual load: a search for boundary conditions. Percept Psychophys. 1991;49(3):270–288. doi: 10.3758/bf03214311. [DOI] [PubMed] [Google Scholar]
- Miniussi C, Wilding EL, Coull JT, Nobre AC. Orienting attention in time. Modulation of brain potentials. Brain. 1999;122(Pt 8):1507–1518. doi: 10.1093/brain/122.8.1507. [DOI] [PubMed] [Google Scholar]
- Nobre AC. Orienting attention to instants in time. Neuropsychologia. 2001;39(12):1317–1328. doi: 10.1016/s0028-3932(01)00120-8. [DOI] [PubMed] [Google Scholar]
- Perlstein WM, Larson MJ, Dotson VM, Kelly KG. Temporal dissociation of components of cognitive control dysfunction in severe TBI: ERPs and the cued-Stroop task. Neuropsychologia. 2006;44(2):260–274. doi: 10.1016/j.neuropsychologia.2005.05.009. [DOI] [PubMed] [Google Scholar]
- Phaf RH, Van der Heijden AH, Hudson PT. SLAM: a connectionist model for attention in visual selection tasks. Cognit Psychol. 1990;22(3):273–341. doi: 10.1016/0010-0285(90)90006-p. [DOI] [PubMed] [Google Scholar]
- Platel H, Price C, Baron JC, Wise R, Lambert J, Frackowiak RS, Eustache F. The structural components of music perception. A functional anatomical study. Brain. 1997;120(Pt 2):229–243. doi: 10.1093/brain/120.2.229. [DOI] [PubMed] [Google Scholar]
- Posner MI, Driver J. The neurobiology of selective attention. Curr Opin Neurobiol. 1992;2(2):165–169. doi: 10.1016/0959-4388(92)90006-7. [DOI] [PubMed] [Google Scholar]
- Rayner K, Springer CJ. Graphemic and semantic similarity effects in the picture-word interference task. Br J Psychol. 1986;77(Pt 2):207–222. doi: 10.1111/j.2044-8295.1986.tb01995.x. [DOI] [PubMed] [Google Scholar]
- Rebai M, Bernard C, Lannou J. The Stroop’s test evokes a negative brain potential, the N400. Int J Neurosci. 1997;91(1-2):85–94. doi: 10.3109/00207459708986367. [DOI] [PubMed] [Google Scholar]
- Reynolds M, Kwan D, Smilek D. To group or not to group: an ecological consideration of the stroop effect. Exp Psychol. 2010;57(4):275–291. doi: 10.1027/1618-3169/a000033. [DOI] [PubMed] [Google Scholar]
- Ridderinkhof KR. Micro- and macro-adjustments of task set: activation and suppression in conflict tasks. Psychol Res. 2002;66(4):312–323. doi: 10.1007/s00426-002-0104-7. [DOI] [PubMed] [Google Scholar]
- Roberts KL, Hall DA. Examining a supramodal network for conflict processing: a systematic review and novel functional magnetic resonance imaging data for related visual and auditory stroop tasks. J Cogn Neurosci. 2008;20(6):1063–1078. doi: 10.1162/jocn.2008.20074. [DOI] [PubMed] [Google Scholar]
- Roelofs A. Goal-referenced selection of verbal action: modeling attentional control in the Stroop task. Psychol Rev. 2003;110(1):88–125. doi: 10.1037/0033-295x.110.1.88. [DOI] [PubMed] [Google Scholar]
- Roelofs A. The visual-auditory color-word stroop asymmetry and its time course. Mem Cognit. 2005;33(8):1325–1336. doi: 10.3758/bf03193365. [DOI] [PubMed] [Google Scholar]
- Roelofs A. Context effects of pictures and words in naming objects, reading words, and generating simple phrases. Q J Exp Psychol (Colchester) 2006;59(10):1764–1784. doi: 10.1080/17470210500416052. [DOI] [PubMed] [Google Scholar]
- Roelofs A. Attention, temporal predictability, and the time course of context effects in naming performance. Acta Psychol (Amst) 2010;133(2):146–153. doi: 10.1016/j.actpsy.2009.11.003. [DOI] [PubMed] [Google Scholar]
- Rosenfeld JP, Skogsberg KR. P300-based Stroop study with low probability and target Stroop oddballs: the evidence still favors the response selection hypothesis. Int J Psychophysiol. 2006;60(3):240–250. doi: 10.1016/j.ijpsycho.2005.05.010. [DOI] [PubMed] [Google Scholar]
- Ruz M, Nobre AC. Attention modulates initial stages of visual word processing. J Cogn Neurosci. 2008;20(9):1727–1736. doi: 10.1162/jocn.2008.20119. [DOI] [PubMed] [Google Scholar]
- Scerif G, Worden MS, Davidson M, Seiger L, Casey BJ. Context modulates early stimulus processing when resolving stimulus-response conflict. J Cogn Neurosci. 2006;18(5):781–792. doi: 10.1162/jocn.2006.18.5.781. [DOI] [PubMed] [Google Scholar]
- Schmidt JR, Besner D. The Stroop effect: why proportion congruent has nothing to do with congruency and everything to do with contingency. J Exp Psychol Learn Mem Cogn. 2008;34(3):514–523. doi: 10.1037/0278-7393.34.3.514. [DOI] [PubMed] [Google Scholar]
- Simon JR. The effects of an irrelevant directional cue on human information processing. In: Proctor RW, Reeve TG, editors. Stimulus-response compatibility. Elsevier; Amsterdam: 1990. pp. 31–86. [Google Scholar]
- Stafford T, Gurney KN. Biologically constrained action selection improves cognitive control in a model of the Stroop task. Philos Trans R Soc Lond B Biol Sci. 2007;362(1485):1671–1684. doi: 10.1098/rstb.2007.2060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starreveld PA, La Heij W. Time-Course Analysis of Semantic and Orthographic Context Effects in Picture Naming. Journal of Experimental Psychology. 1996;22(4):896–918. [Google Scholar]
- Stroop JR. Studies of interference in serial verbal reactions. Journal of Experimental Psychology. 1935;18:643–662. [Google Scholar]
- Sugg MJ, McDonald JE. Time course of inhibition in color-response and word-response versions of the Stroop task. J Exp Psychol Hum Percept Perform. 1994;20(3):647–675. doi: 10.1037//0096-1523.20.3.647. [DOI] [PubMed] [Google Scholar]
- Taylor DA. Time Course of Context Effects. Journal of Experimental Psychology: General. 1977;4:404–426. [Google Scholar]
- Tzelgov J, Henik A, Berger J. Controlling Stroop effects by manipulating expectations for color words. Mem Cognit. 1992;20(6):727–735. doi: 10.3758/bf03202722. [DOI] [PubMed] [Google Scholar]
- Van Veen V, Carter CS. The timing of action-monitoring processes in the anterior cingulate cortex. J Cogn Neurosci. 2002;14(4):593–602. doi: 10.1162/08989290260045837. [DOI] [PubMed] [Google Scholar]
- Weekes NY, Zaidel E. The effects of procedural variations on lateralized Stroop effects. Brain Cogn. 1996;31(3):308–330. doi: 10.1006/brcg.1996.0049. [DOI] [PubMed] [Google Scholar]
- West R. Neural correlates of cognitive control and conflict detection in the Stroop and digit-location tasks. Neuropsychologia. 2003;41(8):1122–1135. doi: 10.1016/s0028-3932(02)00297-x. [DOI] [PubMed] [Google Scholar]
- West R, Alain C. Event-related neural activity associated with the Stroop task. Brain Res Cogn Brain Res. 1999;8(2):157–164. doi: 10.1016/s0926-6410(99)00017-8. [DOI] [PubMed] [Google Scholar]
- West R, Alain C. Effects of task context and fluctuations of attention on neural activity supporting performance of the stroop task. Brain Res. 2000;873(1):102–111. doi: 10.1016/s0006-8993(00)02530-0. [DOI] [PubMed] [Google Scholar]
- West R, Bowry R, McConville C. Sensitivity of medial frontal cortex to response and nonresponse conflict. Psychophysiology. 2004;41(5):739–748. doi: 10.1111/j.1469-8986.2004.00205.x. [DOI] [PubMed] [Google Scholar]
- West R, Jakubek K, Wymbs N, Perry M, Moore K. Neural correlates of conflict processing. Exp Brain Res. 2005;167(1):38–48. doi: 10.1007/s00221-005-2366-y. [DOI] [PubMed] [Google Scholar]
- Woldorff MG. Distortion of ERP averages due to overlap from temporally adjacent ERPs: analysis and correction. Psychophysiology. 1993;30(1):98–119. doi: 10.1111/j.1469-8986.1993.tb03209.x. [DOI] [PubMed] [Google Scholar]
- Woldorff MG, Liotti M, Seabolt M, Busse L, Lancaster JL, Fox PT. The temporal dynamics of the effects in occipital cortex of visual-spatial selective attention. Brain Res Cogn Brain Res. 2002;15(1):1–15. doi: 10.1016/s0926-6410(02)00212-4. [DOI] [PubMed] [Google Scholar]
- Yu Y, Choe Y. Selective attention in time: An extended model of stimulus onset asynchrony (SOA) in Stroop effect. Paper presented at the Proceedings of the fifth international conference on development and learning, Indiana University; Bloomington, IN. 2006. [Google Scholar]
- Zhang HH, Zhang J, Kornblum S. A parallel distributed processing model of stimulus-stimulus and stimulus-response compatibility. Cognit Psychol. 1999;38(3):386–432. doi: 10.1006/cogp.1998.0703. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.