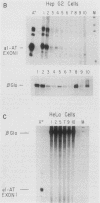
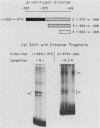
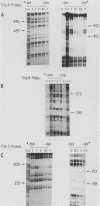
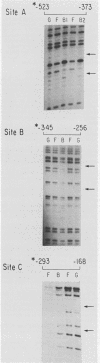
Abstract
We have previously described the isolation and characterization of genomic clones corresponding to the mouse alpha 1-antitrypsin gene (Krauter et al., DNA 5:29-36, 1986). In this report, we have analyzed the DNA sequences upstream of the RNA start site that direct hepatoma cell-specific expression of this gene when incorporated into recombinant plasmids. The 160 nucleotides 5' to the cap site direct low-level expression in hepatoma cells, and sequences between -520 and -160 bp upstream of the RNA start site functioned as a cell-specific enhancer of expression both with the alpha 1-antitrypsin promoter and when combined with a functional beta-globin promoter. Within the enhancer region, three binding sites for proteins present in hepatoma nuclear extracts were identified. The location of each site was positioned, using both methylation protection and methylation interference experiments. Each protein-binding site correlated with a functionally important region necessary for full enhancer activity. These experiments demonstrated a complex arrangement of regulatory elements comprising the alpha 1-antitrypsin enhancer. Significant qualitative differences exist between the findings presented here and the cis-acting elements operative in regulating expression of the human alpha 1-antitrypsin gene (Ciliberto et al., Cell 41:531-540, 1985; De Simone et al., EMBO J. 6:2759-2766, 1987).
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Angel P., Imagawa M., Chiu R., Stein B., Imbra R. J., Rahmsdorf H. J., Jonat C., Herrlich P., Karin M. Phorbol ester-inducible genes contain a common cis element recognized by a TPA-modulated trans-acting factor. Cell. 1987 Jun 19;49(6):729–739. doi: 10.1016/0092-8674(87)90611-8. [DOI] [PubMed] [Google Scholar]
- Babiss L. E., Friedman J. M., Darnell J. E., Jr Cellular promoters incorporated into the adenovirus genome: effects of viral regulatory elements on transcription rates and cell specificity of albumin and beta-globin promoters. Mol Cell Biol. 1986 Nov;6(11):3798–3806. doi: 10.1128/mcb.6.11.3798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babiss L. E., Herbst R. S., Bennett A. L., Darnell J. E., Jr Factors that interact with the rat albumin promoter are present both in hepatocytes and other cell types. Genes Dev. 1987 May;1(3):256–267. doi: 10.1101/gad.1.3.256. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Ciliberto G., Dente L., Cortese R. Cell-specific expression of a transfected human alpha 1-antitrypsin gene. Cell. 1985 Jun;41(2):531–540. doi: 10.1016/s0092-8674(85)80026-x. [DOI] [PubMed] [Google Scholar]
- Citron B. A., Darnell J. E., Jr Mapping a mammalian mRNA cap site by restriction digestion of hybridized cDNA. Gene. 1985;40(1):131–135. doi: 10.1016/0378-1119(85)90032-0. [DOI] [PubMed] [Google Scholar]
- Colantuoni V., Pirozzi A., Blance C., Cortese R. Negative control of liver-specific gene expression: cloned human retinol-binding protein gene is repressed in HeLa cells. EMBO J. 1987 Mar;6(3):631–636. doi: 10.1002/j.1460-2075.1987.tb04801.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa R. H., Lai E., Darnell J. E., Jr Transcriptional control of the mouse prealbumin (transthyretin) gene: both promoter sequences and a distinct enhancer are cell specific. Mol Cell Biol. 1986 Dec;6(12):4697–4708. doi: 10.1128/mcb.6.12.4697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa R. H., Lai E., Grayson D. R., Darnell J. E., Jr The cell-specific enhancer of the mouse transthyretin (prealbumin) gene binds a common factor at one site and a liver-specific factor(s) at two other sites. Mol Cell Biol. 1988 Jan;8(1):81–90. doi: 10.1128/mcb.8.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Onofrio C., Colantuoni V., Cortese R. Structure and cell-specific expression of a cloned human retinol binding protein gene: the 5'-flanking region contains hepatoma specific transcriptional signals. EMBO J. 1985 Aug;4(8):1981–1989. doi: 10.1002/j.1460-2075.1985.tb03881.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dailey L., Hanly S. M., Roeder R. G., Heintz N. Distinct transcription factors bind specifically to two regions of the human histone H4 promoter. Proc Natl Acad Sci U S A. 1986 Oct;83(19):7241–7245. doi: 10.1073/pnas.83.19.7241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darnell J. E., Jr Variety in the level of gene control in eukaryotic cells. Nature. 1982 Jun 3;297(5865):365–371. doi: 10.1038/297365a0. [DOI] [PubMed] [Google Scholar]
- Davidson I., Fromental C., Augereau P., Wildeman A., Zenke M., Chambon P. Cell-type specific protein binding to the enhancer of simian virus 40 in nuclear extracts. Nature. 1986 Oct 9;323(6088):544–548. doi: 10.1038/323544a0. [DOI] [PubMed] [Google Scholar]
- De Simone V., Ciliberto G., Hardon E., Paonessa G., Palla F., Lundberg L., Cortese R. Cis- and trans-acting elements responsible for the cell-specific expression of the human alpha 1-antitrypsin gene. EMBO J. 1987 Sep;6(9):2759–2766. doi: 10.1002/j.1460-2075.1987.tb02570.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dente L., Pizza M. G., Metspalu A., Cortese R. Structure and expression of the genes coding for human alpha 1-acid glycoprotein. EMBO J. 1987 Aug;6(8):2289–2296. doi: 10.1002/j.1460-2075.1987.tb02503.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derman E., Krauter K., Walling L., Weinberger C., Ray M., Darnell J. E., Jr Transcriptional control in the production of liver-specific mRNAs. Cell. 1981 Mar;23(3):731–739. doi: 10.1016/0092-8674(81)90436-0. [DOI] [PubMed] [Google Scholar]
- Dignam J. D., Lebovitz R. M., Roeder R. G. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983 Mar 11;11(5):1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dynan W. S., Tjian R. Control of eukaryotic messenger RNA synthesis by sequence-specific DNA-binding proteins. 1985 Aug 29-Sep 4Nature. 316(6031):774–778. doi: 10.1038/316774a0. [DOI] [PubMed] [Google Scholar]
- Edlund T., Walker M. D., Barr P. J., Rutter W. J. Cell-specific expression of the rat insulin gene: evidence for role of two distinct 5' flanking elements. Science. 1985 Nov 22;230(4728):912–916. doi: 10.1126/science.3904002. [DOI] [PubMed] [Google Scholar]
- Fried M., Crothers D. M. Equilibria and kinetics of lac repressor-operator interactions by polyacrylamide gel electrophoresis. Nucleic Acids Res. 1981 Dec 11;9(23):6505–6525. doi: 10.1093/nar/9.23.6505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman J. M., Babiss L. E., Clayton D. F., Darnell J. E., Jr Cellular promoters incorporated into the adenovirus genome: cell specificity of albumin and immunoglobulin expression. Mol Cell Biol. 1986 Nov;6(11):3791–3797. doi: 10.1128/mcb.6.11.3791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman J. M., Babiss L. E., Weiss M., Darnell J. E., Jr Hepatoma variants (C2) are defective for transcriptional and post-transcriptional actions from both endogenous and viral genomes. EMBO J. 1987 Jun;6(6):1727–1731. doi: 10.1002/j.1460-2075.1987.tb02424.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galas D. J., Schmitz A. DNAse footprinting: a simple method for the detection of protein-DNA binding specificity. Nucleic Acids Res. 1978 Sep;5(9):3157–3170. doi: 10.1093/nar/5.9.3157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garner M. M., Revzin A. A gel electrophoresis method for quantifying the binding of proteins to specific DNA regions: application to components of the Escherichia coli lactose operon regulatory system. Nucleic Acids Res. 1981 Jul 10;9(13):3047–3060. doi: 10.1093/nar/9.13.3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godbout R., Ingram R., Tilghman S. M. Multiple regulatory elements in the intergenic region between the alpha-fetoprotein and albumin genes. Mol Cell Biol. 1986 Feb;6(2):477–487. doi: 10.1128/mcb.6.2.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorski K., Carneiro M., Schibler U. Tissue-specific in vitro transcription from the mouse albumin promoter. Cell. 1986 Dec 5;47(5):767–776. doi: 10.1016/0092-8674(86)90519-2. [DOI] [PubMed] [Google Scholar]
- Hammer R. E., Krumlauf R., Camper S. A., Brinster R. L., Tilghman S. M. Diversity of alpha-fetoprotein gene expression in mice is generated by a combination of separate enhancer elements. Science. 1987 Jan 2;235(4784):53–58. doi: 10.1126/science.2432657. [DOI] [PubMed] [Google Scholar]
- Heard J. M., Herbomel P., Ott M. O., Mottura-Rollier A., Weiss M., Yaniv M. Determinants of rat albumin promoter tissue specificity analyzed by an improved transient expression system. Mol Cell Biol. 1987 Jul;7(7):2425–2434. doi: 10.1128/mcb.7.7.2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herr W., Clarke J. The SV40 enhancer is composed of multiple functional elements that can compensate for one another. Cell. 1986 May 9;45(3):461–470. doi: 10.1016/0092-8674(86)90332-6. [DOI] [PubMed] [Google Scholar]
- Hesse J. E., Nickol J. M., Lieber M. R., Felsenfeld G. Regulated gene expression in transfected primary chicken erythrocytes. Proc Natl Acad Sci U S A. 1986 Jun;83(12):4312–4316. doi: 10.1073/pnas.83.12.4312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelsey G. D., Povey S., Bygrave A. E., Lovell-Badge R. H. Species- and tissue-specific expression of human alpha 1-antitrypsin in transgenic mice. Genes Dev. 1987 Apr;1(2):161–171. doi: 10.1101/gad.1.2.161. [DOI] [PubMed] [Google Scholar]
- Knowles B. B., Howe C. C., Aden D. P. Human hepatocellular carcinoma cell lines secrete the major plasma proteins and hepatitis B surface antigen. Science. 1980 Jul 25;209(4455):497–499. doi: 10.1126/science.6248960. [DOI] [PubMed] [Google Scholar]
- Kovesdi I., Reichel R., Nevins J. R. Identification of a cellular transcription factor involved in E1A trans-activation. Cell. 1986 Apr 25;45(2):219–228. doi: 10.1016/0092-8674(86)90386-7. [DOI] [PubMed] [Google Scholar]
- Krauter K. S., Citron B. A., Hsu M. T., Powell D., Darnell J. E., Jr Isolation and characterization of the alpha 1-antitrypsin gene of mice. DNA. 1986 Feb;5(1):29–36. doi: 10.1089/dna.1986.5.29. [DOI] [PubMed] [Google Scholar]
- Landolfi N. F., Capra J. D., Tucker P. W. Interaction of cell-type-specific nuclear proteins with immunoglobulin VH promoter region sequences. Nature. 1986 Oct 9;323(6088):548–551. doi: 10.1038/323548a0. [DOI] [PubMed] [Google Scholar]
- Lee W., Haslinger A., Karin M., Tjian R. Activation of transcription by two factors that bind promoter and enhancer sequences of the human metallothionein gene and SV40. Nature. 1987 Jan 22;325(6102):368–372. doi: 10.1038/325368a0. [DOI] [PubMed] [Google Scholar]
- Lee W., Mitchell P., Tjian R. Purified transcription factor AP-1 interacts with TPA-inducible enhancer elements. Cell. 1987 Jun 19;49(6):741–752. doi: 10.1016/0092-8674(87)90612-x. [DOI] [PubMed] [Google Scholar]
- Lefevre C., Imagawa M., Dana S., Grindlay J., Bodner M., Karin M. Tissue-specific expression of the human growth hormone gene is conferred in part by the binding of a specific trans-acting factor. EMBO J. 1987 Apr;6(4):971–981. doi: 10.1002/j.1460-2075.1987.tb04847.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legerski R. J., Hodnett J. L., Gray H. B., Jr Extracellular nucleases of pseudomonas BAL 31. III. Use of the double-strand deoxyriboexonuclease activity as the basis of a convenient method for the mapping of fragments of DNA produced by cleavage with restriction enzymes. Nucleic Acids Res. 1978 May;5(5):1445–1464. doi: 10.1093/nar/5.5.1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenardo M., Pierce J. W., Baltimore D. Protein-binding sites in Ig gene enhancers determine transcriptional activity and inducibility. Science. 1987 Jun 19;236(4808):1573–1577. doi: 10.1126/science.3109035. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Melton D. A., Krieg P. A., Rebagliati M. R., Maniatis T., Zinn K., Green M. R. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984 Sep 25;12(18):7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell P. J., Wang C., Tjian R. Positive and negative regulation of transcription in vitro: enhancer-binding protein AP-2 is inhibited by SV40 T antigen. Cell. 1987 Sep 11;50(6):847–861. doi: 10.1016/0092-8674(87)90512-5. [DOI] [PubMed] [Google Scholar]
- Oliviero S., Morrone G., Cortese R. The human haptoglobin gene: transcriptional regulation during development and acute phase induction. EMBO J. 1987 Jul;6(7):1905–1912. doi: 10.1002/j.1460-2075.1987.tb02450.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ornitz D. M., Palmiter R. D., Hammer R. E., Brinster R. L., Swift G. H., MacDonald R. J. Specific expression of an elastase-human growth hormone fusion gene in pancreatic acinar cells of transgenic mice. Nature. 1985 Feb 14;313(6003):600–602. doi: 10.1038/313600a0. [DOI] [PubMed] [Google Scholar]
- Ott M. O., Sperling L., Herbomel P., Yaniv M., Weiss M. C. Tissue-specific expression is conferred by a sequence from the 5' end of the rat albumin gene. EMBO J. 1984 Nov;3(11):2505–2510. doi: 10.1002/j.1460-2075.1984.tb02164.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overbeek P. A., Chepelinsky A. B., Khillan J. S., Piatigorsky J., Westphal H. Lens-specific expression and developmental regulation of the bacterial chloramphenicol acetyltransferase gene driven by the murine alpha A-crystallin promoter in transgenic mice. Proc Natl Acad Sci U S A. 1985 Dec;82(23):7815–7819. doi: 10.1073/pnas.82.23.7815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlino E., Cortese R., Ciliberto G. The human alpha 1-antitrypsin gene is transcribed from two different promoters in macrophages and hepatocytes. EMBO J. 1987 Sep;6(9):2767–2771. doi: 10.1002/j.1460-2075.1987.tb02571.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlino E., Cortese R., Ciliberto G. The human alpha 1-antitrypsin gene is transcribed from two different promoters in macrophages and hepatocytes. EMBO J. 1987 Sep;6(9):2767–2771. doi: 10.1002/j.1460-2075.1987.tb02571.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piette J., Kryszke M. H., Yaniv M. Specific interaction of cellular factors with the B enhancer of polyoma virus. EMBO J. 1985 Oct;4(10):2675–2685. doi: 10.1002/j.1460-2075.1985.tb03987.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinkert C. A., Ornitz D. M., Brinster R. L., Palmiter R. D. An albumin enhancer located 10 kb upstream functions along with its promoter to direct efficient, liver-specific expression in transgenic mice. Genes Dev. 1987 May;1(3):268–276. doi: 10.1101/gad.1.3.268. [DOI] [PubMed] [Google Scholar]
- Powell D. J., Friedman J. M., Oulette A. J., Krauter K. S., Darnell J. E., Jr Transcriptional and post-transcriptional control of specific messenger RNAs in adult and embryonic liver. J Mol Biol. 1984 Oct 15;179(1):21–35. doi: 10.1016/0022-2836(84)90304-8. [DOI] [PubMed] [Google Scholar]
- Rosales R., Vigneron M., Macchi M., Davidson I., Xiao J. H., Chambon P. In vitro binding of cell-specific and ubiquitous nuclear proteins to the octamer motif of the SV40 enhancer and related motifs present in other promoters and enhancers. EMBO J. 1987 Oct;6(10):3015–3025. doi: 10.1002/j.1460-2075.1987.tb02607.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawadogo M., Roeder R. G. Interaction of a gene-specific transcription factor with the adenovirus major late promoter upstream of the TATA box region. Cell. 1985 Nov;43(1):165–175. doi: 10.1016/0092-8674(85)90021-2. [DOI] [PubMed] [Google Scholar]
- Sen R., Baltimore D. Multiple nuclear factors interact with the immunoglobulin enhancer sequences. Cell. 1986 Aug 29;46(5):705–716. doi: 10.1016/0092-8674(86)90346-6. [DOI] [PubMed] [Google Scholar]
- Singh H., Sen R., Baltimore D., Sharp P. A. A nuclear factor that binds to a conserved sequence motif in transcriptional control elements of immunoglobulin genes. Nature. 1986 Jan 9;319(6049):154–158. doi: 10.1038/319154a0. [DOI] [PubMed] [Google Scholar]
- Swift G. H., Hammer R. E., MacDonald R. J., Brinster R. L. Tissue-specific expression of the rat pancreatic elastase I gene in transgenic mice. Cell. 1984 Oct;38(3):639–646. doi: 10.1016/0092-8674(84)90258-7. [DOI] [PubMed] [Google Scholar]
- Walker M. D., Edlund T., Boulet A. M., Rutter W. J. Cell-specific expression controlled by the 5'-flanking region of insulin and chymotrypsin genes. Nature. 1983 Dec 8;306(5943):557–561. doi: 10.1038/306557a0. [DOI] [PubMed] [Google Scholar]
- Wiederrecht G., Shuey D. J., Kibbe W. A., Parker C. S. The Saccharomyces and Drosophila heat shock transcription factors are identical in size and DNA binding properties. Cell. 1987 Feb 13;48(3):507–515. doi: 10.1016/0092-8674(87)90201-7. [DOI] [PubMed] [Google Scholar]
- Wildeman A. G., Zenke M., Schatz C., Wintzerith M., Grundström T., Matthes H., Takahashi K., Chambon P. Specific protein binding to the simian virus 40 enhancer in vitro. Mol Cell Biol. 1986 Jun;6(6):2098–2105. doi: 10.1128/mcb.6.6.2098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L. C., Morley B. J., Campbell R. D. Cell-specific expression of the human complement protein factor B gene: evidence for the role of two distinct 5'-flanking elements. Cell. 1987 Jan 30;48(2):331–342. doi: 10.1016/0092-8674(87)90436-3. [DOI] [PubMed] [Google Scholar]
- Xiao J. H., Davidson I., Ferrandon D., Rosales R., Vigneron M., Macchi M., Ruffenach F., Chambon P. One cell-specific and three ubiquitous nuclear proteins bind in vitro to overlapping motifs in the domain B1 of the SV40 enhancer. EMBO J. 1987 Oct;6(10):3005–3013. doi: 10.1002/j.1460-2075.1987.tb02606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zenke M., Grundström T., Matthes H., Wintzerith M., Schatz C., Wildeman A., Chambon P. Multiple sequence motifs are involved in SV40 enhancer function. EMBO J. 1986 Feb;5(2):387–397. doi: 10.1002/j.1460-2075.1986.tb04224.x. [DOI] [PMC free article] [PubMed] [Google Scholar]