Abstract
Background
Standardized future liver remnant (sFLR) volume and degree of hypertrophy after portal vein embolization (PVE) have been recognized as significant predictors of surgical outcomes after major liver resection. However, regeneration rate of the FLR after PVE varies among individuals and its clinical significance is unknown.
Study Design
Degree of hypertrophy at initial volume assessment divided by number of weeks elapsed after PVE was defined as the kinetic growth rate (KGR). In 107 consecutive patients who underwent liver resection for colorectal liver metastases with a sFLR volume of greater than 20%, the ability of the KGR to predict overall and liver-specific postoperative morbidity and mortality was compared with sFLR volume and degree of hypertrophy.
Results
Using receiver operating characteristic analysis, the best cut-off values for sFLR volume, degree of hypertrophy, and KGR for predicting postoperative hepatic insufficiency were estimated as, respectively, 29.6%, 7.5%, and 2.0% per week. Among these, KGR was the most accurate predictor (area under the curve, 0.830 [0.736-0.923]; asymptotic significance, 0.002). KGR of less than 2% per week vs. ≥2% per week correlate with rates of hepatic insufficiency (21.6% vs. 0%, p = 0.0001) and liver-related 90-day mortality (8.1% vs. 0%, P=0.04). The predictive value of KGR was not influenced by sFLR volume or the timing of initial volume assessment when evaluated within 8 weeks after PVE.
Conclusions
KGR is a better predictor of postoperative morbidity and mortality after liver resection for small FLR than conventional measured volume parameters (sFLR volume and degree of hypertrophy).
Introduction
For patients with colorectal liver metastases (CLM), the introduction of effective systemic therapies and an increase in the utilization of major hepatectomy have led to significant improvements in long-term survivals during the past decade.[1] Our group has utilized and reported various strategies to expand resectability for patients with CLM including perioperative chemotherapy and staged surgical procedures with the use of portal vein embolization (PVE).[2, 3] The trophic effects of pre-operative PVE on the future liver remnant (FLR) allows for safe preservation of hepatic reserve in order to decrease the incidence of postoperative liver failure and death after extended liver resections, which is particularly important in patients with chemotherapy associated liver disease.[4]
As the techniques and indications for PVE have evolved over time, numerous groups have attempted to more accurately predict postoperative outcomes on the basis of PVE-induced preoperative radiographic volume changes.[5] As part of our experience with PVE, we have observed significant variability in the rate of growth of the liver remnant after PVE. In this study, we sought to clarify this clinical observation by determining the relationship between postoperative outcome and kinetic growth rate (KGR), defined as the increase in FLR volume from baseline to first post-PVE volume assessment divided by length of time (weeks) between PVE and first post-PVE volumetry.
Patients and Methods
The Institutional Review Board of The University of Texas, MD Anderson Cancer Center, approved this study protocol (PA12-0225). From a prospective hepatobiliary database maintained by the Department of Surgical Oncology, 194 consecutive patients who underwent right PVE before planned right hemihepatectomy or extended right hepatectomy for small FLR in patients with colorectal metastases between January 1993 and March 2012 were identified.
Of these 194 patients, 44 patients who were unable to proceed to surgery due to progression of disease (n = 30), insufficient regeneration of FLR (n = 5), or other medical issues (n = 9) and 16 patients who were unresectable at laparotomy were excluded from this study. To explore the effect of KGR, patients who did not meet our current minimal FLR volume criteria (>20% of standardized liver volume) [6-8] (n = 19) or who did not undergo initial post-PVE radiographic volume assessment within 8 weeks after PVE (n = 8) were also excluded from the analysis. Thus the remaining 107 patients constituted the current study cohort.
Pre-PVE liver volumetry, calculation of sFLR volume, and PVE
All patients with potentially resectable disease underwent preoperative liver volumetry based on computed tomography (CT) imaging, and sFLR volume was estimated according to the previously reported method.[9] Enhanced CT scans were performed with a multidetector row CT, 4, 16, or 64 slices (Light-Speed; GE Healthcare, Piscataway, NJ), using a triphasic liver protocol or single-phase technique at 2.5 – 5 mm thick slices. The liver volumes were determined by loading the CT images onto an Advantage Workstation 4.1 (GE Medical Systems, Milwaukee, WI). Standardized liver volume was calculated using the following formula: SLV = −794.41 + 1267.28 × body surface area (m2).[10] PVE was considered when sFLR was less than 20% in patients with normal liver, or less than 30% in patients with evidence of fibrosis or severe liver injury.[6, 7, 11] All embolizations were performed by the ipsilateral percutaneous transhepatic approach using tris-acryl microspheres ranging in size from 100-700 microns and coils. The right PVE was specifically expanded to segment IV branches when extended right hepatectomy was considered.[12-15]
Post-PVE liver volumetry and calculation of DH and KGR
Initial post-PVE liver volume assessments were performed with 3-dimensional CT volumetry 2- to 8 weeks after PVE. Degree of hypertrophy (DH) was defined as the percentage point difference between the sFLR volume before and after PVE. The kinetic growth rate (KGR) was calculated by the following formula: KGR = DH at first post-PVE volume assessment (%) ÷ time elapsed since PVE (weeks) at first post-PVE volume assessment. If hypertrophy at the first post-PVE volume assessment was insufficient (i.e., sFLR volume less than 20% in patients with normal liver or sFLR volume less than 30% in patients with injured liver), serial radiographic volumetric assessments were performed and systemic therapy was administered if applicable until the sFLR volume was sufficient to permit resection.
Definitions of outcomes
Postoperative complications were classified using standard criteria, and major complications were defined as grade III or higher complications.[16] Postoperative hepatic insufficiency was defined as a peak total bilirubin level greater than 7mg/dL and/or typical clinical manifestations of hepatic insufficiency, including massive ascites or encephalopathy.[17] The death from liver failure was calculated at 90 days after surgical resection.
Comparison of volume parameters with respect to predicting postoperative hepatic insufficiency, morbidity, and mortality
The performance of sFLR volume, DH, and KGR in predicting hepatic insufficiency was assessed using receiver-operating characteristics (ROC) analysis. The accuracy of each parameter in discriminating patients with and without postoperative hepatic insufficiency was assessed by calculating the area under the curve (AUC) and the asymptotic significance level of each curve compared to the diagonal reference line (AUC=0.500). Using the best cut-off values determined by the ROC analysis, rates of major complications, hepatic insufficiency, and 90-day mortality were compared amongst patients.
Statistical analysis
Statistical analysis was performed using IBM SPSS software (ver19.0. SPSS Inc., IL, USA). Median and ranges of continuous data were compared using the Mann-Whitney U test. Categorical data were compared using Pearson's chi-squared test or Fisher's exact test as appropriate.
Results
Patient characteristics
Baseline characteristics of the 107 studied patients are summarized in Table 1. Right PVE was performed in 40 (37.4%) patients and it was extended to segment IV branch in 67 (62.6%) patients. Of note, 89.7% of the 107 patients included in this study had some histopathological changes in the nontumoral liver, with 92.3% of these patients having received 5-fluorouracil or capecitabine based neoadjuvant chemotherapy.
Table 1. Baseline Characteristics of Study Population (n = 107).
| Age, y, median (range) | 54 (34-76) |
| Males, n (%) | 75 (70.9) |
| Diabetes, n (%) | 7/103 (6.8) |
| BMI, kg/m2, median (range) | 25.6 (17.1 - 40.1) |
| ASA score ≥3, n (%) | 85/103 (82.5) |
| Nontumoral liver pathology, n (%) | |
| None | 77/95 (81.1) |
| Steatosis >30% or | 6/99 (6.1) |
| steatohepatitis* | |
| Fibrosis (F1-4)† | 8/101 (7.9) |
| Cirrhosis (F5-6)† | 0/101 (0) |
| Sinusoidal injury‡ | 8/96 (8.3) |
| Type of PVE, n (%)§ | |
| RPVE | 40/107 (37.4) |
| RPVE extended to segment IV | 67/107 (62.6) |
| 5-FU or capecitabine based preoperative chemotherapy, n (%) | |
| Any | 96/104 (92.3) |
| >12 wk duration | 46/104 (44.2) |
| Oxaliplatin§ | 83/104 (79.8) |
| Irinotecan§ | 16/104 (15.4) |
| Bevacizumab | 72/104 (69.2) |
| Cetuximab or Panitumumab | 3/104 (2.9) |
When 2 numbers are listed, the numerator is the number of patients with the characteristic and the denominator is the number of patients for whom information about this characteristic was available. Values in parentheses are percentage unless indicated otherwise.
Kleiner score 4 or greater.[31]
Fibrosis score according to Ishak et al.[32]
Rubbia-Brandt score 2-3 (moderate to severe).[33]
Three patients had history of both FOLFOX and FOLFIRI.
BMI, body mass index; ASA, American Society of Anesthesiology; RPVE, right portal vein embolization.
Post-PVE liver hypertrophy
Table 2 summarizes volume measurements before and after PVE. Initial volume assessments were performed at a median of 30 days after PVE. Significant increases in both FLR volume and sFLR volume (P < 0.0001 vs. pre-PVE) were apparent at the time of first post-PVE volume assessment with a median DH of 10.1% and KGR of 2.4% per week. Ninety-six patients (89.7%) proceeded to surgery at this point, while 11 (10.3%) patients in whom sFLR volumes were not sufficient for resection underwent additional waiting time (median, 100 days; range, 56 – 254 days) with serial radiographic observation until sFLR volume reached the criteria for resection (ie, sFLR>20%).[18] We found that the KGR was significantly higher for patients who were able to proceed to resection after first post-PVE volume assessment compared to those who required additional waiting time prior to meeting resection criteria, 2.5% per week (range, 0.2-9.4) versus 1.5% per week (range, 0.2-2.7), (P = 0.0056) (Table 3). Among patients with a KGR greater than 2.0% per week, only 4.4% (3/68) required additional waiting time to achieve an acceptable sFLR volume, whereas among patients with a KGR less than 2.0% per week, 20.5% (8/39) required additional waiting time (P = 0.0001).
Table 2. Measures of Future Liver Remnant Volume before and after Portal Vein Embolization.
| Baseline measurement, median (range) | Initial post-PVE measurement (<8 week after PVE), median (range) | Final post-PVE measurement before surgery, median (range) | |
|---|---|---|---|
| No. of days after PVE | 0 | 30 (14 - 54) | 31 (14 - 254) |
| FLR volume, mL | 331 (148 - 927) | 486 (257 - 1187) | 497 (256 - 1186) |
| sFLR volume, % | 20.1 (9.5 - 57.8) | 29.4 (20.1 - 75.1) | 31.2 (20.1 - 75.1) |
| DH, % | - | 10.1 (0.1 - 39.9) | 10.8 (0.1 - 39.9) |
| KGR, % per week | - | 2.4 (0.2 - 9.4) | - |
Eleven patients (10.3%) required additional waiting time at this point due to insufficient volume increase.
FLR, future liver remnant; sFLR, standardized FLR; DH, degree of hypertrophy; KGR, kinetic growth rate, defined as DH per week.
Table 3. Outcomes According to Whether the Standardized Future Liver Remnant Volume was Adequate at Initial Post-Portal Vein Embolization Volume Assessment.
| Adequate sFLR Volume at Initial Assessment (n = 96) | Inadequate sFLR Volume at Initial Assessment (n = 11) | p Value | |
|---|---|---|---|
| Any complication, n (%) | 44/96 (45.8) | 8/11 (72.3) | 0.12 |
| Major complication, n (%) | 20/96 (20.8) | 4/11 (36.4) | 0.26 |
| Peritoneal fluid collection | 5/96 (5.2) | 0/11 (0) | 0.98 |
| Bile leak | 6/96 (6.3) | 0/11 (0) | 0.87 |
| Abscess | 1/96 (1.0) | 0/11 (0) | 0.19 |
| Hepatic insufficiency | 5/96 (5.2) | 3/11 (27.3) | 0.035 |
| Ileus | 1/96 (1.0) | 0/11 (0) | 0.19 |
| Acute cardiac failure | 1/96 (1.0) | 1/11 (9.1) | 0.49 |
| Respiratory failure | 1/96 (1.0) | 0/11 (0) | 0.19 |
| 90-day liver failure death, n (%) | 2/96 (2.1) | 1/11 (9.1) | 0.28 |
| Time from PVE to final CT, d, median (range) | 30 (14-52) | 100 (56-254) | <0.0001 |
| Final sFLR volume, %, median (range) | 31.9 (20.1-75.1) | 28.3 (20.6-41.9) | 0.21 |
| Final DH, %, median (range) | 10.9 (1-39.9) | 6.8 (0.1-23.7) | 0.30 |
| KGR, % per wk, median (range) | 2.5 (0.2-9.4) | 1.5 (0.2-2.7) | 0.0056 |
When 2 numbers are listed, the numerator is the number of patients with the characteristic and the denominator is the number of patients for whom information about this characteristic was available. Values in parentheses are percentage unless indicated otherwise.
CT, computed tomography; DH, degree of hypertrophy; KGR, kinetic growth rate; PVE, ; sFLR,
Regarding the difference between right PVE and right PVE + segment IV, latter procedure showed, higher final standardized volume (25.4% vs. 20.5%, p=0.006), DH (9.5% vs. 6.2%, p=0.07), and KGR (2.3 %/week vs. 1.4%/week, p=0.02) of segment II+III, as reported previously.[15]
Background clinical factors and liver hypertrophy
Influence of background factors on final FLR, DH, and KGR were also investigated regarding patient age, gender, presence of diabetes, BMI, liver fibrosis, steatosis or steatohepatitis, sinusoidal injury, chemotherapy duration, and types of chemotherapy regimens. Among these, presence of diabetes was associated with lower KGR (1.3 %/week vs. 2.6 %/week, p=0.003) and lower DH (5.9% vs. 11.6%, p=0.02). And severe sinusoidal injury was also associated with lower KGR (1.2 %/week vs. 2.5 %/week, p=0.04). In the current population (92.3% received preoperative chemotherapy and 47.9% of them were treated with > 12 weeks of preoperative chemotherapy), no association with duration of chemotherapy or type of chemotherapy regimen was noted.
Postoperative complications, hepatic insufficiency, and mortality
All patients underwent resection without significant intraoperative events. The overall complication rate was 48.6% (55/107), and major complications were observed in 22.4% (24/107) of the cases. The postoperative hepatic insufficiency rate was 7.4% (8/107) and the rate of death from liver failure was 2.8% (3/107).
Accuracy of measured volume parameters for predicting postoperative hepatic insufficiency
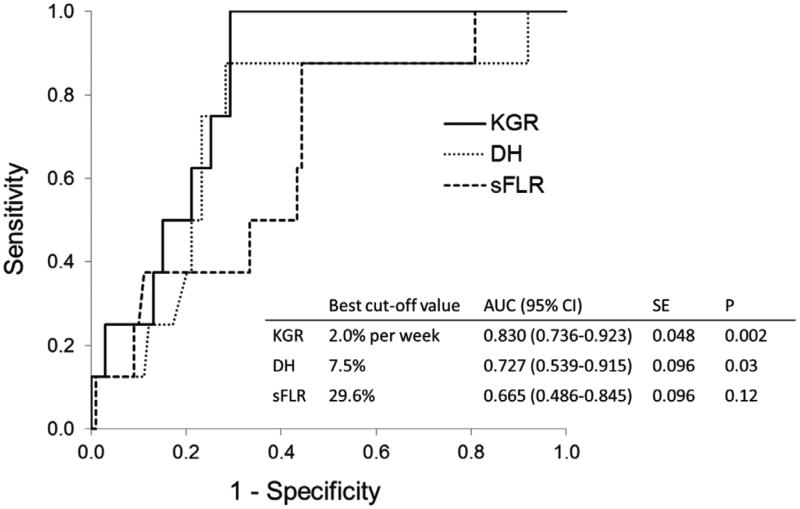
ROC analysis of the 3 tested variables revealed that KGR was the best predictor of postoperative hepatic insufficiency (AUC, 0.830; 95% CI, 0.736-0.923; asymptotic significance level, P = 0.002) (Figure 1). Based on the results of ROC analysis, the best cut-off values for sFLR volume, DH, and KGR to predict postoperative hepatic insufficiency were 29.6%, 7.5%, and 2.0% per week, respectively. In comparison of diagnostic power of these variables, KGR <2.0% per week showed the highest accuracy (81%) with sensitivity of 100% and specificity of 71% in predicting postoperative hepatic insufficiency (Table 4).
Figure 1.
Receiver operating characteristics curves for measured volume parameters in the prediction of postoperative hepatic insufficiency. sFLR, standardized future liver remnant; DH, degree of hypertrophy; and KGR, kinetic growth rate, defined as DH at first volume assessment after portal vein embolization (PVE) divided by weeks between PVE and first post-PVE volume assessment. AUC, area under the curve; CI, confidence interval; SE, standard error; p values represent asymptotic significance (null hypothesis, AUC = 0.500).
Table 4. Diagnostic Characteristics of Kinetic Growth Rate, Degree of Hypertrophy, and Standardized Future Liver Remnant Volume to Predict Postoperative Hepatic Insufficiency.
| KGR <2.0% per wk | DH <7.5% | sFLR <30% | |
|---|---|---|---|
| Sensitivity, n (%) | 8/8 (100) | 7/8 (88) | 7/8 (88) |
| Specificity, n (%) | 70/99 (71) | 71/99 (72) | 55/99 (56) |
| PPV, n (%) | 8/37 (22) | 7/35 (20) | 7/51 (14) |
| NPV, n (%) | 70/70 (100) | 71/72 (99) | 55/56 (98) |
| Accuracy, n (%) | 87/107 (81) | 78/107 (73) | 62/107 (58) |
| LR+ | 3.4 | 3.1 | 2.0 |
| LR− | 0.0 | 0.2 | 0.2 |
KGR, kinetic growth rate; DH, degree of hypertrophy; sFLR, standardized future liver remnant volume; PPV, positive predictive value; NPV, negative predictive value; LR+, likelihood ratio for positive test; LR−, likelihood ratio for negative test.
Accuracy of measured volume parameters for predicting postoperative morbidity and mortality
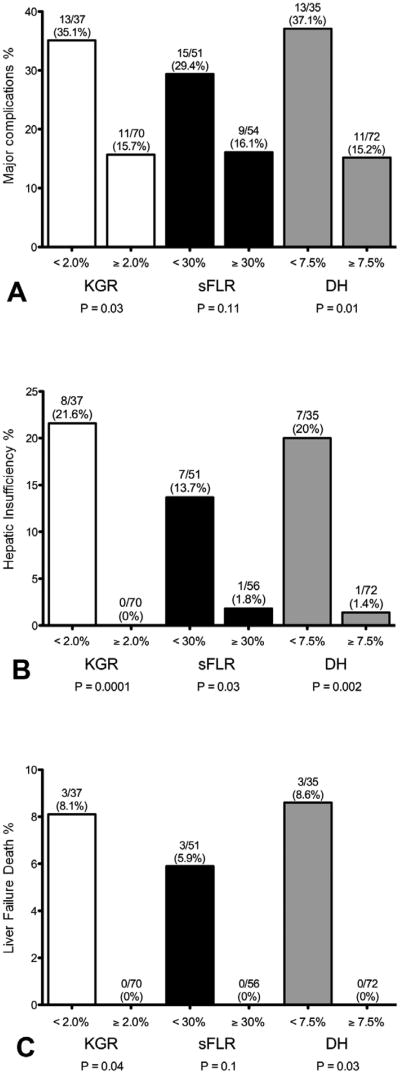
As shown in Figure 2, rates of major complications, hepatic insufficiency, and 90-day liver failure death were well predicted by use of cut-off values determined by the ROC analysis. Among patients with a KGR of at least 2.0% per week, there were no instances of postoperative hepatic insufficiency or liver failure death within 90 days. In contrast, among those patients with a KGR of less than 2.0% per week, rates of hepatic insufficiency and 90-day liver failure death were 21.6% and 8.1%, respectively.
Figure 2.
Rates of (A) major complications, (B) hepatic insufficiency, and (C) 90-day liver-related mortality based on best cut-off values determined with receiver operating characteristics analysis. DH, degree of hypertrophy; KGR, kinetic growth rate, defined as DH at first volume assessment after PVE divided by weeks between PVE and first post-PVE volume assessment; PVE, portal vein embolization; sFLR, standardized future liver remnant.
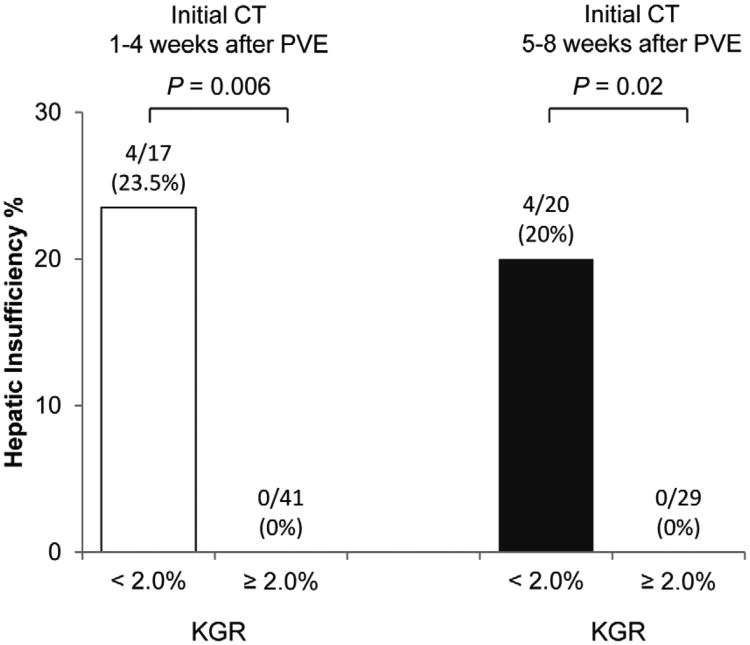
When the discriminatory power of KGR was compared between the patients who underwent initial CT assessment within 4 weeks after PVE and at 5-8 weeks after PVE, rates of postoperative hepatic insufficiency were 24% for KGR <2.0 % per week vs. 0% for KGR ≥2.0% per week (P = 0.006) in patients who underwent initial CT within 4 weeks after PVE, and 20% for KGR <2.0 % per week vs. 0% for KGR ≥2.0% per week (P = 0.02) in patients who underwent initial volume assessment at 5-8 weeks after PVE (Figure 3A).
Figure 3.
Rates of hepatic insufficiency according to kinetic growth rate by timing of (A) initial volume assessment and (B) standardized future liver remnant volume. KGR, kinetic growth rate; PVE, portal vein embolization; sFLR, standardized future liver remnant.
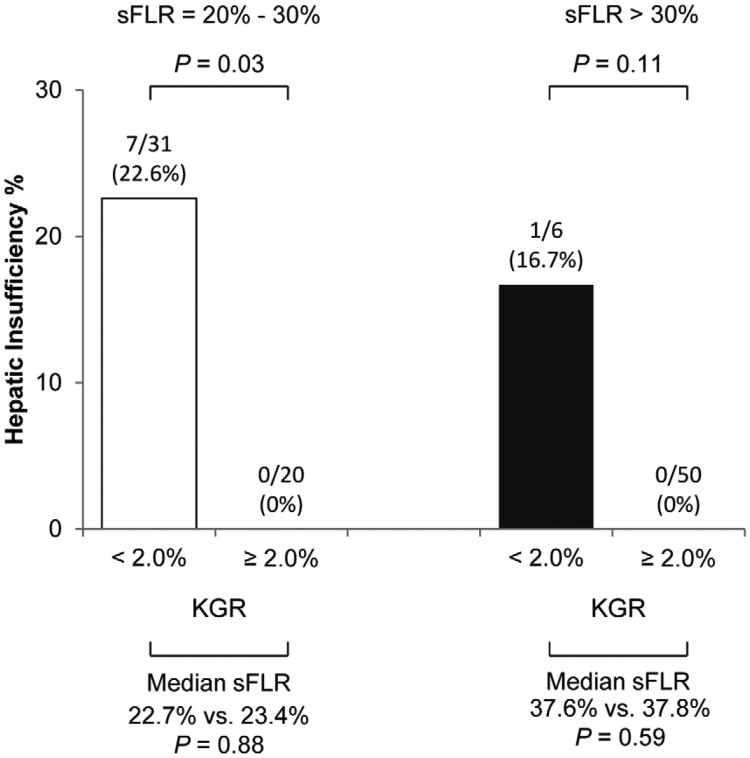
In addition, a subanalysis was performed in which the study population was stratified into 2 groups: patient with a sFLR volume of 20% to 30% and patients with sFLR volume greater than 30% (Figure 3B). In both groups, rates of hepatic insufficiency were higher among patients with a KGR less than 2.0% per week even after adjusting for similar sFLR volumes. This difference was markedly clinically significant, particularly in those patients with marginal sFLR volumes (i.e., 20% - 30%).
Discussion
In this study, we analyzed a novel dynamic measure for post-PVE FLR volume, the kinetic growth ratio (KGR). The analysis indicates that in patients undergoing major hepatectomy for colorectal liver metastases, KGR predicts postoperative hepatic insufficiency more effectively than the conventional measures, sFLR volume and DH. In addition, a KGR of at least 2.0% per week is protective of hepatic complications and liver failure related death. In patients without cirrhotic liver disease, hepatic insufficiency is a strong predictor of death from liver failure.[17, 18] The current study confirms previous studies indicating that approximately one third of patients with postoperative hepatic insufficiency eventually develop liver failure.[17, 18]
With the increasing frequency of complex and extensive procedures for patients pretreated with chemotherapy for colorectal liver metastases, the assessment of sFLR volume has become critical in determining which patients are most likely to benefit from PVE.[2, 3, 19-22] The trophic effects of PVE are specifically beneficial in patients with anticipated marginal sFLR volumes and those with liver injury due to modern systemic therapies.[11, 23, 24] Conventionally, outcomes of liver resection have been predicted mainly by static volumetric assessment of the preoperative sFLR volume.[6, 11, 18, 25, 26] We previously described the minimal necessary sFLR volume as greater than 20% for patients with normal livers, greater than 30% for patients with extensive preoperative chemotherapy or with histopathologic injuries, and greater than 40% for patients with cirrhosis.[4] Although these sFLR cutoffs are useful guide, they do not consistently predict surgical outcome.[27, 28]
For these reasons, we further evaluated the degree of hypertrophy (DH) and showed that a DH of greater than 5 percentage points after PVE along with an sFLR volume of greater than 20% predicted surgical outcomes with high specificity and sensitivity.[29] In this earlier work looking at the steady-state phase of liver growth after PVE, we found that hepatic dysfunction was more common in patients with a small sFLR volume, regardless of DH, and in those patients with a low DH, regardless of sFLR volume. These findings suggested that assessment of the unique patient-specific kinetics of post-PVE liver growth may contribute additional prognostic information beyond that provided by traditional volumetric evaluation in patients undergoing PVE before hepatic resection. The limitation of DH is that it only considers total remnant volume increase from PVE to final CT regardless of the time duration necessary to achieve this volume increase. Although typically most patients at our institution undergo post-PVE volumetry approximately 4 weeks after PVE, the time from PVE to initial post-PVE volume assessment varies among individual patients for a variety of reasons. Degree of hypertrophy alone provides no information about such specific time interval kinetics, and the KGR appears to provide a useful estimation of individual regeneration curves after PVE.
Previous studies indicated that the remnant liver continues to grow when followed over a long duration of time (months) with only a fraction (25%) of the total possible liver growth being achieved within 30 days post-PVE.[30] Therefore, it has been hypothesized that if inadequate growth occurs after the first post-PVE volumetry assessment, patients can be reassessed later and their perioperative morbidity would be equivalent if they eventually reach adequate remnant volume.[29] In the current study, however, the 10% of patients who had not achieved an adequate sFLR volume at the time of initial post-PVE volume assessment showed higher rates of hepatic insufficiency even after achieving our current criteria of sFLR >20% prior to surgery that was well predicted by KGR (Table 4 & Figure 4). Furthermore, KGR completely predicted both postoperative hepatic insufficiency and mortality from liver failure even when the currently accepted sFLR volume criteria failed to discriminate postoperative outcomes.
Figure 4.
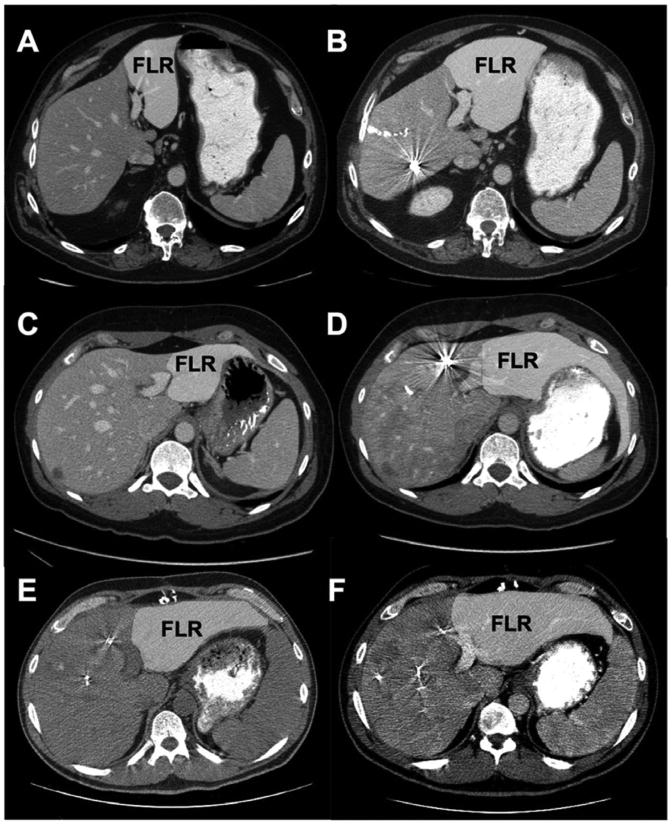
Examples of the clinical utility of kinetic growth rate (KGR). All patients had future liver remnant (FLR) volume ≥30% and degree of hypertrophy (DH) ≥7.5% (suggested eligibility criteria for resection); however, KGR was a more accurate predictor of outcome. (A and B) 60-year-old man. (A) On the basis of the initial CT scan, standardized FLR volume (sFLR) was estimated at 9%. (B) Final CT 35 days after right portal vein embolization (PVE) extended to segment IV indicated an sFLR volume of 33%, DH of 24%, and KGR of 4.8% per week. The patient had an uneventful postoperative course. (C and D) 37-year-old woman. (C) On the basis of the initial CT scan, sFLR volume was estimated at 15%. (D) Final CT 35 days after right PVE extended to segment IV indicated an sFLR volume of 30%, DH of 15%, and KGR of 3.0% per week. The patient had an uneventful postoperative course. (E and F) 43-year-old man. (E) On the basis of the initial CT scan, sFLR volume was estimated at 23%. (F) Final CT 70 days after right PVE extended to segment IV (required additional waiting time to attain adequate remnant volume) indicated an sFLR volume of 31%, a DH of 8%, and a KGR of 0.3% per week (determined after first CT 28 days after PVE). The patient died of postoperative liver failure.
The limitations of this study include its retrospective nature and selected population. However, the study is based on prospectively collected data, and the patients were treated using a similar approach with respect to PVE and sFLR volume.[4, 6, 12, 18] Another limitation is the timing of initial liver volume evaluation after PVE. Earlier studies reported that hypertrophy of the remnant liver follows a non-linear kinetic profile with rapid growth during the first 4 weeks. [29] However, the 2% cut off per week proposed in this study predicted postoperative hepatic insufficiency not only when measured within 4 weeks but also measured between 5-8 weeks after PVE. This suggests that KGR can be used as a reliable volumetric measure at least up to 8 weeks after PVE. Regarding the background factors affecting liver regeneration, the current study indicates that presence of diabetes or severe sinusoidal injury may be associated with impaired initial growth of the liver. However, because most patients included in received preoperative chemotherapy, additional studies investigating the effect of preoperative chemotherapy on liver regeneration are needed.
In conclusion, we present a novel dynamic measure for volume analysis after PVE that predicts surgical outcome. KGR is highly predictive of postoperative hepatic insufficiency in patients undergoing major liver resection for colorectal liver metastases. It provides additive information to the conventional static measure of post-PVE changes (sFLR volume), improving patient selection and outcomes.
Acknowledgments
The University of Texas MD Anderson Cancer Center is supported in part by the National Institutes of Health through Cancer Center Support Grant CA016672.
Footnotes
Disclosure Information: Nothing to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kopetz S, Chang GJ, Overman MJ, et al. Improved survival in metastatic colorectal cancer is associated with adoption of hepatic resection and improved chemotherapy. J Clin Oncol. 2009;27:3677–3683. doi: 10.1200/JCO.2008.20.5278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brouquet A, Abdalla EK, Kopetz S, et al. High survival rate after two-stage resection of advanced colorectal liver metastases: response-based selection and complete resection define outcome. J Clin Oncol. 2011;29:1083–1090. doi: 10.1200/JCO.2010.32.6132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brouquet A, Mortenson MM, Vauthey JN, et al. Surgical strategies for synchronous colorectal liver metastases in 156 consecutive patients: classic, combined or reverse strategy? J Am Coll Surg. 2010;210:934–941. doi: 10.1016/j.jamcollsurg.2010.02.039. [DOI] [PubMed] [Google Scholar]
- 4.Zorzi D, Laurent A, Pawlik TM, et al. Chemotherapy-associated hepatotoxicity and surgery for colorectal liver metastases. Br J Surg. 2007;94:274–286. doi: 10.1002/bjs.5719. [DOI] [PubMed] [Google Scholar]
- 5.Makuuchi M, Thai BL, Takayasu K, et al. Preoperative portal embolization to increase safety of major hepatectomy for hilar bile duct carcinoma: a preliminary report. Surgery. 1990;107:521–527. [PubMed] [Google Scholar]
- 6.Abdalla EK, Barnett CC, Doherty D, et al. Extended hepatectomy in patients with hepatobiliary malignancies with and without preoperative portal vein embolization. Arch Surg. 2002;137:675–680. doi: 10.1001/archsurg.137.6.675. [DOI] [PubMed] [Google Scholar]
- 7.Vauthey JN, Pawlik TM, Abdalla EK, et al. Is extended hepatectomy for hepatobiliary malignancy justified? Ann Surg. 2004;239:722–730. doi: 10.1097/01.sla.0000124385.83887.d5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kishi Y, Zorzi D, Contreras CM, et al. Extended preoperative chemotherapy does not improve pathologic response and increases postoperative liver insufficiency after hepatic resection for colorectal liver metastases. Ann Surg Oncol. 2010;17:2870–2876. doi: 10.1245/s10434-010-1166-1. [DOI] [PubMed] [Google Scholar]
- 9.Vauthey JN, Chaoui A, Do KA, et al. Standardized measurement of the future liver remnant prior to extended liver resection: methodology and clinical associations. Surgery. 2000;127:512–519. doi: 10.1067/msy.2000.105294. [DOI] [PubMed] [Google Scholar]
- 10.Vauthey JN, Abdalla EK, Doherty DA, et al. Body surface area and body weight predict total liver volume in Western adults. Liver Transpl. 2002;8:233–240. doi: 10.1053/jlts.2002.31654. [DOI] [PubMed] [Google Scholar]
- 11.Azoulay D, Castaing D, Krissat J, et al. Percutaneous portal vein embolization increases the feasibility and safety of major liver resection for hepatocellular carcinoma in injured liver. Ann Surg. 2000;232:665–672. doi: 10.1097/00000658-200011000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Madoff DC, Abdalla EK, Gupta S, et al. Transhepatic ipsilateral right portal vein embolization extended to segment IV: improving hypertrophy and resection outcomes with spherical particles and coils. J Vasc Interv Radiol. 2005;16:215–225. doi: 10.1097/01.RVI.0000147067.79223.85. [DOI] [PubMed] [Google Scholar]
- 13.Madoff DC, Hicks ME, Abdalla EK, et al. Portal vein embolization with polyvinyl alcohol particles and coils in preparation for major liver resection for hepatobiliary malignancy: safety and effectiveness--study in 26 patients. Radiology. 2003;227:251–260. doi: 10.1148/radiol.2271012010. [DOI] [PubMed] [Google Scholar]
- 14.Truty MJ, Vauthey JN. Uses and limitations of portal vein embolization for improving perioperative outcomes in hepatocellular carcinoma. Semin Oncol. 2010;37:102–109. doi: 10.1053/j.seminoncol.2010.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kishi Y, Madoff DC, Abdalla EK, et al. Is embolization of segment 4 portal veins before extended right hepatectomy justified? Surgery. 2008;144:744–751. doi: 10.1016/j.surg.2008.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–213. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mullen JT, Ribero D, Reddy SK, et al. Hepatic insufficiency and mortality in 1,059 noncirrhotic patients undergoing major hepatectomy. J Am Coll Surg. 2007;204:854–862. doi: 10.1016/j.jamcollsurg.2006.12.032. [DOI] [PubMed] [Google Scholar]
- 18.Kishi Y, Abdalla EK, Chun YS, et al. Three hundred and one consecutive extended right hepatectomies: evaluation of outcome based on systematic liver volumetry. Ann Surg. 2009;250:540–548. doi: 10.1097/SLA.0b013e3181b674df. [DOI] [PubMed] [Google Scholar]
- 19.de Jong MC, Pulitano C, Ribero D, et al. Rates and patterns of recurrence following curative intent surgery for colorectal liver metastasis: an international multi-institutional analysis of 1669 patients. Ann Surg. 2009;250:440–448. doi: 10.1097/SLA.0b013e3181b4539b. [DOI] [PubMed] [Google Scholar]
- 20.Jarnagin WR, Gonen M, Fong Y, et al. Improvement in perioperative outcome after hepatic resection: analysis of 1,803 consecutive cases over the past decade. Ann Surg. 2002;236:397–406. doi: 10.1097/01.SLA.0000029003.66466.B3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Palavecino M, Kishi Y, Chun YS, et al. Two-surgeon technique of parenchymal transection contributes to reduced transfusion rate in patients undergoing major hepatectomy: analysis of 1,557 consecutive liver resections. Surgery. 2010;147:40–48. doi: 10.1016/j.surg.2009.06.027. [DOI] [PubMed] [Google Scholar]
- 22.Shoup M, Gonen M, D'Angelica M, et al. Volumetric analysis predicts hepatic dysfunction in patients undergoing major liver resection. J Gastrointest Surg. 2003;7:325–330. doi: 10.1016/s1091-255x(02)00370-0. [DOI] [PubMed] [Google Scholar]
- 23.Elias D, Debaere T, Roche A, et al. Preoperative selective portal vein embolizations are an effective means of extending the indications of major hepatectomy in the normal and injured liver. Hepatogastroenterology. 1998;45:170–177. [PubMed] [Google Scholar]
- 24.Wakabayashi H, Ishimura K, Okano K, et al. Application of preoperative portal vein embolization before major hepatic resection in patients with normal or abnormal liver parenchyma. Surgery. 2002;131:26–33. doi: 10.1067/msy.2002.118259. [DOI] [PubMed] [Google Scholar]
- 25.Elias D, Ouellet JF, De Baere T, et al. Preoperative selective portal vein embolization before hepatectomy for liver metastases: long-term results and impact on survival. Surgery. 2002;131:294–299. doi: 10.1067/msy.2002.120234. [DOI] [PubMed] [Google Scholar]
- 26.Kubota K, Makuuchi M, Kusaka K, et al. Measurement of liver volume and hepatic functional reserve as a guide to decision-making in resectional surgery for hepatic tumors. Hepatology. 1997;26:1176–1181. doi: 10.1053/jhep.1997.v26.pm0009362359. [DOI] [PubMed] [Google Scholar]
- 27.Azoulay D, Castaing D, Smail A, et al. Resection of nonresectable liver metastases from colorectal cancer after percutaneous portal vein embolization. Ann Surg. 2000;231:480–486. doi: 10.1097/00000658-200004000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Clavien PA, Petrowsky H, DeOliveira ML, Graf R. Strategies for safer liver surgery and partial liver transplantation. N Engl J Med. 2007;356:1545–1559. doi: 10.1056/NEJMra065156. [DOI] [PubMed] [Google Scholar]
- 29.Ribero D, Abdalla EK, Madoff DC, et al. Portal vein embolization before major hepatectomy and its effects on regeneration, resectability and outcome. Br J Surg. 2007;94:1386–1394. doi: 10.1002/bjs.5836. [DOI] [PubMed] [Google Scholar]
- 30.Correa D, Schwartz L, Jarnagin WR, et al. Kinetics of liver volume changes in the first year after portal vein embolization. Arch Surg. 2010;145:351–354. doi: 10.1001/archsurg.2010.42. [DOI] [PubMed] [Google Scholar]
- 31.Kleiner DE, Brunt EM, Van Natta M, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313–1321. doi: 10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
- 32.Ishak K, Baptista A, Bianchi L, et al. Histological grading and staging of chronic hepatitis. J Hepatol. 1995;22:696–699. doi: 10.1016/0168-8278(95)80226-6. [DOI] [PubMed] [Google Scholar]
- 33.Rubbia-Brandt L, Audard V, Sartoretti P, et al. Severe hepatic sinusoidal obstruction associated with oxaliplatin-based chemotherapy in patients with metastatic colorectal cancer. Ann Oncol. 2004;15:460–466. doi: 10.1093/annonc/mdh095. [DOI] [PubMed] [Google Scholar]