Abstract
In Drosophila melanogaster, genes of the sex-determination hierarchy orchestrate the development and differentiation of sex-specific tissues, establishing sex-specific physiology and neural circuitry. One of these sex-determination genes, fruitless (fru), plays a key role in the formation of neural circuits underlying Drosophila male courtship behavior. Conservation of fru gene structure and sex-specific expression has been found in several insect orders, though it is still to be determined whether a male courtship role for the gene is employed in these species due to the lack of mutants and homologous experimental evidence. We have isolated the fru ortholog (Md-fru) from the common housefly, Musca domestica, and show the gene’s conserved genomic structure. We demonstrate that male-specific Md-fru transcripts arise by conserved mechanisms of sex-specific splicing. Here we show that Md-fru, is similarly involved in controlling male courtship behavior. A male courtship behavioral function for Md-fru was revealed by the behavioral and neuroanatomical analyses of a hypomorphic allele, Md-traman, which specifically disrupted the expression of Md-fru in males, leading to severely impaired male courtship behavior. In line with a role in nervous system development, we found that expression of Md-fru was confined to neural tissues in the brain, most prominently in optic neuropil and in peripheral sensory organs. We propose that, like in Drosophila, overt sexual differentiation of the housefly depends on a sex-determining pathway that bifurcates downstream of the Md-tra gene to coordinate dimorphic development of non-neuronal tissues mediated by Md-dsx with that of neuronal tissues largely mediated by Md-fru.
Introduction
The genetic pathway controlling sex-specific behaviors in the invertebrate model system Drosophila melanogaster has been elucidated in great detail [1], [2]. These studies revealed that the genetic control of male mating behavior in Drosophila melanogaster is largely governed by the transcription factor fruitless (fru) [3], [4]. This gene acts as a pivotal switch for the correct developmental implementation of male-specific behaviors. A specific class of fru alleles have been identified which impairs courtship performance of males, but leaves females unaffected [5], [6], [7]. Moreover, it was demonstrated that expression of the male-specific fru functions in genotypically female flies elicits some aspects of male courtship behavior [8], [9], [10], [11]. However, the conclusion that fru is not only necessary but also sufficient for implementing male mating behavior in Drosophila has been challenged in recent studies [12], [13], [14], [15], [16]. These studies suggest that the sex-determining double-switch gene doublesex (dsx) also contributes to the regulation of male mating behavior.
The fru gene in Drosophila melanogaster is very large in size spanning approximately 150 kb of genomic sequences. Transcription initiates from four independent promoters (P1–P4) and the resulting transcripts undergo a complex pattern of alternative splicing events at both the 5′ and the 3′ ends, generating 18 different transcript variants which code for a family of transcription factors containing a common BTB domain and one of four alternative zinc-finger DNA binding domains [17]. The distal most promoter P1 gives rise to transcripts, which are implicated in regulating sex-specific behaviors [3], [4]. Transcripts derived from the other three promoters are non sex-specific and have vital developmental functions in both males and females [18]. Sex-specific expression is achieved by alternative splicing at the 5′ end of P1 transcripts mediated by the splice regulator transformer (tra), a key component of the sex-determining pathway. In females, TRA protein forms a splice enhancer complex with TRA2 and RBP1 (RNA Binding Protein 1) binding to nascent fru RNAs where they act to alter splice site choice, resulting in the inclusion of a pre-mature stop codon signal and thereby generating female-specific transcripts (fruF) with a severely truncated ORF [3], [19]. In males, where the tra gene is functionally OFF, the default splice-site is used and the resulting male-specific transcripts (fruM) encode functional full-length BTB-Zn-finger type transcription factors. These sex-specific transcripts incorporate alternate 3′ exons A, B, or C to encode the FruM isoforms: FruMA, FruMB and FruMC. FruM isoforms are translated only in males and are expressed in all regions of the nervous system implicated in male courtship behavior [12], [20], [21], [22], [23]. These FRUM positive neurons located in widely distributed clusters throughout the male brain and nerve chord are key parts of the neuronal circuitry required for behavioral responses to sex pheromones and the display of proper male courtship behavior.
Given the critical role of fru in controlling sex-specific behaviors the question arises whether this function is conserved in other insects. The existence of sex-specifically expressed fru homologs in various dipteran and in hymenopteran species (Anopheles gambiae [24], Aedes aegypti [25], Ceratitis capitata [26] and Nasonia vitripennis [27]) suggests that fru has a conserved male-specific function in holometabolous insects. However, functional studies have yet to establish a definitive role in male courtship behavior in these species.
In our study we used the common housefly, Musca domestica, as an experimental system to investigate the genetic control underlying male courtship behavior. In previous studies we showed that all aspects of sexually dimorphic development including behavior are governed by a key switch gene, previously referred to as F and more recently identified as an ortholog of the transformer gene, Md-tra [28], [29]. A male-determining M-factor, which is located on the Y chromosome in standard strains but can also be found on autosomes in natural populations, serves as the primary instructive signal [29]. When M is absent, zygotic Md-tra is spliced in a mode that generates transcripts with an intact ORF. These Md-tra messages code for a RS-type of splicing regulator, which through an autocatalytic function upholds the productive splicing of its own pre-mRNA. Maternally deployed Md-tra activity is required to engage this positive feedback loop in the early zygote [28]. Presence of M in the zygote invokes a change in splicing of Md-tra, which results in the production of messages with a truncated ORF. Likewise, a previously identified target of Md-tra, the Musca doublesex homologue, Md-dsx, is regulated by sex-specific processing of its pre-mRNA [30]. Md-TRA together with its co-factor Md-TRA2 directs the female-specific splice mode of Md-dsx generating a female-specific variant which instructs female differentiation [31]. In M containing individuals, where Md-tra remains OFF, the male-specific splice mode of Md-dsx is established by default and a male-specific isoform is generated thereby ensuring male development.
Here we report the identification of the housefly ortholog of fruitless, Md-fru, and show that this gene is a downstream target of Md-tra. In an analogous way to Drosophila, Md-fru expresses functional splice variants only in males where Md-tra is OFF. Residual levels of Md-tra activity in males homozygous for the hypomorphic Md-traman allele, prevents expression of functional Md-fru products leading to sterility. Our findings suggest that the correct implementation of male mating behavior is governed by a specific branch of the sex-determining pathway mediated by fru in both Musca and Drosophila.
Results
Md-fru is Sex-specifically Regulated and a Target of Md-tra
In Drosophila, the pathway downstream of tra bifurcates and employs the gene fruitless (fru) to control various aspects of sexually dimorphic CNS development [18], [32]. To establish if Md-tra also regulates housefly courtship through a fru-like downstream effector we set out to isolate the fru ortholog in Musca. PCR amplification with degenerate primers flanking the conserved BTB domain of fru yielded a genomic fragment of 190 bp in length. Subsequent extension by 3′ RACE of cDNAs prepared from adult houseflies revealed the existence of four alternative mRNA transcripts (Fig. 1). All 4 variants were present in female and male cDNA preparations and each encoded a different zinc finger type motif at its carboxy-terminal end. The same complex pattern of 3′ end processing has been reported in fru orthologs of Drosophila melanogaster, Anopheles gambiae, Aedes aegypti, Nasonia vitripennis, and Tribolium castaneum, [24], [25], [27]; Nicole Meier and Daniel Bopp, unpublished results). Sequence alignments of the differentially processed exons showed a high degree of similarity to the corresponding Drosophila exons (Fig. S1), so the genomic order of the differentially spliced 3′ exons is additionally conserved.
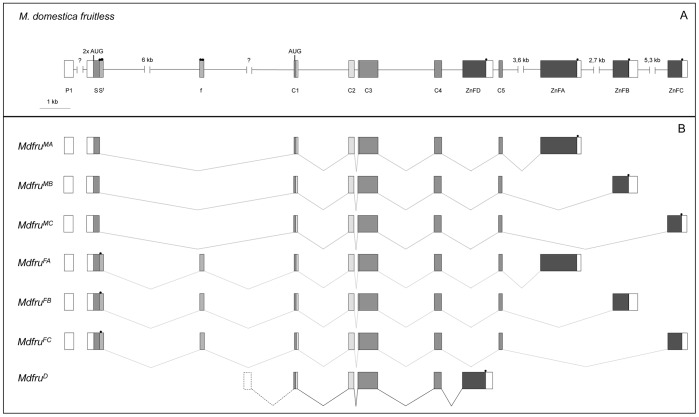
Figure 1. Genomic organisation and multiple transcript variants of Md-fru.
(A) Schematic drawing of the Md-fru locus. Coding exons are indicated as grey shaded boxes, while 5′ and 3′ UTRs are shown as white boxes. Sequences in C1, C2 and C3 exons encoding the BTB domain are labelled in light grey and exons encoding the zinc finger-like domains (ZnFA, ZnFB, ZnFC, ZnFD) are shown in dark grey. P1 labels the most distally identified exon followed by the common exon S, and the two female-specific exons Sf and f. Small black dots mark the positions of in-frame stop codons (two in each female-specific exon, one in each zinc finger encoding exon). Two potential translational start codons are present in the exon S, and one in exon C1. (B) Multiple transcript variants identified by RT-PCR analysis in male and female head RNA samples. In male samples, three variants were detected (Md-fruMA Md-fruMB and Md-fruMC) each of which includes a different zinc finger exon. None of them include the female-specific exons Sf and f and. The splice variants Md-fruFA Md-fruFB and Md-fruFC were only detected in female head samples, all included the female-specific exons Sf and/or f which introduce several in-frame translational stop codons that prematurely terminate the ORF. In addition, we identified a nonsex-specific transcript variant (Md-fruD) which incorporates exon ZnFD but does not originate from P1. Preliminary results from 5′ RACE studies indicate that the 5′ end of this transcript is located in the intron sequences between exons f and C1 and thus may derive from a different promoter.
In Drosophila, the genes acting immediately before fru in the sex determination hierarchy direct sex-specific splicing of transcripts derived from the most distally located P1 fru promoter [3], [19]. To examine the corresponding upstream sequences of the putative housefly fru ortholog, 5′ RACE was performed on cDNA templates prepared from RNA extracted from adult male and female heads. In male preparations, we detected transcripts that contained two additional exons (P1 and S) upstream of the BTB coding exons (C1–C3) extending the ORF by an additional 72 amino acids at the amino terminal end (Figs. 1, S1). In female transcripts we observed that exon S was significantly larger in size due to the utilization of a more downstream located splice donor site (Sf). In addition, we identified a female-specific exon (f) downstream of exon Sf (Fig. 1). These additional exonic sequences, which are absent in male transcripts, contain several in-frame stop codons, which prematurely truncate the long ORF as defined by the male transcripts (dots in Fig. 1A). Four repeat elements were identified in these female-specific sequences that perfectly match the cis-regulatory elements (TRA/TRA2 binding sites) known to play an important role in sex-specific splicing of Drosophila fru (Fig. 2). The male-specific transcripts encode full-length products, which show a high degree of protein sequence similarity to those of P1-derived male transcripts in Drosophila and are therefore referred to as Md-fruM (GenBank Accession Number KC750909, KC750911 and KC750913). The female-specific transcripts, on the other hand, have a severely truncated ORF and thus are likely to represent non-functional splice variants of the same locus. Because of their structural correspondence to the non-functional products of P1-derived female transcripts in Drosophila they are collectively named Md-fruF (GenBank Accession Number KC750910, KC750912 and KC750914). Taken together we conclude that the Musca genome harbors an ortholog of fru, Md-fru, which expresses a set of male-specific products and hence is likely to be a target of the sex determining pathway.
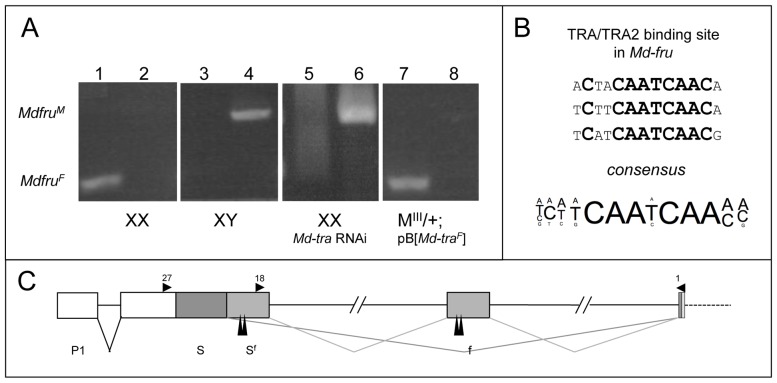
Figure 2. Sex-specific splicing of Md-fru is mediated by Md-tra.
(A) RT-PCR results with sex-specific sets of Md-fru primer pairs. Amplification with Md-fruF specific primers Md-fru-18 and Md-fru-1 in wildtype XX females (1), in wildtype XY males (3), in Md-tra silenced XX females (5) and in ectopically Md-traF expressing MIII males (7). Amplification with Md-fruM specific primers Md-fru-27 and Md-fru-1 in wildtype XX females (2), in wildtype XY males (4), in Md-tra silenced XX females (6) and in ectopically Md-traF expressing MIII males (8). In wildtype XX flies, where Md-tra is active, only Md-fruF but no Md-fruM transcripts can be detected (1 and 2). A clear shift to Md-fruM splicing can be observed in XX individuals when Md-tra is silenced (5 and 6). Likewise, only Md-fruM transcripts are present in wildtype XY males, where Md-tra is not active (3 and 4). Md-fru splicing shifts to the female mode in MIII males when continuous Md-traF activity is provided by a transgene [28] (7+8). (B) Direct splicing regulation by Md-tra is suggested by the presence of putative TRA/TRA2 binding sites in Md-fru. Comparison of three different sequences found in Md-fru with the TRA/TRA2 binding site consensus derived from sequence alignments of binding sites found in the fru and dsx orthologs of Drosophila melanogaster, Musca, domestica and Anopheles gambiae. (C) Putative TRA/TRA2 binding sites (long vertical arrowheads) are found in close vicinity to the regulated splicing sites in the Md-fru locus. Positions of the primers used in this expression analysis are indicated by small horizontal arrowheads.
The presence of conserved TRA/TRA2 binding sites in Md-fru, suggested that it is sex-specifically spliced and directly targeted like Md-dsx, by Md-tra. To explore this possibility, we tested expression of Md-fru in different genetic backgrounds in which Md-tra activity was experimentally altered (Fig. 2). Previous studies demonstrated that silencing by early dsRNA injections is an effective means to deplete Mdtra activity in XX embryos, resulting in a complete shift of Md-dsx splicing from female to male mode [28]. Likewise, we observed that the splicing mode of Md-fru changed from female to male in Md-tra silenced XX individuals (Fig. 2A). The same result was obtained when Md-tra2, an essential co-factor of Md-tra, was depleted by RNAi (data not shown). We conclude that female splicing of Md-fru depends on the presence of Md-tra and Md-tra2 activity supporting our notion that Md-fru is a downstream target of Md-tra in the sex determination pathway. To extend this type of epistatic analysis we investigated whether ectopic expression of the female activity of Md-tra is sufficient to direct female-specific splicing of Md-fru in genotypic males. To this end, we used a transgenic line in which Md-traF is constitutively expressed in flies carrying the male determining factor MIII on the third chromosome [28]. These individuals develop into fully fertile females demonstrating that forced Md-traF expression overrides repression by MIII and is sufficient to direct all aspects of female development. RNA samples analyzed from the heads of these sex-reverted MIII/+; p[Md-traF] individuals showed the presence of only female-specific splice variants of Md-fru (Fig. 2A). Hence, Md-tra activity is not only required but also sufficient to impose female splicing mode of Md-fru. The presence of well-conserved TRA/TRA2 binding sites around the critically involved splice sites suggested that Md-fru is a direct target of Md-tra (Fig. 2B,C).
Md-traman Males Express Female Md-fru Transcripts and are Impaired in Courtship
We previously described the masculinizer (man) allele of Md-tra to be a hypomorphic mutation with residual Md-tra activity [28], [33]. Flies homozygous for Md-traman develop into phenotypically normal looking males (from here on referred to as Md-traman1 males). However, they are reduced in fertility, as only a fraction (about 10%) produces progeny in single mating tests. Sterility is 100% in homozygous Md-traman males when their mother carried the dominant gain-of-function allele Md-traD in trans [28], [34], [35]. Males with this maternal background were referred to as Md-traman2 males. The genital apparatus of Md-traman1 and Md-traman2 males looked indistinguishable in morphology from those of wildtype males, and normal amounts of motile sperm were present in squashed preparations of mutant testes. To test for gonad and sperm functionality, the larval anlagen of Md-traman2 testes were transplanted into wildtype male hosts (Fig. S2). As these mosaic animals were capable of siring a large number of donor-derived offspring, we concluded that sperm maturation was normal in Md-traman2 mutant testes.
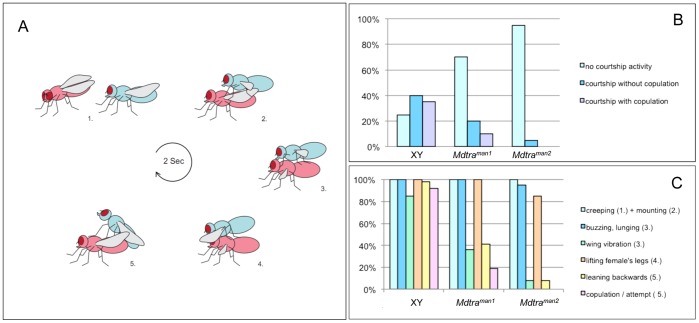
Mutations affecting neuronal development can disturb behavioral outputs such as male courtship and, thus, can lead to sterility. Therefore, we decided to test courtship performance of Md-traman1 and Md-traman2 males with wild-type females. Meffert [36] described courtship in the housefly as a very brief and stereotypical ritual (see Fig. 3A). Behavioral analysis of Md-traman mutant males showed that their overall levels of courtship were substantially lower than those of wild-type XY males (30% of Md-traman1 and 5% of Md-traman2 males compared to 80% in wild-type males) (Fig 3B). In single courtship assays, only half of the Md-traman1 males attempted copulation, while no copulation attempts were observed when Md-traman2 males were tested. In contrast, 80% of courting wild-type males attempted copulation and almost 50% successfully copulated during the 20 minutes observation period (Fig. 3B). Of the mutant males which did engage in courting, courtship latency was comparable to that of wild-type males, but copulation duration was on average three times longer than wild-type males (Table S1). To assess whether specific parts of the mating ritual were affected, we performed a second set of courtship assays using the courting scheme described by Meffert and Hagenbuch [37]. Md-traman1 and Md-traman2 males which engaged in courting performed two of the last three steps with substantially reduced frequencies, in contrast to control wild-type males which performed each step of the sequence at comparable rates (Fig. 3C and Table S2). In particular the latter steps involving wing vibrations and backward leaning of the males on top of the females are severely affected in Md-traman males. Premature termination of the courting ritual was more often observed in Md-traman2 males than in Md-traman1 males of which 19% performed the last steps and even attempted copulation. These data suggest that the infertility observed in homozygous Md-traman males was due to failure to properly complete the sequence of courting steps prior to copulation.
Figure 3. Impaired courtship performance of Md-traman males.
(A) Sketch of the housefly courtship ritual as described by [36]: (1) The male fly (blue) approaches the female (pink) and performs an abortive form of walking and stalking towards the female. The male fly will interdigitate his legs with those of the female while making tapping movements. The female may respond with similar fencing. (2) Next the male will mount the female and flutter his wings. (3) While he is lunging forward to the head of the female he will continue to flutter. He will then lift the female’s forelegs. At this point, he holds his wings in a position that is roughly 180° from their resting orientations. (4) When mounted, the female thrusts her wings 90° to her body and perpendicular to the substrate. (5) Finally, the male will lean backward and attempt to copulate. The whole ritual lasts around 2 seconds and will be repeated by the male when the first attempt fails. (B) Courtship activity of standard wild-type XY males, Md-traman1 males, and Md-traman2 males in single mating assays with wild-type females; percentage of males which courted at least once during a 20 minutes observation period (total number of males per genotype observed n = 20). A marked reduction of courting activity is observed for Md-traman1 males and almost no activity is seen for Md-traman2 males. (C) Percentage of males performing individual steps of the courtship ritual (total number of courting males per genotype observed n = 19). While the percentage of wildtype XY males initiating courtship remains equally high throughout the various steps of the ritual, the percentage of Md-traman males engaged in courtship declines precipitously during the last steps, in particular step 3 (fluttering) and step 5 (copulation attempt).
We hypothesized that residual feminizing activity of the Md-traman allele caused male courtship failure. We tested this hypothesis by injecting dsRNA of Md-tra into syncytial Md-traman2 embryos. Silencing of Md-tra at this early stage leads to a complete and lasting loss of Md-tra activity [28]. Of 52 injected Md-traman2 males, 41 were fertile in single mating tests and, due to the absence of an M factor, produced only female offspring when outcrossed with wild-type females. The percentage of successful matings (about 80%) was comparable to those of wild-type control males in single crosses. In contrast, all 20 Md-traman2 males injected with Ringer’s buffer remained infertile. Hence, the sterility of Md-traman2 homozygous males can be rescued by targeted silencing of Md-tra. In a previous study we showed that silencing of Md-tra2, a required co-regulator for female expression of Md-tra, could also completely restore fertility of Md-traman2 mutant males [28], [31]. These results suggested that the residual feminizing activity of Md-traman is sufficient to cause male courtship failure.
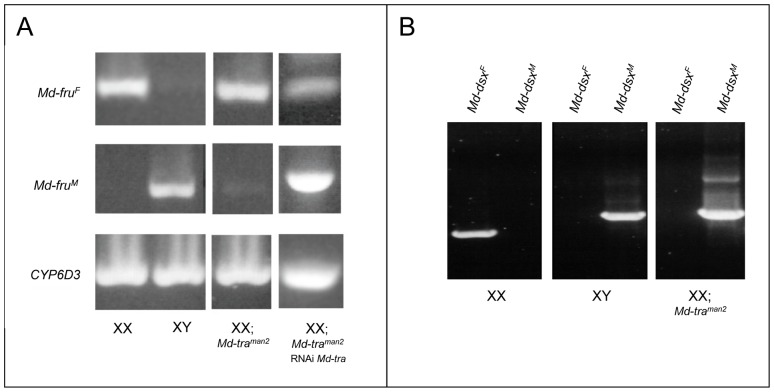
This result prompted us to test whether this phenotype may be caused by misregulation of the newly identified Md-tra target Md-fru. The sex-specific splicing of Md-fru was investigated in Md-traman2 males. RT-PCR experiments on RNA extracted from Md-traman2 male heads detected only non-functional Md-fruF transcripts (Fig. 4A), suggesting residual Md-traF activity in Md-traman males is sufficient to impose female-specific splicing of Md-fru. In contrast, the previously described target of Md-tra, Md-dsx, seems to be unaffected as only male products were detected in Md-traman2 males (Fig. 4B). It seems likely that, in Md-traman2 males, lack of functional Md-fruM products is the primary cause of impaired courtship performance. If normal male courtship indeed depends on presence of Md-fruM we expected that male splicing of Md-fru must be restored in rescued Md-traman2 males. Consistent with this interpretation, we detected substantial levels of Md-fruM transcripts in rescued males (Fig. 4A) indicating a shift in the splicing mode from female back to male. We conclude that normal male behavior correlates with presence of functional Md-fruM transcripts suggesting that this gene plays a crucial role in the correct implementation of male behavior in the housefly.
Figure 4. Md-fru but not Md-dsx is spliced in the female mode in Md-traman2 males.
(A) Md-fru splicing: sex-specific primer pairs were used to detect Md-fruF transcripts (Md-fru-18 and Md-fru-1) and Md-fruM transcripts (Md-fru-27 and Md-fru-1) in wildtype XX females, in XY males, in XX; Md-traman2 males and in XX; Md-traman2 males in which Md-tra was silenced by embryonic dsRNA injections (RNAi Md-tra). As an internal control CYP6D3 transcripts were amplified from the same RNA samples. In XX; Md-traman2 males the splicing mode of Md-fru P1 transcripts is predominantly female whereas after silencing of Md-tra it shifts back into the functional male mode. (B) Md-dsx splicing: sex-specific primer pairs were used to detect Md-dsxF transcripts (Md-dsx-6S and Md-dsx-11AS) and Md-dsxM transcripts (Md-dsx-6S and Md-dsx-46AS) in wildtype XX females, in XY males and in XX; Md-traman2 males. Primer sequences are published in [28]. In contrast to Md-fru, Md-dsx is almost exclusively spliced in the male mode in Md-traman2 males.
Md-fru is Expressed in the CNS and Peripheral Sensory Neurons
In Drosophila sex-specific fru expression is confined to neuronal tissues [12], [20], [21], [22], [23]. To investigate the spatial expression pattern of Md-fru in houseflies we performed in situ hybridization assays. Whole mount preparations of third instar larval tissues were initially investigated. We detected ubiquitous expression of Md-fru P1 transcripts in both larval brain hemispheres and in the ventral ganglion. Staining was neither observed in imaginal discs nor in other non-neural tissues (data not shown). If Md-fru were indeed part of a developmental program involved in establishing the neuronal circuitry involved in courtship behavior, we would expect a locally restricted expression pattern in the adult CNS similar to that reported in Drosophila. RNA in situ hybridizations were performed on cryosections of adult female and male housefly heads with an antisense probe against 5′ sequences (P1-derived) of Md-fru (Fig. 5). Strong ubiquitous staining was detected in areas which process visual information, namely in and around the four optic neuropils lamina, medulla, lobula and lobula plate (Fig. 5A-C). Prominent fru expression has also been detected in the visual systems of D. suzukii and D. virilis, in particularly in the lamina, but is completely absent in D. melanogaster [38]. Overall, P1-specific transcripts of Md-fru appeared to be more broadly expressed in the mid brain than the corresponding P1 transcripts in D. melanogaster (Ryner et al., 1996). The Md-fru probe also labeled neurons located in the base of taste sensilla of the labellum (Fig. 5D–F). We propose these neurons belong to the gustatory system, as corresponding gustatory receptor neurons in Drosophila are known to express FRUM [22].
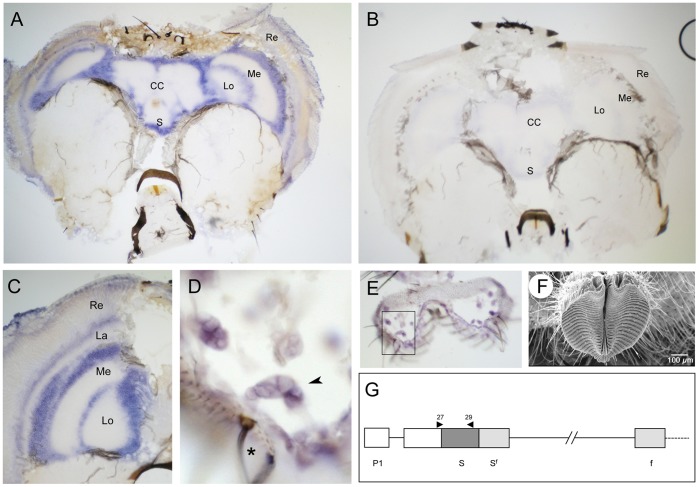
Figure 5. Md-fru P1 transcripts are expressed in the CNS and in peripheral sensory organs.
(A) Frontal section of a male head hybridised with Md-fru P1-specific antisense RNA probe. Strong ubiquitous staining is observed in the layers below the retina and around the optic lobes (Re: Retina, La: Lamina, Me: Medulla, Lo; Lobula), as wells as around the central complex (CC) and the subesophageal ganglion (S). (B) Frontal section of a male head hybridised with the Md-fru P1 sense RNA probe. (C) Higher magnification of the stained areas in the optic lobes. (D) Close-up of basal neurons (arrowhead) connected to a sensory bristle (black star) located in the labellum and expressing Md-fru P1 transcripts. (E) Overview of the sectioned labellum shown in D (boxed). (F) SEM imaging of the fly’s mouthpart (labellum) and surrounding sensory bristles. (G). Primers used to prepare templates for P1-specific sense and antisense RNA probes are indicated as black triangles (Md-fru-27 and Md-fru-29).
Within the sexually dimorphic neural circuitry identified in the CNS of Drosophila melanogaster is a cluster of 10 serotonergic neurons located in the posterior dorsal part of the abdominal ganglion which are present in males but not in females. These SAbg neurons send their projections to the main trunk that innervates the male internal reproductive organs such as the testicular ducts, seminal vesicles, accessory glands, and ejaculatory duct [39]. In fru mutant males these SAbg clusters are substantially diminished or completely absent suggesting that FRUM is responsible for the formation and/or neurotransmitter specification of these neurons [12]. This well-documented example of fru function in the male CNS led us to investigate whether a similar neuroanatomical dimorphism exists in the housefly. We performed anti-5HT (serotonin) antibody staining on whole mount preparations of adult male and female CNS ventral ganglions (Fig. 6). As in Drosophila, we identified a small cluster of serotonergic neurons which are present at the posterior end of the abdominal ganglion in male flies, but absent in the corresponding region of the female ganglion (Fig. 6B–C). Also, projections of these serotonergic neurons are sent to the main trunk and innervate male internal reproductive organs (Figure 6E,F). Significantly, this cluster of serotonergic neurons and its projections to the male internal reproductive organs were absent in abdominal ganglions dissected from behaviourally sterile Md-traman2 mutant males (Fig. 6D,G). There was a complete lack of serotonin immunoreactivity in neurons innervating the male reproductive organs in 75% of Md-traman2 mutants (n = 12), compared with 100% immunoreactivity in wild-type controls and in Md-traman1 (n = 10 each). The loss of serotonin staining of this cluster correlates with the lack of Md-fruM expression suggesting that, just as Drosophila, Md-FRUM plays a key role in the specification of these male serotonergic cells [13].
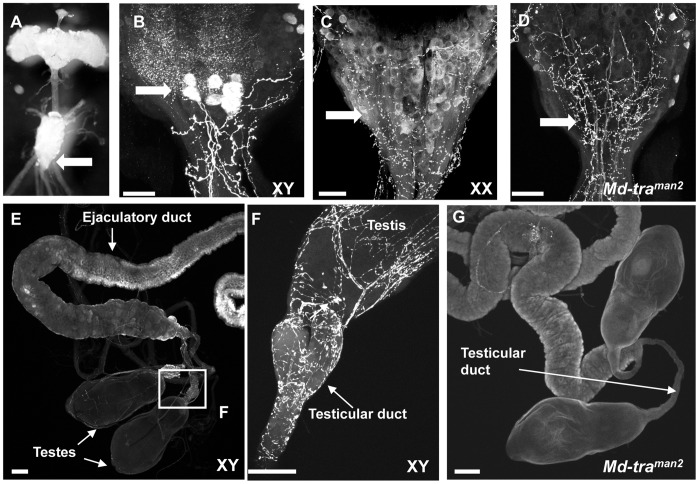
Figure 6. Male-specific serotonergic cluster in the ventral abdominal ganglion is absent in Md-traman2 males.
(A) Whole mount CNS of an adult housefly visualized by light stereomicroscopy. The white arrow indicates the abdominal ganglion, where the SAbg cluster shown in the next panels is located. (B) Abdominal ganglion of a wild type male (XY) stained with an anti 5-HT (serotonin) antibody. The immunoreactive cell bodies of the SAbg serotonergic neurons in this region are indicated by a white arrow. (C) Abdominal ganglion of a wildtype female (XX) stained with anti 5-HT antibody. Arrow points to the corresponding area where SAbg neurons are located in males (B). (D) Abdominal ganglion of a behaviourally sterile Mdtraman2 male. In contrast to wildtype males (B), there is no serotonin immunoreactivity in the corresponding area of the abdominal ganglion (white arrow). (E) Internal male reproductive organs are innervated by serotonergic projections. The white box indicates area of the testicular duct magnified in (F) showing serotonin immunoreactive varicosities. (G) Internal male reproductive organs of Mdtraman2 do not show serotonin immunoreactivity. Images B-G are maximum projection of a confocal stack. Scale bars: 50 µm.
Discussion
Male Mating Behavior in Houseflies is Controlled by Md-tra
We previously reported that the gene Md-tra acts as the key switch in the sex determining hierarchy of the house fly governing every aspect of somatic dimorphic development [28]. In this study we show that courtship of male houseflies is an innate behavior that is also under the control of Md-tra. Our analysis of Mdtraman males revealed that infertility is primarily a result of anomalous behavior rather than of aberrant differentiation of reproductive tissues. We propose that the primary cause for anomalous courtship behavior in mutant males is the presence of female Md-tra activity. When Md-tra is knock downed by RNAi, fertility of these males is restored suggesting the absence of Md-tra activity is a pre-requisite for proper implementation of male courtship behavior. In females, on the other hand, Md-tra is activated in order to prevent the establishment of these behaviors. We propose that Md-tra acts as an upstream switch, coordinating dimorphic differentiation of both morphological and behavioral traits. The central role of tra in coordinating these two facets of dimorphic development has been extensively investigated in Drosophila. For instance, genotypically female XX animals which are mutant for tra develop into morphologically normal males which behave and court like males [40]. Reciprocally, feminization of XY individuals by activating tra results in the development of pseudofemales, which do not display male behaviors. Morphological and behavioral dimorphic development, however, differ with regard to the spatial and temporal requirements of tra. While tra’s functions in somatic differentiation are needed as early as during embryonic development (e.g., gonad differentiation), the critical developmental window for the control of sex-specific behaviors was mapped to much later stages of development around larval-pupal transition [41]. When tra is activated at these stages in genotypically male individuals, they will develop into normal looking males however courtship behavior is severely impaired. It suffices to target expression of tra to specific areas of the brain to accomplish a complete loss of courtship behavior in otherwise normally developed males [42]. This phenotype is reminiscent of the situation in Md-traman males suggesting that residual Md-tra activity in these males is primarily affecting late events in dimorphic development in particular those during the critical stages when male courtship behavior is established. As feminization is only observed at the behavior level this suggests that the female activity provided by the Md-traman alleles is either below a threshold needed to differentiate female structures or is confined to neural tissues where it specifically affects CNS related aspects of dimorphic development.
Md-fru is a Target of the Sex-determining Pathway Downstream of Md-tra
In this study we have identified the house fly homologue of the key Drosophila sex-determination gene fru, Md-fru. We have shown that, in an analogous manner to Drosophila, Md-fru RNAs are alternatively spliced leading to male-specific expression of Md-FRUM proteins from the P1 promoter. The presence of four putative TRA/TRA2 bindings sites located close to the regulated splice sites of the female-specific exons of Md-fru suggests that this regulation is due to its targeting by the female-specific splicing factor Md-tra. This is similar to the regulation of the previously proposed targets of Md-tra, Md-dsx and Md-tra itself [28], [30]. We show that changes in Md-tra activity lead to corresponding changes in Md-fru expression. For instance, ablating Md-tra function by RNAi in karyotypically female individuals shifts the mode of Md-fru splicing from female to male. Reciprocally, when Md-tra functions are ectopically expressed in karyotypic males, Md-fru expression switches to the female nonfunctional mode. We additionally show that Md-tra2, a functional splicing co-factor with tra in Drosophila, is involved in Md-fru splicing regulation, by silencing Md-tra2 in Md-traman2 males and showing a shift of Md-fru splicing from the female to the male mode (data not shown) [31].
Taken together we propose that, like Md-dsx and Md-tra, Md-fru is another downstream and possibly direct target of Md-tra in the house fly sex determination pathway. In D. melanogaster, male-specific FRUM products are required for the proper display of male courtship behavior. When tra is ectopically activated in the developing male CNS, it prevents the production of FRUM and, as a consequence, these males exhibit impaired and anomalous courtship [3], [43], [44]. Along the same lines, we propose that Md-fru is a target of Md-tra and that misregulation of Md-fru by residual Md-tra activity is the primary cause for courtship failure of Md-traman males. Interestingly, the sex-specific splicing of Md-fru, and not Md-dsx, seems to be affected by the hypomorphic activity produced by the Md-traman alleles. In Md-traman mutant males the splicing of Md-fru shifts from the male to female mode, resulting in the loss of ability to produce male-specific FRU proteins.
The disparity in splicing behavior of Md-dsx and Md-fru in the hypomorphic Md-traman background can be explained by either different thresholds requirements or by differences in temporal and spatial requirements for activating the female splicing mode. For instance, Md-fru pre-mRNAs may have a higher affinity for Md-TRA binding than Md-dsx pre-mRNAs or may require lower levels of bound complexes to activate the female-specific splice sites. On the other hand, the differential effects on Md-dsx and Md-fru may result from discontinuous and/or tissue-specific Md-traman expression during development. In either case, its prominent impact on behavioral dimorphic development suggests that Md-traman must be expressed in the nervous system around the time when the CNS shapes the neuronal circuits required for proper male behavior [41]. Consistent with this interpretation, we detected female fru transcripts (Md-fruF) in the heads of male Md-traman adults. We propose that Md-fru is a direct target of Md-tra thereby defining an Md-dsx independent branch in the sex-determining pathway, which specifies the fates or activities of neurons that carry out higher order control functions to elicit and coordinate the activities comprising male reproductive behavior.
Is Md-fru Functionally Conserved?
We propose that male-specific Md-fruM expression is required for the correct display of male courtship in the housefly. This conclusion is based on the strict correlation between proper courtship performance and male expression of Md-fru. In our courtship assays we found that only males that expressed the Md-fruM variants performed as well as normal males, while those that expressed non-functional Md-fruF variants either performed poorly or did not engage in courtship at all. The most compelling argument comes from the observation that silencing of Md-tra in Md-traman2 males not only shifts Md-fru expression from the female to male mode but also completely restores fertility. A more direct test to confirm the role of Md-fru in courtship behavior would require a targeted disruption of Md-fruM function (cf. [8]). We expect that silencing of Md-fruM causes behavioral phenotypes similar to those observed in Md-traman males. Unfortunately, several attempts to specifically disrupt Md-fruM functions have thus far failed. Injections of dsRNA against P1 derived sequences of Md-fru into early syncytial blastoderm embryos failed to downregulate Md-fru expression in larval and pupal stages (data not shown). To overcome this problem we introduced a construct into the housefly genome which contains inverted repeats against P1 derived Md-fru transcripts driven by a constitutively expressed promoter. Unfortunately, none of the tested transgenic lines effectively suppressed Md-fru (data not shown). On the other hand, downregulation of the respective fru homolog in the hemimetabolous species, Blatetella germanica and Schistocerca gregaria, both of which are responsive to systemic effects of dsRNA silencing, have been shown to disrupt proper male behavior suggesting that fru plays an important and widely conserved role in male behavior [45], [46], [47]. However, it is not known whether the fru homologs in these species are direct targets of the sex-determining cascade and whether of not these homologs are specifically expressed in the CNS.
The functions of fru in male courtship have been studied in great detail at the cellular level in Drosophila. FRUM is expressed in about 1700 neurons which appear to be involved in various aspects of male courtship including olfactory and gustatory neurons required for behavioral responses to sex pheromones. Likewise, we observe that expression of Md-fru is confined to specific areas of the CNS and to neurons of the peripheral nervous system. Though expression appears much broader than in Drosophila and apparent in the optic neuropils, its neural-specific expression suggests that Md-fru is also involved in building and specifying neuronal circuits required for male behavior. High levels of expression in the neuropils which process visual cues hint at a prevalent role of the visual system in male behavior of the housefly. Previous studies showed that visually guided behavior patterns in houseflies differ between the sexes. Male houseflies chase females in the air using visual cues, but are not chased by females [48], coinciding with this sex-specific behavior are the presence of male-specific neurons in the visual system [49], [50]. It is thus possible that expression of Md-fru in the optic neuropils is involved in establishing the sexual difference of neural architecture involved in motion detection.
One striking dimorphic neural trait common to houseflies and fruit flies is the presence of a male-specific cluster of serotonergic neurons in the dorsal posterior part of the abdominal ganglion. In both types of dipterans these neurons send projections to the male internal reproductive organs where they innervate contractile muscles. It has been proposed that these muscles are required for transferring sperm and seminal fluids during copulation [51], [52]. In semi-fertile fru mutant males this cluster is substantially reduced in size and projection patterns are aberrant [51]. A similar phenotype was found in Md-traman2 housefly males where the cluster is substantially diminished in size and projections to the internal reproductive organs are lost. In Drosophila the formation and/or specification of the male-specific serotonergic cluster depends on presence of FRUM and DSXM activity [12], [21]. It is thus conceivable that lack of Md-FRUM is the cause for their absence in Md-traman2 males, suggesting a conserved mechanism in the specification of these male-specific neurons.
Our study in the housefly suggests that merging the control of the two terminal pathway effectors, fru and dsx, at the level of tra is a widely used strategy in holometabolous insects (Fig. 7). We propose that this linked control ensures that sex-determining instructions are coordinately relayed and implemented in neural and non-neural tissues.
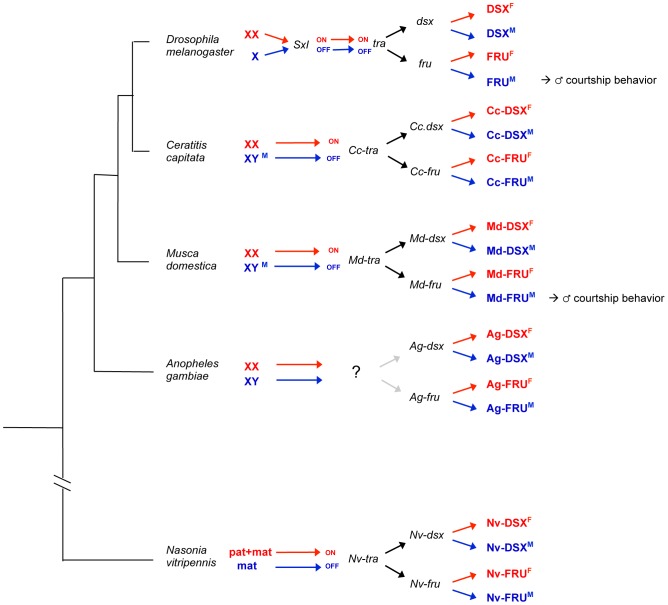
Figure 7. Bifurcation of the sex-determination pathway: a general principle in holometabolous insects?
The phylogenetic relationship and the sex-determining pathways of several dipteran species and the hymenopteran species Nasonia are depicted in this scheme. Despite considerable differences regarding the nature of the instructive sex-determining signal (X-counting in Drosophila, male [M] dominant determiners in Musca and Ceratitis, haplo-maternal [mat] versus diplo-maternal-paternal [pat+mat] in Nasonia) many insects use tra as the binary on/off switch downstream of the signal [54], though the molecular basis of this switch in A. gambiae remains still elusive [24], [55], [56]. The dsx gene, expressing sex-specific variants DSXF and DSXM, appears to be a conserved target of the binary switch and its use as a bifunctional executor of the instructive signal was validated by gene function studies in the depicted insect species [26], [30], [56], [57], [58] Also, homologs of fru were identified in these species that express sex-specific variants FRUF and FRUM [24], [26], [27], [59]. For Musca we here present evidence that fru is a downstream target of the tra branch which is involved in controlling proper display of courtship. Though no functional fru data from other holometabolous insects are available as yet, we propose that fru independently from dsx represents another conserved effector of the instructive signal. Hence, bifurcation of the pathway downstream of tra may have already existed before the divergence of holometabolous insect orders.
Materials and Methods
Rearing of the Flies
Rearing of Musca domestica larvae and flies has been described previously [33]. Since low density of larvae on standard medium can cause substantial decrease in survival rates, we reared transplanted host larvae and larvae from injected embryos on porcine manure. To dispose of mites and other parasites and to avoid contamination with eggs or larvae from wild-type populations, manure was stored at −70°C for at least two weeks prior to use.
Strains of Musca Domestica
(1) Wild-type strain: females XX, males XY; (2) autosomal M strain: females XX; pw, bwb/pw, bwb, males XX; MIII, pw+, bwb+/pw, bwb; (3) Md-traman1 strain: females XX, Md-traman/Md-tra+, males XX, Md-traman/Md-traman [33]; (4) Md-traman2 strain: females XX; MdtraD, Ba/Mdtraman, Ba+, males XX; Mdtraman, Ba+/Mdtraman, Ba+; (5) multimarked strain: females XX; ac/ac; ar/ar; bwb/bwb; ye/ye; snp/snp, males XY; ac/ac; ar/ar; bwb/bwb; ye/ye; snp/snp.
Transplantation of Larval Gonads
Crossing schemes and used genotypes are shown in Fig. S2. Larval anlagen of Mdtraman2, bwb + testes were dissected and transplanted into the posterior region of bwb male host larvae in Ringer’s solution. These larvae were then reared on porcine manure and surviving adults were outcrossed with bwb females of the host strain. In the progeny we screened for presence of donor derived bwb + individuals.
Sperm Analysis
Testes of 5-days old adult males were dissected in Ringer’s solution and transferred to a coverslip where they were gently squeezed to release sperm which was then dispersed with a needle. Morphology and mobility of sperm were examined by phase-contrast microscopy.
Courtship Assays
Male and female flies collected for courtship assays were separated shortly after hatching and kept at 25°C for 6 days in plastic beakers with sugar water and milk powder. The air humidity was kept between 65% and 75% and flies were exposed to a 12 hours light/dark cycle. The courtship assays were performed at the same time of the day. The courtship arena consisted of a see-through plastic cylinder with a height of 40 mm and a length of 160 mm. For each couple courtship activities were examined during a period of at least 20 minutes.
Md-tra dsRNA Injections
The same primers were used for synthesis of Md-tra dsRNA as described in [28]: Md-tra forward primer located in the 5′UTR of exon 1 linked to T7 promoter sequences (small letters): (5′ gtaatacgactcactatagggTGGTGTAATATGGCTCTATCG 3′) and Md-tra reverse primer located in exon 5 linked to T7 promoter sequences (small letters): (5′ gtaatacgactcactatagggGCTGCCATACAAACGTGTC 3′).
Total RNA from adult females was reverse transcribed and the resulting cDNA was amplified with this primer pair to generate a female-specific template of 620 bp in length. In vitro transcription of this template with T7 RNA polymerase yielded dsRNA that was ethanol precipitated and resuspended in injection buffer to a final concentration of 1 µg/µl. Embryos were collected within 1 h after egg laying and dechorionated. Then 1–2 nl of the dsRNA solution was injected into the posterior pole of syncytial blastoderm embryos [28]. As a negative control, embryos of the same stage were injected with a similar volume of Ringer’s solution. Injected embryos were allowed to develop at room temperature and placed in porcine manure for further development. Hatching flies were mated to virgins of the multimarked strain.
Isolation of Md-fru
A small Md-fru fragment spanning the conserved BTB domain was previously recovered by David Dolezel (University of Ceske Budejovice, Czech Republic) using degenerated primers and made available to us for further investigation. Based on sequence alignments between fru sequences of Drosophila and Anopheles gambiae [24] we generated degenerated primers for the different zinc finger-domains:
domain A: Md-fru-6 (5′ RCAATGNGCYTTCATRTTATC 3′),
domain B: Md-fru-7 (5′ ATGNSWATGRAAATGATGCCA 3′),
domain C: Md-fru-8 (5′ CATNGGATGYTTRAAYTTRCA 3′).
These reverse primers were used in combination with the forward primer MdFRU-3E (5′CAGTTAAGGCTCATCAAGCC3′) located in the BTB domain to amplify cDNA variants containing zincfinger domains B and C. Forward primer MdFRU-15s (5′CATGGGCCTCAATCAGTCG3′) was used to amplify the variant which contains zinc finger domain A. The zinc finger domain D was identified by bioinformatics in the genomic region between exon C4 and exon C5.
5′ and 3′ RACE of Md-fru cDNA
Female and male heads were collected from freshly hatched adults and total RNA was prepared according to the protocol of the manufacturer TRI REAGENT (SIGMA-ALDRICH, St Louis, USA) or to the protocol of the “NucleoSpin RNAII” kit (Macherey-Nagel, Düren, Germany). For long term storage, total RNA was dissolved in 70% ethanol and stored at −80°C.
3′ RACE was performed according to the protocol “5′/3′ RACE Kit, 2nd Generation” (Roche Applied Sciences, Rotkreuz, Switzerland). For 3′ RACE, the supplied oligo-dT primers were used for reverse strand cDNA synthesis. Forward primers located in the zincfinger encoding exons A, B, and C, were used for second strand synthesis and subsequent amplification: Md-fru-70B in domain A (5′ GCGATGACTTGAGTCTGACA 3′), Md-fru-35 in domain B (5′ CAACCTGAGGACACATTGCA 3′), Md-fru-36 in domain C (5′ GGACGTATGTCAAGAACCTC 3′) in combination with the shortened PCR-anchor primer A-3 (5′ ACGCGTATCGATGTCGAC 3′). For 5′ RACEs, gene-specific primer Md-fru-1 (5′ TCTGAGTATCTGACATCCTTC 3′) was used for reverse transcription. First round PCR amplification used gene-specific primer Md-fru-4E (5′ GCAGGCTAGAGTAACATCAC 3′) and the oligo-dT primer supplied with the Kit. Second round PCR amplification used gene-specific primer Md-fru-23 (5′ GACACCGGTTAAATTTGTCGG 3′) and a shortened PCR-anchor primer A-3 provided by the manufacturer Boehringer. In the first round PCR, the polymerase and Buffer 3 of the “Expand Long Template PCR System” kit by the manufacturer Roche Diagnostics (Risch, Switzerland) was used. For the second round PCR, the “Taq DNA Polymerase in Storage Buffer B” by the manufacturer Promega (Madison, USA) was used.
RT-PCR Analysis of Sex-specific Md-fru Splice Variants
The following primers were used to monitor amplify the sex-specific splice variants of Md-fru transcripts. Forward primers: Md-fru-18 (5′ TCCATCATGAGCGAACTCTGT 3′) is located in the female-specific sequences of the P1 transcripts, Md-fru-27 (5′ GTGAAGTGAACTTAGTACATGG 3′) is located in the common sequences of the P1 transcripts; reverse primer: Md-fru-1 (5′ TCTGAGTATCTGACATCCTTC 3′), located in the C1 exon. As an internal control the cytochromatic gene CYP6D3 was amplified with the following primers: CYP6D3-1 (5′ GTTCGGTAATATTTGGCTTGG 3′) and CYP6D3-2 (5′ CCCGTATTCCGTAGTTGAATT 3′). Primer pair Md-fru-18 and Md-fru-1 yields a fragment of 289 bp in females, and primer pair Md-fru-27 and Md-fru-1 generates a 602 bp long PCR product in males.
When testing the splicing mode of Md-fru in Mdtra RNAi treated XX males, the following primer pair was used for reamplification: Md-fru-2 (5′ TCGAAATATGGACTGCATGCTG 3′; in exon C2) and Mdfru-28 (5′ GTTTGTTCTCTGTCCAGTGTT 3′; in the common part of exon P1).
Multiple Sequence Alignments
For multiple sequence alignments the ClustalW algorithm provided by EMBL (http://www.ebi.ac.uk/Tools/msa/clustalw2/) was used.
In situ Hybridisation of Md-fruP1 Transcripts on Adult Head Sections
Probes were derived from templates generated by PCR with primer Md-fru-27 (forward; 5′ GTGAAGTGAACTTAGTACATGG 3′) and Md-fru-29 (reverse; 5′ CTGTCAAGGGTTCTCGGCTT 3′): A T7 promoter sequence (GTAATACGACTCACTATAGGG) was added to the Md-fru-27 primer for preparing sense probes (control) and to the Md-fru-29 primer for preparing antisense probes. Both amplicons were transcribed with T7 polymerase to generate labelled single stranded RNA probes (Roche, Madison, USA). Heads were dissected from adult flies, mounted in O.C.T medium and frozen at −20°C prior cryosectioning with a Leica rotary microtome. In situ hybridizations were carried out on adult head sections according to the protocol of [53].
Serotonin Staining of Abdominal Ganglions
Abdominal ganglions were dissected in PBS solution and fixed in 4% paraformaldehyde in PBS for 1 hour on ice while gently shaking. Then the tissues were washed three times in PBS for 15 minutes each. Following a brief 5 minutes incubation in 1 N HCl, the tissues were washed in PBS for 15 minutes and three times in 0.4% PBT for 10 minutes each. Afterwards the tissue samples were incubated for 1 hour in PTN (0.4% PBT, 5% (v/v) normal goat serum), followed by the incubation with the primary antibody for 24–36 hours at 4°C. The rabbit anti-serotonin (5-HT) antibody was purchased from SIGMA-ALDRICH (cat # S5545). Dilution of the antibody was 1/400 in PTN. After the incubation of the primary antibody the ganglions were washed four times in PBT for 30 minutes each, followed by the incubation of the secondary antibody at 4°C overnight or at room temperature for 3 to 4 hours. Dilution of the secondary antibody (anti-rabbit FITC, SIGMA-ALDRICH, anti-rabbit AlexaFluor 488, Molecular Probes Eugene, USA) was 1/600 in PTN. To remove unbound secondary antibody the samples were washed four times in PBT for 1 hour each. Thereafter, the samples were washed several times in PBS for several hours each (1–2 days in total). Finally, the samples were mounted in VectaShield medium (Vector Labs, Burlingame, USA) on glass slides and covered with cover slips. The mounted samples were examined and analysed by confocal laser scanning microscopy (Zeiss LSM 510). The genotypes of all animals were coded before dissections to avoid bias in either preparation or scoring of the samples.
Supporting Information
Amino acid sequence alignments of fru homologs in different dipterans and in Tribolium and Nasonia. (A) Alignments of the male-specific N-terminal extension of fru in M.domestica, D. melanogaster, A. gambiae, N. vitripennis. The putative start codon is labelled in bold. (B) Alignment of the BTB domain of fru homologs from the same species as in (A) with the addition of in silico identified fru sequences of T. castaneum. The BTB domain overall shows a high degree of sequence similarity. (C) Alignment of the zinc-finger A encoding exon. (D) Alignment of the zinc-finger B encoding exon. (E) Alignment of the zinc-finger C encoding exon. (F) Alignment of the zinc-finger D encoding exons. Identical amino acids are indicated with an asterisk (*). Highly similar amino acids are labelled with a colon (:) while amino acids with weak similarity are indicated with a dot (.). Similarity scores between the different domains of Musca and Drosophila fru are: 54% (A), 98% (B), 90% (C), 74% (D), 54% (E) and 54% (F).
(TIF)
Larval testes transplantations. Male Md-traman2 progeny (donor) was generated by crossing bwb +; Md-traman males with bwb +; Md-traman/Md-traD females. Gonads were dissected from these male larvae and transplanted into male bwb, MIII; Md-tra+ larvae (host). These larvae were reared to adulthood and outcrossed with females of the host strain. Three different phenotypes of progeny were expected, bwb males and females (light brown) from host derived sperm and bwb + females (dark brown) from donor derived sperm. We recovered 7 successfully transplanted males which sired in total 728 individuals with a host genotype (bwb) and 154 individuals with a donor genotype (bwb +).
(TIF)
Courtship latency and duration of wild-type males and mutant Md-traman males.
(DOCX)
Frequencies of discrete courtship elements observed in courting wild-type males and Md-traman males.
(DOCX)
Acknowledgments
David Dolozel is gratefully acknowledged for providing the initial clone of Md-fru. We thank Claudia Brunner for technical assistance, Christina Siegenthaler for larval testes transplantations, Raymond Grunder and Hanna Nägeli for rearing of the housefly cultures.
Funding Statement
This work was supported by the Swiss National Science Foundation (31003A-109690 to D.B.) and by the Wellcome Trust (GR069590MA to J-C.B. and S.F.G.). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Villella A, Hall JC (2008) Neurogenetics of courtship and mating in Drosophila . Adv Genet 62: 67–184. [DOI] [PubMed] [Google Scholar]
- 2. Ferveur JF (2010) Drosophila female courtship and mating behaviors: sensory signals, genes, neural structures and evolution. Curr Opin in Neurobiol 20: 764–769. [DOI] [PubMed] [Google Scholar]
- 3. Ryner LC, Goodwin SF, Castrillon DH, Anand A, Villella A, et al. (1996) Control of male sexual behavior and sexual orientation in Drosophila by the fruitless gene. Cell 87: 1079–1089. [DOI] [PubMed] [Google Scholar]
- 4. Ito H, Fujitani K, Usui K, Shimizu-Nishikawa K, Tanaka S, et al. (1996) Sexual orientation in Drosophila is altered by the satori mutation in the sex-determination gene fruitless that encodes a zinc finger protein with a BTB domain. P Natl Acad of Sci USA 93: 9687–9692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gailey DA, Hall JC (1989) Behavior and cytogenetics of fruitless in Drosophila melanogaster: different courtship defects caused by separate, closely linked lesions. Genetics 121: 773–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hall JC (1994) The mating of a fly. Science 264: 1702–1714. [DOI] [PubMed] [Google Scholar]
- 7. Villella A, Gailey DA, Berwald B, Ohshima S, Barnes PT, et al. (1997) Extended reproductive roles of the fruitless gene in Drosophila melanogaster revealed by behavioral analysis of new fru mutants. Genetics 147: 1107–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Demir E, Dickson BJ (2005) fruitless splicing specifies male courtship behavior in Drosophila . Cell 121: 785–794. [DOI] [PubMed] [Google Scholar]
- 9. Manoli DS, Meissner GW, Baker BS (2006) Blueprints for behavior: genetic specification of neural circuitry for innate behaviors. Trends Neurosci 29: 444–451. [DOI] [PubMed] [Google Scholar]
- 10. Vrontou E, Nilsen SP, Demir E, Kravitz EA, Dickson BJ (2006) fruitless regulates aggression and dominance in Drosophila . Nat Neurosci 9: 1469–1471. [DOI] [PubMed] [Google Scholar]
- 11. Clyne JD, Miesenbock G (2008) Sex-specific control and tuning of the pattern generator for courtship song in Drosophila . Cell 133: 354–363. [DOI] [PubMed] [Google Scholar]
- 12. Billeter JC, Villella A, Allendorfer JB, Dornan AJ, Richardson M, et al. (2006) Isoform-specific control of male neuronal differentiation and behavior in Drosophila by the fruitless gene. Curr Biol 16: 1063–1076. [DOI] [PubMed] [Google Scholar]
- 13. Rideout EJ, Billeter JC, Goodwin SF (2007) The sex-determination genes fruitless and doublesex specify a neural substrate required for courtship song. Curr Biol 17: 1473–1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sanders LE, Arbeitman MN (2008) Doublesex establishes sexual dimorphism in the Drosophila central nervous system in an isoform-dependent manner by directing cell number. Dev Biol 320: 378–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Shirangi TR, Dufour HD, Williams TM, Carroll SB (2009) Rapid evolution of sex pheromone-producing enzyme expression in Drosophila . PLoS Biol 7: e1000168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rideout EJ, Dornan AJ, Neville MC, Eadie S, Goodwin SF (2010) Control of sexual differentiation and behavior by the doublesex gene in Drosophila melanogaster . Nat Neurosci 13: 458–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zollman S, Godt D, Prive GG, Couderc JL, Laski FA (1994) The BTB domain, found primarily in zinc finger proteins, defines an evolutionarily conserved family that includes several developmentally regulated genes in Drosophila . P Natl Acad of Sci USA 91: 10717–10721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Anand A, Villella A, Ryner LC, Carlo T, Goodwin SF, et al. (2001) Molecular genetic dissection of the sex-specific and vital functions of the Drosophila melanogaster sex determination gene fruitless. Genetics 158: 1569–1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Heinrichs V, Ryner LC, Baker BS (1998) Regulation of sex-specific selection of fruitless 5′ splice sites by transformer and transformer-2 . Mol Cell Biol 18: 450–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lee G, Foss M, Goodwin SF, Carlo T, Taylor BJ, et al. (2000) Spatial, temporal, and sexually dimorphic expression patterns of the fruitless gene in the Drosophila central nervous system. J Neurobiol 43: 404–426. [DOI] [PubMed] [Google Scholar]
- 21. Billeter JC, Goodwin SF (2004) Characterization of Drosophila fruitless-gal4 transgenes reveals expression in male-specific fruitless neurons and innervation of male reproductive structures. J Comp Neurol 475: 270–287. [DOI] [PubMed] [Google Scholar]
- 22. Stockinger P, Kvitsiani D, Rotkopf S, Tirian L, Dickson BJ (2005) Neural circuitry that governs Drosophila male courtship behavior. Cell 121: 795–807. [DOI] [PubMed] [Google Scholar]
- 23. Manoli DS, Foss M, Villella A, Taylor BJ, Hall JC, et al. (2005) Male-specific fruitless specifies the neural substrates of Drosophila courtship behaviour. Nature 436: 395–400. [DOI] [PubMed] [Google Scholar]
- 24. Gailey DA, Billeter JC, Liu JH, Bauzon F, Allendorfer JB, et al. (2006) Functional conservation of the fruitless male sex-determination gene across 250 Myr of insect evolution. Mol Biol Evol 23: 633–643. [DOI] [PubMed] [Google Scholar]
- 25. Salvemini M, D’Amato R, Petrella V, Aceto S, Nimmo D, et al. (2013) The Orthologue of the Fruitfly Sex Behaviour Gene Fruitless in the Mosquito Aedes aegypti: Evolution of Genomic Organisation and Alternative Splicing. PLoS ONE 8: e48554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Salvemini M, Robertson M, Aronson B, Atkinson P, Polito LC, et al. (2009) Ceratitis capitata transformer-2 gene is required to establish and maintain the autoregulation of Cctra, the master gene for female sex determination. Int J Dev Biol 53: 109–120. [DOI] [PubMed] [Google Scholar]
- 27. Bertossa RC, van de Zande L, Beukeboom LW (2009) The Fruitless gene in Nasonia displays complex sex-specific splicing and contains new zinc finger domains. Mol Biol Evol 26: 1557–1569. [DOI] [PubMed] [Google Scholar]
- 28. Hediger M, Henggeler C, Meier N, Perez R, Saccone G, et al. (2010) Molecular Characterization of the Key Switch F Provides a Basis for Understanding the Rapid Divergence of the Sex-determining Pathway in the Housefly. Genetics 184: 155–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Dubendorfer A, Hediger M, Burghardt G, Bopp D (2002) Musca domestica, a window on the evolution of sex-determining mechanisms in insects. Int J Dev Biol 46: 75–79. [PubMed] [Google Scholar]
- 30. Hediger M, Burghardt G, Siegenthaler C, Buser N, Hilfiker-Kleiner D, et al. (2004) Sex determination in Drosophila melanogaster and Musca domestica converges at the level of the terminal regulator doublesex. Dev Genes Evol 214: 29–42. [DOI] [PubMed] [Google Scholar]
- 31. Burghardt G, Hediger M, Siegenthaler C, Moser M, Dubendorfer A, et al. (2005) The transformer2 gene in Musca domestica is required for selecting and maintaining the female pathway of development. Dev Genes Evol. 215: 165–176. [DOI] [PubMed] [Google Scholar]
- 32. Dauwalder B (2011) The roles of fruitless and doublesex in the control of male courtship. Int Rev Neurobiol 99: 87–105. [DOI] [PubMed] [Google Scholar]
- 33. Schmidt R, Hediger M, Nöthiger R, Dübendorfer A (1997) The mutation masculinizer (man) defines a sex determining gene with maternal and zygotic functions in Musca domestica L. Genetics. 145: 173–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. McDonald IC, Evenson P, Nickel CA, Johnson OA (1978) House fly genetics: Isolation of a female determining factor on chromosome 4. Ann Entomol Soc Amer 71: 692–694. [Google Scholar]
- 35. Dübendorfer A, Hilfiker-Kleiner D, Nöthiger R (1992) Sex determination mechanisms in dipteran insects: the case of Musca domestica. Sem Dev Biol 3: 349–356. [Google Scholar]
- 36. Meffert LM (1995) Bottleneck effects on genetic variance for courtship repertoire. Genetics 139: 365–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Meffert LM, Hagenbuch KL (2005) The genetic architecture of house fly mating behavior. Curr Top Dev Biol 66: 189–213. [DOI] [PubMed] [Google Scholar]
- 38. Usui-Aoki K, Mikawa Y, Yamamoto D (2005) Species-specific patterns of sexual dimorphism in the expression of fruitless protein, a neural musculinizing factor in Drosophila . J Neurogenet 19: 109–121. [DOI] [PubMed] [Google Scholar]
- 39. Lee G, Hall JC (2001) Abnormalities of male-specific FRU protein and serotonin expression in the CNS of fruitless mutants in Drosophila . J Neurosci 21: 513–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. McRobert SP, Tompkins L (1985) The effect of transformer, doublesex and intersex mutations on the sexual behavior of Drosophila melanogaster . Genetics 111: 89–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Arthur BI Jr, Jallon JM, Caflisch B, Choffat Y, Nothiger R (1998) Sexual behaviour in Drosophila is irreversibly programmed during a critical period. Curr Biol 8: 1187–1190. [DOI] [PubMed] [Google Scholar]
- 42. Ferveur JF, Stortkuhl KF, Stocker RF, Greenspan RJ (1995) Genetic feminization of brain structures and changed sexual orientation in male Drosophila . Science 267: 902–905. [DOI] [PubMed] [Google Scholar]
- 43. Nagoshi RN, McKeown M, Burtis KC, Belote JM, Baker BS (1988) The control of alternative splicing at genes regulating sexual differentiation in D. melanogaster . Cell 53: 229–236. [DOI] [PubMed] [Google Scholar]
- 44. Baker BS, Ridge KA (1980) Sex and the single cell. I. On the action of major loci affecting sex determination in Drosophila melanogaster . Genetics 94: 383–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Clynen E, Ciudad L, Belles X, Piulachs MD (2011) Conservation of fruitless’ role as master regulator of male courtship behaviour from cockroaches to flies. Dev Genes Evol 221: 43–48. [DOI] [PubMed] [Google Scholar]
- 46. Boerjan B, Tobback J, De Loof A, Schoofs L, Huybrechts R (2011) Fruitless RNAi knockdown in males interferes with copulation success in Schistocerca gregaria . Insect Biochem Molec 41: 340–347. [DOI] [PubMed] [Google Scholar]
- 47. Boerjan B, Tobback J, Vandersmissen HP, Huybrechts R, Schoofs L (2012) Fruitless RNAi knockdown in the desert locust, Schistocerca gregaria, influences male fertility. J Insect Physiol 58: 265–269. [DOI] [PubMed] [Google Scholar]
- 48. Wehrhahn C (1979) Sex-specific differences in the chasing behavior of houseflies (Musca). Biol Cybern 32: 239–241. [Google Scholar]
- 49. Strausfeld NJ (1980) Male and female visual neurones in dipterous insects. Nature 283: 381–383. [Google Scholar]
- 50. Sukontason KL, Chaiwong T, Piangjai S, Upakut S, Moophayak K, et al. (2008) Ommatidia of blow fly, house fly, and flesh fly: implication of their vision efficiency. Parasitol Res 103: 123–131. [DOI] [PubMed] [Google Scholar]
- 51. Lee G, Villella A, Taylor BJ, Hall JC (2001) New reproductive anomalies in fruitless-mutant Drosophila males: extreme lengthening of mating durations and infertility correlated with defective serotonergic innervation of reproductive organs. J Neurobiol 47: 121–149. [DOI] [PubMed] [Google Scholar]
- 52. Villella A, Peyre JB, Aigaki T, Hall JC (2006) Defective transfer of seminal-fluid materials during matings of semi-fertile fruitless mutants in Drosophila. J Comp Physiol A Neuroethol Sens Neural Behav Physiol 192: 1253–1269. [DOI] [PubMed] [Google Scholar]
- 53. Tautz D, Pfeifle C (1989) A non-radioactive in situ hybridization method for the localization of specific RNAs in Drosophila embryos reveals translational control of the segmentation gene hunchback. Chromosoma 98: 81–85. [DOI] [PubMed] [Google Scholar]
- 54. Verhulst EC, van de Zande L, Beukeboom LW (2010) Insect sex determination: it all evolves around transformer . Curr Opin Genet Dev 20: 376–383. [DOI] [PubMed] [Google Scholar]
- 55. Suzuki MG (2010) Sex determination: insights from the silkworm. J Genet 89: 357–363. [DOI] [PubMed] [Google Scholar]
- 56. Salvemini M, Mauro U, Lombardo F, Milano A, Zazzaro V, et al. (2011) Genomic organization and splicing evolution of the doublesex gene, a Drosophila regulator of sexual differentiation, in the dengue and yellow fever mosquito Aedes aegypti . BMC evolutionary biology 11: 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Oliveira DC, Werren JH, Verhulst EC, Giebel JD, Kamping A, et al. (2009) Identification and characterization of the doublesex gene of Nasonia . Insect Mol Biol 18: 315–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Saccone G, Salvemini M, Pane A, Polito LC (2008) Masculinization of XX Drosophila transgenic flies expressing the Ceratitis capitata DoublesexM isoform. Int J Dev Biol 52: 1051–1057. [DOI] [PubMed] [Google Scholar]
- 59. Salvemini M, Polito C, Saccone G (2010) Fruitless alternative splicing and sex behaviour in insects: an ancient and unforgettable love story? J Genet 89: 287–299. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Amino acid sequence alignments of fru homologs in different dipterans and in Tribolium and Nasonia. (A) Alignments of the male-specific N-terminal extension of fru in M.domestica, D. melanogaster, A. gambiae, N. vitripennis. The putative start codon is labelled in bold. (B) Alignment of the BTB domain of fru homologs from the same species as in (A) with the addition of in silico identified fru sequences of T. castaneum. The BTB domain overall shows a high degree of sequence similarity. (C) Alignment of the zinc-finger A encoding exon. (D) Alignment of the zinc-finger B encoding exon. (E) Alignment of the zinc-finger C encoding exon. (F) Alignment of the zinc-finger D encoding exons. Identical amino acids are indicated with an asterisk (*). Highly similar amino acids are labelled with a colon (:) while amino acids with weak similarity are indicated with a dot (.). Similarity scores between the different domains of Musca and Drosophila fru are: 54% (A), 98% (B), 90% (C), 74% (D), 54% (E) and 54% (F).
(TIF)
Larval testes transplantations. Male Md-traman2 progeny (donor) was generated by crossing bwb +; Md-traman males with bwb +; Md-traman/Md-traD females. Gonads were dissected from these male larvae and transplanted into male bwb, MIII; Md-tra+ larvae (host). These larvae were reared to adulthood and outcrossed with females of the host strain. Three different phenotypes of progeny were expected, bwb males and females (light brown) from host derived sperm and bwb + females (dark brown) from donor derived sperm. We recovered 7 successfully transplanted males which sired in total 728 individuals with a host genotype (bwb) and 154 individuals with a donor genotype (bwb +).
(TIF)
Courtship latency and duration of wild-type males and mutant Md-traman males.
(DOCX)
Frequencies of discrete courtship elements observed in courting wild-type males and Md-traman males.
(DOCX)