Abstract
The number of fully active antibiotic options that treat nosocomial infections due to multidrug-resistant Acinetobacter baumannii (A. baumannii) is extremely limited. Magnolia officinalis, Mahonia bealei, Rabdosia rubescens, Rosa rugosa, Rubus chingii, Scutellaria baicalensis, and Terminalia chebula plant extracts were previously shown to have growth inhibitory activity against a multidrug-resistant clinical strain of A. baumannii. In this study, the compounds responsible for their antimicrobial activity were identified by fractionating each plant extract using high performance liquid chromatography, and determining the antimicrobial activity of each fraction against A. baumannii. The chemical structures of the fractions inhibiting >40% of the bacterial growth were elucidated by liquid chromatography/mass spectrometry analysis and nuclear magnetic resonance spectroscopy. The six most active compounds were identified as: ellagic acid in Rosa rugosa; norwogonin in Scutellaria baicalensis; and chebulagic acid, chebulinic acid, corilagin, and terchebulin in Terminalia chebula. The most potent compound was identified as norwogonin with a minimum inhibitory concentration of 128 µg/mL, and minimum bactericidal concentration of 256 µg/mL against clinically relevant strains of A. baumannii. Combination studies of norwogonin with ten anti-Gram negative bacterial agents demonstrated that norwogonin did not enhance the antimicrobial activity of the synthetic antibiotics chosen for this study. In conclusion, of all identified antimicrobial compounds, norwogonin was the most potent against multidrug-resistant A. baumannii strains. Further studies are warranted to ascertain the prophylactic and therapeutic potential of norwogonin for infections due to multidrug-resistant A. baumannii.
Introduction
Outbreaks of infections due to Acinetobacter baumannii (A. baumannii) have been reported worldwide [1], and have been attributed to contamination of inanimate objects in the hospital setting and facilitated by healthcare workers who may transmit this organism via direct person-to-person contact [2], [3]. Today, fully active antibiotic options available to treat nosocomial infections due to multidrug-resistant (MDR) A. baumannii are extremely limited [1].
Chemotherapeutic agents against MDR A. baumannii currently in the pharmaceutical pipeline do not appear to hold promise [3]. In order to identify novel treatment options, commercially available plant extracts were previously screened for their ability to inhibit MDR A. baumannii in vitro. The extracts showing the most potent inhibitory effects against a clinical strain of MDR A. baumannii in vitro were: Magnolia officinalis, Mahonia bealei, Rabdosia rubescens, Rosa rugosa, Rubus chingii, Scutellaria baicalensis, and Terminalia chebula [4].
Tannins, flavones, and phenolic compounds are reported to have low to moderate inhibitory effects on A. baumannii in vitro [5], [6]. Tannins are a group of polymerized phenolic substances shown to inhibit a variety of microorganisms [7], [8]. Flavones are phenolic structures that are synthesized by some plants in response to microbial infections [7]. We hypothesized that the anti-MDR A. baumannii activity of these plant extracts may result from the combination of tannins and non-tannins that may be present in the extracts. Here, we identified active chemical compounds in the anti-MDR A. baumannii plant extracts and characterized their antimicrobial properties in vitro.
Materials and Methods
Bacterial strains and ethics statement
MDR A. baumannii strains 31P, 125P and 152P were isolated from blood (31P) and respiratory (125P and 152P) cultures of three different patients at Cedars-Sinai Medical Center in Los Angeles, California, USA. The strains belonged to different clones based on repetitive-polymerase chain reaction amplification, and their dendrogram is shown in Figure S1 [9]. 31P was determined to be resistant to piperacillin/tazobactam, anti-pseudomonal cephalosporins (ceftazidime and cefepime), carbapenems (imipenem and meropenem), aminoglycosides (tobramycin and amikacin), and fluoroquinolones (ciprofloxacin and levofloxacin) by VITEK®2 (bioMérieux, Durham, North Carolina, USA); and sensitive to colistin by Etest (bioMérieux) based on interpretations according to Clinical and Laboratory Standards Institute (CLSI) breakpoints [10]. The strain was intermediate to tigecycline by Etest with results interpreted per the United States Food and Drug Administration's breakpoint recommendations for Enterobacteriaceae. 125P was intermediate to piperacillin/tazobactam, resistant to anti-pseudomonal cephalosporins, carbapenems, fluoroquinolones, colistin and tigecycline, and sensitive to aminoglycosides. 152P was resistant to piperacillin/tazobactam, anti-pseudomonal cephalosporins, aminoglycosides, fluoroquinolones and colistin, and intermediate to carbapenems and tigecycline. We obtained an exempt status from the Cedars-Sinai Institutional Review Board to use these strains to perform all experiments in this study (Protocol number 15767).
A. baumannii strain, BAA-1605 and Escherichia coli strain, 25922 (E. coli 25922) were obtained from the American Type Culture Collection (ATCC, Manassas, Virginia, USA).
Plant extracts
Seven plant extracts with the most potent inhibitory activity against 31P were selected for further characterization [4]. Dry powders of the plant extracts were obtained as follows: Magnolia officinalis, Rubus chingii, Scutellaria baicalensis and Terminalia chebula were obtained from Sun Ten Laboratories, Inc. (Irvine, California, USA); those of Mahonia bealei and Rosa rugosa were obtained from Bio Essence Corporation (Richmond, California, USA); and those of Rabdosia rubescens were obtained from Mayway Corporation (Torrance, California, USA). The minimum inhibitory concentrations (MICs) of these extracts against 31P were identical to those previously reported [4].
Dimethyl sulfoxide (DMSO) tolerance test
DMSO (Sigma-Aldrich, St. Louis, Missouri, USA) was sterilized using a 0.2 µm Acrodisc nylon membrane syringe filter (Pall, Ann Arbor, Michigan, USA). A 5 mL culture of 31P was grown in cation-adjusted Mueller-Hinton (CAMH) broth (Beckton-Dickenson, Franklin Lakes, New Jersey, USA) with agitation at 37°C. The culture was diluted to 106 CFU/mL in fresh medium. A 100 µL aliquot of CAMH broth with DMSO (concentration range: 0% to 15%) and 100 µL of 31P suspension were mixed in each well of a sterile 96-well polystyrene assay plate (Corning, Lowell, Massachusetts, USA). Negative controls consisted of non-inoculated media. A colistin (Sigma-Aldrich) dose response (0.0625–8 µg/mL) was included as a positive control. Assay plates were incubated without agitation for 16 h at 37°C, and optical density at 600 nm (OD600 mm) was measured. Percent growth inhibition (% growth inhibition) for each replicate (n = 8) was calculated as follows: [1−([OD600 nm of a sample – average OD600 nm of negative controls]/[average OD600 nm of positive controls - average OD600 nm of negative controls])]×100. Results were presented as the mean and standard deviation of eight replicates at each DMSO concentration.
De-tanninization of the plant extracts
1,000 mg of each plant extract powder was solubilized in a solution of 75°C water and DMSO (3∶1 [vol/vol]) at a concentration of 20 mg/mL. Each solution was stirred for 30 min and was centrifuged for 15 min at 7,500 rpm to remove insoluble polysaccharide excipients. The solution was dried down using a Genevac sample concentrator (Genevac Inc, Gardiner, New York, USA) under reduced pressure at 30°C. The sample was re-suspended at a concentration of 10 mg/mL in water and methanol (1∶1 [vol/vol]). 300 mg of polyvinyl pyrrolidone (Crescent Chemical Company, Islandia, New York, USA) was added. The solution was stirred for 30 min and was centrifuged. The supernatant was removed and dried down as described above to yield 150–350 mg of de-tanninized plant extracts.
Dose response testing of plant extracts before and after de-tanninization
The plant extracts were two-fold serially diluted in water, and 20 µL of solubilized extract and 80 µL of CAMH broth were mixed in an untreated, sterile 96-well plate. This was mixed with 100 µL of a 106 CFU/mL suspension of 31P from a cryopreserved stock. The final concentration of the plant extracts ranged from 7.8125 to 1,000 µg/mL. Negative and positive controls were prepared as described above. Assay plates were incubated without agitation at 37°C, and OD600 mm was measured at 16 h and 24 h. The % growth inhibition was calculated as described above. The same procedure was repeated using the de-tanninized plant extracts. All the samples, crude and de-tanninized, were tested in triplicate.
Fractionation of de-tanninized plant extracts
Each de-tanninized plant extract was re-suspended at a concentration of 10 mg/mL in water and DMSO (2∶1 [vol/vol]), and a 1 mg aliquot was fractionated using a liquid chromatography/mass spectrometer (LC/MS) system with an ultraviolet (UV), evaporative light scattering detector (ELSD) and MS detectors. 1H and 13C nuclear magnetic resonance (NMR), correlation spectroscopy (COSY), heteronuclear single-quantum correlation spectroscopy (HSQC) and heteronuclear multiple-bond correlation spectroscopy (HMBC) spectra were recorded using a Bruker DRX 500 NMR spectrometer (Bruker Corporation, Billerica, Massachusetts, USA) in DMSO-d6 at 320K at 500 MHz for 1H and 125 MHz for 13C NMR, respectively. MS was performed on a Sciex API 150 EX single quadrupole (AB SCIEX, Framingham, Massachusetts, USA) with an ion spray ionization source operating in positive mode; capillary voltage, 5.0 kV; declustering potential 35.0. High resolution mass spectra were gathered with a Waters Premier Q-Tof mass spectrometer (Waters, Milford, Massachusetts, USA) equipped with an electrospray ionization source operated in the positive-ion mode; capillary voltage, 3.5 kV; source temperature, 80°C; desolvation temperature, 200°C; nitrogen desolvation flow, 200 l/h. Samples were diluted with water: acetonitrile (1∶1 [vol/vol]) containing 0.1% formic acid and introduced via infusion using the onboard syringe pump. Semi-preparative high performance liquid chromatography (HPLC) was performed using a Waters system (Waters) with a 600 pump connected to a 996 diode-array detector and controlled by Empower software (Empower Software Solutions, Inc., Orlando, Florida, USA).
Each plant extract was fractionated as follows: chromatographic separation was performed at room temperature on a C18 Luna 5 µm (100 mm×4.6 mm, inside diameter [i.d.]) column (Phenomenex, Torrance, California, USA). The mobile phase was initially composed of water with trifluoroacetic acid (0.05%) (Solvent A)/acetonitrile with trifluoroacetic acid (0.05%) (Solvent B), 95∶5. The compounds were eluted using an isocratic hold (95∶5, A∶B) until 5 min and then were ramped to 50∶50 from 5 to 15 min, after which a final isocratic step of 50∶50 from 15 to 25 min was used as a wash.
Identification of active antibacterial compounds
The fractions of all de-tanninized extracts except Scutellaria baicalensis were solubilized in 20 µL warm water mixed with 80 µL CAMH broth. The fractions of de-tanninized Scutellaria baicalensis were solubilized in 10 µL DMSO, and 2 µL of each fraction was transferred to 98 µL of CAMH broth in a 96-well plate. All wells were mixed with 100 µL of a 106 CFU/mL suspension of 31P. Controls were the same as described above. The assay plates were incubated without agitation for 16 h at 37°C, and OD600 nm was measured. The % growth inhibition was calculated as described above. The experiments were done in triplicate.
The chemical structures of the fractions resulting in >40% of the bacterial growth inhibition were identified on the basis of MS and UV data. Rosa rugosa, Scutellaria baicalensis and Terminalia chebula extracts contained fractions showing >40% growth inhibition. Therefore, 200 mg each of these extracts was prepared at a concentration of 10 mg/mL in water and DMSO (2∶1 [vol/vol]), and chromatographic separation was performed at room temperature on a C18 Luna 5 µm (250 mm×10 mm, i.d.) column (Phenomenex). The mobile phase was initially composed of 95∶5 water with trifluoroacetic acid (0.05%) (Solvent A)/acetonitrile with trifluoroacetic acid (0.05%) (Solvent B) and then was ramped to 40∶60 (A∶B) over 30 min. The flow rate was set at 5 mL/min. A complete set of 1H NMR, 13C NMR, and high resolution mass spectrometry (HRMS) (+electrospray ionization [ESI] time-of-flight mass spectrometry [TOFMS] [M+H]) data were acquired for the peaks of interest. UV spectra, molecular weight and NMR data were used to search internal and external databases (Dictionary of Natural Products, Chapman & Hall/CRC Chemical Database, Version 16∶2, Boca Raton, Florida, USA) to identify precise chemical structures of the target compounds.
The following procedures were conducted to isolate more material of the target metabolites from extracts of Rosa rugosa, Scutellaria baicalensis and Terminalia chebula. Preparative HPLC chromatographic separation was performed at room temperature on a C18 Prodigy 5 µm (250 mm×21 mm, i.d.) column (Phenomenex). The mobile phase was initially composed of water with trifluoroacetic acid (0.05%) (Solvent A)/acetonitrile with trifluoroacetic acid (0.05%) (Solvent B), 95∶5. The compounds were eluted using an isocratic hold (95∶5, A∶B) until 4 min and then were ramped to 50∶50 from 5 to 25 min, after which a final isocratic step of 50∶50 from 25 to 30 min was used as a wash. The flow rate was set at 20 mL/min. Plant fractions from several HPLC runs were combined and collected into vials, and dried down using a Genevac sample concentrator under reduced pressure at 30°C. The purity of the isolated fractions was confirmed by HPLC and/or 1H NMR.
Determination of the minimum inhibitory concentration (MIC)90, and minimum bactericidal concentration (MBC), time-kill kinetic analysis, and resazurin reduction assay
The MIC90 was defined as the lowest concentration of a compound which inhibited ≥90% of bacterial growth compared to an untreated control. Purified forms of the following compounds were solubilized at 10 mg/mL in warm water: ellagic acid from Rosa rugosa; chebulagic acid, chebulinic acid, corilagin and terchebulin from Terminalia chebula. The solutions were two-fold serially diluted in water, and 20 µL per well was transferred to 80 µL CAMH broth in a 96-well plate. Each solution was mixed with 100 µL of either 31P or BAA-1605 suspension (5×105 CFU/mL final). Assay plates including negative control (CAMH broth only) and positive control (bacterial suspension at a final concentration of 5×105 CFU/mL) were incubated without agitation for 16 h at 37°C, and OD600 nm was measured. The % growth inhibition was calculated as described above. Purified norwogonin was insoluble in water and was solubilized at 25.6 mg/mL in DMSO and two-fold serially diluted. One µL of norwogonin dilution was transferred to 99 µL of CAMH broth in a 96-well plate, mixed, and 20 µL per well were transferred to 4 wells of a 384-well plate. Negative controls (CAMH broth plus 1% DMSO) and positive controls (same as above) were included. Norwogonin, having the lowest MIC90, was tested against BAA-1605 in the same fashion.
Next, MBC testing and time-kill kinetic assays of norwogonin were performed on 31P. The bactericidal effect was defined as a 99.9% decrease in CFU (3 logs) in the starting inoculum during a 24 h incubation in the presence of antibiotic. The MBC was determined by transferring 1 µL from each well of an overnight MIC plate to 63 µL of sterile CAMH broth in a fresh 384-well plate. OD600 nm was measured after 20 h incubation at 37°C. The % growth inhibition was calculated as described above. For both the MIC and MBC assays, 12 replicate wells were tested. For the time-kill kinetic analysis, a bacterial overnight culture was diluted (5×105 CFU/mL final) using CAMH broth supplemented with DMSO (1% final) and 1× or 2× MIC of norwogonin. Cultures were grown with agitation at 37°C, and aliquots were collected at the indicated time intervals, serially diluted in 0.9% sterile saline solution and plated onto CAMH agar plates. Viable colonies were enumerated after 24 h at 37°C. The limit of detection for this preliminary assay was 101 CFU/mL.
Finally, the growth inhibitory effect of norwogonin against 31P, 125P and 152P was determined by measuring both turbidity and respiration. Purified norwogonin was solubilized at 12.8 mg/mL in DMSO and triplicate two-fold serial dilutions (0.003 mg/mL final concentration) were performed. Five hundred nL of each dilution was transferred to three 384-well assay plates. Each assay plate was inoculated with 50 µL of 31P, 125P, or 152P diluted to 5×105 CFU/mL in CAMH broth, and incubated without agitation for 16 h at 37°C. Negative controls (CAMH broth plus 1% DMSO) and positive controls (inoculum plus 1% DMSO) were included in each assay plate. Turbidity was assessed by reading OD600 nm. After determining turbidity, 5 µL of a 0.001% aqueous resazurin solution (Sigma-Aldrich) was added to each well and assay plates were incubated at room temperature for 30 min. Resazurin reduction to resorfurin was determined by measuring fluorescence (530 nm excitation/590 nm emission). These assays were performed in triplicate on three separate days.
Dose response testing of norwogonin in combination with synthetic anti-Gram negative bacterial agents
Stock solutions of ampicillin, cefepime, sulbactam, sulfamethoxazole (Fisher Scientific, Pittsburgh, Pennsylvania, USA), azithromycin, levofloxacin, minocycline, rifampin and trimethoprim (Sigma-Aldrich) were prepared at 12.8 mg/mL in DMSO. Stock solutions of colistin (Sigma-Aldrich) and tobramycin (Fisher) were dissolved in water. Imipenem (USP, Rockville, Maryland, USA) solution was warmed to 50°C for 5 min to facilitate solubilization, aliquoted, and stored at −20°C. Ampicillin and sulbactam were combined in a ratio of 2∶1 while trimethoprim and sulfamethoxazole were combined in a ratio of 5∶1. The same ratios are used for commercially available co-formulated ampicillin/sulbactam (2∶1) and trimethoprim and sulfamethoxazole (5∶1). The MIC90 values of antibiotics against E. coli 25922 were determined in triplicate experiments on two separate days, and were consistent with CLSI performance standards [10].
MIC90 of eight synthetic antibiotics and two synthetic antibiotic combinations against 31P, BAA-1605 and E. coli 25922 were determined using a modified broth micro-dilution methods as described by CLSI [11]. 20 µL of bacterial culture diluted to 106 CFU/mL in CAMH broth were dispensed to 384-well plates containing 20 µL of two-fold serial dilutions of the antibiotics in CAMH broth. The antibiotics were tested in the following 12-point two-fold serial dilutions series: 0.016–64 µg/mL for ampicillin/sulbactam, azithromycin, colistin, imipenem, levofloxacin, minocycline and rifampin; 0.031–128 µg/mL for trimethoprim/sulfamethoxazole; and 0.063–256 µg/mL for cefepime and tobramycin. Final DMSO concentration in the assay was 1%. Positive and negative controls were included as described above. Plates were incubated without agitation for 16 h at 37°C, and OD600 nm was measured. The % growth inhibition was calculated as described above. Combinations of norwogonin and chemotherapeutic agents were tested as follows: half maximal inhibitory concentration (IC50) of norwogonin was extrapolated from the graph of bacterial growth inhibition. Testing of norwogonin in combination with each of the above synthetic antibiotics and antibiotic combinations against 31P was performed in the same fashion, except that in addition to the synthetic antibiotic dose response, CAMH broth was supplemented with norwogonin either at its IC50 (16 µg/mL) or a concentration one step below the IC50 (8 µg/mL). The experiment was performed in duplicate on two separate days. The combination study was repeated with the same strain using each of the above synthetic antibiotics at a concentration that was one step below their respective IC50 and a dose response of norwogonin. The experiment was performed in triplicate on one day.
Results
Dose response testing of crude and de-tanninized plant extracts
As DMSO was used to solubilize Scutellaria baicalensis extracts, norwogonin and many of the synthetic antibiotics, the DMSO tolerance of 31P was evaluated prior to MIC and MBS determination. As shown in Figure S2, growth of 31P decreased with increasing DMSO concentration. The reduction in growth was most pronounced above 2% DMSO in the medium. Therefore, all subsequent assays were performed at or below a 1% DMSO final concentration.
Due to their non-specific protein binding capacity, tannins are known to interfere with the isolation and purification of bioactive compounds [12]; therefore, the antibacterial effect of the various plant extracts was determined before and after de-tanninization. Results of dose response testing of our plant extracts before and after de-tanninization are shown in Figure S3. At concentrations between 7.81 and 1,000 µg/mL, the antimicrobial potency of the four extracts (Rosa rugosa, Rubus chingii, Scutellaria baicalensis and Terminalia chebula) was reduced after de-tanninization by 13.5 to 39.3% (Figures S3D–G). In this concentration range, Magnolia officinalis, Mahonia bealei and Rabdosia rubescens showed no inhibitory activity before or after de-tanninization (Figures S3A–C).
Isolation and characterization of antimicrobial compounds
UV chromatograms of the seven extracts from the LC/MS system are shown in Figure S4. Eighty fractions from each extract were tested against 31P in vitro, and the chemical structures of the fractions inhibiting >40% of the bacterial growth were identified on the basis of MS and UV data as follows: ellagic acid, which is a phenolic natural antioxidant in Rosa rugosa; norwogonin, which is a flavonoid in Scutellaria baicalensis; chebulinic acid, corilagin and terchebulin, all of which are ellagitannins in Terminalia chebula; and chebulagic acid, which is a benzopyran tannin antioxidant in Terminalia chebula. Chromatograms of Rosa rugosa, Scutellaria baicalensis and Terminalia chebula extracts from preparative HPLC are shown in Figure S5. Peaks with a retention time of 13.783 min corresponded to ellagic acid (Figure S5A); retention time of 16.958 min corresponded to norwogonin (Figure S5B); and retention times of 9.129, 10.946, 12.931 and 14.443 min corresponded to terchebulin, corilagin, chebulagic acid and chebulinic acid, respectively (Figure S5C).
The purity of norwogonin was confirmed to be >95% by HPLC and 1H NMR, and that of ellagic acid, chebulagic acid, chebulinic acid, corilagin and terchebulin was confirmed to be >85% by HPLC.
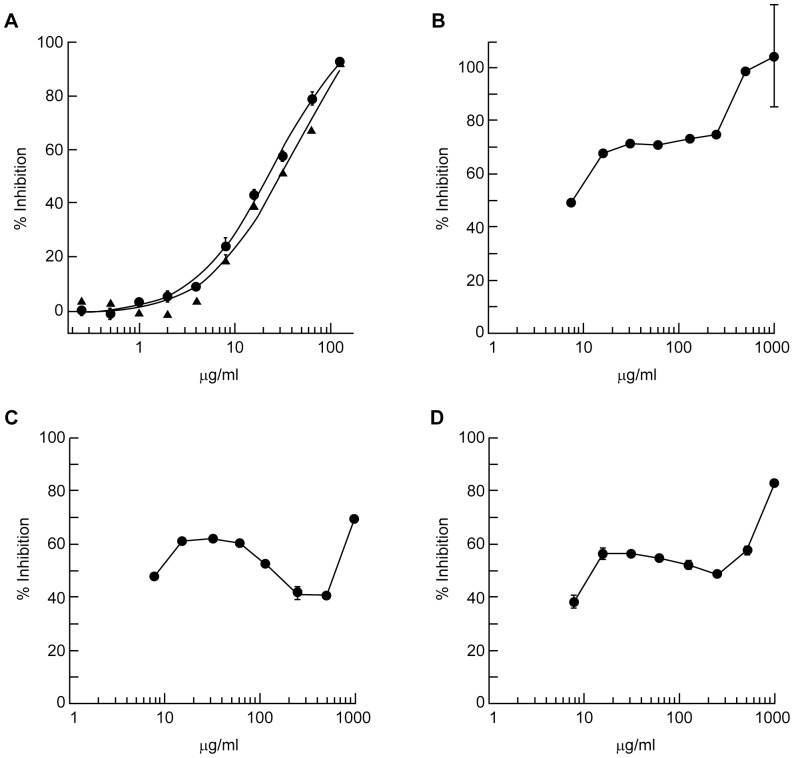
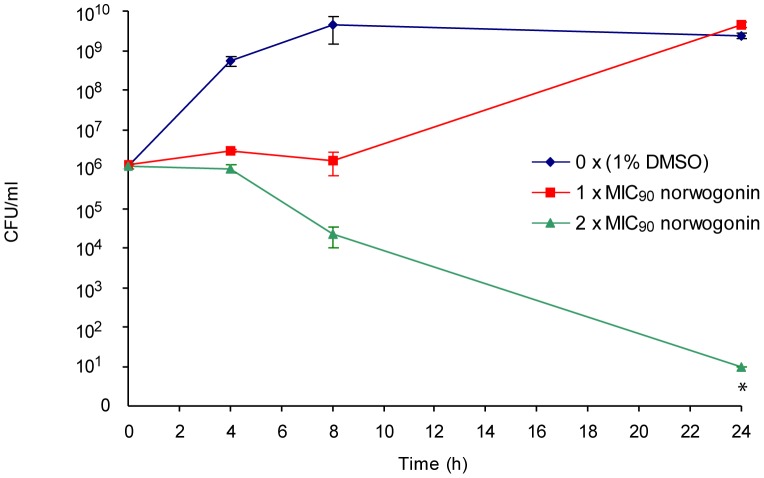
The most potent plant-derived compound was determined to be norwogonin with an MIC90 of 128 µg/mL against both 31P and BAA-1605 (Figure 1A). The MIC90 of terchebulin, the second most potent compound, was 500 µg/mL (Figure 1B). The chemical formula and structures of norwogonin and terchebulin are shown in Figure S6. Time-kill kinetic analysis of norwogonin against 31P showed complete growth inhibition at 2×MIC (256 µg/mL), and no re-growth was observed at 24 h (Figure 2).
Figure 1. Determination of antimicrobial activity of purified compounds from plant extracts against two A. baumannii strains.
Two-fold serially diluted norwogonin (A), terchebulin (B), chebulagic acid (C) and corilagin (D) suspensions were prepared in cation-adjusted Mueller-Hinton broth and mixed with an equal volume of either strain 31P or BAA-1605 suspension (5×105 CFU/mL final). Bacterial growth was measured after a 16 h incubation at 37°C. The final test concentration for each compound ranged from 0.25 to 128 µg/mL for norwogonin (MIC90 = 128 µg/mL), and 7.8 to 1,000 µg/mL for terchebulin (MIC90 = 500 µg/mL), chebulagic acid and corilagin. • 31P and ▴ BAA-1605.
Figure 2. Time-kill kinetic analysis of norwogonin against 31P.
The time-kill kinetics of 31P by norwogonin at 1× and 2× MIC was studied over a 24 h incubation. Aliquots were collected at 0, 4, 8 and 24 h, serially diluted in phosphate buffered saline before plating on Mueller-Hinton agar plates. Surviving colonies were enumerated after an 18 h incubation at 37°C. * Estimate: no colonies were observed at the highest concentration plated.
The MIC testing of norwogonin was repeated against 31P, 125P and 152P to confirm that it had the same growth inhibitory effects on A. baumannii strains that are clonally distinct and that have different antimicrobial susceptibility profile. In this experiment, MIC90 was determined by measuring turbidity. A resazurin reduction assay was performed in parallel to confirm MIC90 as, in the absence of cell lysis, turbidity cannot distinguish between live and dead bacteria [13]. Resazurin, an oxidation-reduction indicator, has been used to assess bacterial viability and to test for antimicrobial activity [14]–[16]. MIC90 of norwogonin against 31P, 125P and 152P was 128 µg/mL in all experiments by turbidity measurement (Figure 3A) and resazurin reduction assay (Figure 3B).
Figure 3. MIC90 determination of purified norwogonin against three clonally distinct strains of A. baumannii.
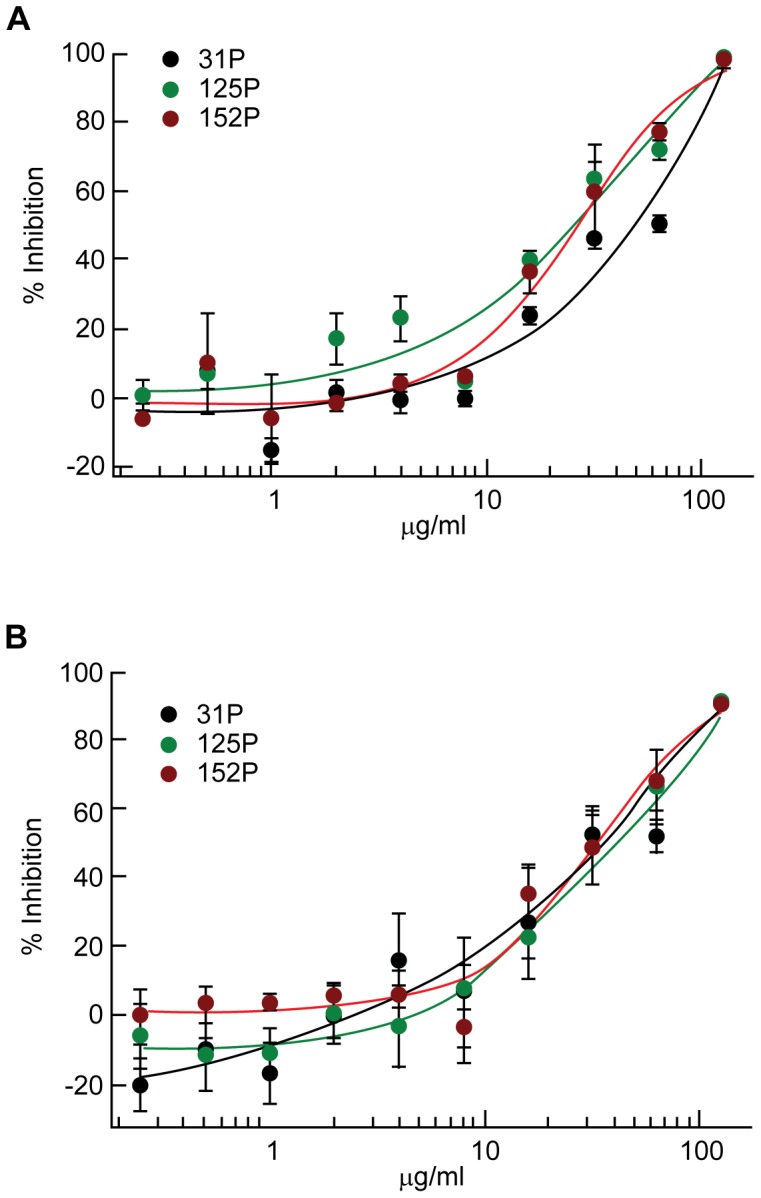
MIC90 of norwogonin against 31P, 125P and 152P was determined by measuring OD600 nm (A) and was confirmed by resazurin reduction assay (B).
As shown in Figure 1A, dose-response testing of norwogonin demonstrated a sigmoidal growth inhibition curve (Figure 1A). Terchebulin demonstrated a biphasic growth inhibition curve (Figure 1B). Growth inhibition reached 70% at 31.25 µg/mL and remained steady up to 250 µg/mL before increasing to 90% inhibition at concentrations greater than 250 µg/mL. This biphasic growth inhibition curve was also seen in chebulagic acid and corilagin (Figures 1CD).
The following compounds did not reach the 90% inhibition threshold: ellagic acid inhibited 67% at 250 µg/mL; chebulagic acid inhibited 60.39% at 62.5 µg/mL and 88% at 1,000 µg/mL; chebulinic acid inhibited 65% at 62.5 µg/mL; and corilagin inhibited 56% at 15.625 µg/mL and 83% at 1,000 µg/mL.
Dose response testing of norwogonin in combination with anti-Gram negative bacterial agents
Ten synthetic antibiotics and antibiotic combinations belonging to different classes and clinically relevant for the treatment of a variety of Gram-negative bacterial infections were selected and tested alone or in combination with norwogonin for growth inhibitory activity against 31P.
When tested alone at 8 and 16 µg/mL against 31P, norwogonin resulted in 40% and 53% growth inhibition, respectively. The IC90 values for synthetic antibiotic alone or in combinations with either 8 or 16 µg/mL of norwogonin are shown in Table 1. None of the ten synthetic antibiotics or antibiotic combinations displayed significant enhancement in their anti-A. baumannii activity when tested in the presence of norwogonin. Similarly, dose response testing of norwogonin in the presence of a fixed dose of synthetic antibiotics did not demonstrate a shift in inhibitory activity (data not shown).
Table 1. Dose response testing of synthetic anti-Gram negative bacterial agents in combination with norwogonin.
| Anti-Gram negative bacterial agents | IC90 of antibacterial agents alone | IC90 in the presence of 8 µg/mL norwogonin | IC90 in the presence of 16 µg/mL norwogonin |
| Ampicillin/sulbactam | 5.8 | 4.8 | 4.9 |
| Azithromycin | 2.1 | 2.0 | 2.7 |
| Cefepime | 31 | 23 | 30 |
| Colistin | 0.66 | 1.6 | 1.2 |
| Imipenem | 3.6 | 2.6 | 3.9 |
| Levofloxacin | 31 | 27 | 38 |
| Minocycline | 1.0 | 0.71 | 0.78 |
| Rifampin | 2.0 | 1.2 | 2.0 |
| Tobramycin | 80 | 69 | 89 |
| Trimethoprim/sulfamethoxazole* | - | - | - |
The IC90 (µg/mL) of each antibiotic either alone or in combination with 8 or 16 µg/mL norwogonin against strain 31P were determined. Results are presented as the average IC90 for two experiments each done in duplicate.
IC90 for trimethoprim/sulfamethoxazole could not be determined as maximum inhibition remained below 90% at all concentrations tested.
Discussion
In this study, the most potent non-tannin fraction was identified as norwogonin (5,6,7-trihydroxyflavone) from Scutellaria baicalensis, with an MIC90 of 128 µg/mL against clonally distinct clinical strains of A. baumannii as well as the ATCC strain BAA-1605. Ellagic acid, chebulinic acid, chebulagic acid, corilagin and terchebulin had low to moderate anti-A. baumannii activity in vitro.
To our knowledge this is the first report of anti-A. baumannii activities for norwogonin although inhibitory activities of Scutellaria baicalensis and its constituents, baicalin and baicalein, against other bacteria are documented in the medical literature. For example, baicalin was shown to inhibit Chlamydia trachomatis by down-regulating the expression of its serine protease, Chlamydia protease-like activity factor [17], [18]; ethanol extract of Scutellaria baicalensis was reported to have mild growth inhibitory effects on Salmonella enterica serovars Typhimurium, Kentucky, Senftenberg, and Enteritidis in vitro [19]; baicalin and Scutellaria baicalensis were found subsequently to be bactericidal against Helicobacter pylori based on broth dilution assays [20]; the mechanism of growth inhibition in the latter two studies remains unknown. A study by Chan et al (2011) demonstrated that a combination of baicalein and ciprofloxacin synergistically inhibited quinolone-resistant strains of Methicillin-resistant Staphylococcus aureus in vitro, and baicalein was shown to inhibit enzymatic activity of staphylococcal pyruvate kinase [21].
Terchebulin, chebulagic acid and corilagin in Terminalia chebula demonstrated a two-step killing kinetic. We have previously demonstrated the skip-well phenomenon of Terminalia chebula (i.e. an observation of regrowth after a clearly defined point of bacterial inhibition in broth dilution) [4]. However, at this time, it is unclear whether the seemingly biphasic nature of growth inhibition with terchebulin, chebulagic acid and corilagin in Terminalia chebula, is related to the previously observed skip-well phenomenon.
In the medical literature, several phenolic compounds from plant extracts are reported to enhance the potency of synthetic antibiotics against A. baumannii in vitro. For example, ellagic and tannic acids were reported to enhance the activity of novobiocin, coumermycin, chlorobiocin, rifampicin and fusidic acid against A. baumannii in vitro [22]. Synergy was noted between a purified polyphenol in green tea and topical mafenide against a clinical strain of MDR A. baumannii in vitro [5]. In contrast, we observed no evidence of additivity or synergy in the activity of combinations of norwogonin and anti-Gram negative antibiotics. In this study, combining norwogonin with ellagic acid, chebulagic acid, chebulinic acid, corilagin or terchebulin did not produce synergy against 31P in vitro either (data not shown).
This research project was a critical initial part of our effort to develop new therapeutics and infection-control modalities for MDR A. baumannii. Development of norwogonin as a systemic therapeutic may be limited by its high MIC against A. baumannii. In drug development where unfavorable factors (e.g. limited bioavailability and high toxicity) limit systemic use of novel compounds, their topical application has been tested to address superficial infections and colonization as exemplified by synthetic antimicrobial peptides [23]. A key to controlling MDR A. baumannii outbreaks is identification and elimination of its source [2], [3], [24]. Studies have shown that antibacterial prophylaxis with topical and systemic agents can decrease respiratory tract infections in critically ill patients [25]. Indeed, it is suggested that adjunctive control measures to decolonize MDR A. baumannii from patients' skin should be explored [2]. Colistin, which is considered a last resort systemic antibiotic for infections due to MDR A. baumannii and is available in a topical form at a 1,000× the systemic concentration, can still result in resistance in A. baumannii, as colistin usage increases [1]. It would seem to be more prudent to reserve colistin for the treatment of life-threatening infections due to MDR A. baumannii, and to utilize different antimicrobial agents for its decolonization. Therefore, further studies are warranted to ascertain the prophylactic and therapeutic potential of norwogonin for infections due to MDR A. baumannii.
Supporting Information
Dendrogram of 31P, 125P and 152P. The strains were analyzed by repetitive-polymerase chain reaction amplification (PCR); PCR products were separated by a gel matrix. Band patterns for each strain were aligned and interpreted as described in our previous study [9].
(TIF)
Dose response testing of dimethyl sulfoxide (DMSO) against 31P. Growth of 31P was measured after a 16 h incubation at 37°C in cation-adjusted Mueller-Hinton broth supplemented with increasing concentration of DMSO.
(TIF)
Dose response testing of crude and de-tanninized extracts against A. baumannii strain 31P. The ability of crude and de-tanninized extracts of Magnolia officinalis (A) Mahonia bealei (B), Rabdosia rubescens (C), Rosa rugosa (D), Rubus chingii (E), Scutellaria baicalensis (F), and Terminalia chebula (G) to inhibit growth of 31P was evaluated by measuring optical density at 600 nm (OD600 nm) after a 16 h incubation of 5×105 CFU/mL suspension in cation-adjusted Mueller-Hinton broth supplemented with increasing concentration (7.8125–1,000°g/mL) of each extract
(TIF)
Ultraviolet chromatogram of the seven extracts from the liquid chromatography/mass spectrometry system. Magnolia officinalis (A), Mahonia bealei (B), Rabdosia rubescens (C), Rosa rugosa (D), Rubus chingii (E), Scutellaria baicalensis (F), and Terminalia chebula (G).
(TIF)
Chromatograms of Rosa rugosa , Scutellaria baicalensis and Terminalia chebula extracts from high performance liquid chromatography. The chemical structures of the fractions that resulted in >40% of the bacterial growth inhibition corresponded to peaks with retention times of 13.783 min from Rosa rugosa (A); 16.958 min from Scutellaria baicalensis (B); and 9.129, 10.946, 12.931 and 14.443 min from Terminalia chebula (C). The precise chemical structures were elucidated by liquid chromatography/mass spectrometry analysis and nuclear magnetic resonance spectroscopy.
(TIF)
Chemical structure of the most potent compounds in this study. Norwogonin (A) in Scutellaria baicalensis (chemical formula C15H10O5), and terchebulin (B) in Terminalia chebula (chemical formula C48H28O30).
(TIF)
Acknowledgments
We express our gratitude to Raymond C. Chan, Ph.D. who analyzed our three clinical strains of A. baumannii by repetitive-polymerase chain reaction amplification in our previous study and supplied the dendrogram in Figure S1.
Funding Statement
This research study was funded by Cedars-Sinai Medical Center in Los Angeles, California, USA. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Perez F, Hujer AM, Hujer KM, Decker BK, Rather PN, et al. (2007) Global challenge of multidrug-resistant Acinetobacter baumannii . Antimicrob Agents Chemother 51: 3471–3484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Munoz-Price LS, Weinstein RA (2008) Acinetobacter infection. N Engl J Med 358: 1271–1281. [DOI] [PubMed] [Google Scholar]
- 3. Karageorgopoulos DE, Falagas ME (2008) Current control and treatment of multidrug-resistant Acinetobacter baumannii infections. Lancet Infect Dis 8: 751–762. [DOI] [PubMed] [Google Scholar]
- 4. Miyasaki Y, Nichols WS, Morgan MA, Kwan JA, Van Benschoten MM, et al. (2010) Screening of herbal extracts against multi-drug resistant Acinetobacter baumannii . Phytother Res 24: 1202–1206. [DOI] [PubMed] [Google Scholar]
- 5. Osterburg A, Gardner J, Hyon SH, Neely A, Babcock G (2009) Highly antibiotic-resistant Acinetobacter baumannii clinical isolates are killed by the green tea polyphenol (-)-epigallocatechin-3-gallate (EGCG). Clin Microbiol Infect 15: 341–346. [DOI] [PubMed] [Google Scholar]
- 6. Sukumaran S, Kiruba S, Mahesh M, Nisha SR, Miller PZ, et al. (2011) Phytochemical constituents and antibacterial efficacy of the flowers of Peltophorum pterocarpum (DC.) Baker ex Heyne. Asian Pac J Trop Med 4: 735–738. [DOI] [PubMed] [Google Scholar]
- 7. Cowan MM (1999) Plant products as antimicrobial agents. Clin Microbiol Rev 12: 564–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Scalbert A (1999) Antimicrobial properties of tannins. Phytochemistry 30: 3875–3883. [Google Scholar]
- 9. Miyasaki Y, Morgan MA, Chan RC, Nichols WS, Hujer KM, et al. (2012) In vitro activity of antibiotic combinations against multidrug-resistant strains of Acinetobacter baumannii and the effects of their antibiotic resistance determinants. FEMS Microbiol Lett 328: 26–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clinical and Laboratory Standards Institute (2009) Performance standards for antimicrobial susceptibility testing; nineteenth informational supplement M100-S19. Wayne: Clinical and Laboratory Standards Institute. pp. 46–47, 100–101.
- 11.Clinical and Laboratory Standards Institute (2006) Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard–seventh edition M07-A7. Wayne: Clinical and Laboratory Standards Institute. pp. 16–18.
- 12.Jones WP, Kinghorn AD (2010) Extraction of plant secondary metabolites. In: Sarker SD, Latif Z, Gray AI, editors. Natural Product Isolation. 2nd ed. Totowa: Humana Press. pp. 323–351.
- 13. Carroll P, Schreuder LJ, Muwanguzi-Karugaba J, Wiles S, Robertson BD, et al. (2010) Sensitive detection of gene expression in mycobacteria under replicating and non-replicating conditions using optimized far-red reporters. PLoS One 5 ((3)) e9823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mann CM, Markham JL (1998) A new method for determining the minimum inhibitory concentration of essential oils. J Appl Microbiol 84 ((4)) 538–44. [DOI] [PubMed] [Google Scholar]
- 15. Palomino JC, Martin A, Camacho M, Guerra H, Swings J, et al. (2002) Resazurin microtiter assay plate: simple and inexpensive method for detection of drug resistance in Mycobacterium tuberculosis . Antimicrob Agents Chemother 46 ((8)) 2720–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Smith CF, Townsend DE (1999) A new medium for determining the total plate count in food. J Food Prot 62 ((12)) 1404–10. [DOI] [PubMed] [Google Scholar]
- 17. Hao H, Aixia Y, Dan L, Lei F, Nancai Y, et al. (2009) Baicalin suppresses expression of Chlamydia protease-like activity factor in Hep-2 cells infected by Chlamydia trachomatis . Fitoterapia 80: 448–452. [DOI] [PubMed] [Google Scholar]
- 18. Hao H, Aixia Y, Lei F, Nancai Y, Wen S (2010) Effects of baicalin on Chlamydia trachomatis infection in vitro . Planta Med 76: 76–78. [DOI] [PubMed] [Google Scholar]
- 19. Lu Y, Joerger R, Wu C (2011) Study of the chemical composition and antimicrobial activities of ethanolic extracts from roots of Scutellaria baicalensis Georgi. J Agric Food Chem 59 ((20)) 10934–42. [DOI] [PubMed] [Google Scholar]
- 20. Wu J, Hu D, Wang KX (2008) Study of Scutellaria baicalensis and Baicalin against antimicrobial susceptibility of Helicobacter pylori strains in vitro . Zhong Yao Cai 31: 707–710. [PubMed] [Google Scholar]
- 21. Chan BC, Ip M, Lau CB, Lui SL, Jolivalt C, et al. (2011) Synergistic effects of baicalein with ciprofloxacin against NorA over-expressed methicillin-resistant Staphylococcus aureus (MRSA) and inhibition of MRSA pyruvate kinase. J Ethnopharmacol 137 ((1)) 767–73. [DOI] [PubMed] [Google Scholar]
- 22. Chusri S, Villanueva I, Voravuthikunchai SP, Davies J (2009) Enhancing antibiotic activity: a strategy to control Acinetobacter infections. J Antimicrob Chemother 64: 1203–1211. [DOI] [PubMed] [Google Scholar]
- 23. Hancock RE, Sahl HG (2006) Antimicrobial and host-defense peptides as new anti-infective therapeutic strategies. Nat Biotechnol 24: 1551–1557. [DOI] [PubMed] [Google Scholar]
- 24. Urban C, Segal-Maurer S, Rahal JJ (2003) Considerations in control and treatment of nosocomial infections due to multidrug-resistant Acinetobacter baumannii . Clin Infect Dis 36: 1268–1274. [DOI] [PubMed] [Google Scholar]
- 25. Krueger WA, Lenhart FP, Neeser G, Ruckdeschel G, Schreckhase H, et al. (2002) Influence of combined intravenous and topical antibiotic prophylaxis on the incidence of infections, organ dysfunctions, and mortality in critically ill surgical patients: a prospective, stratified, randomized, double-blind, placebo-controlled clinical trial. Am J Respir Crit Care Med 166: 1029–1037. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Dendrogram of 31P, 125P and 152P. The strains were analyzed by repetitive-polymerase chain reaction amplification (PCR); PCR products were separated by a gel matrix. Band patterns for each strain were aligned and interpreted as described in our previous study [9].
(TIF)
Dose response testing of dimethyl sulfoxide (DMSO) against 31P. Growth of 31P was measured after a 16 h incubation at 37°C in cation-adjusted Mueller-Hinton broth supplemented with increasing concentration of DMSO.
(TIF)
Dose response testing of crude and de-tanninized extracts against A. baumannii strain 31P. The ability of crude and de-tanninized extracts of Magnolia officinalis (A) Mahonia bealei (B), Rabdosia rubescens (C), Rosa rugosa (D), Rubus chingii (E), Scutellaria baicalensis (F), and Terminalia chebula (G) to inhibit growth of 31P was evaluated by measuring optical density at 600 nm (OD600 nm) after a 16 h incubation of 5×105 CFU/mL suspension in cation-adjusted Mueller-Hinton broth supplemented with increasing concentration (7.8125–1,000°g/mL) of each extract
(TIF)
Ultraviolet chromatogram of the seven extracts from the liquid chromatography/mass spectrometry system. Magnolia officinalis (A), Mahonia bealei (B), Rabdosia rubescens (C), Rosa rugosa (D), Rubus chingii (E), Scutellaria baicalensis (F), and Terminalia chebula (G).
(TIF)
Chromatograms of Rosa rugosa , Scutellaria baicalensis and Terminalia chebula extracts from high performance liquid chromatography. The chemical structures of the fractions that resulted in >40% of the bacterial growth inhibition corresponded to peaks with retention times of 13.783 min from Rosa rugosa (A); 16.958 min from Scutellaria baicalensis (B); and 9.129, 10.946, 12.931 and 14.443 min from Terminalia chebula (C). The precise chemical structures were elucidated by liquid chromatography/mass spectrometry analysis and nuclear magnetic resonance spectroscopy.
(TIF)
Chemical structure of the most potent compounds in this study. Norwogonin (A) in Scutellaria baicalensis (chemical formula C15H10O5), and terchebulin (B) in Terminalia chebula (chemical formula C48H28O30).
(TIF)