Abstract
Chytridiomycosis, a disease caused by Batrachochytrium dendrobatidis, has contributed to worldwide amphibian population declines; however, the pathogenesis of this disease is still somewhat unclear. Previous studies suggest that infection disrupts cutaneous sodium transport, which leads to hyponatremia and cardiac failure. However, infection is also correlated with unexplained effects on appetite, skin shedding, and white blood cell profiles. Glucocorticoid hormones may be the biochemical connection between these disparate effects, because they regulate ion homeostasis and can also influence appetite, skin shedding, and white blood cells. During a laboratory outbreak of B. dendrobatidis in Australian Green Tree Frogs, Litoria caerulea, we compared frogs showing clinical signs of chytridiomycosis to infected frogs showing no signs of disease and determined that diseased frogs had elevated baseline corticosterone, decreased plasma sodium and potassium, and altered WBC profiles. Diseased frogs also showed evidence of poorer body condition and elevated metabolic rates compared with frogs showing no signs of disease. Prior to displaying signs of disease, we also observed changes in appetite, body mass, and the presence of shed skin associated with infected but not yet diseased frogs. Collectively, these results suggest that elevated baseline corticosterone is associated with chytridiomycosis and correlates with some of the deleterious effects observed during disease development.
Introduction
Emerging infectious diseases (EIDs) of wildlife can have profound effects on animal biodiversity [1], [2]; however, little is known about the pathogenesis of most wildlife EIDs [3]. Since wildlife EIDs are often associated with anthropogenic and environmental stressors, pathogenesis is likely influenced by the host’s response to stressors [3]–[6]. The evolutionarily conserved stress response is one of the mechanisms by which vertebrates modulate responses to these stressors [7]. The stress response is of interest in a disease context, because it is mediated by glucocorticoid (GC) hormones that are known to affect susceptibility to infection [8].
GCs influence a suite of physiological functions in vertebrates, including reproduction, development, blood ion homeostasis, metabolism, appetite, growth, and, importantly in the context of disease, immunity [9]. While much is known about how GCs influence physiological function in non-diseased animals, much less is known about how GCs influence the same physiological functions in diseased animals. To our knowledge only one such study has been conducted in wild vertebrates. Warne et al. [10] exposed Rana sylvatica to ranaviruses and observed an increase in corticosterone (CORT; the most abundant amphibian GC) concentration and accelerated developmental changes consistent with the effects of endogenous and exogenous elevations of CORT in non-diseased amphibians.
Chytridiomycosis, a disease caused by the amphibian chytrid fungus Batrachochytrium dendrobatidis (Bd) [11] has contributed to worldwide amphibian population declines. It is considered to be a significant threat to global amphibian biodiversity [2], [12]–[14]. Chytridiomycosis, like CORT, influences blood ion homeostasis, appetite, skin shedding, and immunity. Specifically, Bd disrupts sodium transport in the host’s epidermis, which leads to hyponatremia and cardiac failure [15]. Bd also suppresses appetite [15], [16], disrupts normal skin shedding [15], [16], and causes alterations in white blood cell (WBC) abundance [17], [18]. Yet there are no studies that have attempted to document what hormones may be mediating these changes in blood ions, behavior, shedding, and WBC abundance.
GCs may mediate the aforementioned effects of Bd infection. In amphibians, GCs are critical regulators of blood ion homeostasis [19]–[24], appetite [25], [26], skin shedding [27]–[30], and WBC numbers and immune function [31]–[37]. A normal, adaptive, regulatory mechanism to maintain sodium homeostasis is likely a moderate, transitory elevation in CORT secretion to increase cutaneous uptake of sodium as well as digestive uptake (facilitated by increased appetite) [23], [24], [26]. Because Bd infection directly compromises cutaneous sodium transport, a sustained elevation of CORT could occur to maintain ion homeostasis. However, high concentrations of GCs may become maladaptive, altering immune responses [36]–[39], increasing metabolic rate [40], as well as actually suppressing appetite [41]. The suppression of appetite may further exacerbate ion imbalance, leading to unsustainable blood sodium levels and cardiac failure. Thus, CORT, in its regulatory role of maintaining ion homeostasis, may be elevated in response to disease caused by infection, and could contribute to Bd-induced mortality.
The overall aims of our study were to determine whether Bd infection influences CORT levels and whether CORT profiles are associated with previously undescribed, as well as previously described effects of Bd infection. We documented the relationship of Bd infection to plasma CORT, sodium, and potassium concentrations; food intake; skin shedding; and WBC profiles during an outbreak of Bd in a laboratory colony of Australian Green Tree Frogs (Litoria caerulea). Because both CORT and disease influence energy balance, we also monitored resting metabolic rate (RMR), body condition, and body mass.
Results
Evaluation of Pre-diseased Frogs
Individuals that eventually displayed clinical signs of disease (e.g. listlessness, odd body posture, and skin discoloration) consumed significantly less food one week prior to displaying clinical signs of disease compared to individuals that displayed no signs of disease (ANOVA, P = 0.022, F1,21 = 6.16; Fig. 1). During the week prior to sacrifice, shed skin was found on significantly more days within bins of frogs that eventually became diseased than within the bins of non-diseased frogs (ANOVA, P<0.001, F1,21 = 38.11; Fig. 1). Frogs that eventually became diseased also lost significantly more weight than frogs that remained non-diseased in the weeks prior to sacrifice (Repeated measures ANOVA, Disease status: P<0.001, F1,21 = 38.06, Time: P<0.001, F1,42 = 12.25, Disease status × Time: P = 0.2; Fig. 2).
Figure 1. Evaluation of pre-diseased frogs.
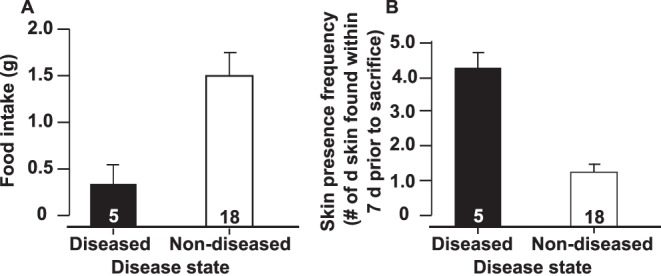
Average food intake (Fig. 1A) and skin presence frequency (Fig. 1B) +1 standard error of Litoria caerulea that eventually became diseased or remained non-diseased at one week prior to sacrifice (Fig. 1A) and within the week leading up to sacrifice (Fig. 1B). Disease states were statistically different (Food intake: ANOVA, P = 0.022, F1,21 = 6.16; skin presence frequency: ANOVA, P<0.001, F1,21 = 38.11).
Figure 2. Change in body mass throughout infection.
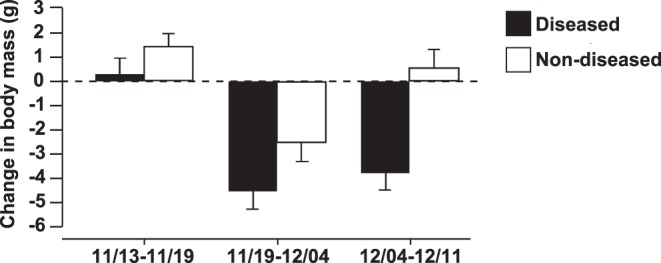
Average change in body mass (±1 standard error) of Litoria caerulea that eventually became diseased (n = 9) or remained non-diseased (n = 14) for chytridiomycosis between dates leading up to sacrifice on 12/12/09. Disease states were statistically different (Repeated measures ANOVA, Disease status: P<0.001, F1,21 = 38.06, Time: P<0.001, F1,42 = 12.25, Disease status × Time: P = 0.2).
Evaluation of Diseased Frogs
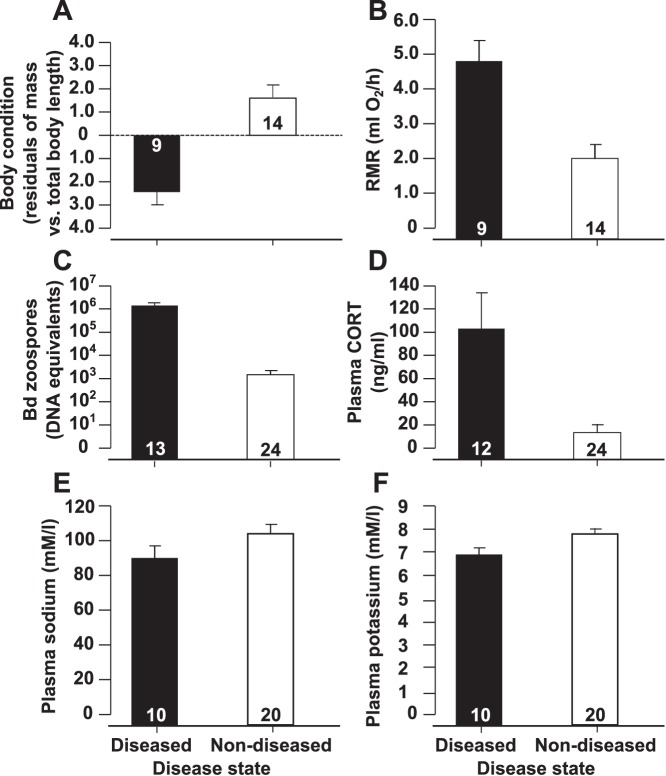
Approximately 24 hours prior to sacrifice, frogs that displayed clinical signs of chytridiomycosis had significantly lower body conditions (ANCOVA, Disease status: P<0.001, F1,20 = 19.02; Total body length: P<0.001, F1,20 = 273.43). There was no disease status by total body length interaction (P = 0.22). For ease of interpretation, these data are visually presented as average residuals from a regression of body mass by total body length (Fig. 3). Diseased individuals also consumed significantly more oxygen compared to non-diseased frogs (ANCOVA, Disease status: P<0.001, F1,20 = 24.52, Body mass: P = 0.010, F1,20 = 7.99; Fig. 3). There was no disease status by body mass interaction (P = 0.3).
Figure 3. Evaluation of diseased frogs.
Mean body condition (Fig. 3A), resting metabolic rate (RMR, Fig. 3B), log transformed Bd zoospore equivalents +1 (Fig. 3C), plasma corticosterone (CORT, Fig. 3D), plasma sodium (Fig. 3E), and plasma potassium (Fig. 3F) ±1 standard error for Litoria caerulea that displayed clinical signs of disease (diseased) or did not display clinical signs of disease (non-diseased). Disease states were significantly different for all measures (ANOVA/ANCOVA, P<0.05). Sample sizes vary among measures because of sampling limitations.
When sacrificed, swabs taken from diseased frogs contained significantly more Bd zoospore equivalents than swabs taken from non-diseased individuals (ANOVA, P<0.001, F1,35 = 19.66; Fig. 3). Although non-diseased individuals all had detectable levels of Bd, they contained approximately 1,000 times fewer zoospore equivalents than diseased individuals, on average.
Blood parameters determined at sacrifice also differed with disease status. Diseased frogs contained significantly fewer plasma electrolytes (ANOVA, Sodium: P = 0.049, F1,28 = 4.24, Potassium: P = 0.049, F1,28 = 4.24; Fig. 3) and significantly greater concentrations of plasma CORT (ANOVA, P = 0.001, F1,34 = 18.73; Fig. 3) compared with non-diseased frogs. Additionally, WBC profiles differed significantly between diseased and non-diseased individuals (MANOVA, P<0.001, F1,20 = 12.26; Fig. 4). Blood smears from diseased frogs contained significantly fewer lymphocytes and eosinophils and significantly more neutrophils among 100 WBCs counted than smears from non-diseased frogs (Sheffe’s range tests, P≤0.002).
Figure 4. White blood cell counts of diseased frogs.
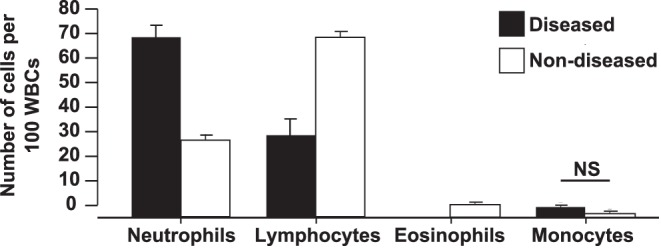
Average relative abundance of neutrophils, lymphocytes, eosinophils, and monocytes per 100 white blood cells (WBC) +1 standard error from Litoria caerulea displaying clinical signs of chytridiomycosis (diseased, n = 7) or not displaying clinical signs of disease (non-diseased, n = 19). WBC abundance profiles were significantly different between disease states (MANOVA, P<0.001, F1,20 = 12.26). Blood smears from diseased frogs contained significantly fewer lymphocytes and eosinophils and significantly more neutrophils than smears from non-diseased frogs (Sheffe’s range test, P<0.001). Disease states had similar numbers of monocytes (NS) and basophils (not shown).
Changes in CORT, RMR, and Lymphocyte Abundance at different Bd Burdens
Because all individuals in the study contained different Bd burdens and were, thus, apparently at different points in infection we used segmented regression to estimate the zoospore intensity at which CORT, RMR, and lymphocyte abundances changed significantly (the zoospore breakpoints). The zoospore breakpoints for CORT, RMR, and lymphocytes were 4,940; 4,066; and 10,778 zoospores, respectively (Fig. 5). Linear regressions suggested significant relationships between Bd burden and CORT (R2 = 0.25, P = 0.002, F1,35 = 11.40), RMR (R2 = 0.46, P<0.001, F1,22 = 18.16), and lymphocytes (R2 = 0.20, P = 0.022, F1,25 = 5.96).
Figure 5. Changes in variables at different Bd burdens.
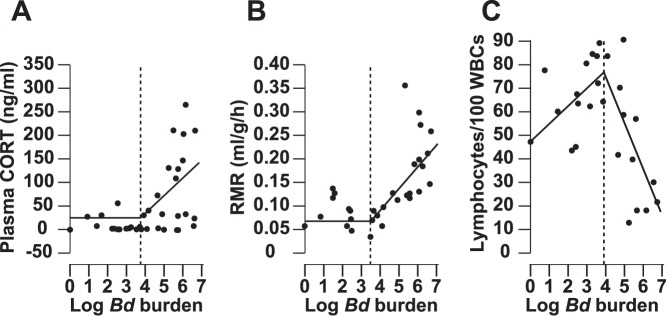
Segmented regressions of plasma corticosterone (CORT, Fig. 5A), resting metabolic rate (RMR, Fig. 5B), and lymphocyte abundances (Fig. 5C) of Litoria caerulea during an outbreak of chytridiomycosis. Horizontal and vertical dotted lines indicate X and Y axes, respectively. Vertical dashed lines indicate breakpoints at which the dependent variables changed significantly. Black lines indicate the two segments fit to the data before and after the breakpoint. The zoospore breakpoints for CORT, RMR, and lymphocytes were 4,940; 4,066; and 10,778 zoospores, respectively. Data before and after the breakpoint were significantly different for all three variables (Segmented regression, P<0.05). Linear regressions suggested significant relationships between Bd burden and CORT (R2 = 0.25, P = 0.002, F1,35 = 11.40), RMR (R2 = 0.46, P<0.001, F1,22 = 18.16), and lymphocytes (R2 = 0.20, P = 0.022, F1,25 = 5.96).
Discussion
Individuals that displayed clinical signs of chytridiomycosis had significantly elevated baseline CORT, decreased plasma sodium and potassium, altered WBC profiles, increased RMR, and decreased body condition compared with non-diseased individuals. WBC profiles and RMR changes in diseased frogs parallel those observed following treatment with glucocorticoids in other studies (e.g. increased neutrophils and oxygen consumption and decreased lymphocytes and eosinophils in relation to total WBC) [31]–[35], [40], [42]. It is important to note that non-diseased individuals were also infected, but released thousands of Bd zoospores while diseased individuals released millions of zoospores, on average. When we plotted this broad range of Bd burdens (regardless of disease status) against CORT, RMR, and lymphocytes, segmented regressions indicated these three variables changed significantly at similar breakpoints (4,000–10,000 zoospores; Fig. 5).
Appetite suppression likely contributed to other effects we observed. For example, appetite suppression likely exacerbated hyponatremia because amphibians take up sodium via digestive as well as cutaneous routes [43]. Additionally, amphibians usually consume their shed skin, so appetite suppression also could explain why we observed shed skin more often in the containers of frogs that eventually became diseased [43]. Finally, appetite suppression, coupled with an increased RMR, may have contributed to the weight loss and poor body condition observed in frogs that eventually became diseased. With no input of food, sick frogs must catabolize body tissues to meet their elevated respiratory demand, which results in weight loss and reduced body condition.
The levels of CORT in plasma were determined after development of several effects, so although infection was associated with decreased food intake, increased presence of shed skin, and weight loss, it is unclear whether increased CORT secretion was a cause or consequence of these parameters. We happened to be monitoring food intake, skin shedding, and weight loss as part of a separate experiment when the outbreak occurred, thus we did not monitor CORT along with these other variables. Although we observed changes in CORT, RMR, and lymphocytes at similar Bd burdens, future studies are needed to determine the timing of when key physiological variables change during infection and whether CORT manipulation can alter pathogenesis.
It is unclear whether infection-induced GC secretion is beneficial or maladaptive in vertebrates. Few studies have tested the effects of disease on GC levels in vertebrates [44]–[57]. Even fewer have observed how this may then lead to beneficial or deleterious physiological effects. To our knowledge, this is the first study that has assessed the effects of disease on baseline CORT levels in an adult amphibian (however CORT levels have been assessed in tadpoles) [10], [56], [57]. Our data suggest that disease, at least chytridiomycosis, may be a potent modulator of baseline plasma CORT. Frogs displaying clinical signs of disease contained eight times more plasma CORT than non-diseased frogs. Average plasma CORT was 104 ng/ml in symptomatic frogs (maximum level of 270 ng/ml), rivaling the highest average levels of CORT observed in amphibians in response to other stressors [58]–[62]. Given these findings and the large number of emerging diseases in wildlife, we suggest that more studies focus on post-infection stress responses in wild animals.
Better understanding of the physiological effects of CORT, and its involvement in mediating factors that threaten the conservation status of amphibians (e.g. habitat destruction, global climate change, pollution, etc.) is needed. Specifically, there is a lack of data on how CORT influences metabolic rate and appetite in amphibians [25], [63]. Our study provides data to suggest that CORT is associated with these factors, but controlled laboratory data are needed to complement this study. Understanding how amphibians respond to environmental change has become more urgent given recent amphibian population declines [64]. Several perturbations that potentially contribute to amphibian population declines have been linked to stress physiology (e.g. anthropogenic contaminants [62], [65]–[72], disease [10], [56], [57], low habitat quality [73], and habitat desiccation [74]). Though the influence of anthropogenic contaminants on the stress axis has been relatively well studied in amphibians, far less is known about how disease, habitat destruction, invasive predators, and climate change may influence stress physiology. Given the powerful and far reaching effects of GCs on wildlife life histories, understanding how these hormones mediate the interplay between environmental perturbations and life histories is essential to future conservation efforts.
Materials and Methods
Ethics Statement
All methods were approved by Auburn University Institutional Animal Care and Use Committee (Permit Number: 2009-1620). All surgery was performed following euthanasia and all efforts were made to minimize suffering.
Laboratory Outbreak and Experimental Design
Ninety eight Litoria caerulea were obtained commercially from an amphibian trader (Tri Reptile, Miami, FL) who imported the frogs from Indonesia, in autumn of 2009. Within a month, the frogs became ill and died at a rapid rate (i.e., 13 died within 13 days). Shed skin from individuals showing clinical signs of chytridiomycosis (e.g., listlessness, odd body posture, and skin discoloration) [75] was viewed under a light microscope and Bd was detected by visual inspection of the skin in all samples viewed. It is unclear whether infection originated from the wild or from our laboratory.
At this point, disease status (i.e., individuals displaying clinical signs of chytridiomycosis [diseased] or individuals not displaying clinical signs [non-diseased]) was monitored daily and food intake was assessed in all remaining non-diseased individuals (n = 79). When an individual became diseased, the diseased frog and two randomly selected non-diseased individuals were swabbed (to quantify Bd zoospores), pithed, and bled (within 3 min of handling). Several drops of whole blood were used to make blood smears for enumeration of WBCs. The remaining blood was centrifuged for 4 min at 3,500×g and the plasma was frozen and stored at −20°C for later use in ion and CORT assays.
Animal Care
Amphibians were housed individually in plastic containers (17×17×17 cm) in which paper towels saturated with well water were used as substrate. Wet paper towels were replaced twice each week for the duration of the study. Light was provided by full spectrum light bulbs on a 12∶12 light/dark cycle. Room temperature was maintained by a thermostat at ∼22°C.
Bd Zoospore Burden
Frogs were swabbed in a standardized fashion by lightly brushing a sterile cotton swab (Medical Wire & Equipment, MW113) 10 times over the sides, venter, and ventral surface of the thighs and 5 times over the underside of each foot [76]. Zoospore equivalents were determined by standard extraction and quantitative PCR techniques [77], [78]. Nucleic acids were extracted by adding 60 µl of PrepMan Ultra (Applied Biosystems, Foster City, CA) and 30–35 mg of Zirconium/silica beads (0.5 mm, Biospec Products, Bartlesville, OK) to the tip of each swab. Samples were homogenized for 45 s in a Mini Beadbeater (MP Bio, Solon, OH) and centrifuged for 30 s at 15,000×g. After a second homogenization and centrifugation, the samples were boiled for 10 min, returned to room temperature for 2 min, and centrifuged at 15,000×g for 3 min. Nucleic acids in the supernatant were removed for real-time PCR. Samples were loaded into an Mx3000P Real-Time PCR system (Stratagene, La Jolla, CA) for 40 cycles of 95°C for 10 min, 95°C for 30 s, 55°C for 1 min, and 72°C for 1 min. Zoospore equivalents were determined using a standard curve and indicate Bd burden.
Ion Analyses
Plasma prepared by centrifugation of whole blood for 4 min at 3,500×g to remove red blood cells, was analyzed by Inductively Coupled Plasma with Optical Emission Spectroscopy (ICP-OES, Perkin Elmer 7100 DV, Waltham, MA) with simultaneous measurement of Ca, Co, Cu, Fe, K, Mg, Mn, Mo, Na, P, S, Zn [79]. Equal volume of plasma was diluted into ultra-pure, metal-free water (MilliQ, Millipore) then centrifuged at 13,000×g to remove particulates and then introduced directly into the instrument argon plasma using a cyclonic nebulizer. Metal concentrations are determined comparing emission intensities to a standard curve created from certified metal standards (SPEX, Metuchen, NJ). Standard curves were confirmed by re-analysis of standard solutions diluted in a matrix equivalent to the sample. Individual readings are the average of two intensity measurements varied by less than 5%. Repeated analysis of individual samples showed less than 5% variability.
Radioimmunoassay
Plasma CORT concentrations were determined by radioimmunoassay as described by Mendonça et al. [80]. All samples were run in one assay. Extraction efficiency was 81% and intraassay variation was 19.8%.
Food Intake and Body Mass
Frogs were weighed weekly from the time they arrived in the laboratory. Once a week, for two weeks prior to sacrifice, each animal was blotted dry, weighed, and fed approximately 10% of their body weight with 2.5 week old crickets coated in vitamin dust. All crickets not consumed were weighed after 24 hours. Food intake was determined as the mass of the crickets not consumed subtracted from the original mass of crickets given to the frog.
Shed Skin Collection
A subsample of frogs and bins were examined daily for the presence of shed skin. Shed skin was removed if it was observed on amphibians or within their containers. Dates in which skin was found on a frog or within its container were recorded. When frogs were sacrificed, the number of days, within the previous seven days, the frogs had shed skin on their body or within their container was determined. This value is referred to hereafter as “skin presence frequency”.
Relative WBC Numbers
Dried blood smears were stained with a Hema 3 kit (Fisher scientific, Kalamazoo, MI) and viewed under a light microscope. Slides were read in a standard zig-zag fashion. One hundred WBCs were observed and the number of neutrophils, lymphocytes, eosinophils, monocytes, and basophils were recorded.
Respirometry and Body Condition
Closed system respirometry was used to measure RMR (oxygen consumption) 1 day prior to sacrifice following the methods of Ward et al. [81]. Frogs were first acclimated in their respirometry chamber (140 ml syringes served as the respirometry chambers, Monoject, Sherwood Medical Industries, Ballymoney, UK) for at least 45 min in a darkened incubator (22°C). For the respirometry measurements, the frogs were then incubated for ∼50 min in a darkened incubator (22°C). Any frogs that urinated or defecated during incubation were excluded from analyses. Frogs were weighed and their total body length was determined following respirometry to determine body condition [82].
Statistical Analyses
Bd burden; plasma CORT, sodium, and potassium; food intake; and skin presence frequency were compared relative to disease status with analyses of variance (ANOVAs). RMR was compared between disease states (i.e. diseased or non-diseased) with an analysis of covariance (ANCOVA) with disease status as the independent variable, RMR as the dependent variable, and body mass as the covariate. Since body mass influences RMR, RMR is presented as least squared means, corrected for body mass. Body condition was estimated as the residuals obtained by regressing body mass against total body length. Body condition was compared between disease states with an ANCOVA, with disease status as the independent variable, body mass as the dependent variable, and total body length as the covariate. Change in body mass was compared relative to disease status with a repeated measures ANOVA, because the same individuals were sampled multiple times. Relative WBC numbers were compared relative to disease status with a multivariate analysis of variance (MANOVA). Sheffe’s range tests were conducted for all a posteriori comparisons. We were unable to monitor changes over time for several variables; however, when diseased and non-diseased frogs were sacrificed Bd burden was highly variable across all frogs (ranging from 0 to millions of zoospores per frog), suggesting that each frog was at a different point within disease progression. We used segmented regression to determine the threshold Bd burden at which the trend of CORT, RMR, and lymphocytes changed significantly (breakpoints), regardless of disease status [83]. We also used linear regression to determine whether there were linear relationships between Bd burden and CORT, RMR, and lymphocytes. SAS (SAS institute, version 9.2) was used for the RMR ANCOVA (PROC GLM) and all segmented regressions (PROC NLIN). StatView for Windows (SAS institute, version 5.0.1) was used for all other statistical analyses. All data met the assumptions of normality and significance was determined as P≤0.05.
Acknowledgments
C. Guyer, W. Hopkins, R. Lishak, and J. Terhune provided valuable comments on this manuscript.
Funding Statement
This research was supported by the National Science Foundation Grants IOS-0619536 and IOS-0843207 (to LR-S). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Harvell CD, Kim K, Burkholder JM, Colwell RR, Epstein PR, et al. (1999) Emerging Marine Diseases–Climate Links and Anthropogenic Factors. Science 285(5433): 1505–1510. [DOI] [PubMed] [Google Scholar]
- 2. Lips KR, Brem F, Brenes R, Reeve JD, Alford RA, et al. (2006) Emerging infectious disease and the loss of biodiversity in a Neotropical amphibian community. Proc Natl Acad Sci USA 103(9): 3165–3170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Daszak P, Cunningham AA, Hyatt AD (2001) Anthropogenic environmental change and the emergence of infectious diseases in wildlife. Acta Trop 78(2): 103–116. [DOI] [PubMed] [Google Scholar]
- 4. Acevedo-Whitehouse K, Duffus ALJ (2009) Effects of environmental change on wildlife health. Phil Trans R Soc B 364(1534): 3429–3438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dobson A, Foufopoulos J (2001) Emerging infectious pathogens of wildlife. Phil Trans R Soc B 356(1411): 1001–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rachowicz LJ, Hero J, Alford RA, Taylor JW, Morgan JAT, et al. (2005) The Novel and Endemic Pathogen Hypothesis: Competing Explanations for the Origin of Emerging Infectious Diseases of Wildlife. Conserv Biol 19(5): 1441–1448. [Google Scholar]
- 7. Wingfield JC, Maney DL, Breuner CW, Jacobs JD, Lynn S, et al. (1998) Ecological Bases of Hormone–Behavior Interactions: The “Emergency Life History Stage”. Amer Zool 38(1): 191–206. [Google Scholar]
- 8. Elenkov IJ, Chrousos GP (1999) Stress Hormones, Th1/Th2 patterns, Pro/Anti-inflammatory Cytokines and Susceptibility to Disease. Trends Endocrinol Metabol 10(9): 359–368. [DOI] [PubMed] [Google Scholar]
- 9. Sapolsky RM, Romero LM, Munck AU (2000) How Do Glucocorticoids Influence Stress Responses? Integrating Permissive, Suppressive, Stimulatory, and Preparative Actions. Endocr Rev 21(1): 55–89. [DOI] [PubMed] [Google Scholar]
- 10. Warne RW, Crespi EJ, Brunner JL (2011) Escape from the pond: stress and developmental responses to ranavirus infection in wood frog tadpoles. Funct Ecol 25(1): 139–146. [Google Scholar]
- 11. Longcore JE, Pessier AP, Nichols DK (1999) Batrachochytrium dendrobatidis gen. et sp. nov., a chytrid pathogenic to amphibians. Mycologia 91(2): 219–227. [Google Scholar]
- 12. Berger L, Speare R, Daszak P, Green DE, Cunningham AA, et al. (1998) Chytridiomycosis causes amphibian mortality associated with population declines in the rain forests of Australia and Central America. Proc Natl Acad Sci USA 95(15): 9031–9036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Skerratt L, Berger L, Speare R, Cashins S, McDonald KR, et al. (2007) Spread of Chytridiomycosis Has Caused the Rapid Global Decline and Extinction of Frogs. EcoHealth 4(2): 125–134. [Google Scholar]
- 14. Kilpatrick AM, Briggs CJ, Daszak P (2010) The ecology and impact of chytridiomycosis: an emerging disease of amphibians. Trends Ecol Evolut 25(2): 109–118. [DOI] [PubMed] [Google Scholar]
- 15. Voyles J, Young S, Berger L, Campbell C, Voyles WF, et al. (2009) Pathogenesis of Chytridiomycosis, a Cause of Catastrophic Amphibian Declines. Science 326(5952): 582–585. [DOI] [PubMed] [Google Scholar]
- 16. Nichols D, Lamirande E, Pessier A, Longcore J (2001) Experimental transmission of cutaneous chytridiomycosis in dendrobatid frogs. J Wildl Dis 37(1): 1–11. [DOI] [PubMed] [Google Scholar]
- 17. Woodhams DC, Ardipradja K, Alford RA, Marantelli G, Reinert LK, et al. (2007) Resistance to chytridiomycosis varies among amphibian species and is correlated with skin peptide defenses. Anim Conserv 10(4): 409–417. [Google Scholar]
- 18. Davis A, Keel M, Ferreira A, Maerz J (2010) Effects of chytridiomycosis on circulating white blood cell distributions of bullfrog larvae (Rana catesbeiana). Comp Clin Path 19(1): 49–55. [Google Scholar]
- 19. Middler SA, Kleeman CR, Edwards E, Brody D (1969) Effect of adenohypophysectomy on salt and water metabolism of the toad Bufo marinus with studies on hormonal replacement. Gen Comp Endocrinol 12(2): 290–304. [DOI] [PubMed] [Google Scholar]
- 20. Yorio T, Bentley PJ (1978) Stimulation of the short-circuit current (sodium transport) across the skin of the forg (Rana pipiens) by corticosteroids: structure-activity relationships. J Endocrinol 79(3): 283–290. [DOI] [PubMed] [Google Scholar]
- 21. Brewer KJ, Hoyt BJ, McKeown BA (1980) The effects of prolactin, corticosterone and ergocryptine on sodium balance in the urodele, Ambystoma gracile . Comp Biochem Physiol C 66(2): 203–208. [Google Scholar]
- 22. De Ruyter ML, Stiffler DF (1986) Interrenal function in larval Ambystoma tigrinum: II. Control of aldosterone secretion and electrolyte balance by ACTH. Gen Comp Endocrinol 62(2): 298–305. [DOI] [PubMed] [Google Scholar]
- 23. Stiffler DF, De Ruyter ML, Hanson PB, Marshall M (1986) Interrenal function in larval Ambystoma tigrinum: I. Responses to alterations in external electrolyte concentrations. Gen Comp Endocrinol 62(2): 290–297. [DOI] [PubMed] [Google Scholar]
- 24. Heney HW, Stiffler DF (1983) The effects of aldosterone on sodium and potassium metabolism in larval Ambystoma tigrinum . Gen Comp Endocrinol 49(1): 122–127. [DOI] [PubMed] [Google Scholar]
- 25. Crespi EJ, Vaudry H, Denver RJ (2004) Roles of Corticotropin-Releasing Factor, Neuropeptide Y and Corticosterone in the Regulation of Food Intake In Xenopus laevis . J Neuroendocrinol 16(3): 279–288. [DOI] [PubMed] [Google Scholar]
- 26. Crespi EJ, Denver RJ (2005) Roles of stress hormones in food intake regulation in anuran amphibians throughout the life cycle. Comp Biochem Physiol A 141(4): 381–390. [DOI] [PubMed] [Google Scholar]
- 27. Jørgensen CB, Larsen LO (1964) Further observations on molting and its hormonal control in Bufo bufo (L.). Gen Comp Endocrinol 4(4): 389–400. [DOI] [PubMed] [Google Scholar]
- 28. Jørgensen CB, Larsen LO (1961) Molting and its hormonal control in toads. Gen Comp Endocrinol 1(2): 145–153. [DOI] [PubMed] [Google Scholar]
- 29. Stefano FJE, Donoso AO (1964) Hypophyso-adrenal regulation of moulting in the toad. Gen Comp Endocrinol 4(5): 473–480. [DOI] [PubMed] [Google Scholar]
- 30. Budtz PE (1979) Epidermal structure and dynamics of the toad, Bufo bufo, deprived of the pars distalis of the pituitary gland. J Zool 189(1): 57–92. [Google Scholar]
- 31. Bennett MF, Gaudio CA, Johnson AO, Spisso JH (1972) Changes in the blood of newts, Notophthalmus viridescens, following the administration of hydrocortisone. J Comp Physiol A 80(2): 233–237. [Google Scholar]
- 32. Bennett MF, Harbottle JA (1968) The effects of hydrocortisone on the blood of tadpoles and frogs, Rana catesbeiana . Biol Bull 135(1): 92–95. [DOI] [PubMed] [Google Scholar]
- 33. Garrido E, Gomariz RP, Leceta J, Zapata A (1987) Effects of dexamethasone on the lymphoid organs of Rana perezi . Dev Comp Immunol 11(2): 375–384. [DOI] [PubMed] [Google Scholar]
- 34. Belden LK, Kiesecker JM (2005) Glucocorticosteroid Hormone Treatment of Larval Treefrogs Increases Infection by Alaria Sp. Trematode Cercariae. J Parasitol 91(3): 686–688. [DOI] [PubMed] [Google Scholar]
- 35.Davis AK, Maerz JC (2010) Effects of Exogenous Corticosterone on Circulating Leukocytes of a Salamander (Ambystoma talpoideum) with Unusually Abundant Eosinophils. Int J Zool. doi:10.1155/2010/735937.
- 36. Rollins LA, McKinnell RG (1980) The influence of glucocorticoids on survival and growth of allografted tumors in the anterior eye chamber of leopard frogs. Dev Comp Immunol 4: 283–294. [DOI] [PubMed] [Google Scholar]
- 37. Ramakrishnan L, Valdivia RH, McKerrow JH, Falkow S (1997) Mycobacterium marinum causes both long-term subclinical infection and acute disease in the leopard frog (Rana pipiens). Infect Immun 65(2): 767–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Munck A, Guyre PM, Holbrook NJ (1984) Physiological Functions of Glucocorticoids in Stress and Their Relation to Pharmacological Actions. Endocr Rev 5(1): 25–44. [DOI] [PubMed] [Google Scholar]
- 39. Dhabhar FS, McEwen BS (1997) Acute Stress Enhances while Chronic Stress Suppresses Cell-Mediated Immunityin Vivo: A Potential Role for Leukocyte Trafficking. Brain Behav Immun 11(4): 286–306. [DOI] [PubMed] [Google Scholar]
- 40. DuRant SE, Romero LM, Talent LG, Hopkins WA (2008) Effect of exogenous corticosterone on respiration in a reptile. Gen Comp Endocrinol 156(1): 126–133. [DOI] [PubMed] [Google Scholar]
- 41. Bernier NJ (2006) The corticotropin-releasing factor system as a mediator of the appetite-suppressing effects of stress in fish. Gen Comp Endocrinol 146(1): 45–55. [DOI] [PubMed] [Google Scholar]
- 42. Wack CL, DuRant SE, Hopkins WA, Lovern MB, Feldhoff RC, et al. (2012) Elevated plasma corticosterone increases metabolic rate in a terrestrial salamander. Comp Biochem Physiol A 161(2): 153–158. [DOI] [PubMed] [Google Scholar]
- 43.Feder ME, Burggren WW (1992) Environmental physiology of the amphibians. Chicago: University of Chicago Press. p 646.
- 44. Pickering AD, Christie P (1981) Changes in the concentrations of plasma cortisol and thyroxine during sexual maturation of the hatchery-reared brown trout, Salmo trutta L. Gen Comp Endocrinol. 44(4): 487–496. [DOI] [PubMed] [Google Scholar]
- 45. Laidley CW, Woo PTK, Leatherland JF (1988) The stress-response of rainbow trout to experimental infection with the blood parasite Cryptobia salmositica Katz, 1951. J Fish Biol 32(2): 253–261. [Google Scholar]
- 46. Hermann G, Beck FM, Sheridan JF (1995) Stress-induced glucocorticoid response modulates mononuclear cell trafficking during an experimental influenza viral infection. J Neuroimmunol 56(2): 179–186. [DOI] [PubMed] [Google Scholar]
- 47. Al-Afaleq AI (1998) Biochemical and Hormonal Changes Associated with Experimental Infection of Chicks with Infectious Bursal Disease Virus. J Vet Med B 45(1–10): 513–517. [DOI] [PubMed] [Google Scholar]
- 48. Finstad B, Bjørn PA, Grimnes A, Hvidsten NA (2000) Laboratory and field investigations of salmon lice [Lepeophtheirus salmonis (Krøyer)] infestation on Atlantic salmon (Salmo salar L.) post-smolts. Aquacult Res 31(11): 795–803. [Google Scholar]
- 49. Fleming MW (1997) Cortisol as an Indicator of Severity of Parasitic Infections of Haemonchus contortus in Lambs (Ovis aries). Comp Biochem Physiol B 116(1): 41–44. [DOI] [PubMed] [Google Scholar]
- 50. Fleming MW (1998) Experimental Inoculations with Ostertagia ostertagi or Exposure to Artificial Illumination Alter Peripheral Cortisol in Dairy Calves (Bos taurus). Comp Biochem Physiol A 119(1): 315–319. [DOI] [PubMed] [Google Scholar]
- 51. Hanley KA, Stamps JA (2002) Does corticosterone mediate bidirectional interactions between social behaviour and blood parasites in the juvenile black iguana, Ctenosaura similis? Anim Behav 63(2): 311–322. [Google Scholar]
- 52. Sures B, Knopf K, Kloas W (2001) Induction of stress by the swimbladder nematode Anguillicola crassus in European eels, Anguilla anguilla, after repeated experimental infection. Parasitol 123(02): 179–184. [DOI] [PubMed] [Google Scholar]
- 53. Sures B, Scheef G, Klar B, Kloas W, Taraschewski H (2002) Interaction between cadmium exposure and infection with the intestinal parasite Moniliformis moniliformis (Acanthocephala) on the stress hormone levels in rats. Environ Pollut 119(3): 333–340. [DOI] [PubMed] [Google Scholar]
- 54. Fast MD, Muise DM, Easy RE, Ross NW, Johnson SC (2006) The effects of Lepeophtheirus salmonis infections on the stress response and immunological status of Atlantic salmon (Salmo salar). Fish Shellfish Immun 21(3): 228–241. [DOI] [PubMed] [Google Scholar]
- 55. Raouf SA, Smith LC, Brown MB, Wingfield JC, Brown CR (2006) Glucocorticoid hormone levels increase with group size and parasite load in cliff swallows. Anim Behav 71(1): 39–48. [Google Scholar]
- 56. Kindemann C, Narayan EJ, Hero J (2012) Urinary corticosterone metabolites and chytridiomycosis disease prevalence in a free-living population of male Stony Creek frogs (Litoria wilcoxii). Comp Biochem Physiol A 162(3): 171–176. [DOI] [PubMed] [Google Scholar]
- 57. Gabor C, Fisher MC, Bosch J (2013) A non-invasive stress assay shows that tadpole populations infected with Batrachochytrium dendrobatidis have elevated corticosterone levels. PLOS ONE 8(2): e56054 doi:10.1371/journal.pone.0056054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Jurani M, Murgas K, Mikulaj L, Babusikova F (1973) Effect of stress and environmental temperature on adrenal function in Rana esculenta . J Endocrinol 57(3): 385–391. [DOI] [PubMed] [Google Scholar]
- 59. Zerani M, Amabili F, Mosconi G, Gobbetti A (1991) Effects of captivity stress on plasma steroid levels in the green frog, Rana esculenta, during the annual reproductive cycle. Comp Biochem Physiol A 98(3–4): 491–496. [Google Scholar]
- 60. Coddington EJ, Cree A (1995) Effect of Acute Captivity Stress on Plasma Concentrations of Corticosterone and Sex Steroids in Female Whistling Frogs, Litoria ewingi . Gen Comp Endocrinol 100(1): 33–38. [DOI] [PubMed] [Google Scholar]
- 61. Gobbetti A, Zerani M (1996) Possible mechanism for the first response to short captivity stress in the water frog, Rana esculenta . J Endocrinol 148(2): 233–239. [DOI] [PubMed] [Google Scholar]
- 62. Hopkins WA, Mendonça MT, Congdon JD (1997) Increased Circulating Levels of Testosterone and Corticosterone in Southern Toads, Bufo terrestris, Exposed to Coal Combustion Waste. Gen Comp Endocrinol 108(2): 237–246. [DOI] [PubMed] [Google Scholar]
- 63. Carr JA, Brown CL, Mansouri R, Venkatesan S (2002) Neuropeptides and amphibian prey-catching behavior. Comp Biochem Physiol B 132(1): 151–162. [DOI] [PubMed] [Google Scholar]
- 64. Stuart SN, Chanson JS, Cox NA, Young BE, Rodrigues ASL, et al. (2004) Status and Trends of Amphibian Declines and Extinctions Worldwide. Science 306(5702): 1783–1786. [DOI] [PubMed] [Google Scholar]
- 65. Gendron AD, Bishop CA, Fortin R, Hontela A (1997) In vivo testing of the functional integrity of the corticosterone-producing axis in mudpuppy (amphibia) exposed to chlorinated hydrocarbons in the wild. Environ Toxicol Chem 16(8): 1694–1706. [Google Scholar]
- 66. Larson DL, McDonald S, Fivizzani AJ, Newton WE, Hamilton SJ (1998) Effects of the herbicide atrazine on Ambystoma tigrinum metamorphosis: duration, larval growth, and hormonal response. Physiol Zool 71(6): 671–679. [DOI] [PubMed] [Google Scholar]
- 67. Hopkins WA, Mendonça MT, Congdon JD (1999) Responsiveness of the hypothalamo–pituitary–interrenal axis in an amphibian (Bufo terrestris) exposed to coal combustion wastes. Comp Biochem Physiol C 122(2): 191–196. [DOI] [PubMed] [Google Scholar]
- 68. Glennemeier KA, Denver RJ (2001) Sublethal effects of chronic exposure to an organochlorine compound on northern leopard frog (Rana pipiens) tadpoles. Environ Toxicol 16(4): 287–297. [PubMed] [Google Scholar]
- 69. Goulet BN, Hontela A (2003) Toxicity of cadmium, endosulfan, and atrazine in adrenal steroidogenic cells of two amphibian species, Xenopus laevis and Rana catesbeiana . Environ Toxicol Chem 22(9): 2106–2113. [DOI] [PubMed] [Google Scholar]
- 70. Hayes TB, Case P, Chui S, Chung D, Haeffele C, et al. (2006) Pesticide Mixtures, Endocrine Disruption, and Amphibian Declines: Are We Underestimating the Impact? Environ Health Perspect 114(S-1): 40–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Ward CK, Fontes C, Breuner CW, Mendonça MT (2007) Characterization and quantification of corticosteroid-binding globulin in a southern toad, Bufo terrestris, exposed to coal-combustion-waste. Gen Comp Endocrinol 152(1): 82–88. [DOI] [PubMed] [Google Scholar]
- 72. Peterson JD, Peterson VA, Mendonça MT (2009) Exposure to coal combustion residues during metamorphosis elevates corticosterone content and adversely affects oral morphology, growth, and development in Rana sphenocephala . Comp Biochem Physiol C 149(1): 36–39. [DOI] [PubMed] [Google Scholar]
- 73. Homan RN, Regosin JV, Rodrigues DM, Reed M, Windmiller BS, et al. (2003) Impacts of varying habitat quality on the physiological stress of spotted salamanders (Ambystoma maculatum). Anim Conserv 6(01): 11–18. [Google Scholar]
- 74. Denver RJ (1998) Hormonal Correlates of Environmentally Induced Metamorphosis in the Western Spadefoot Toad, Scaphiopus hammondii . Gen Comp Endocrinol 110(3): 326–336. [DOI] [PubMed] [Google Scholar]
- 75. Berger L, Marantelli G, Skerratt LF, Speare R (2005) Virulence of the amphibian chytrid fungus Batrachochytium dendrobatidis varies with the strain. Dis Aquat Org 68(1): 47–50. [DOI] [PubMed] [Google Scholar]
- 76. Kriger KM, Hero JM, Ashton KJ (2006) Cost efficiency in the detection of chytridiomycosis using PCR assay. Dis Aqua Org 71(2): 149–154. [DOI] [PubMed] [Google Scholar]
- 77. Ramsey JP, Reinert LK, Harper LK, Woodhams DC, Rollins-Smith LA (2010) Immune defenses against Batrachochytrium dendrobatidis, a fungus linked to global amphibian declines, in the South African clawed frog, Xenopus laevis . Infect Immun 78(9): 3981–3992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Boyle DG, Boyle DB, Olsen V, Morgan JAT, Hyatt AD (2004) Rapid quantitative detection of chytridiomycosis (Batrachochytrium dendrobatidis) in amphibian samples using real-time Taqman PCR assay. Dis Aqua Org 60(2): 141–148. [DOI] [PubMed] [Google Scholar]
- 79. Cruz LF, Cobine PA, De La Fuente L (2012) Calcium Increases Xylella fastidiosa Surface Attachment, Biofilm Formation, and Twitching Motility. Appl Environ Microbiol 78(5): 1321–1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Mendonça MT, Chernetsky SD, Nester KE, Gardner GL (1996) Effects of Gonadal Sex Steroids on Sexual Behavior in the Big Brown Bat, Eptesicus fuscus, Upon Arousal from Hibernation. Horm Behav 30(2): 153–161. [DOI] [PubMed] [Google Scholar]
- 81. Ward CK, Appel AG, Mendonça MT (2006) Metabolic measures of male southern toads (Bufo terrestris) exposed to coal combustion waste. Comp Biochem Physiol A 143(3): 353–360. [DOI] [PubMed] [Google Scholar]
- 82. Bancila RL, Hartel T, Plaiasu R, Smets J, Cogalniceanu D (2010) Comparing three body condition indices in amphibians: a case study of yellow-bellied toad Bombina variegata . Amphibia-Reptilia 31(4): 558–562. [Google Scholar]
- 83.Seber GAF, Wild CJ (1989) Nonlinear regression. New York: John Wiley and Sons.