Abstract
The aim of this study was to investigate macrophage reverse cholesterol transport (RCT) in hamster, a CETP-expressing species, fed omega 3 fatty acids (ω3PUFA) supplemented high fat diet (HFD). Three groups of hamsters (n = 6/group) were studied for 20 weeks: 1) control diet: Control, 2) HFD group: HF and 3) HFD group supplemented with ω3PUFA (EPA and DHA): HFω3. In vivo macrophage-to-feces RCT was assessed after an intraperitoneal injection of 3H-cholesterol-labelled hamster primary macrophages.
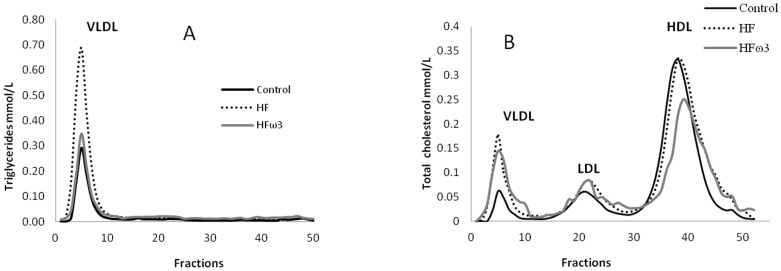
Compared to Control, HF presented significant (p<0.05) increase in body weight, plasma TG (p<0.01) and cholesterol (p<0.001) with an increase in VLDL TG and in VLDL and LDL cholesterol (p<0.001).
Compared to HF, HFω3 presented significant decrease in body weight. HFω3 showed less plasma TG (p<0.001) and cholesterol (p<0.001) related to a decrease in VLDL TG and HDL cholesterol respectively and higher LCAT activity (p<0.05) compared to HF. HFω3 showed a higher fecal bile acid excretion (p<0.05) compared to Control and HF groups and higher fecal cholesterol excretion (p<0.05) compared to HF. This increase was related to higher gene expression of ABCG5, ABCA1 and SR-B1 in HFω3 compared to Control and HF groups (<0.05) and in ABCG1 and CYP7A1 compared to HF group (p<0.05). A higher plasma efflux capacity was also measured in HFω3 using 3H- cholesterol labeled Fu5AH cells.
In conclusion, EPA and DHA supplementation improved macrophage to feces reverse cholesterol transport in hamster fed HFD. This change was related to the higher cholesterol and fecal bile acids excretion and to the activation of major genes involved in RCT.
Introduction
Metabolic syndrome is a common pathological situation leading to an increase in cardiovascular disease. Dyslipidemia (higher triglyceride (TG) and lower HDL-cholesterol plasma concentrations) is frequently associated with metabolic syndrome [1]. Plasma HDL-cholesterol (HDL-c) levels are known to be inversely correlated with the risk of atherosclerotic cardiovascular diseases [1], however, this inverse relationship between HDL and cardiovascular disease reported in epidemiological studies is not confirmed in subgroups of patients with specific apoA-I mutations as ApoA1 Milano [2] or CETP polymorphism [3]. Then, a low plasma HDL-cholesterol concentration does not always predict an increase of the cardiovascular risk and deeper understanding of HDL metabolism could help to define the critical situations. The protective effects of HDL are mainly due to their central role in the reverse cholesterol transport (RCT), a process mediating the transport of cholesterol excess by HDL from peripheral tissues back to the liver for excretion into the bile and ultimately in the feces. In human, the cholesterol ester transfer protein (CETP) plays a critical role in RCT and performs, in parallel to direct uptake of HDL cholesterol by liver, transfer of cholesterol from HDL to LDL followed by liver LDL uptake. Thus, this metabolism is very complex and to study its modulation, it is easier to use animal models. Molecular mechanisms of RCT have been extensively studied in mouse [4], [5]. However, this animal model does not have any CETP and when it was over expressed in transgenic animals the rate of RCT was accelerated [6]. Therefore, CETP pathway would represent a major route for human RCT [7] and CETP expressing species, as hamster, represents a better model to investigate lipoprotein metabolism [8].
Omega-3 fatty acids such as docosahexaenoic acid (DHA) or eicosapentaenoic acid (EPA), abundant in fish oil, reduce clinical cardiovascular complications of atherosclerotic disease [9]. Several mechanisms have been proposed by which ω3PUFA reduce cardiovascular events, including triglyceride-lowering, anti-inflammatory, antithrombotic and anti-arrhythmic effects [10]. The effect of omega 3 fatty acid on reverse cholesterol transport has been tested in mice by Nichimoto et al, [5]. In this later study, authors showed that fish oil decreased HDL cholesterol and accelerated RCT by increasing excretion of HDL-derived 3H cholesterol recovered in fecal neutral sterols.
In our study, we investigated in hamster CETP species, whether omega 3 fatty acids supplemented high fat diet can modulate in vivo macrophage-to-feces RCT using hamster primary macrophages.
Materials and Methods
Ethics statement
All experiments were performed according to the regulations for animal welfare of the French Ministry of Food, Agriculture and Fisheries. The experimental protocol was adhered to European Union guidelines and was approved by the local Animal Used and Care Advisory Committee (Bretagne-Pays de la Loire committee). All animal trial was carried out under isofluran anesthesia. Animals were sacrificed by intra-cardiac injection of lethal dose of pentobarbital.
Animal
18 males golden Syrian hamsters were obtained from Janvier (Le Genest-St-Isle, France) at 8 weeks of age weighting 80 to 90 g. They were housed in colony cages with wood litter (3 hamsters/cage) in controlled environment (22°C, 12/12 h light/dark cycle) and received water and diet ad libitum.
Diets
Two high fat diet (HFD, 21% fat w/w), either enriched (HFω3) or not (HF) in ω3PUFA, and a control chow diet (5% fat w/w; Control) were used. Each of both high-fat diets were designed with the same composition and contained 38.7% starch, 24.7% proteins, 21% lipids, 8.5% minerals, 6% cellulose and 1.2% vitamins. The lipid mixture consisted of 15% lard, 3.5% palm oil, and 2.5% corn oil for HF and 1.5 g of lard was replaced with 1.5 g ω3PUFA oil mixture (Pierre Fabre Santé, Castres, France) for HFω3 diet. Both HF diets contained a low amount 0.014 g/100 g of cholesterol (from lard) and total lipids provided about 45% of total energy content. Control diet contained 23% protein, 58% starch, 5% lipids (2% corn oil and 3% palm oil), 8.5% minerals, 6% cellulose and 1.2% vitamins. 13% of energy intake was provided by lipids. Lipid composition of the 3 diets has been previously detailed [11] and contained respectively for Control, HF and HFω3 diets: SFA (39, 44.5 and 41%), MUFA (36, 46.5 and 41%), PUFA ω6 (24.5, 9 and 16.5%) and PUFA ω3 (0.5, 0.5 and 4.5%). For Control, HF and HFω3 diets, ω6/ω3 ratio was 50, 20 and 4 respectively. Experiments were performed in the three groups of animals at the end of a 20-week period of diet consumption.
Biochemical analysis
After 20 weeks of diet, hamsters were fasted for 18 hours and blood was obtained by retro-orbital under isofluran anesthesia to determine plasma lipids. Plasma was then separated by centrifugation (4°C, 10 min, 3000 g). Total cholesterol and triglyceride were assayed using commercial kits (Biomerieux, France). Separation of HDL was performed using fast protein liquid chromatography (AKTA FPLC SYSTEM, GE Healthcare, USA). CETP activity was measured using a commercial kit (Roar Biomedical, NY, USA). Lecithin cholesterol acyl transferase (LCAT) activity was evaluated by measurement of free cholesterol content at T0 and after 1 hour of plasma incubation at 37°C. The decrease of free cholesterol concentration is expressed as the percentage of free cholesterol transformed into cholesteryl ester by hour [12].
Isolation of peritoneal-elicited macrophages
Peritoneal macrophage were isolated as described in our previous study [13]. Hamsters were injected intraperitoneally (IP) with 15 mL of 3% Brewer thioglycollate medium (Sigma). Three days after injection, hamsters are sacrificed with lethal injection of pentobarbital and macrophages were collected by peritoneal washing with 10 mL ice-cold PBS. After centrifugation (1500 rpm for 5 minutes at 4°C), the pelleted macrophages were suspended in 5 mL of red blood cell lysis buffer (0.15 M NH4Cl, 10 mM KHCO3, EDTA 0.5 M) and incubated 15 minutes in ice. The macrophages were collected after centrifugation (1500 rpm for 5 minutes at 4°C) and suspended in RPMI culture medium supplemented with 10% FBS (Gibco). The cells were then plated in flasks in RPMI complete medium and allowed to adhere for 4 h in a 37°C humidified 5% CO2 incubator. Non adherent cells were removed with two washes of RPMI.
In vivo macrophage-to-feces reverse cholesterol transport
Hamster macrophages were radiolabeled as described previously [13]. Briefly, 5 µCi/mL medium 3H-cholesterol and cholesterol loaded with 50 µg/mL acetylated LDL over 48 hours. Radiolabeled cells were then washed with RPMI and equilibrated for 4 hours in fresh RPMI supplemented with 0.2% BSA. Cells were pelleted by low speed centrifugation and suspended in PBS prior to injection into hamsters (n = 6/group). 3H-cholesterol–labeled and acetylated LDL-loaded cells (typically, 2.5 million of cells and 5×106 cpm in 2.5 mL PBS) were injected IP into individually caged hamsters. Blood was collected via the retro-orbital sinus at 24, 48 and 72 hours and radioactivity in 20 µL of plasma was counted in a liquid scintillation counter. Hamsters were then sacrificed and liver was collected from each animal. Approximately 50 mg-piece of liver was homogenized in 400 µL water then lipids were extracted.
Fecal cholesterol and bile acid extraction was performed as previously described [13]. The total feces collected over 72 hours were weighed and soaked in Millipore water. One mL of the homogenized samples was used to extract the 3H-cholesterol and 3H-bile acid fractions. The extracts were evaporated, resuspended in toluene, and then radioactivity determined. Results were expressed as a percent of the radioactivity injected recovered in plasma, liver and feces (in cholesterol and bile acids fractions). The plasma volume was estimated as 3.5% of the body weight.
In vitro measurement of cholesterol efflux
The ability of animals' serum to efflux cell unesterified cholesterol was measured by a procedure previously described [14] using [3H]-cholesterol–labeled Fu5AH. Briefly, Fu5AH was cultured in Eagle's minimum essential medium (EMEM) (Sigma, Saint-Quentin Fallavier, France) containing 10% fetal calf serum (FCS) (Sigma, Saint-Quentin Fallavier, France). Penicillin, streptomycin, and glutamine were present in all media. For efflux experiments, cells were plated in costar 24-well plates and grown in appropriate medium containing 10% FCS at 37°C in a humidified 5% CO2 atmosphere. When they were nearly confluent, cells were incubated for 24 hours at 37°C with 1 µCi/mL of [1,2-3H] cholesterol (10% FCS in EMEM). To ensure the label was evenly distributed among cellular pools, the labeling medium was replaced with EMEM containing 1% BSA (Sigma), and cells were incubated in albumin for 18 to 20 hours before measuring cholesterol efflux. The cells were then washed and incubated with the indicated serum prepared in EMEM (5% [vol/vol]), and efflux was performed for 4 hours. After efflux period, media were collected and counted for radioactivity by liquid scintillation counting. The residual radioactivity in the cells was determined after extraction with isopropanol. The results are expressed as 3H-cholesterol efflux into the culture medium as a percent of the initial 3H-cholesterol inside the cells.
RNA extraction and gene expression analysis
Liver samples for mRNA analysis were homogenized, and RNA was isolated using Trizol reagent (Invitrogen, France). Real-time quantitative polymerase chain reaction (PCR) analysis was performed as follows: 1 µg of total RNA was reverse-transcribed using 100 units of MML-V reverse transcriptase (Promega, France). Real time quantitative PCR was performed on the 7000 Sequence Detection System with SYBR green MESAGREEN Master Mix Plus (Eurogentec, Angers, France). The reaction contained 10 ng of reverse-transcripted total RNA, 500 nM forward and reverse primers, 5× Sybr green Mix. Primers sequences are available on request. All reactions were performed at least in duplicate and Cyclophilin RNA amplification was used as a reference. Each couple of primers was tested in successive dilutions of cDNA to analyze and validate its efficiency. The expression of ABCA1, ATP binding cassette protein A1 (ABCA1), G1 (ABCG1), G5 (ABCG5), G8 (ABCG8); Cytochrome P450, family 7 , subfamily A, polypeptide 1 (CYP7A1), scavenger receptor class B type 1 (SR-B1), and low density lipoprotein receptor (LDLr) was measured (6 hamsters/group).
Western blot analysis
For in vivo expression of SR-BI in hamster, proteins were harvested from liver of each group. The protein concentration was determined using the BCA Protein Assay method (Sigma Aldrich). For quantitative purposes, the same amount of protein (30 µg) was loaded for each liver sample. Proteins were separated on 4–15% SDS-PAGE and transferred to a nitrocellulose membrane. Membranes were blocked overnight with 5% milk TBS-Tween solution at 4°C, then washed and incubated for 2 h with primary polyclonal antibody against rabbit SR-BI (PA1-16803, Thermo Scientific). A second 2 h-incubation with a peroxidase-conjugated IgG secondary antibody was performed. Bands were visualized by Uptilight US Blot chemiluminescent substrate kit (Interchim). The abundance of β-actin was used as control (monoclonal antibody from Sigma Aldrich). Densitometric analyses of protein bands in the Western blots were done using G: Box Imager software (Syngene). Relative band intensities were derived by using the software to calculate integrated optical density for each band, and then each band was normalized with the integrated optical density of the corresponding β-actin band.
Measurement of lipids content in liver
About 30 mg of hepatic tissue were used to extract lipid using Folch method [15]. Briefly the tissue was homogenized with chloroform/methanol (2/1), and then centrifuged to recover the liquid phase. The solvent was washed with 0.9% NaCl solution. After centrifugation the lower chloroform phase containing lipids was evaporated and lipids were suspended in 0.5 ml of ethanol. TG and cholesterol were then measured using Biomérieux kits reagents (TG: PAP 150, cholesterol RTU; Biomérieux, Marcy-l'Etoile, France) (n = 6/group).
Statistical analysis
Results were expressed as means ± S.E.M. Statistical analyses were performed using Statview software (SAS Institute Inc., SAS campus drive, Cary, NC, USA). The ANOVA followed by PLSD Fisher's test was performed to estimate the effect of group and ω3PUFA supplementation (n = 6/group of hamster). Differences were considered significant at p<0.05.
Results
Effect of diets on body weight, plasma lipid parameters and LCAT and CETP activities (Table 1)
Table 1. Effect of different diets on body weight, plasma lipid parameters and CETP and LCAT activities.
| Control | HF | HFω3 | ANOVA | |
| Body weight (g) | 120.0 | 134.3 * | 123.8† | p<0.05 |
| Plasma | ||||
| Triglyceride mmol/L | 1.40±0.16 | 2.2097±0.19** | 1.49±0.13 | p<0.01 |
| Total cholesterol mmol/L | 3.35±0.08 | 4.02±0.06*** | 3.47±0.12††† | p<0.001 |
| VLDL cholesterol mmol/L | 0.209±0.005 | 0.581±0.008 *** | 0.624±0.023*** | p<0.0001 |
| LDL cholesterol mmol/L | 0.522±0.012 | 0.679±0.010*** | 0.681±0.025*** | p<0.0001 |
| HDL cholesterol mmol/L | 2.621±0.064 | 2.760±0.042 | 2.097±0.077†††*** | p<0.0001 |
| LCAT activity (%/h) | 20.28±1.19 | 17.19±1.0 | 21.02±1.5 † | p<0.05 |
| CETP activity (pmol/h/µl) | 60.1±4.1 | 61.3±4.01 | 52.6±4.11 | NS |
Values are mean ± SEM, n = 6 per group.
different from Control (*p<0.05 ** p<0.01; ***p<0.001).
different from HF († p<0.05, †† p<0.01; ††† p<0.001).
HFD led to an increase of body weight compared to Control (12%, p<0.05), while HFω3 did not. Plasma and VLDL triglyceride (TG) were higher in HF group compared to Control (57%, p<0.01), related to higher TG content of VLDL (figure 1A), but lower in HFω3 compared to HF (46%, p<0.001). Cholesterol was higher in plasma (20%, p<0.001) and in VLDL and LDL (30%, p<0.001) in HF compared to Control, (figure 1B). Plasma total cholesterol did not differ in HFω3 compared to Control but significantly lower compared to HF (14%, p<0.001). This change was related to lower HDL cholesterol (24%, p<0.001) also obvious in figure 1B. LCAT activity did not differ between Control and HF and was higher in HFω3 compared to HF (22%, p<0.05). CETP activity did not differ among the three groups.
Figure 1. Representative TG (A) and cholesterol (B) profiles of Control, HF and HFω3 groups performed by FPLC.
Effect of diets on tissues lipids content
Liver TG content was higher in HF compared to Control group (14.2±2.1 mg/g vs. 8.5±1.1, P<0.05) and lower in HFω3 (11.1±2.6 mg/g) compared to HF group (P<0.05). No difference was observed in hepatic cholesterol content among the three groups (data not shown).
Effect of diets on in vivo reverse cholesterol transport
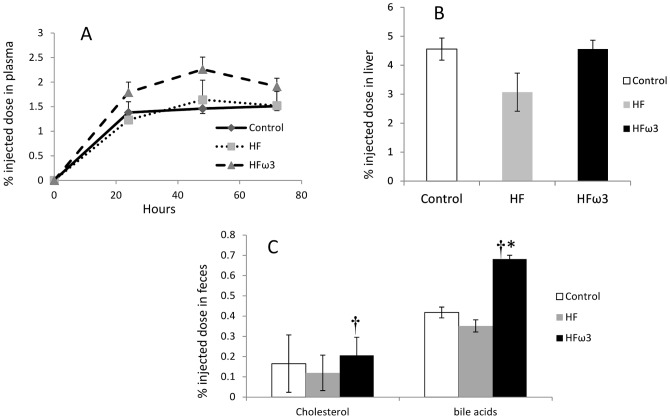
To investigate whether reverse cholesterol transport is affected in vivo, Control, HF and HFω3 hamsters were injected with 3H-cholesterol labelled and cholesterol loaded hamster primary macrophages. As shown in figure 2A, plasma 3H-tracer appearance at 24, 40 and 72 h after macrophage injection did not differ among the three groups. No difference was showed in tracer recovery in liver among groups (Figure 2B). No significant change was observed between HF and Control for fecal cholesterol and bile acids excretion (Figure 2C). A higher 3H-tracer recovery was observed in fecal bile acids (60%, p<0.05) in HFω3 compared to Control. The tracer recovery in fecal bile acid (90%, p<0.05) and cholesterol (70%, p<0.05) was higher in HFω3 compared to HF.
Figure 2. Effect of the three diets on reverse cholesterol transport.
A: 3H-tracer appearance in plasma at time 24, 48 and 72 hours after injection. B: Liver 3H-tracer recovery at 72 h after injection of labeled and acetylated LDL-loaded macrophages. C: 3H-tracer recovery in fecal cholesterol and bile acids. Data are expressed as percent cpm injected and mean ± SEM (n = 6 per group; * p<0.05 different from Control; † p<0.05 different from HF.
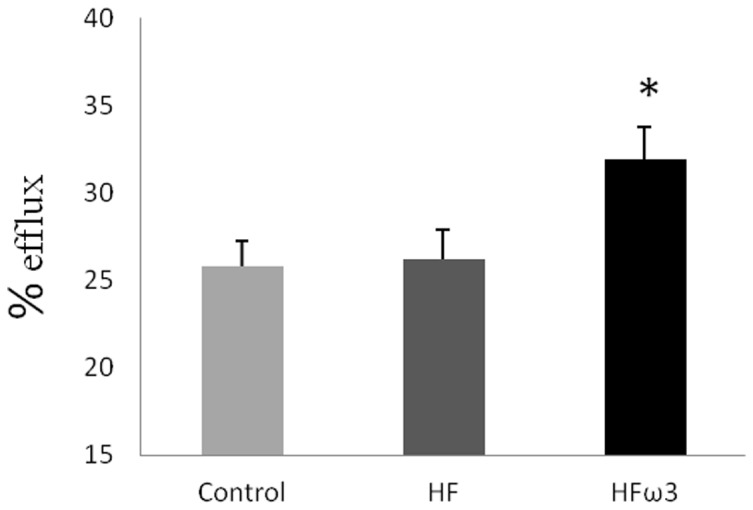
Effect of diets on in vitro reverse cholesterol efflux
We tested the ability of plasma from hamsters to promote cholesterol efflux from Fu5AH cells. After 4H incubation, HFω3 hamster plasma showed an increase in cholesterol efflux (30.99±1.99%) compared to HF and Control groups (24.57±0.42 and 25.79±1.48% respectively). No difference was observed between HF and Control group (figure 3).
Figure 3. [3H]-cholesterol efflux from Fu5AH.
[3H]-cholesterol labeled Fu5AH cells were incubated with hamster serum and efflux was performed for 4 h. Data are mean ± SEM. n = 6 per group.
Effect of diets on genes involved in cholesterol metabolism
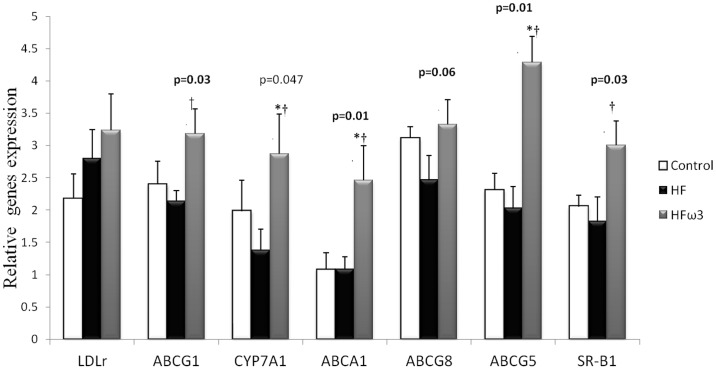
Liver genes expression in the three groups is shown in figure 4. No change was observed for any studied gene between HF and Control groups. ω3PUFA supplemented high fat diet increased expression of the ABCA1 (127%, p<0.05), ABCG1 (49%, p<0.05), SR-B1 (65%, p<0.05), ABCG8 (35%, p = 0.06), ABCG5 (111%, p<0.05) and CYP7A1 (108%, p<0.05) compared to HF group. The ω3PUFA supplementation increased significantly the gene expression of CYP7A1, ABCA1 and ABCG5 compared to Control group. Expression of LDLr gene did remain unchanged among groups.
Figure 4. Effect of the three diets on hepatic gene expression.
Relative gene expression in liver in Control, HF and HFω3 groups. Values are means ± SEM (n = 6 per group; * p<0.05 different from Control; † p<0.05 different from HF). (ABCA1, ATP binding cassette protein A1, CYP7A1, Cytochrome P450 family 7 subfamily A polypeptide 1, SR-B1, scavenger receptor class B type 1, and LDLr low density lipoprotein receptor).
Effect of diets on SR-B1 protein abundance
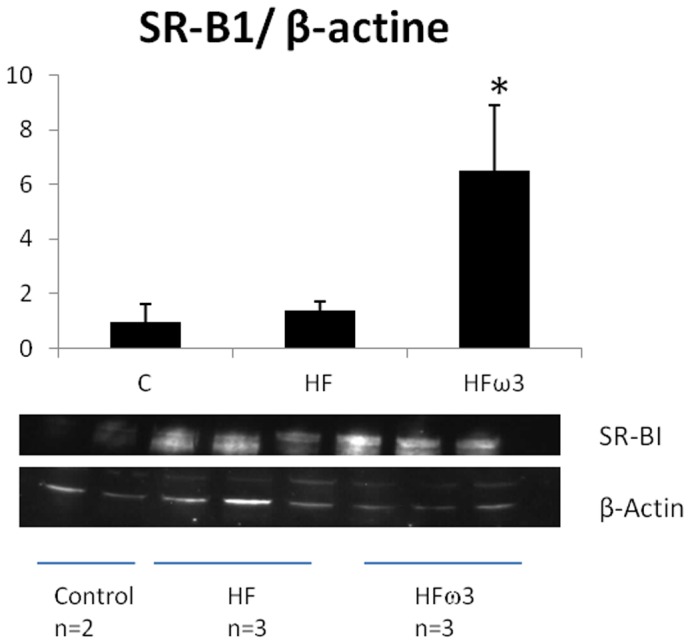
Hepatic tissue SR-B1 protein abundance in FHω3 animals was significantly higher than that found in HF and Control groups (p<0.05) (figure 5).
Figure 5. Western blot analysis of hepatic SR-B1 protein in hamster fed control, HF or HF diet supplemented with omega 3 fatty acids.
Values are means ± SEM; n = 2 for Control group; n = 3 for HF and HFω3 groups.
Discussion
In the present study performed in hamster, we showed that ω3PUFA prevented the increase of plasma TG and cholesterol by decreasing VLDL TG and HDL cholesterol concentrations respectively. These changes were related to increase of RCT efficiency as showed by a higher fecal bile acid and cholesterol elimination.
We have recently studied [13] the RCT in hamster using labelled primary hamster macrophages contrarily to more classical protocols using murine cells [16], [17]. This new protocol avoids the cross-species inflammatory reaction which could potentially lead to an immunological targeted destruction of macrophages when using J774 murine cells. Moreover, the J774 murine cells does not express endogenous apolipoprotein E (apoE) gene [18] involved in the removal of excess cholesterol from macrophage foam cells [19]. Hence, primary macrophages from hamster donors may be more reliable for assessing in vivo reverse cholesterol transport in hamsters [13]. But the probable pro-inflammatory reaction to this macrophage injection cannot be totally excluded.
In our study ω3PUFA supplementation had significant preventing effect against weight gain induced by high fat diet. The same results were obtained during ω3PUFA supplementation in rat [20] mice [21] and in hamster [11]. In this latter study, the expression of SCD1 decreased with omega 3 fatty acids supplementation. The inhibition of SCD1 is described to protect from HFD-induced obesity related to an up-regulation of genes involved in beta-oxidation and a down-regulation of lipogenesis gene expression as shown in mice SCD1−/− [22].
Comparing TG and cholesterol profile in HF and HFω3 groups, it can be established that ω3PUFA prevented the increase of plasma TG and cholesterol induced by high fat diet by decreasing VLDL TG and HDL cholesterol concentrations. A similar effect of ω3PUFA supplemented HFD on lipid profiles was previously reported in mice [23] and hamster [24]. Inversely, studies in rat [25] or in human [26] did not show any change in HDL cholesterol levels with ω3PUFA supplementation. This data divergence between studies could be mainly explained by difference in the diet (high or low fat, enriched or not in cholesterol). Improvement by ω3PUFA of plasma TG potentially disturbed by high fat diet is well known [27]. Such an improvement was also observed in our study concerning liver TG content. The normalization of VLDL TG concentration, potentially increased by production rate by high fat diet, suggests also normalization of VLDL TG production by liver. This improvement has been previously observed in hypertriglyceridemic human [28] or hamster [11] by ω3PUFA supplementation.
Cholesterol export from peripheral cells reduces intracellular cholesterol accumulation and atherosclerosis [29]. We reported that under ω3PUFA supplementation in vivo macrophage-to-feces reverse cholesterol transport was significantly accelerated, as shown by a significantly higher fecal cholesterol and bile acid excretion. This stimulated RCT was related to an increase in expression of most key genes involved in this process (ABCA1, ABCG1, SR-B1, ABCG5 and Cyp7A1). We therefore reported an enhancement of the ability of plasma from omega 3 fatty acid supplemented hamster to promote cholesterol efflux from Fu5AH cells. As in our study we have used Fu5AH for this assay, only the effect of omega 3 on the capacity of HDL to mobilize cholesterol from cells was measured. Similar to our findings, Montoya et al. found a positive effect of ω3PUFA rich diet on cholesterol efflux in-vitro using the same cell model [30]. Thus, the increased efflux measured is totally explained by the capacity of HDL from omega 3 supplemented diet to facilitate the cholesterol efflux. The HDL composition, not measured in our study, is probably affected by omega 3 fatty acids which known to influence HDL phospholipid acyl-chain composition [31], [32] and so cholesterol efflux capability [33], [34]. As mentioned higher, our essay did not allow to measure the capacity of macrophages to evacuate cholesterol but we cannot exclude a possible increase as shown by an enhanced ABCAI expression. ABCA1 and ABCG1 are key mediators of cholesterol efflux from macrophages to HDL particle, a first step of RCT in vivo. The fluxed cholesterol is then esterified in HDL by LCAT action which maintains this efflux [35]. We found an increase of ABCA1 and ABCG1 expression with ω3PUFA in good agreement with an increase of RCT and cholesterol efflux. Sheril et al. [36] reported in genetically dyslipidemic rat that ω3PUFA increase reverse cholesterol transport but with no change in ABCA1 expression. The same result was reported by Nishimoto et al. in mice [5] with no change in ABCA1 and ABCG1. Using apolipoprotein E-null mice, Zhang et al. described a decrease in the expression of ABCA1 and ABCG1 gene expression with a low ω6/ω3 ratio [37]. In vitro on murine macrophage cells, ω3PUFA regulate the activity of ABCA1 and ABCG1 by a mechanism involving LXR/RXR and alter cholesterol efflux [38]. Then, data on the effect of ω3PUFA on these receptors remains controversial related probably to different experimental conditions and animal or cell models. The scavenger receptor class B type I (SR-B1) plays an important role in meditating the uptake of HDL-derived cholesterol and cholesteryl ester in the liver. This receptor is also described as facilitating the initial step of HDL-mediated cholesterol efflux [39]. In our study, SR-B1was not measured in macrophage but its up-regulation in liver is described to promote macrophage RCT [40]. We reported here that ω3PUFA increased SR-B1 gene expression and protein abundance. In agreement with our study, omega 3 PUFA increased SR-B1 gene expression in hamster [24] and mice [23]. While Nishimoto et al. did not report any change in this gene expression [5]. Contradiction between these studies could be explained by the difference in the diet composition especially the amount of added cholesterol.
In our study we observed a higher LCAT activity in HFω3 in accord with a higher cholesterol efflux. In apoE−/− mice study [37], serum LCAT activity increased with decreased ω6/ω3 PUFA ratio. In human, alpha linolenic acid increase LCAT activity [41]. Conversely, Parks et al. have reported in monkey an inhibitory effect of dietary EPA and DHA on LCAT activity [42]. These inconsistencies could be related to the animal model or to the difference in composition of diets.
The stimulation of this initial RCT step was reported to be antiatherogenic [43]. It was also shown that dyslipidemia may contribute to the pathogenesis of mellitus diabetes type II. Loss-of-function mutations in ABCA1 show impaired insulin secretion [44] and increased cholesterol efflux improve insulin sensitivity [45]. Improvement of insulin sensitivity was observed in rats submitted to high fat diet enriched with ω3PUFA [46] and in our previous study [11].
The CETP activity did not change with omega 3 fatty acid supplemented diet. There are few studies on the effect of ω3PUFA on CETP activity. Data from literature are scarce and divergent with no change [47] increase [48] or a reduction [49] in CETP activity with ω3PUFA. Differences in the methods and diet used and the animal species studied may explain this discrepancies.
In the present study, hamsters fed ω3PUFA supplemented diet showed a higher cholesterol fecal excretion. The gene expression was significantly higher for ABCG5 and tended to be significantly higher for ABCG8. The same results were shown in mice [5] and rat [50], while in vitro study did not confirm these data [38].
The increase in cholesterol fecal excretion can also resulted from a decrease in intestinal absorption of cholesterol. NPC1L1 is indisputably one protein that plays a fundamental role in sterol absorption [51]. Nishimoto et al. reported an acceleration in macrophage to feces RCT with non-significant decrease in NPC1L1 gene expression [5]. However studies in hamster reported no effect or up-regulation on NPC1L1 gene expression with omega 3 fatty acids [52], [53] while in vitro studies showed a down regulation of this gene in Caco2 in the presence of omega 3 fatty acids [54], [55]. These divergent findings are not sufficient to conclude about the effect of omega 3 fatty acids on NPC1L1 modulation. As the implication of the intestinal absorption is not to be excluded in what we observed with omega 3 fatty acids, the effect of these fatty acids on cholesterol absorption must be investigated with specific method [56].
In our study, HFω3 showed a higher bile acids fecal excretion. This result was related to significantly higher gene expression of Cyp7A1. Cyp7A1 is the limited enzyme in bile acids synthesis and could be also involved in the increase of RCT. This effect was already reported in human [57] and in mice [58].
In the present study we reported a stimulated RCT by EPA and DHA as attested by an increased cholesterol and bile acids fecal excretion. As RCT is accomplished essentially by LDL and HDL cholesterol uptake by liver, the elevated cholesterol excretion in presence of ω3PUFA could be related to an increase in these two lipoproteins catabolism. But as the level of plasma LDL cholesterol and mRNA of LDLr gene did not change, any change in catabolism is unlikely. The measured increase in SR-B1 expression suggested an increase in the catabolism of the HDL which could explain a decrease of their concentration. Then our results suggest that, although conditions providing increased HDL cholesterol are generally required to prevent cardiovascular disease, decrease of HDL cholesterol as a consequence of stimulated RCT is atheroprotective index.
In conclusion, dietary ω3PUFA supplementation in high fat diet fed hamsters improved body weight, plasma TG and cholesterol and promoted excretion of cholesterol and bile acid into the feces through RCT. This probably results of an increase in the efflux of cholesterol from peripheral tissue to HDL particle, due to the activation of ABCA1 and ABCG1, and an increase of cholesterol movements from HDL into feces due to the activation of SR-B1, ABCG5 and CYP7A1. Our results may contribute to explain the anti-atherogenic effect of ω3PUFA and also underline the complexity of considering HDL cholesterol concentration as atheroprotective index.
Acknowledgments
Authors thank Jean Michel Huvelin for performing the WB analysis and Audrey Aguesse for her technical assistance.
Funding Statement
This work was supported by CRNH (Centre de Recherche en Nutrition Humaine, Nantes) and research program NUPEM supported by Region Pays de la Loire. The ω3 polyunsaturated fatty acids were provided by Pierre Fabre Santé. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Ginsberg HN, Zhang YL, Hernandez-Ono A (2006) Metabolic syndrome: focus on dyslipidemia. Obesity (Silver Spring) 14 Suppl 1: 41S–49S. [DOI] [PubMed] [Google Scholar]
- 2. Calabresi L, Sirtori CR, Paoletti R, Franceschini G (2006) Recombinant apolipoprotein A-IMilano for the treatment of cardiovascular diseases. Curr Atheroscler Rep 8: 163–167. [DOI] [PubMed] [Google Scholar]
- 3. Sakai N, Yamashita S, Hirano K, Menju M, Arai T, et al. (1995) Frequency of exon 15 missense mutation (442D:G) in cholesteryl ester transfer protein gene in hyperalphalipoproteinemic Japanese subjects. Atherosclerosis 114: 139–145. [DOI] [PubMed] [Google Scholar]
- 4. deGoma EM, deGoma RL, Rader DJ (2008) Beyond high-density lipoprotein cholesterol levels evaluating high-density lipoprotein function as influenced by novel therapeutic approaches. J Am Coll Cardiol 51: 2199–2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Nishimoto T, Pellizzon MA, Aihara M, Stylianou IM, Billheimer JT, et al. (2009) Fish oil promotes macrophage reverse cholesterol transport in mice. Arterioscler Thromb Vasc Biol 29: 1502–1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. nTanigawa H, Billheimer JT, Tohyama J, Zhang Y, Rothblat G, et al. (2007) Expression of cholesteryl ester transfer protein in mice promotes macrophage reverse cholesterol transport. Circulation 116: 1267–1273. [DOI] [PubMed] [Google Scholar]
- 7. Ouguerram K, Krempf M, Maugeais C, Maugere P, Darmaun D, et al. (2002) A new labeling approach using stable isotopes to study in vivo plasma cholesterol metabolism in humans. Metabolism 51: 5–11. [DOI] [PubMed] [Google Scholar]
- 8. Briand F (2010) The use of dyslipidemic hamsters to evaluate drug-induced alterations in reverse cholesterol transport. Curr Opin Investig Drugs 11: 289–297. [PubMed] [Google Scholar]
- 9. Yokoyama M, Origasa H, Matsuzaki M, Matsuzawa Y, Saito Y, et al. (2007) Effects of eicosapentaenoic acid on major coronary events in hypercholesterolaemic patients (JELIS): a randomised open-label, blinded endpoint analysis. Lancet 369: 1090–1098. [DOI] [PubMed] [Google Scholar]
- 10. Harrison N, Abhyankar B (2005) The mechanism of action of omega-3 fatty acids in secondary prevention post-myocardial infarction. Curr Med Res Opin 21: 95–100. [DOI] [PubMed] [Google Scholar]
- 11. Kasbi Chadli F, Andre A, Prieur X, Loirand G, Meynier A, et al. (2011) n-3 PUFA prevent metabolic disturbances associated with obesity and improve endothelial function in golden Syrian hamsters fed with a high-fat diet. Br J Nutr 1–11. [DOI] [PubMed] [Google Scholar]
- 12. Nagasaki T, Akanuma Y (1977) A new colorimetric method for the determination of plasma lecithin-cholesterol acyltransferase activity. Clin Chim Acta 75: 371–375. [DOI] [PubMed] [Google Scholar]
- 13. Treguier M, Briand F, Boubacar A, Andre A, Magot T, et al. (2011) Diet-induced dyslipidemia impairs reverse cholesterol transport in hamsters. Eur J Clin Invest [DOI] [PubMed] [Google Scholar]
- 14. Ripolles Piquer B, Nazih H, Bourreille A, Segain JP, Huvelin JM, et al. (2006) Altered lipid, apolipoprotein, and lipoprotein profiles in inflammatory bowel disease: consequences on the cholesterol efflux capacity of serum using Fu5AH cell system. Metabolism 55: 980–988. [DOI] [PubMed] [Google Scholar]
- 15. Folch J, Lees M, Sloane Stanley GH (1957) A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem 226: 497–509. [PubMed] [Google Scholar]
- 16. Castro-Perez J, Briand F, Gagen K, Wang SP, Chen Y, et al. (2011) Anacetrapib promotes reverse cholesterol transport and bulk cholesterol excretion in Syrian golden hamsters. J Lipid Res 52: 1965–1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tchoua U, D'Souza W, Mukhamedova N, Blum D, Niesor E, et al. (2008) The effect of cholesteryl ester transfer protein overexpression and inhibition on reverse cholesterol transport. Cardiovasc Res 77: 732–739. [DOI] [PubMed] [Google Scholar]
- 18. Bernard DW, Rodriguez A, Rothblat GH, Glick JM (1991) cAMP stimulates cholesteryl ester clearance to high density lipoproteins in J7774 macrophages. J Biol Chem 266: 710–716. [PubMed] [Google Scholar]
- 19. Mazzone T, Reardon C (1994) Expression of heterologous human apolipoprotein E by J774 macrophages enhances cholesterol efflux to HDL3. J Lipid Res 35: 1345–1353. [PubMed] [Google Scholar]
- 20. Okuno M, Kajiwara K, Imai S, Kobayashi T, Honma N, et al. (1997) Perilla oil prevents the excessive growth of visceral adipose tissue in rats by down-regulating adipocyte differentiation. J Nutr 127: 1752–1757. [DOI] [PubMed] [Google Scholar]
- 21. Rossmeisl M, Jelenik T, Jilkova Z, Slamova K, Kus V, et al. (2009) Prevention and reversal of obesity and glucose intolerance in mice by DHA derivatives. Obesity (Silver Spring) 17: 1023–1031. [DOI] [PubMed] [Google Scholar]
- 22. Ntambi JM, Miyazaki M, Stoehr JP, Lan H, Kendziorski CM, et al. (2002) Loss of stearoyl-CoA desaturase-1 function protects mice against adiposity. Proc Natl Acad Sci U S A 99: 11482–11486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. le Morvan V, Dumon MF, Palos-Pinto A, Berard AM (2002) n-3 FA increase liver uptake of HDL-cholesterol in mice. Lipids 37: 767–772. [DOI] [PubMed] [Google Scholar]
- 24. Spady DK, Kearney DM, Hobbs HH (1999) Polyunsaturated fatty acids up-regulate hepatic scavenger receptor B1 (SR-BI) expression and HDL cholesteryl ester uptake in the hamster. J Lipid Res 40: 1384–1394. [PubMed] [Google Scholar]
- 25. Hassanali Z, Ametaj BN, Field CJ, Proctor SD, Vine DF (2010) Dietary supplementation of n-3 PUFA reduces weight gain and improves postprandial lipaemia and the associated inflammatory response in the obese JCR:LA-cp rat. Diabetes Obes Metab 12: 139–147. [DOI] [PubMed] [Google Scholar]
- 26. Skulas-Ray AC, Kris-Etherton PM, Harris WS, Vanden Heuvel JP, Wagner PR, et al. (2011) Dose-response effects of omega-3 fatty acids on triglycerides, inflammation, and endothelial function in healthy persons with moderate hypertriglyceridemia. Am J Clin Nutr 93: 243–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Harris W (2010) Omega-6 and omega-3 fatty acids: partners in prevention. Curr Opin Clin Nutr Metab Care 13: 125–129. [DOI] [PubMed] [Google Scholar]
- 28. Ouguerram K, Maugeais C, Gardette J, Magot T, Krempf M (2006) Effect of n-3 fatty acids on metabolism of apoB100-containing lipoprotein in type 2 diabetic subjects. Br J Nutr 96: 100–106. [DOI] [PubMed] [Google Scholar]
- 29. Ozasa H, Ayaori M, Iizuka M, Terao Y, Uto-Kondo H, et al. (2011) Pioglitazone enhances cholesterol efflux from macrophages by increasing ABCA1/ABCG1 expressions via PPARgamma/LXRalpha pathway: findings from in vitro and ex vivo studies. Atherosclerosis 219: 141–150. [DOI] [PubMed] [Google Scholar]
- 30. Montoya MT, Porres A, Serrano S, Fruchart JC, Mata P, et al. (2002) Fatty acid saturation of the diet and plasma lipid concentrations, lipoprotein particle concentrations, and cholesterol efflux capacity. Am J Clin Nutr 75: 484–491. [DOI] [PubMed] [Google Scholar]
- 31. Ottestad I, Hassani S, Borge GI, Kohler A, Vogt G, et al. (2012) Fish oil supplementation alters the plasma lipidomic profile and increases long-chain PUFAs of phospholipids and triglycerides in healthy subjects. PLoS One 7: e42550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Davidson WS, Gillotte KL, Lund-Katz S, Johnson WJ, Rothblat GH, et al. (1995) The effect of high density lipoprotein phospholipid acyl chain composition on the efflux of cellular free cholesterol. J Biol Chem 270: 5882–5890. [DOI] [PubMed] [Google Scholar]
- 33. Holzer M, Wolf P, Curcic S, Birner-Gruenberger R, Weger W, et al. (2012) Psoriasis alters HDL composition and cholesterol efflux capacity. J Lipid Res 53: 1618–1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Fournier N, Atger V, Cogny A, Vedie B, Giral P, et al. (2001) Analysis of the relationship between triglyceridemia and HDL-phospholipid concentrations: consequences on the efflux capacity of serum in the Fu5AH system. Atherosclerosis 157: 315–323. [DOI] [PubMed] [Google Scholar]
- 35. Rousset X, Vaisman B, Amar M, Sethi AA, Remaley AT (2009) Lecithin: cholesterol acyltransferase–from biochemistry to role in cardiovascular disease. Curr Opin Endocrinol Diabetes Obes 16: 163–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sheril A, Jeyakumar SM, Jayashree T, Giridharan NV, Vajreswari A (2009) Impact of feeding polyunsaturated fatty acids on cholesterol metabolism of dyslipidemic obese rats of WNIN/GR-Ob strain. Atherosclerosis 204: 136–140. [DOI] [PubMed] [Google Scholar]
- 37. Zhang L, Geng Y, Xiao N, Yin M, Mao L, et al. (2009) High dietary n-6/n-3 PUFA ratio promotes HDL cholesterol level, but does not suppress atherogenesis in apolipoprotein E-null mice 1. J Atheroscler Thromb 16: 463–471. [DOI] [PubMed] [Google Scholar]
- 38. Uehara Y, Miura S, von Eckardstein A, Abe S, Fujii A, et al. (2007) Unsaturated fatty acids suppress the expression of the ATP-binding cassette transporter G1 (ABCG1) and ABCA1 genes via an LXR/RXR responsive element. Atherosclerosis 191: 11–21. [DOI] [PubMed] [Google Scholar]
- 39. Ji Y, Jian B, Wang N, Sun Y, Moya ML, et al. (1997) Scavenger receptor BI promotes high density lipoprotein-mediated cellular cholesterol efflux. J Biol Chem 272: 20982–20985. [DOI] [PubMed] [Google Scholar]
- 40. Zhang Y, Da Silva JR, Reilly M, Billheimer JT, Rothblat GH, et al. (2005) Hepatic expression of scavenger receptor class B type I (SR-BI) is a positive regulator of macrophage reverse cholesterol transport in vivo. J Clin Invest 115: 2870–2874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Vaysse-Boue C, Dabadie H, Peuchant E, Le Ruyet P, Mendy F, et al. (2007) Moderate dietary intake of myristic and alpha-linolenic acids increases lecithin-cholesterol acyltransferase activity in humans. Lipids 42: 717–722. [DOI] [PubMed] [Google Scholar]
- 42. Parks JS, Bullock BC, Rudel LL (1989) The reactivity of plasma phospholipids with lecithin:cholesterol acyltransferase is decreased in fish oil-fed monkeys. J Biol Chem 264: 2545–2551. [PubMed] [Google Scholar]
- 43. Yvan-Charvet L, Ranalletta M, Wang N, Han S, Terasaka N, et al. (2007) Combined deficiency of ABCA1 and ABCG1 promotes foam cell accumulation and accelerates atherosclerosis in mice. J Clin Invest 117: 3900–3908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Vergeer M, Brunham LR, Koetsveld J, Kruit JK, Verchere CB, et al. (2010) Carriers of loss-of-function mutations in ABCA1 display pancreatic beta-cell dysfunction. Diabetes Care 33: 869–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. von Eckardstein A, Sibler RA (2011) Possible contributions of lipoproteins and cholesterol to the pathogenesis of diabetes mellitus type 2. Curr Opin Lipidol 22: 26–32. [DOI] [PubMed] [Google Scholar]
- 46. Storlien LH, Kraegen EW, Chisholm DJ, Ford GL, Bruce DG, et al. (1987) Fish oil prevents insulin resistance induced by high-fat feeding in rats. Science 237: 885–888. [DOI] [PubMed] [Google Scholar]
- 47. Thomas TR, Smith BK, Donahue OM, Altena TS, James-Kracke M, et al. (2004) Effects of omega-3 fatty acid supplementation and exercise on low-density lipoprotein and high-density lipoprotein subfractions. Metabolism 53: 749–754. [DOI] [PubMed] [Google Scholar]
- 48. Sugano M, Makino N, Yanaga T (1997) Effect of dietary omega-3 eicosapentaenoic acid supplements on cholesteryl ester transfer from HDL in cholesterol-fed rabbits. Biochim Biophys Acta 1346: 17–24. [DOI] [PubMed] [Google Scholar]
- 49. Abbey M, Clifton P, Kestin M, Belling B, Nestel P (1990) Effect of fish oil on lipoproteins, lecithin:cholesterol acyltransferase, and lipid transfer protein activity in humans. Arteriosclerosis 10: 85–94. [DOI] [PubMed] [Google Scholar]
- 50. Pawar A, Botolin D, Mangelsdorf DJ, Jump DB (2003) The role of liver X receptor-alpha in the fatty acid regulation of hepatic gene expression. J Biol Chem 278: 40736–40743. [DOI] [PubMed] [Google Scholar]
- 51. Wang LJ, Song BL (2012) Niemann-Pick C1-Like 1 and cholesterol uptake. Biochim Biophys Acta 1821: 964–972. [DOI] [PubMed] [Google Scholar]
- 52. Lecker JL, Matthan NR, Billheimer JT, Rader DJ, Lichtenstein AH (2011) Changes in cholesterol homeostasis modify the response of F1B hamsters to dietary very long chain n-3 and n-6 polyunsaturated fatty acids. Lipids Health Dis 10: 186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Chen J, Jiang Y, Liang Y, Tian X, Peng C, et al. (2012) DPA n-3, DPA n-6 and DHA improve lipoprotein profiles and aortic function in hamsters fed a high cholesterol diet. Atherosclerosis 221: 397–404. [DOI] [PubMed] [Google Scholar]
- 54. Mathur SN, Watt KR, Field FJ (2007) Regulation of intestinal NPC1L1 expression by dietary fish oil and docosahexaenoic acid. J Lipid Res 48: 395–404. [DOI] [PubMed] [Google Scholar]
- 55. Alvaro A, Rosales R, Masana L, Vallve JC (2010) Polyunsaturated fatty acids down-regulate in vitro expression of the key intestinal cholesterol absorption protein NPC1L1: no effect of monounsaturated nor saturated fatty acids. J Nutr Biochem 21: 518–525. [DOI] [PubMed] [Google Scholar]
- 56. Zilversmit DB (1983) A model for cholesterol absorption: isotope vs. mass; single dose vs. constant infusion. J Lipid Res 24: 297–302. [PubMed] [Google Scholar]
- 57. Jonkers IJ, Smelt AH, Princen HM, Kuipers F, Romijn JA, et al. (2006) Fish oil increases bile acid synthesis in male patients with hypertriglyceridemia. J Nutr 136: 987–991. [DOI] [PubMed] [Google Scholar]
- 58. Berard AM, Dumon MF, Darmon M (2004) Dietary fish oil up-regulates cholesterol 7alpha-hydroxylase mRNA in mouse liver leading to an increase in bile acid and cholesterol excretion. FEBS Lett 559: 125–128. [DOI] [PubMed] [Google Scholar]