Abstract
Yellow-seed (i.e., yellow seed coat) is one of the most important agronomic traits of Brassica plants, which is correlated with seed oil and meal qualities. Previous studies on the Brassicaceae, including Arabidopsis and Brassica species, proposed that the seed-color trait is correlative to flavonoid and lignin biosynthesis, at the molecular level. In Arabidopsis thaliana, the oxidative polymerization of flavonoid and biosynthesis of lignin has been demonstrated to be catalyzed by laccase 15, a functional enzyme encoded by the AtTT10 gene. In this study, eight Brassica TT10 genes (three from B. napus, three from B. rapa and two from B. oleracea) were isolated and their roles in flavonoid oxidation/polymerization and lignin biosynthesis were investigated. Based on our phylogenetic analysis, these genes could be divided into two groups with obvious structural and functional differentiation. Expression studies showed that Brassica TT10 genes are active in developing seeds, but with differential expression patterns in yellow- and black-seeded near-isogenic lines. For functional analyses, three black-seeded B. napus cultivars were chosen for transgenic studies. Transgenic B. napus plants expressing antisense TT10 constructs exhibited retarded pigmentation in the seed coat. Chemical composition analysis revealed increased levels of soluble proanthocyanidins, and decreased extractable lignin in the seed coats of these transgenic plants compared with that of the controls. These findings indicate a role for the Brassica TT10 genes in proanthocyanidin polymerization and lignin biosynthesis, as well as seed coat pigmentation in B. napus.
Introduction
Brassica species belong to the same taxonomic family Brassicaceae as Arabidopsis, representing the closest relatives to Arabidopsis thaliana. The genus Brassica contains many oilseed, vegetable and ornamental crops that are important sources of cooking oil, vegetables, and protein-rich meal for livestock feed. Among these species, Brassica napus is one of the most important oilseed crops cultivated, worldwide. The yellow-seeded B. napus varieties possess several advantages over black-seeded varieties, such as thinner seed coat, lower husk proportion and fiber content, and higher oil and protein content [1]–[3]. Extensive studies have established that the B. napus yellow-seed trait is highly correlated with high oil and meal qualities [1]–[3]. Unfortunately, currently, seed stock for the natural B. napus yellow-seeded genotype is unavailable. The yellow-seed trait was strongly influenced by environmental conditions [4]–[6]. Consequently, development of yellow-seeded B. napus cultivars and selection of stable yellow-seed traits has been a long-term breeding objective [7], [8]. However, a lack of information concerning the molecular basis controlling yellow-seed trait inheritance has seriously hampered progress in the breeding of yellow-seeded genotypes.
B. napus (2n = 38, AACC) is an amphidiploid species, originated from interspecific crosses of the diploid species Brassica rapa (2n = 20, AA) and Brassica oleracea (2n = 18, CC) [9]. The close phylogenetic relationship within these three Brassica species provides an ideal model for analyzing genetic evolution and practical implications for Brassica crop improvement by comparative studies. The high level of synteny and remarkably conserved genome structure between Arabidopsis and Brassica genomes [10] also enables comparative gene cloning and functional analysis in Brassica using Arabidopsis sequences as a reference.
A stable major quantitative trait locus (QTL) affecting seed coat color of B. napus in different generations and environments was earlier identified [7]. Based on microsynteny of this major QTL with Arabidopsis genome sequences, the functional gene TT10 was considered as a potential candidate for this QTL. In Arabidopsis, the AtTT10 (At5g48100) gene is one of 22 TT-type loci (TT1-19, BAN, TTG1 and TTG2) identified by the transparent testa (tt) mutants that are affected in seed coat pigmentation, a process involved in flavonoid biosynthesis [11]–[14]. At harvest, seeds of the tt10 mutant display pale-brown seed coat coloration with a dark-brown chalaza zone. After 6 to 12 months of storage, the seed coat turns brown and eventually it resembles the wild-type [15].
AtTT10 encodes the laccase 15 (AtLAC15), an enzyme involved in polymerization of proanthocyanidin (PA) into larger polymers and probable further oxidation of PA, which confers brown color to the seed coat [15]. Additionally, AtTT10 was also involved in lignin synthesis and polymerization of monolignols in Arabidopsis seeds [16]. PA was dramatically reduced in the seed coat of yellow-seeded rapeseed and seems to be the source of black-seed color in B. napus cv. Tower [17]. In B. napus, lignin content is an important trait of seed quality [8] and it is reported that yellow-seeded accessions, among the Brassicaceae, have significantly lower lignin content than those of brown- or dark-seeded accessions [17].
The correlation between PA polymerization and lignin synthesis with B. napus seed coat color, and the function of the AtTT10 gene in seed coat pigmentation, strongly suggests that systematic cloning and functional identification of the Brassica TT10 would provide a means to uncover the molecular mechanism of yellow-seed trait inheritance in Brassica species. In this study, 8 Brassica TT10 genes were cloned from B. napus and its two parental species, B. rapa and B. oleracea. Expression studies and functional analyses performed on transgenic TT10 gene-silenced plant lines established that BnTT10 is involved in PA metabolism, lignin synthesis and seed coats pigmentation. These findings are discussed in terms of clues to the nature of the molecular mechanisms underlying the establishment of yellow seed traits in Brassica species.
Materials and Methods
Plant Materials and Nucleic Acid Extraction
Typical black-seeded B. napus line 5B, B. rapa line 06K130 and B. oleracea line 06K158 were used for gene cloning. B. napus NILs 09L588 (black-seed) and 09L587 (yellow-seed), B. rapa NILs 09L597 (black-seed) and 09L600 (yellow-seed), and B. oleracea NILs 09Bo-1 (black-seed) and 09Bo-4 (yellow-seed) were used for expression pattern detection of Brassica TT10 genes by quantitative RT-PCR (qRT-PCR). Root (Ro), hypocotyl (Hy), cotyledon (Co), stem (St), leaf (Le), silique pericarp (SP) from each black-seeded line and bud (Bu), flower (Fl), seeds of 10 to 15, 25 to 30, 40 to 45 and 50 to 55 DAF of each black- and yellow-seeded lines were sampled for total RNA extraction using the CTAB method, with slight modifications [18]. Three independent plants from each line were sampled for RNA extraction. RNA aliquots were treated with RNase-free DNase I (TaKaRa, Dalian) to remove genomic DNA. Fresh leaves of each line were sampled to extract total genomic DNA, according to the CTAB method [19]. B. napus cv. Westar, Zhongyou821 and Zhongshuang10 were used for transgenic assays.
Cloning of TT10 Genes and Bioinformatics Analysis
The cDNAs of Brassica TT10 genes were cloned by RACE with GeneRacer kit (Invitrogen, USA), according to manufacturer's instructions. For each of 5B, 06K130, and 06K158 lines, a 5-µg equally proportioned (w/w) mixture of total RNA extracted from Bu, Fl, and seeds sampled 10, 20, and 30 DAF was used for first-strand cDNA synthesis. Gene-specific primers were designed based on the conservative region of AtTT10 and are listed in Table S1 [15], [20]. Primer combinations of RTT10-51, RTT10-52 and 5′-end cloning primers, and FTT10-31, FTT10-32 and 3′-end cloning primers were used for amplification of 5′- and 3′-ends of TT10 genes. PCR products were cloned into the pMD18-T vectors (TaKaRa, Dalian) and sequenced. The cDNAs of Brassica TT10 genes were amplified with combinations of 5′ primers (FBNTT10, FBRTT10 and FBOTT10) and 3′ primers (RBNTT10, RBRTT10, RBOTT10 and RBRTT10). Primers successful in cDNA amplifications were used to amplify corresponding genomic DNA sequences.
Open reading frame (ORF), parameter calculation and sequence alignment were performed with Vector NTI Advance 10.3 (Invitrogen). Sequence alignments and protein structure predictions were performed on Expasy (http://www.expasy.org). Multiple sequence alignments results from Clustal ×1.83 were subjected for phylogenetic tree construction by the Neighbor-Joining method with MEGA 3.1 [21], [22]. The reliability of the tree was measured by bootstrap analysis with 1,000 replicates.
Southern Hybridization
For each Brassica species, 50-µg genomic DNA was fully digested at 37°C with DraI, EcoRI and EcoRV (Fermentas, Lithuania), respectively, separated in 0.8% agarose gels, and transferred onto positively charged nylon membrane (Roche, Germany). A 951-bp fragment, mainly composed of the fifth exon of TT10 and no cutting sites of restriction enzymes for genomic DNA digestion, was used as probe, which was then amplified with primers FTT10A and RTT10A using BnTT10-2 cDNA as template. Probe labeling was carried out using a PCR DIG Probe Synthesis Kit (Roche) and hybridization was then performed with the DIG Easy Hyb Kit (Roche), and immunological detection was implemented with DIG Wash and Block Buffer Set and DIG Nucleic Acid Detection Kit (Roche), according to the manufacturer's instructions.
Expression Pattern Assay
Expression patterns of TT10 genes in various organs of three Brassica species were detected by qRT-PCR. One µg of each total RNA sample was reverse-transcribed in a 10-µl volume, using RNA PCR Kit (AMV) Ver.3.0 (TaKaRa, Dalian) and 0.5 µl of the RT product was used as template for a 25-µl standard Taq-PCR. A 193-bp conserved region of 18S rRNA was amplified as an internal control [23]. Primers for qRT-PCR were: FBnT10Q and RBnT10Q for detection of overall TT10 gene expression; FBnT10-1Q and RBnT10-1Q for detection of BnTT10-1 and BrTT10-1A; FBnT10-2Q and RBnT10-2Q for detection of BnTT10-2 and BoTT10-1; FBnT10-3Q and RBnT10-3Q for detection of BnTT10-3 and BRTT10-2; FBrT10-1BQ and RBrT10-1BQ for detection of BrTT10-1B; and FBoT10-1pseQ and RBoT10-1pseQ for detection of BoTT10-1pse. Reactions were performed in triplicate from 3 independent samples, with a negative water control in each run. The specificity of qRT-PCR products was confirmed by agarose gel electrophoresis followed by sequencing.
Transgenic Plant Development
The 951-bp conserved fragment used for Southern hybridization was cloned in antisense orientation into the binary vector, pCAMBIA2301G. This antisense TT10 expression construct was transferred into B. napus cv. Westar, Zhongyou821 and Zhongshuang10 using Agrobacterium tumefaciens strain LBA4404, as previously described [24].
GUS staining and PCR identification of leaf pieces was used for selection of T0 generation transgenic plants [25]. To assess the inhibition of BnTT10 gene expression in seeds of transgenic plants, qRT-PCR was applied to measure the overall TT10 and individual genes BnTT10-1, BnTT10-2 and BnTT10-3 expression in seeds at 35 DAF with the above-mentioned gene-specific primers. Negative plants were kept as controls.
Seed Pigmentation Observation
To observe seed coat color during seed development, pods were regularly sampled at 40, 45, 50, 55 and 60 DAF, and the stripped pods were visualized under a low-power stereoscope. For observation of seed coat color during seed after-ripening, pods sampled at 40, 50, and 55 DAF were also stripped and surveyed after 5 and 15 days of in vitro storage at 4°C.
Quantification of Proanthocyanidin (PA) and Extractable Lignin Content
Soluble and insoluble PA content was determined using the butanol-HCl method, as previously described [16], [26]. Assessment of extractable lignin content was carried out according to the acetyl bromide method, as previously described [27], [28], with slight modifications. The assessment was conducted using three independent batches of seed samples. Each sample was assayed in triplicate to obtain a mean value. Proanthocyanidin and extractable lignin content are shown in absorbance value (OD550 and OD280, respectively) per sample weight unit.
High Performance Liquid Chromatography (HPLC)-MS-UV Analysis
Liquid chromatography-mass spectrometry (LC-MS) solvents were obtained from Fisher Science (Rockford, IL, USA). Flavonoid standards were obtained from Indofine (Somerville, NJ, USA), Sigma-Aldrich (St. Louis, MO) and Chromax (Irvine, CA). Freshly harvested seed coats (100 mg fresh weight) were homogenized in 80% methanol (1 ml), and the suspension was placed in an ultrasonic bath for 1 h. The extract was centrifuged (1.0×104 g, 20 min) and the supernatant was then filtered.
Analyses of plant extracts were performed with an Agilent 1100 HPLC system (Hewlett-Packard, Palo Alto, CA) combined with an ion trap mass spectrometer (model Bruker Esquire 3000, Bruker Daltonics, Bremen Germany). Instrument analyses were carried out using a Grace Column (2.0×250 mm, grain diameter 4.6 µm). UV-visible spectra were obtained by scanning from 200 to 600 nm. The mobile phase consisted of (A) water containing 0.1% (v/v) formic acid and (B) acetonitrile, using the following binary gradient: 0 to 5 min, isocratic 95% A and 5% B; 5 to 10 min, isocratic 10% B; 10 to 17 min, isocratic 17% B; 17 to 25 min, isocratic 25% B; 25 to 30 min, isocratic 30% B, 30 to 55 min, isocratic 55% B, 55 to 65 min, isocratic 70% B. 65 to 70 min, isocratic 5% B, 70 to 75 min, isocratic 95% A and 5% B. Flow rate was 0.8 ml min−1. Negative-ion ESI mass spectra were employed, using an ion source voltage of 3.5 kV, a counter current nitrogen flow set at a pressure of 12 psi, and a capillary temperature of 350°C. Mass spectra were recorded over the range 50–2,200 m z−1. The Bruker ion-trap mass spectrometer (ITMS) was operated using an ion current control (ICC) of approximately 1×104 with a maximum acquisition time of 100 ms. Tandem mass spectra were obtained in manual mode for targeted masses using an isolation width 2.0, fragmentation amplitude of 2.2 and threshold set at 6×103.
Accession Numbers: HM805058 (BnTT10-1 gene), HM805059 (BnTT10-1 mRNA), HM805060 (BnTT10-2 gene), HM805061 (BnTT10-2 mRNA), HM805062 (BnTT10-3 gene), HM805063 (BnTT10-3 mRNA), HM805064 (BrTT10-1A gene), HM805065 (BrTT10-1A mRNA), HM805066 (BrTT10-1B gene), HM805067 (BrTT10-1B mRNA), HM805068 (BrTT10-2 gene), HM805069 (BrTT10-2 mRNA), HM805070 (BoTT10-1 gene), HM805071 (BoTT10-1 mRNA), HM805072 (BoTT10-1pse mRNA).
Results
Cloning and Sequence Analysis of TT10 Genes from B. napus and Its Two Parental Species
Three full-length cDNAs, designated as BnTT10-1, BnTT10-2 and BnTT10-3, were cloned from B. napus (Fig. S1), two full-length cDNAs BoTT10-1, BoTT10-1pse were isolated from B. oleracea, and three full-length cDNAs BrTT10-1A, BrTT10-2, BrTT10-1B were obtained from B. rapa (Table S2). Corresponding genomic DNA of these cDNAs were obtained by PCR amplification with genomic DNA as template. Unfortunately, genomic DNA sequences for BnTT10-2 and BoTT10-1pse were not obtained.
Southern blot hybridization against genomic DNA of B. napus, B. oleracea and B. rapa showed 4, 1 and 1–2 bands in all lanes, respectively (Fig. S2), indicating the presence of minimum number of TT10 genes. Taken together, our results of gene cloning and Southern blot hybridization suggest that the eight cloned Brassica TT10 genes comprise the majority of TT10 genes in B. napus and its two parental species.
Based on transcript sequence similarity and the theoretical protein translations, the Brassica TT10 genes could be divided into two groups (Table S3 and S4). Group I included BnTT10-3 and BrTT10-2 that share 99.7% similarity and encode identical proteins of 560 amino acids (aa), Group II contains the remaining six Brassicaceae TT10 genes with similarity of 94.6% to 99.8%, whereas their similarity to Group I members was only 85.2% to 86.3%. Among Group II, BnTT10-1, BrTT10-1A and BrTT10-1B showed 99.3% to 99.8% identity at the mRNA level, and the deduced proteins of the three members were all 559 aa in length and shared 99.3% to 99.8% similarity. BnTT10-2 and BoTT10-1 have identical cDNA sequences and encode identical proteins of 563 aa; BoTT10-1pse does not encode a fully functional protein, due to deletion mutations leading to a frame-shift and premature termination of translation. Pairwise alignment indicates that either cDNA or protein sequences of Brassica TT10 genes are approximately 80% identical to that of AtTT10.
SignalP 3.0 (http://www.cbs.dtu.dk/services/SignalP/) predicted that BnTT10 proteins contain a signal peptide most likely cleaved at A21–H22. PSORT (http://psort.ims.u-tokyo.ac.jp/) also predicted that Group I proteins would be secreted to the cell wall. In contrast, Group II proteins appear to have an uncleavable N-terminal signal sequence and may well be endoplasmic reticulum-located proteins. Brassica TT10 proteins were as conserved as AtTT10 and other laccases. In addition, as previous reported, they contain four His-rich copper binding domains, L1 to L4 [29], and two signatures of the multicopper oxidase family, the 21-aa multicopper oxidase signature 1 and 12-aa signature 2 (Fig. S3), corresponding to the putative catalytic sites conserved in laccases [29]. Phylogenetic analysis indicated that these Brassica TT10 proteins (excluding BoTT10-1pse) formed a small branch clustered with AtTT10 and diverged from other analyzed laccases (Fig. S4).
Spatial and Temporal Expression Patterns of Brassica TT10 Genes
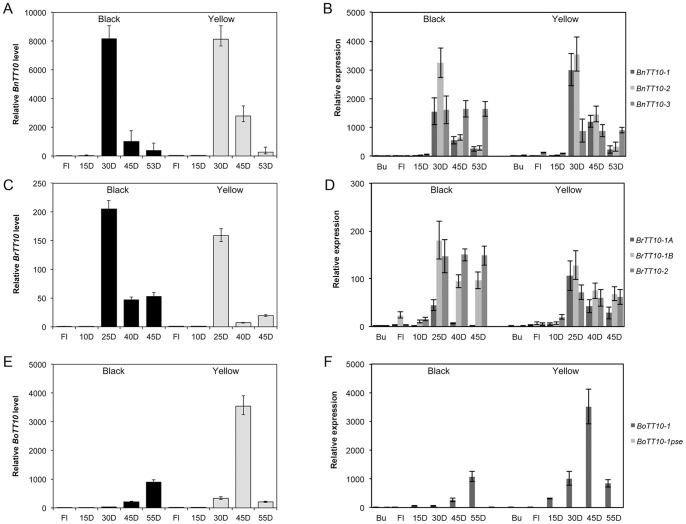
AtTT10 was mainly expressed in developing siliques and seeds, specifically in the seed coat [15], [16], [20], [30]. To verify the organ-specific function of Brassica TT10 genes, qRT-PCR was used to determine their mRNA levels in different organs of the black-seeded lines from the three Brassica species. As shown in Fig. 1 and S5, Brassica TT10 genes were predominantly expressed in developing seeds, low level transcripts of BnTT10-3, BrTT10-2 and BrTT10-1B were observed in flowers, whereas expression of TT10 genes was hardly detected in silique pericarp, hypocotyl, stem or root tissues. Transcripts for BoTT10-1pse, the B. oleracea TT10 gene with deletion mutations, was undetectable in all organs examined. We also found that BnTT10 genes were expressed at very low levels in hypocotyls and roots; here, expression was almost 5×10−4 below that in seeds.
Figure 1. Expression patterns of BnTT10, BrTT10 and BoTT10 genes in black- and yellow-seeded lines.
QRT-PCR detection of BnTT10 (A), BrTT10 (C) and BoTT10 (E) family mRNA, and transcript levels of BnTT10 (B), BrTT10 (D) and BoTT10 (F) family members in reproductive organs of B. napus, B. rapa and B. oleracea black- and yellow-seeded near-isogenic lines. Error bars represent standard deviations (n = 3 for A, C and E).
Temporal expression of Brassica TT10 genes was analyzed with developing seeds from the three Brassica species. The Group I members, BnTT10-3 and BrTT10-2, showed similar expression patterns through middle (25 to 30 days after flowering [DAF]), late (40 to 45 DAF) and pigmented (45 to 53 DAF) stages (Fig. 1 and S5). The Group II members, BnTT10-1, BnTT10-2 and BrTT10-1B, were found to be expressed only weakly at the early stage, at highest levels during the middle stage, and strongly at late and pigmented stages. BrTT10-1A was expressed strongly at middle stage, moderately at late stage, but transcripts were undetectable at the early and weakly in pigmented stages. Expression of BoTT10-1 showed a seed-development-stage-dependent increase with weakest expression during the early stage and highest expression at the pigmented stage (Fig. 1 and S5).
Correlation Between TT10 Expression and Yellow-seed Trait of B. napus and Its Two Parental Species
To examine the correlation between yellow-seed trait and TT10 gene expression in Brassica species, expression of Brassica TT10 genes was detected in developing seeds and flowers from black- and yellow-seeded lines of the three Brassica species. In developing seeds of B. rapa and B. oleracea, yellow-seeded lines showed down- and up-regulation of overall expression of TT10 genes compared with the black-seeded lines, respectively (Fig. 1C and 1E). The yellow-seeded lines of B. napus showed slight up-regulation of overall expression of TT10 genes in seeds (45 DAF) compared with the black-seeded lines (Fig. 1A). Moreover, individual members also showed different expression patterns (Fig. 1B, 1D and 1F). Group I genes (BnTT10-3, BrTT10-2) and a Group II member (BrTT10-1B) showed higher expression levels in developing seeds of black-seeded lines compared to yellow-seeded lines, whereas transcription of other Group II members (BnTT10-1, BnTT10-2, BrTT10-1A and BoTT10-1) exhibited the opposite trend. Additionally, in developing seeds of B. oleracea, BoTT10-1 showed the highest expression level in pigmented (55 DAF) stage of black-seeded lines, but in late (45 DAF) stage of yellow-seeded lines (Fig. 1F).
Antisense Suppression of TT10 Genes Retards Pigmentation in the Seed Coat of Black-Seeded B. napus
To investigate the function of Brassica TT10 genes in seed coat pigmentation, a 951-bp DNA fragment conserved in the Brassica TT10 genes was expressed in antisense orientation to suppress TT10 genes in black-seeded B. napus. A total of 14, 8 and 9 T0 plants of Westar, Zhongyou821 and Zhongshuang10 were identified as positive transgenic plants by GUS staining and qRT-PCR analysis, in which the overall expression of TT10 genes was repressed up to 83% (Fig. S6), and suppression level of individual members were similar (Fig. S6). For each cultivar, three T0 plants with suppressed TT10 expression were selected to generate progenies for seed coat pigmentation analysis.
Seeds of T2 generation from Zhongyou821 and Zhongshuang10 transgenic plants exhibit various degrees of retarded seed coat pigmentation at the maturing stage when compared with control plants (Fig. 2). To explore the association of autoxidation with seed pigmentation, the in vitro seed pigmentation process of transgenic plants was also investigated. When pods were picked at 40 DAF, seed pigmentation of control plants was initiated rapidly and the seed coat color turned to black in 5 days of storage (DS) (Fig. S7). In contrast, under these conditions, seeds of transformants showed retarded pigmentation after 5 DS (Fig. S7) and they displayed homogeneous red-brown color after 15 DS, with no trend to turn black after a month of storage under ambient temperature conditions.
Figure 2. Seed pigmentation observation.
Seedpods were sampled at 42, 50, 55, 60 and 45 DAF and the opened pods were observed under a low-power stereoscope. 5 DAH: five days after harvest. Seed coat pigmentation in the T2 transgenic and control B. napus cv. Zhongyou821 (A) and Zhongshuang10 (B).
Suppression of TT10 Genes Increased Soluble PAs and Resulted in Reduction of Lignin Content in Seed Coats
To understand the roles of Brassica TT10 genes in PA polymerization, we measure the PA content in seed coats of transgenic B. napus. The acetone-soluble PA content in seed coats of T2 transgenic Westar plants was more than 3 fold higher than that detected in the control, and this increase was highly correlated with the degree to which the TT10 transcripts were lowered in these transgenic lines (Fig. 3A). Interestingly, the acetone-insoluble PA of seed coats, measured in the remaining residue after solvent extraction, also showed 1.2 to 1.9 fold increase in these transgenic Westar lines compared with the control (Fig. 3B). However, here, the difference of insoluble PA content was smaller than for soluble PA-content.
Figure 3. Soluble and insoluble PAs measured after acid-catalyzed hydrolysis in seed coats transgenic and control lines.

The P-value is for a t-test for means of paired samples. W-10, W-12, W-13: transgenic lines with inhibited BnTT10 expression; W-22: transgenic lines with no inhibition in BnTT10 expression; W-24: control lines with normal BnTT10 expression. T2-P: positive T2 progenies; T2-N: negative T2 progenies after separation. Soluble (A) and insoluble PA (B) content in seed coats of T2 transgenic and control B. napus cv. Westar plants. Each value represents the means of three independent experiments +/− SD.
Seed coats of T2 transgenic lines contained less extractable lignin than the controls, being decreased by 5%, 12% and 16% in the three transgenic lines (Fig. 4). Transgenic line W-12 exhibited close to 83% reduction in overall TT10 mRNA level and showed the highest decrease in lignin content compared with the other lines. Consistent with this notion, soluble and insoluble PA content accumulation (Fig. S8 and S9), and reduction in lignin content in seed coats was also verified in Zhongyou821 and Zhongshuang10 transgenic lines (Fig. S10 and S11).
Figure 4. Lignin content in seed coats of transgenic and control lines.
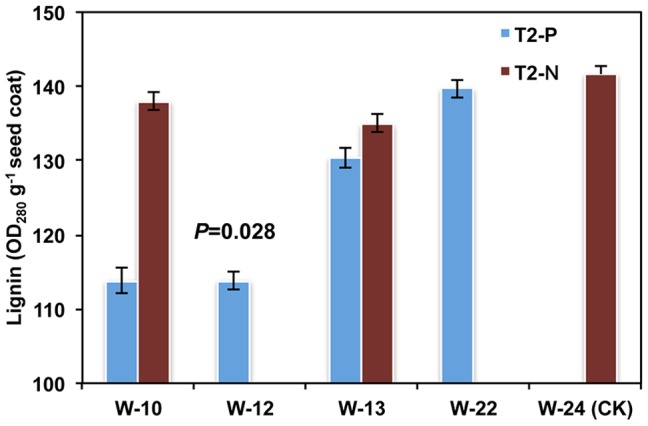
Lignin content was analyzed for seed coats of T2 antisense BnTT10 transgenic and control lines using the acetyl bromide method. Data are means for three T2 progenies of each line, with triplicate measurements in each sample. The P-value is for a t-test for means of paired samples. W-10, W-12 and W-13: transgenic lines with inhibited BnTT10 expression; W-22: transgenic lines with no inhibition in BnTT10 expression; W-24: control lines with normal BnTT10 expression. T2-P: positive T2 progenies; T2-N: negative T2 progenies after separation. Each value represents the means of three independent experiments +/− SD.
Negative T2 progenies generated from positive T1 plants and the transgenic lines without repression of TT10 gene expression (W-22) showed no significant difference with the control with respect to PA and lignin content of the seed coat. This strengthened our conclusion that the observed decrease in PA content and reduction in lignin content in seed coats from transgenic plants was due to a reduction in the transcript levels for the TT10 genes.
Suppression of TT10 Genes Changed Flavonoid Composition in the Seed Coat
Epicatechin and procyanidin polymers were detected by liquid chromatography- mass spectrometry (LC-MS) analysis of the soluble fractions (Fig. 5 and S12). Compared with seed coats from control plants, monomer PA epicatechin and procyanidin dimer B2 (epicatechin-[4β-8]-epicatechin) were 1.5 and 2.8 fold more abundant in transgenic lines with reduced BnTT10 transcript levels. We also carried out LC-MS analysis of anthocyanins and/or other flavonoids. Seed coats of transgenic lines contained more kaempferol-3-O-glucoside-7-O-glucoside and isorhamnetin-dihexoside compared with the control. On the other hand, quercetin-3-glucoside was about 2.3 fold lower than in the control seed coats. The abundance of other flavonols was not significantly changed by the antisense suppression of TT10 genes (Fig. 5).
Figure 5. Detection of flavonoid composition in seed coats from transgenic and control B. napus plants.
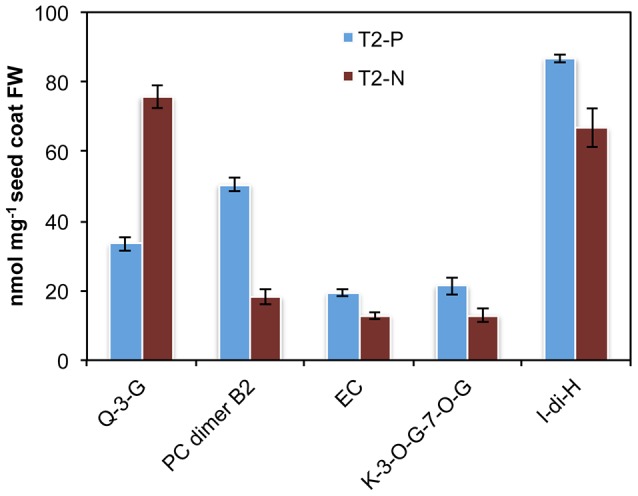
Analyses were performed by LC-UV-MS on seed coats of T2 antisense BnTT10 transgenic and control lines of B. napus cv. Zhongyou821. Q-3-G, Quercetin-3-glucoside; PC dimer B2, [DP2]-B2, epicatechin-(4β-8)-epicatechin; EC, epicatechin; K-3-O-G-7-O-G, kaempferol-3-O-glucoside-7-O-glucoside; I-di-H, isorhamnetin-dihexoside. Each value represents the means of three independent experiments +/− SD.
Discussion
Brassica TT10 Genes Show Differential Involvement in the Yellow Seed Trait
Our studies established that the BrTT10 and BoTT10 genes are donors of BnTT10 genes. Highly homologous mRNA sequences, identical protein sequences and similar expression patterns indicated that BrTT10-1A, BoTT10-1 and BrTT10-2 are progenitors of BnTT10-1, BnTT10-2 and BnTT10-3, respectively. Moreover, individual BnTT10 genes probably inherited the expression patterns of its donor gene, for spatial and temporal characteristics, as well as association with the yellow seed trait. BnTT10-2 and its donor gene, BoTT10-1, BnTT10-1 and its donor gene, BrTT10-1A, showed higher expression in yellow-seeded lines, whereas BrTT10-2 and its acceptor gene, BnTT10-3, showed down-regulated expression in yellow-seeded lines, indicating that individual gene members of a gene family may play individual roles in terms of the yellow seed trait. However, overall TT10 expression in developing seeds of yellow- and black-seeded near-isogenic lines (NILs) of B. napus showed no significant difference. Therefore, the features of amphidiploid B. napus and the TT10 multi-gene family should be considered in studies to elucidate the molecular basis of yellow seed traits.
Involvement of BnTT10 Genes in PA Accumulation in Seed Coats
PAs are oligomers and polymers of flavan-3-ols ((+)-catechin and (–)-epicatechin) units, also called condensed tannins, and known as end products of the flavonoid biosynthetic pathway [31], [32]. Based on studies in Arabidopsis, it has been proposed that AtTT10 functions in PA polymerization [15], [16]. Our result also showed that suppression of TT10 genes results in an increase in the level of soluble PAs (i.e. monomers or oligomers of PAs) in seed coat, indicating that BnTT10 probably performs the same function as AtTT10 at the step of oxidative polymerization of PAs. Our LC-MS analysis confirmed this result. Seed coats of transgenic plant lines accumulated more epicatechin and PA dimer B2 (epicatechin-[4β-8]-epicatechin) than that of controls, suggesting that BnTT10 genes are involved in polymerization of these two types of soluble PAs, the monomer and B2-type dimer epicatechin.
In addition, the PA content in the seed coat residue remaining after solvent extraction was also higher in the transgenic lines compared with the control. These results are similar to the findings of Pourcel et al. [15] and Liang et al. [16], in that tt10 mutant seeds showed increases in total PA content. According to the report of Marles and Gruber [17], PA was tightly bound in mature Brassicaceae seed coats and only a very small amount of PA could be extracted from the seed coat of these species and detected as released anthocyanidin subunits. Thus, the increase in PA content, in the seed coat residue, can be regarded as a decrease in larger polymers, which could not be acid hydrolyzed. Presumably, then, BnTT10 is able to further oxidize PAs into larger polymers in seed coat of B. napus.
Our studies indicated that functional divergence occurred between the two groups of Brassica TT10 genes in PAs oxidative polymerization. Group II members, excluding BoTT10-1 and BoTT10-1pse, achieved their highest level of expression in middle-stage (25 to 30 DAF) seeds, with a decreasing trend during the gradual seed maturing process. In addition, the predicted subcellular localization of Group II proteins is the endoplasmic reticulum (ER) and in ER-derived vesicles in which flavan-3-ols are synthesized as colorless polymers on Arabidopsis testa [15], [33], [34]. In this scenario, Group II genes probably act at the early step of PA polymerization, though the product may not be colorless [15]. In contrast, the Group I members, BnTT10-3 and BrTT10-2, exhibited strong expression during the entire process of seed development. As with AtTT10, the Group I genes are predicted to be secreted into the apoplast, where epicatechin and PAs would interact with TT10 proteins to become oxidized and polymerized during the seed desiccation period [15], [35]. Hence, it is possible that the TT10 Group I genes participate primarily in the further oxidation of PAs.
TT10 Genes Affect the Pigmentation Process of Seed Coats in B. napus
In brown or dark seed coats, the pigmentation process is thought to be initiated by the polymerization of flavan-3-ol monomers into colorless PAs polymers, which then undergo successive oxidation reactions leading to more complex oxidation levels of PAs during seed maturation. The final product is the insoluble complexes within the cell wall, with the resultant browning or darkening of the seed coat [4], [15]. TT10 has been shown to catalyze the oxidative polymerization of epicatechin into yellow to brown PA derivatives that differ from the colorless PAs in Arabidopsis [15], our study also established that BnTT10 genes act probably at the step of polymerization of monomers or oligomeric PA into polymers, thus, the functional defect caused by our transgenic mediated reduction in BnTT10 transcript level may affect the accumulation of pigments in seed coats and, furthermore, caused the observed delay in the onset of discoloration.
Our results establish that the BnTT10 play a key role in the initiation of seed coat browning or darkening in B. napus. This finding is also corroborated by in vitro observations of seed pigmentation. Compared with seed pigmentation of control plants, seeds of transgenic plants, harvested at 40 DAF (here, seed coat pigmentation has not yet been initiated), also showed pigmentation delay after 5 days storage at 4°C. The fact that seed coat color did not turn black, after a month of after-ripening, revealed that the role of BnTT10 in pigment precursor accumulation within the seed coat could not be compensated for by another enzymatic reaction and autoxidation. Then, seeds from transgenic plants, harvested at 50 DAF and 55 DAF (late stage of pigmentation), became completely discolored during a 5-day-storage period, implying that there is enough pigment precursors had accumulated in the seed coat and are readily oxidized to form pigment that confers browning or darkening of the seed coat, and then, the seed then accomplished pigmentation by autoxidation (a condition resembling the after-ripening process of rapeseed). Given that PA polymers may have multiple stereochemistries [31], the forms of the pigments associated with dark seed coat coloration need to be studied to determine the role of both enzymatic reaction and auto-oxidation in the further oxidation of PA polymers.
Involvement of BnTT10 Genes in Lignin Synthesis in Seed Coats
One of the functions of plant laccases is in the polymerization of monolignol to lignin polymers, which are constituents of the plant cell wall [36], [37]. It has been reported that laccases are involved in lignin synthesis [36], [38], and Liang et al. [16] showed that lignin content in mature seeds of the Arabidopsis tt10 mutant is decreased by some 30% and, further, developing seeds of the tt10 mutant also have reduced activity in terms of monolignol polymerization. Consistent with a role for BnTT10 genes in lignin sysnthesis, seed coats of transgenic B. napus plants had reduced levels of lignin. Although lignin content was reported to be significantly lower in yellow-seeded samples compared with the dark-seeded accessions, and low lignin is strongly associated with the unpigmented seed coat trait in Brassicaceae [17], the correlation of lignin synthesis with formation of seed coat color needs to be further investigated. Since lignin content is an important trait that needs to be considered when selecting for low-phenolic and low-fibre rapeseed [17], BnTT10 genes may provide an avenue for further quality improvement.
TT10 May Not Be the Major Locus for the Yellow-Seed Trait
The seed coat of yellow-seeded rapeseed is uncolored and the associated degree of transparency allows the underlying yellow-colored embryo to be visible. Although the transgenic plants with suppressed BnTT10 expression showed reduced seed pigmentation, the T2 progeny of these transgenic lines showed no difference in seed coat color with that of the control plants. Furthermore, the seed coat of the Arabidopsis tt10 mutant has an unstable pale brown color, but is not completely colorless. Thus, neither a complete nor incomplete functional deficiency of the BnTT10 genes would give rise to yellow seed color in B. napus. Taken together, these findings suggest that the BnTT10 genes may not function as the major locus that is crucial for development of the yellow-seed trait.
Based on previous studies from our group, the yellow-seed trait may represent a QTL that is controlled by a combination of a major gene and several minor genes. Considering that lignin and PA synthesis are two diverged branches of the phenylpropanoid metabolic pathway, in conjunction with our finding that both extractable and unextractable PA, as well as lignin content, are dramatically reduced in seed coats of yellow-seeded rapeseed [17], [39], the possibility exists that the major gene responsible for the yellow-seed trait is an upstream gene acting on a common step, or affecting the accumulation of a common precursor of PA and lignin synthesis. Alternatively, as genes acting at the terminal steps of the two branches, BnTT10 may be one of the downstream genes regulated by this major gene of the yellow-seed trait. Investigations on biosynthesis and deposition of pigments and lignin, within the seed coat, may provide further insight into the molecular basis of this important trait.
Supporting Information
Alignment of the mRNA sequences of BnTT10 genes. Conservative similar residues were displayed in dark background. The 951-bp conserved fragment used for Southern hybridization and antisense suppression was highlighted by red line.
(PDF)
Southern blot hybridization detection of TT10 genes in B. napus , B. oleracea and B. rapa .
(TIF)
Alignment of the amino acid sequences of BnTT10 proteins. BrTT10 and BoTT10-1 proteins share high identity with BnTT10 proteins and were not shown in the alignment. Non-similar, weakly similar, block of similar, conservative/strongly similar residues are displayed in dark, dark gray, gray and light gray background, respectively. The four His-rich copper binding domains, L1-L4, are highlighted by line segments, and the amino acids involved in binding copper are boxed. Two predicted multicopper oxidase signature 1 and 2 are identified by single and double dotted lines, respectively.
(TIF)
Phylogenetic relationship of inferred Brassica TT10 proteins and other plant laccases. Acer pseudoplatanus ApLAC1 (AAB09228); Aspergillus flavus AfLAC (XP_002378028); Arabidopsis thaliana AtTT10 (NP_199621), AtLAC4 (NP_565881), AtLAC12 (NP_196158), AtLAC13 (NP_196330), AtLAC14 (NP_196498); AtLAC17 (NP_200810), Castanea mollissima CmLAC (ACI46953); Oryza sativa OsLAC2 (Q8RYM9), OsLAC9 (Q6Z8L2); Pinus taeda PtLAC1 (AAK37823), PtLAC2 (AAK37824); Ricinus communis RcLAC (XP_002527130); Solanum lycopersicum ascorbate oxidase SlAOX (AAY47050); Zea mays ZmLAC3 (NP_001105915). This tree was constructed by the Neighbor-Joining method with p-distance. The number for each interior branch is the percent bootstraps value (1,000 replicates), and only values greater than 50% are shown. Scale bar indicates the estimated number of amino acid substitutions per site.
(TIF)
QRT-PCR detection of transcription levels for BnTT10 (A), BrTT10 (B) and BoTT10 (C) genes in various organs of black-seeded B. napus , B. rapa and B. oleracea , respectively. Ro: root; Hy: hypocotyl; Co: cotyledon; St: stem; Le: leaf; Bu: bud; Fl: flower; SP: silique pericarp; 10, 15, 25, 30, 40, 45, 50, 52 and 55 D: seeds at 10, 15, 25, 30, 40, 45, 50, 52 and 55 days after flowering.
(TIF)
Expression of BnTT10 genes in the seeds of transgenic and control B. napus . QRT-PCR analysis of overall BnTT10 expression in seeds of T1 transgenic and control lines of Westar (A), Zhongyou821 (C) and Zhongshuang10 (E); qRT-PCR analysis of member-specific expression of BnTT10 genes in seeds of transgenic and control lines of Westar (B), Zhongyou821 (D) and Zhongshuang10 (F). Error bars indicate SD.
(TIF)
In vitro seed pigmentation of T2 transgenic and control of B. napus . Siliques of T2 transgenic and control lines of B. napus were regularly sampled at 40, 50 and 55 DAF, and the pods opened 5 days later for observation under a low-power stereoscope. In vitro seed pigmentation of T2 transgenic and control B. napus cv. Zhongyou821 (A) and Zhongshuang10 (B) plants.
(TIF)
Analysis of soluble (A) and insoluble (B) PAs measured after acid-catalyzed hydrolysis of seed coats from seeds of T2 transgenic and control B. napus cv. Zhongyou821 plants. ZY-1, ZY-3 and ZY-17: transgenic lines with inhibited BnTT10 expression; ZY-13: control lines with normal BnTT10 expression. Values are means +/− SD from triplicate measurements in each sample.
(TIF)
Analysis of soluble (A) and insoluble (B) PAs measured after acid-catalyzed hydrolysis of seed coats from seeds of T2 transgenic and control B. napus cv. Zhongshuang10 plants. ZS-4, ZS-8 and ZS-12: transgenic lines with inhibited BnTT10 expression; ZS-2: control lines with normal BnTT10 expression. Values are means +/− SD from triplicate measurements in each sample.
(TIF)
Lignin content in seed coats of transgenic and control B. napus cv. Zhongyou821 plants. Lignin content was analyzed on seed coats from seeds of T2 transgenic and control lines using the acetyl bromide method. Values are means +/− SD from triplicate measurements in each sample.
(TIF)
Lignin content in seed coats of transgenic and control B. napus cv. Zhongshuang10 plants. Lignin content was analyzed on seed coats from seeds of T2 transgenic and control lines using the acetyl bromide method. Error bars indicate SD of three biological replicates.
(TIF)
LC-UV-MS chromatograms of flavonoid compounds identified from seed coats extracts from control (A) and transgenic (B) B. napus cv. Zhongyou821 plants.
(TIF)
Primers used in this study.
(DOC)
Structure parameters of BnTT10 , BrTT10 and BoTT10 genes.
(DOC)
Percentages of full-length mRNA identities for Brassica TT10 genes and AtTT10 .
(DOC)
Identities and positives between deduced Brassica TT10 proteins and AtTT10 (%).
(DOC)
Acknowledgments
We thank Professor William Lucas, University of California-Davis and Dr. Hongbo Zhang, Southwest University for their critical readings of the manuscript, and Dr. Fu-you Fu, Plant Gene Resources of Canada, Agriculture and Agri-Food Canada for LC-MS assays.
Funding Statement
This work was supported by National Natural Science Foundation of China [31071450, 31171619, 31101175 and 31171177, http://www.nsfc.gov.cn], the “111” Project [B12006, http://www.moe.edu.cn], the Fundamental Research Funds for the Central Universities [XDJK2012A009, http://www.moe.edu.cn], the Specialized Research Fund for the Doctoral Program of Higher Education of China [20100182120016, http://www.moe.edu.cn], and the Chongqing Program for Key Science and Technology Project [2009AB1030, http://www.ctin.ac.cn]. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Stringam GR, Mcgregor DI, Pawlowski SH (1974) Chemical and morphological characteristics associated with seed coat colour in rapeseed. Proceedings of the 4th International Rapeseed Conference, Giessen, Germany 4–8 June 99–108. [Google Scholar]
- 2. Tang ZL, Li JN, Zhang XK, Chen L, Wang R (1997) Genetic variation of yellow-seeded rapeseed lines (Brassica napus L.) from different genetic sources. Plant Breeding 116: 471–474. [Google Scholar]
- 3. Meng JL, Shi SW, Gan L, Li ZY, Qu XS (1998) The production of yellow-seeded Brassica napus (AACC) through crossing interspecific hybrids of B. campestris (AA) and B. carinata (BBCC) with B. napus . Euphytica 103: 329–333. [Google Scholar]
- 4. Marles MAS, Gruber MY, Scoles GJ, Muir AD (2003) Pigmentation in the developing seed coat and seedling leaves of Brassica carinata is controlled at the dihydroflavonol reductase locus. Phytochemistry 62: 663–672. [DOI] [PubMed] [Google Scholar]
- 5. Burbulis N, Kott LS (2005) A new yellow-seeded canola genotype originating from double low black-seeded Brassica napus cultivars. Canadian Journal of Plant Science 85: 109–114. [Google Scholar]
- 6. Liu XP, Tu JX, Chen BY, Fu TD (2005) Identification and inheritance of a partially dominant gene for yellow seed colour. Plant Breeding 124: 9–12. [Google Scholar]
- 7. Fu FY, Liu LZ, Chai YR, Chen L, Yang T, et al. (2007) Localization of QTLs for seed color using recombinant inbred lines of Brassica napus in different environments. Genome 50: 840–854. [DOI] [PubMed] [Google Scholar]
- 8. Chai YR, Lei B, Huang HL, Li JN, Yin JM, et al. (2009) TRANSPARENT TESTA12 genes from Brassica napus and parental species: cloning, evolution, and differential involvement in yellow seed trait. Molecular Genetics and Genomics 281: 109–123. [DOI] [PubMed] [Google Scholar]
- 9. U N (1935) Genome-analysis in Brassica with special reference to the experimental formation of B. napus and its peculiar mode of fertilization. Japanese Journal of Botany 7: 389–452. [Google Scholar]
- 10. Mun JH, Kwon SJ, Yang TJ, Seol YJ, Jin M, et al. (2009) Genome-wide comparative analysis of the Brassica rapa gene space reveals genome shrinkage and differential loss of duplicated genes after whole genome triplication. Genome Biology 10 (10) R111 doi: 10.1186/gb-2009-10-10-r111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Debeaujon I, Peeters AJM, Léon-Kloosterziel KM, Koornneef M (2001) The TRANSPARENT TESTA12 gene of Arabidopsis encodes a multidrug secondary transporter-like protein required for flavonoid sequestration in vacuoles of the seed coat endothelium. The Plant Cell 13: 853–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nesi M, Lucas MO, Auger B, Baron C, Lécureuil A, et al. (2009) The promoter of the Arabidopsis thaliana BAN gene is active in proanthocyanidin-accumulating cells of the Brassica napus seed coat. Plant Cell Reports 28: 601–617. [DOI] [PubMed] [Google Scholar]
- 13. Routaboul JM, Kerhoas L, Debeaujon I, Pourcel L, Caboche M, et al. (2006) Flavonoid diversity and biosynthesis in seed of Arabidopsis thaliana . Planta 224: 96–107. [DOI] [PubMed] [Google Scholar]
- 14. Pourcel L, Routaboul JM, Cheynier V, Lepiniec L, Debeaujon I (2007) Flavonoid oxidation in plants: from biochemical properties to physiological functions. Trends in Plant Science 12: 29–36. [DOI] [PubMed] [Google Scholar]
- 15. Pourcel L, Routaboul JM, Kerhoas L, Caboche M, Lepiniec L, et al. (2005) TRANSPARENT TESTA10 encodes a laccase-like enzyme involved in oxidative polymerization of flavonoids in Arabidopsis seed coat. The Plant Cell 17: 2966–2980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Liang MX, Davis E, Gardner D, Cai XN, Wu YJ (2006) Involvement of AtLAC15 in lignin synthesis in seeds and in root elongation of Arabidopsis . Planta 224: 1185–1196. [DOI] [PubMed] [Google Scholar]
- 17. Marles MAS, Gruber MY (2004) Histochemical characterisation of unextractable seed coat pigments and quantification of extractable lignin in the Brassicaceae. Journal of the Science of Food and Agriculture 84: 251–262. [Google Scholar]
- 18. Lu K, Chai YR, Zhang K, Wang R, Chen L, et al. (2008) Cloning and characterization of phosphorus starvation inducible Brassica napus PURPLE ACID PHOSPHATASE12 gene family, and imprinting of a recently evolved MITE-minisatellite twin structure. Theoretical and Applied Genetics 117: 963–975. [DOI] [PubMed] [Google Scholar]
- 19. Saghai-Maroof MA, Soliman KM, Jorgensen RA, Allard RW (1984) Ribosomal DNA spacer-length polymorphisms in barley: Mendelian inheritance, chromosomal location, and population dynamics. Proceedings of the National Academy of Sciences, USA 81: 8014–8018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. McCaig BC, Meagher RB, Dean JFD (2005) Gene structure and molecular analysis of the laccase-like multicopper oxidase (LMCO) gene family in Arabidopsis thaliana . Planta 221: 619–636. [DOI] [PubMed] [Google Scholar]
- 21. Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG (1997) The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Research 25: 4876–4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kumar S, Tamura K, Nei M (2004) MEGA3: integrated software for molecular evolutionary genetics analysis and sequence alignment. Briefings in Bioinformatics 5: 150–163. [DOI] [PubMed] [Google Scholar]
- 23. Lai DY, Li HZ, Fan SL, Song MZ, Pang CY, et al. (2011) Generation of ESTs for flowering gene discovery and SSR marker development in upland cotton. PLOS ONE 6 (12) e28676 doi:10.1371/journal.pone.0028676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cardoza V, Stewart CN (2003) Increased Agrobacterium-mediated transformation and rooting efficiencies in canola (Brassica napus L.) from hypocotyl segment explants. Plant Cell Reports 21: 599–604. [DOI] [PubMed] [Google Scholar]
- 25. Jefferson R, Kavanagh T, Bevan M (1987) GUS fusions: β-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. The EMBO Journal 6: 3901–3907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Dalzell SA, Kerven GL (1998) A rapid method for the measurement of Leucaena spp proanthocyanidins by the proanthocyanidin (butanol/HCl) assay. Journal of the Science of Food and Agriculture 78: 405–416. [Google Scholar]
- 27. Morrison IM (1972) A semi-micro method for the determination of lignin and its use in predicting the digestibility of forage crops. Journal of the Science of Food and Agriculture 23: 455–463. [DOI] [PubMed] [Google Scholar]
- 28. Hatfield R, Fukushima RS (2005) Can lignin be accurately measured? Crop Science 45: 832–839. [Google Scholar]
- 29. Kumar SVS, Phale PS, Durani S, Wangikar PP (2003) Combined sequence and structure analysis of the fungal laccase family. Biotechnology and Bioengineering 83: 386–394. [DOI] [PubMed] [Google Scholar]
- 30. El-Mezawy A, Wu L, Shah S (2009) A seed coat-specific promoter for canola. Biotechnology Letters 31: 1961–1965. [DOI] [PubMed] [Google Scholar]
- 31. Xie DY, Dixon RA (2005) Proanthocyanidin biosynthesis - still more questions than answers? Phytochemistry 66: 2127–2144. [DOI] [PubMed] [Google Scholar]
- 32. Dixon RA, Xie DY, Sharma SB (2005) Proanthocyanidins: a final frontier in flavonoid research? New Phytologist 165: 9–28. [DOI] [PubMed] [Google Scholar]
- 33. Stafford HA (1988) Proanthocyanidins and the lignin connection. Phytochemistry 27: 1–6. [Google Scholar]
- 34. Kitamura S, Shikazono N, Tanaka A (2004) TRANSPARENT TESTA 19 is involved in the accumulation of both anthocyanins and proanthocyanidins in Arabidopsis . The Plant Journal 37: 104–114. [DOI] [PubMed] [Google Scholar]
- 35. Zhao J, Pang YZ, Dixon RA (2010) The mysteries of proanthocyanidin transport and polymerization. Plant Physiology 153: 437–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Mayer AM, Staples RC (2002) Laccase: new functions for an old enzyme. Phytochemistry 60: 551–565. [DOI] [PubMed] [Google Scholar]
- 37. Nakamura K, Go N (2005) Function and molecular evolution of multicopper blue proteins. Cellular and Molecular Life Sciences 62: 2050–2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lise J, Serge B, Julien M, Nathalie DC, Brice A, et al. (2011) Identification of laccases involved in lignin polymerization and strategies to deregulate their expression in order to modify lignin content in Arabidopsis and poplar. BMC Proceedings 5 (Suppl 7) O39. [Google Scholar]
- 39. Akhov L, Ashe P, Tan Y, Datla R, Selvaraj G (2009) Proanthocyanidin biosynthesis in the seed coat of yellow-seeded, canola quality Brassica napus YN01-429 is constrained at the committed step catalyzed by dihydroflavonol 4-reductase. Botany 87: 616–625. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Alignment of the mRNA sequences of BnTT10 genes. Conservative similar residues were displayed in dark background. The 951-bp conserved fragment used for Southern hybridization and antisense suppression was highlighted by red line.
(PDF)
Southern blot hybridization detection of TT10 genes in B. napus , B. oleracea and B. rapa .
(TIF)
Alignment of the amino acid sequences of BnTT10 proteins. BrTT10 and BoTT10-1 proteins share high identity with BnTT10 proteins and were not shown in the alignment. Non-similar, weakly similar, block of similar, conservative/strongly similar residues are displayed in dark, dark gray, gray and light gray background, respectively. The four His-rich copper binding domains, L1-L4, are highlighted by line segments, and the amino acids involved in binding copper are boxed. Two predicted multicopper oxidase signature 1 and 2 are identified by single and double dotted lines, respectively.
(TIF)
Phylogenetic relationship of inferred Brassica TT10 proteins and other plant laccases. Acer pseudoplatanus ApLAC1 (AAB09228); Aspergillus flavus AfLAC (XP_002378028); Arabidopsis thaliana AtTT10 (NP_199621), AtLAC4 (NP_565881), AtLAC12 (NP_196158), AtLAC13 (NP_196330), AtLAC14 (NP_196498); AtLAC17 (NP_200810), Castanea mollissima CmLAC (ACI46953); Oryza sativa OsLAC2 (Q8RYM9), OsLAC9 (Q6Z8L2); Pinus taeda PtLAC1 (AAK37823), PtLAC2 (AAK37824); Ricinus communis RcLAC (XP_002527130); Solanum lycopersicum ascorbate oxidase SlAOX (AAY47050); Zea mays ZmLAC3 (NP_001105915). This tree was constructed by the Neighbor-Joining method with p-distance. The number for each interior branch is the percent bootstraps value (1,000 replicates), and only values greater than 50% are shown. Scale bar indicates the estimated number of amino acid substitutions per site.
(TIF)
QRT-PCR detection of transcription levels for BnTT10 (A), BrTT10 (B) and BoTT10 (C) genes in various organs of black-seeded B. napus , B. rapa and B. oleracea , respectively. Ro: root; Hy: hypocotyl; Co: cotyledon; St: stem; Le: leaf; Bu: bud; Fl: flower; SP: silique pericarp; 10, 15, 25, 30, 40, 45, 50, 52 and 55 D: seeds at 10, 15, 25, 30, 40, 45, 50, 52 and 55 days after flowering.
(TIF)
Expression of BnTT10 genes in the seeds of transgenic and control B. napus . QRT-PCR analysis of overall BnTT10 expression in seeds of T1 transgenic and control lines of Westar (A), Zhongyou821 (C) and Zhongshuang10 (E); qRT-PCR analysis of member-specific expression of BnTT10 genes in seeds of transgenic and control lines of Westar (B), Zhongyou821 (D) and Zhongshuang10 (F). Error bars indicate SD.
(TIF)
In vitro seed pigmentation of T2 transgenic and control of B. napus . Siliques of T2 transgenic and control lines of B. napus were regularly sampled at 40, 50 and 55 DAF, and the pods opened 5 days later for observation under a low-power stereoscope. In vitro seed pigmentation of T2 transgenic and control B. napus cv. Zhongyou821 (A) and Zhongshuang10 (B) plants.
(TIF)
Analysis of soluble (A) and insoluble (B) PAs measured after acid-catalyzed hydrolysis of seed coats from seeds of T2 transgenic and control B. napus cv. Zhongyou821 plants. ZY-1, ZY-3 and ZY-17: transgenic lines with inhibited BnTT10 expression; ZY-13: control lines with normal BnTT10 expression. Values are means +/− SD from triplicate measurements in each sample.
(TIF)
Analysis of soluble (A) and insoluble (B) PAs measured after acid-catalyzed hydrolysis of seed coats from seeds of T2 transgenic and control B. napus cv. Zhongshuang10 plants. ZS-4, ZS-8 and ZS-12: transgenic lines with inhibited BnTT10 expression; ZS-2: control lines with normal BnTT10 expression. Values are means +/− SD from triplicate measurements in each sample.
(TIF)
Lignin content in seed coats of transgenic and control B. napus cv. Zhongyou821 plants. Lignin content was analyzed on seed coats from seeds of T2 transgenic and control lines using the acetyl bromide method. Values are means +/− SD from triplicate measurements in each sample.
(TIF)
Lignin content in seed coats of transgenic and control B. napus cv. Zhongshuang10 plants. Lignin content was analyzed on seed coats from seeds of T2 transgenic and control lines using the acetyl bromide method. Error bars indicate SD of three biological replicates.
(TIF)
LC-UV-MS chromatograms of flavonoid compounds identified from seed coats extracts from control (A) and transgenic (B) B. napus cv. Zhongyou821 plants.
(TIF)
Primers used in this study.
(DOC)
Structure parameters of BnTT10 , BrTT10 and BoTT10 genes.
(DOC)
Percentages of full-length mRNA identities for Brassica TT10 genes and AtTT10 .
(DOC)
Identities and positives between deduced Brassica TT10 proteins and AtTT10 (%).
(DOC)