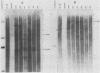
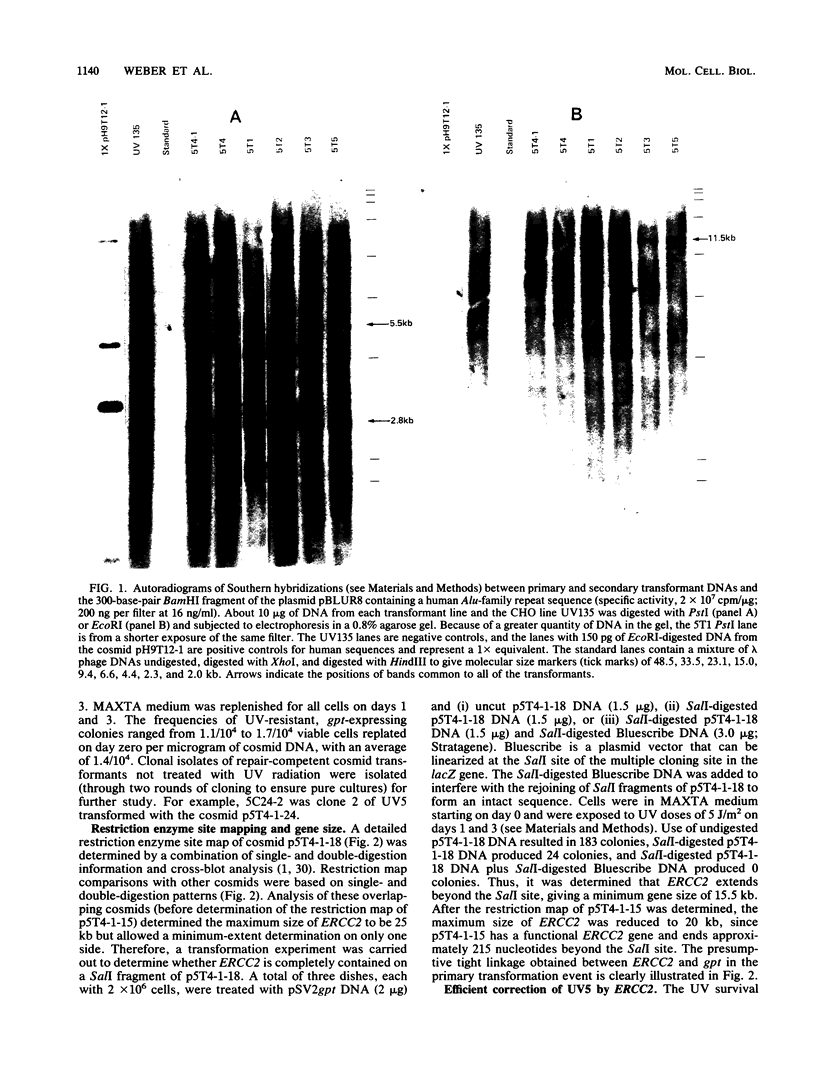
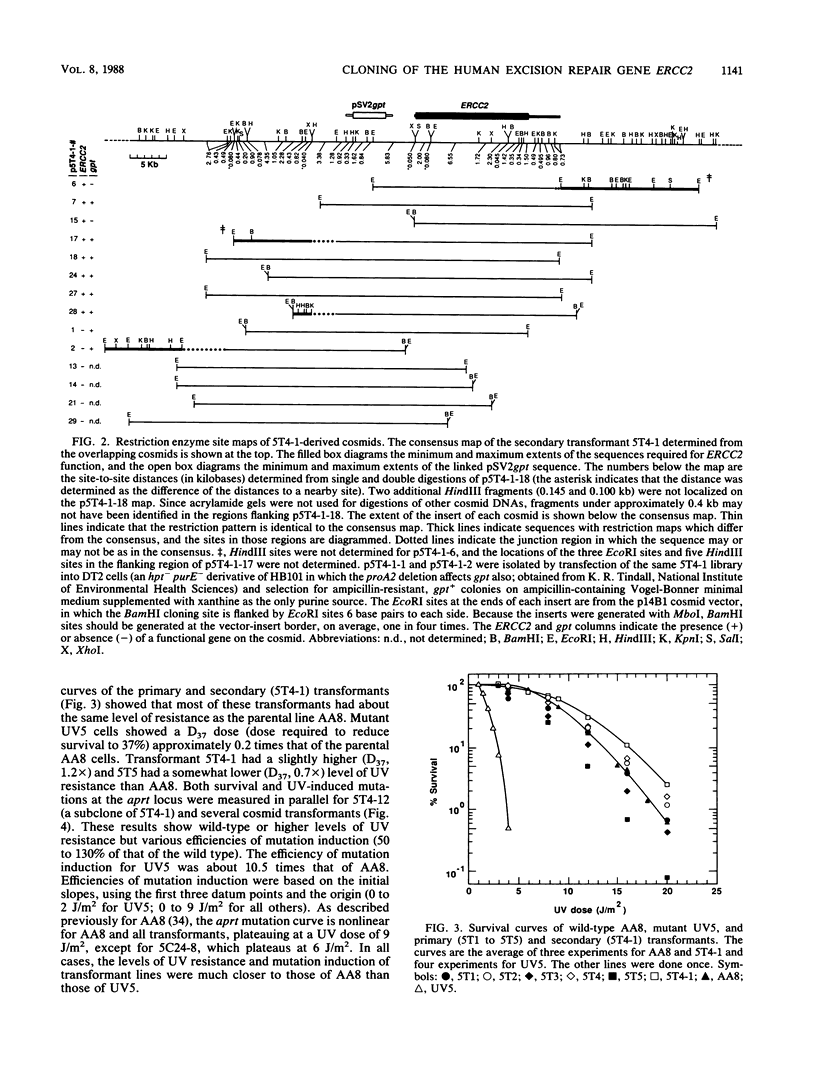
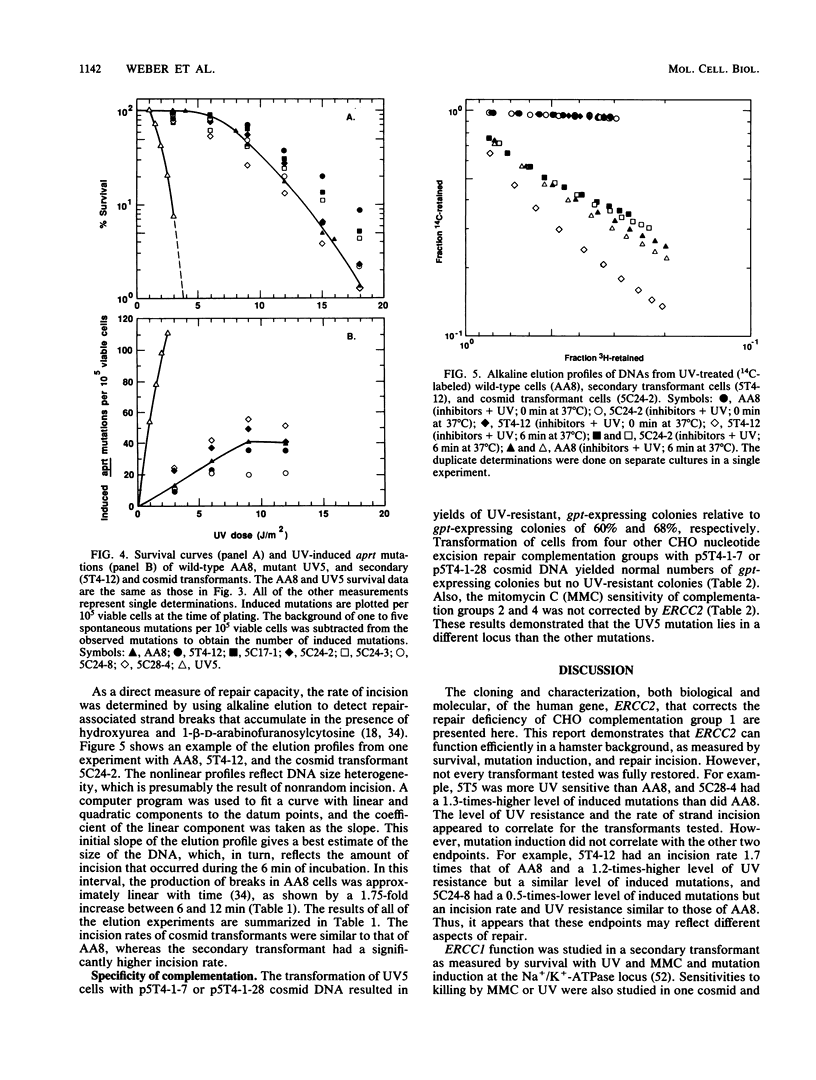
Abstract
The UV-sensitive Chinese hamster ovary (CHO) cell line UV5, which is defective in the incision step of nucleotide excision repair, was used to identify and clone a complementing human gene, ERCC2, and to study the repair process. Genomic DNA from a human-hamster hybrid cell line was sheared and cotransferred with pSV2gpt plasmid DNA into UV5 cells to obtain five primary transformants. Transfer of sheared DNA from one primary transformant resulted in a secondary transformant expressing both gpt and ERCC2. The human repair gene was identified with a probe for Alu-family repetitive sequences. For most primary, secondary, and cosmid transformants, survival after UV exposure showed a return to wild-type levels of resistance. The levels of UV-induced mutation at the aprt locus for secondary and cosmid transformants varied from 50 to 130% of the wild-type level. Measurements of the initial rate of UV-induced strand incision by alkaline elution indicated that, whereas the UV5 rate was 3% of the wild-type level, rates of cosmid-transformed lines were similar to that of the wild type, and the secondary transformant rate was about 165% of the wild-type rate. Analysis of overlapping cosmids determined that ERCC2 is between 15.5 and 20 kilobases and identified a closely linked gpt gene. Cosmids were obtained with functional copies of both ERCC2 and gpt. ERCC2 corrects only the first of the five CHO complementation groups of incision-defective mutants.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baas F., van Ommen G. J., Bikker H., Arnberg A. C., de Vijlder J. J. The human thyroglobulin gene is over 300 kb long and contains introns of up to 64 kb. Nucleic Acids Res. 1986 Jul 11;14(13):5171–5186. doi: 10.1093/nar/14.13.5171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohr V. A., Smith C. A., Okumoto D. S., Hanawalt P. C. DNA repair in an active gene: removal of pyrimidine dimers from the DHFR gene of CHO cells is much more efficient than in the genome overall. Cell. 1985 Feb;40(2):359–369. doi: 10.1016/0092-8674(85)90150-3. [DOI] [PubMed] [Google Scholar]
- Brown W. M. Polymorphism in mitochondrial DNA of humans as revealed by restriction endonuclease analysis. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3605–3609. doi: 10.1073/pnas.77.6.3605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busch D. B., Cleaver J. E., Glaser D. A. Large-scale isolation of UV-sensitive clones of CHO cells. Somatic Cell Genet. 1980 May;6(3):407–418. doi: 10.1007/BF01542792. [DOI] [PubMed] [Google Scholar]
- Collins A., Johnson R. T. DNA repair mutants in higher eukaryotes. J Cell Sci Suppl. 1987;6:61–82. doi: 10.1242/jcs.1984.supplement_6.4. [DOI] [PubMed] [Google Scholar]
- Corsaro C. M., Pearson M. L. Enhancing the efficiency of DNA-mediated gene transfer in mammalian cells. Somatic Cell Genet. 1981 Sep;7(5):603–616. doi: 10.1007/BF01549662. [DOI] [PubMed] [Google Scholar]
- Deininger P. L., Jolly D. J., Rubin C. M., Friedmann T., Schmid C. W. Base sequence studies of 300 nucleotide renatured repeated human DNA clones. J Mol Biol. 1981 Sep 5;151(1):17–33. doi: 10.1016/0022-2836(81)90219-9. [DOI] [PubMed] [Google Scholar]
- Fischer E., Keijzer W., Thielmann H. W., Popanda O., Bohnert E., Edler L., Jung E. G., Bootsma D. A ninth complementation group in xeroderma pigmentosum, XP I. Mutat Res. 1985 May;145(3):217–225. doi: 10.1016/0167-8817(85)90030-6. [DOI] [PubMed] [Google Scholar]
- Hanahan D., Meselson M. Plasmid screening at high colony density. Methods Enzymol. 1983;100:333–342. doi: 10.1016/0076-6879(83)00066-x. [DOI] [PubMed] [Google Scholar]
- Hanahan D., Meselson M. Plasmid screening at high colony density. Gene. 1980 Jun;10(1):63–67. doi: 10.1016/0378-1119(80)90144-4. [DOI] [PubMed] [Google Scholar]
- Hoeijmakers J. H. Characterization of genes and proteins involved in excision repair of human cells. J Cell Sci Suppl. 1987;6:111–125. doi: 10.1242/jcs.1984.supplement_6.7. [DOI] [PubMed] [Google Scholar]
- Hoeijmakers J. H., Odijk H., Westerveld A. Differences between rodent and human cell lines in the amount of integrated DNA after transfection. Exp Cell Res. 1987 Mar;169(1):111–119. doi: 10.1016/0014-4827(87)90230-8. [DOI] [PubMed] [Google Scholar]
- Hoy C. A., Thompson L. H., Mooney C. L., Salazar E. P. Defective DNA cross-link removal in Chinese hamster cell mutants hypersensitive to bifunctional alkylating agents. Cancer Res. 1985 Apr;45(4):1737–1743. [PubMed] [Google Scholar]
- Kafatos F. C., Jones C. W., Efstratiadis A. Determination of nucleic acid sequence homologies and relative concentrations by a dot hybridization procedure. Nucleic Acids Res. 1979 Nov 24;7(6):1541–1552. doi: 10.1093/nar/7.6.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karentz D., Cleaver J. E. Repair-deficient xeroderma pigmentosum cells made UV light resistant by fusion with X-ray-inactivated Chinese hamster cells. Mol Cell Biol. 1986 Oct;6(10):3428–3432. doi: 10.1128/mcb.6.10.3428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konze-Thomas B., Hazard R. M., Maher V. M., McCormick J. J. Extent of excision repair before DNA synthesis determines the mutagenic but not the lethal effect of UV radiation. Mutat Res. 1982 Jun;94(2):421–434. doi: 10.1016/0027-5107(82)90305-0. [DOI] [PubMed] [Google Scholar]
- Lehmann A. R. Use of recombinant DNA techniques in cloning DNA repair genes and in the study of mutagenesis in mammalian cells. Mutat Res. 1985 Jun-Jul;150(1-2):61–67. doi: 10.1016/0027-5107(85)90101-0. [DOI] [PubMed] [Google Scholar]
- Mellon I., Bohr V. A., Smith C. A., Hanawalt P. C. Preferential DNA repair of an active gene in human cells. Proc Natl Acad Sci U S A. 1986 Dec;83(23):8878–8882. doi: 10.1073/pnas.83.23.8878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyerowitz E. M., Guild G. M., Prestidge L. S., Hogness D. S. A new high-capacity cosmid vector and its use. Gene. 1980 Nov;11(3-4):271–282. doi: 10.1016/0378-1119(80)90067-0. [DOI] [PubMed] [Google Scholar]
- Mitchell D. L., Haipek C. A., Clarkson J. M. (6-4)Photoproducts are removed from the DNA of UV-irradiated mammalian cells more efficiently than cyclobutane pyrimidine dimers. Mutat Res. 1985 Jul;143(3):109–112. doi: 10.1016/s0165-7992(85)80018-x. [DOI] [PubMed] [Google Scholar]
- Mulligan R. C., Berg P. Expression of a bacterial gene in mammalian cells. Science. 1980 Sep 19;209(4463):1422–1427. doi: 10.1126/science.6251549. [DOI] [PubMed] [Google Scholar]
- Mulligan R. C., Berg P. Factors governing the expression of a bacterial gene in mammalian cells. Mol Cell Biol. 1981 May;1(5):449–459. doi: 10.1128/mcb.1.5.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulligan R. C., Berg P. Selection for animal cells that express the Escherichia coli gene coding for xanthine-guanine phosphoribosyltransferase. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2072–2076. doi: 10.1073/pnas.78.4.2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perucho M., Hanahan D., Wigler M. Genetic and physical linkage of exogenous sequences in transformed cells. Cell. 1980 Nov;22(1 Pt 1):309–317. doi: 10.1016/0092-8674(80)90178-6. [DOI] [PubMed] [Google Scholar]
- Perucho M., Wigler M. Linkage and expression of foreign DNA in cultured animal cells. Cold Spring Harb Symp Quant Biol. 1981;45(Pt 2):829–838. doi: 10.1101/sqb.1981.045.01.101. [DOI] [PubMed] [Google Scholar]
- Potter H., Dressler D. A 'Southern Cross' method for the analysis of genome organization and the localization of transcription units. Gene. 1986;48(2-3):229–239. doi: 10.1016/0378-1119(86)90081-8. [DOI] [PubMed] [Google Scholar]
- Soberon X., Covarrubias L., Bolivar F. Construction and characterization of new cloning vehicles. IV. Deletion derivatives of pBR322 and pBR325. Gene. 1980 May;9(3-4):287–305. doi: 10.1016/0378-1119(90)90328-o. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Thompson L. H., Brookman K. W., Carrano A. V., Dillehay L. E. Role of DNA repair in mutagenesis of Chinese hamster ovary cells by 7-bromomethylbenz[a]anthracene. Proc Natl Acad Sci U S A. 1982 Jan;79(2):534–538. doi: 10.1073/pnas.79.2.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson L. H., Brookman K. W., Dillehay L. E., Mooney C. L., Carrano A. V. Hypersensitivity to mutation and sister-chromatid-exchange induction in CHO cell mutants defective in incising DNA containing UV lesions. Somatic Cell Genet. 1982 Nov;8(6):759–773. doi: 10.1007/BF01543017. [DOI] [PubMed] [Google Scholar]
- Thompson L. H., Brookman K. W., Mooney C. L. Repair of DNA adducts in asynchronous CHO cells and the role of repair in cell killing and mutation induction in synchronous cells treated with 7-bromomethylbenz[a]anthracene. Somat Cell Mol Genet. 1984 Mar;10(2):183–194. doi: 10.1007/BF01534907. [DOI] [PubMed] [Google Scholar]
- Thompson L. H., Brookman K. W., Salazar E. P., Fuscoe J. C., Weber C. A. DNA repair genes of mammalian cells. Basic Life Sci. 1986;39:349–358. doi: 10.1007/978-1-4684-5182-5_30. [DOI] [PubMed] [Google Scholar]
- Thompson L. H., Busch D. B., Brookman K., Mooney C. L., Glaser D. A. Genetic diversity of UV-sensitive DNA repair mutants of Chinese hamster ovary cells. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3734–3737. doi: 10.1073/pnas.78.6.3734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson L. H., Fong S., Brookman K. Validation of conditions for efficient detection of HPRT and APRT mutations in suspension-cultured Chinese hamster ovary cells. Mutat Res. 1980 Feb;74(1):21–36. doi: 10.1016/0165-1161(80)90188-0. [DOI] [PubMed] [Google Scholar]
- Thompson L. H., Mooney C. L., Brookman K. W. Genetic complementation between UV-sensitive CHO mutants and xeroderma pigmentosum fibroblasts. Mutat Res. 1985 Jun-Jul;150(1-2):423–429. doi: 10.1016/0027-5107(85)90139-3. [DOI] [PubMed] [Google Scholar]
- Thompson L. H., Mooney C. L., Burkhart-Schultz K., Carrano A. V., Siciliano M. J. Correction of a nucleotide-excision-repair mutation by human chromosome 19 in hamster-human hybrid cells. Somat Cell Mol Genet. 1985 Jan;11(1):87–92. doi: 10.1007/BF01534738. [DOI] [PubMed] [Google Scholar]
- Thompson L. H., Rubin J. S., Cleaver J. E., Whitmore G. F., Brookman K. A screening method for isolating DNA repair-deficient mutants of CHO cells. Somatic Cell Genet. 1980 May;6(3):391–405. doi: 10.1007/BF01542791. [DOI] [PubMed] [Google Scholar]
- Thompson L. H., Salazar E. P., Brookman K. W., Collins C. C., Stewart S. A., Busch D. B., Weber C. A. Recent progress with the DNA repair mutants of Chinese hamster ovary cells. J Cell Sci Suppl. 1987;6:97–110. doi: 10.1242/jcs.1984.supplement_6.6. [DOI] [PubMed] [Google Scholar]
- Thompson L. H., Salazar E. P., Brookman K. W., Hoy C. A. Hypersensitivity to cell killing and mutation induction by chemical carcinogens in an excision repair-deficient mutant of CHO cells. Mutat Res. 1983 Dec;112(6):329–344. doi: 10.1016/0167-8817(83)90027-5. [DOI] [PubMed] [Google Scholar]
- Valerie K., de Riel J. K., Henderson E. E. Genetic complementation of UV-induced DNA repair in Chinese hamster ovary cells by the denV gene of phage T4. Proc Natl Acad Sci U S A. 1985 Nov;82(22):7656–7660. doi: 10.1073/pnas.82.22.7656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijg J., Mullaart E., van der Schans G. P., Lohman P. H., Knook D. L. Kinetics of ultraviolet induced DNA excision repair in rat and human fibroblasts. Mutat Res. 1984 Sep-Oct;132(3-4):129–138. doi: 10.1016/0167-8817(84)90007-5. [DOI] [PubMed] [Google Scholar]
- Westerveld A., Hoeijmakers J. H., van Duin M., de Wit J., Odijk H., Pastink A., Wood R. D., Bootsma D. Molecular cloning of a human DNA repair gene. Nature. 1984 Aug 2;310(5976):425–429. doi: 10.1038/310425a0. [DOI] [PubMed] [Google Scholar]
- Wood R. D., Burki H. J. Repair capability and the cellular age response for killing and mutation induction after UV. Mutat Res. 1982 Aug;95(2-3):505–514. doi: 10.1016/0027-5107(82)90281-0. [DOI] [PubMed] [Google Scholar]
- Zdzienicka M. Z., Roza L., Westerveld A., Bootsma D., Simons J. W. Biological and biochemical consequences of the human ERCC-1 repair gene after transfection into a repair-deficient CHO cell line. Mutat Res. 1987 Jan;183(1):69–74. doi: 10.1016/0167-8817(87)90047-2. [DOI] [PubMed] [Google Scholar]
- Zelle B., Reynolds R. J., Kottenhagen M. J., Schuite A., Lohman P. H. The influence of the wavelength of ultraviolet radiation on survival, mutation induction and DNA repair in irradiated Chinese hamster cells. Mutat Res. 1980 Aug;72(3):491–509. doi: 10.1016/0027-5107(80)90121-9. [DOI] [PubMed] [Google Scholar]
- van Duin M., Westerveld A., Hoeijmakers J. H. UV stimulation of DNA-mediated transformation of human cells. Mol Cell Biol. 1985 Apr;5(4):734–741. doi: 10.1128/mcb.5.4.734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Duin M., de Wit J., Odijk H., Westerveld A., Yasui A., Koken M. H., Hoeijmakers J. H., Bootsma D. Molecular characterization of the human excision repair gene ERCC-1: cDNA cloning and amino acid homology with the yeast DNA repair gene RAD10. Cell. 1986 Mar 28;44(6):913–923. doi: 10.1016/0092-8674(86)90014-0. [DOI] [PubMed] [Google Scholar]
- van Zeeland A. A., Smith C. A., Hanawalt P. C. Sensitive determination of pyrimidine dimers in DNA of UV-irradiated mammalian cells. Introduction of T4 endonuclease V into frozen and thawed cells. Mutat Res. 1981 Jun;82(1):173–189. doi: 10.1016/0027-5107(81)90148-2. [DOI] [PubMed] [Google Scholar]