Abstract
Background
Cardiovascular disease is one of the major causes of death worldwide.
Assessing the risk for cardiovascular disease is an important aspect in clinical decision making and setting a therapeutic strategy, and the use of serological biomarkers may improve this. Despite an overwhelming number of studies and meta-analyses on biomarkers and cardiovascular disease, there are no comprehensive studies comparing the relevance of each biomarker. We performed a systematic review of meta-analyses on levels of serological biomarkers for atherothrombosis to compare the relevance of the most commonly studied biomarkers.
Methods and Findings
Medline and Embase were screened on search terms that were related to “arterial ischemic events” and “meta-analyses”. The meta-analyses were sorted by patient groups without pre-existing cardiovascular disease, with cardiovascular disease and heterogeneous groups concerning general populations, groups with and without cardiovascular disease, or miscellaneous. These were subsequently sorted by end-point for cardiovascular disease or stroke and summarized in tables. We have identified 85 relevant full text articles, with 214 meta-analyses. Markers for primary cardiovascular events include, from high to low result: C-reactive protein, fibrinogen, cholesterol, apolipoprotein B, the apolipoprotein A/apolipoprotein B ratio, high density lipoprotein, and vitamin D. Markers for secondary cardiovascular events include, from high to low result: cardiac troponins I and T, C-reactive protein, serum creatinine, and cystatin C. For primary stroke, fibrinogen and serum uric acid are strong risk markers. Limitations reside in that there is no acknowledged search strategy for prognostic studies or meta-analyses.
Conclusions
For primary cardiovascular events, markers with strong predictive potential are mainly associated with lipids. For secondary cardiovascular events, markers are more associated with ischemia. Fibrinogen is a strong predictor for primary stroke.
Introduction
Atherothrombosis is one of the major causes of death worldwide [1]. Upon rupture of an atherosclerotic plaque, a hemostatic response is initiated that could lead to infarction causing ischemia downstream. Assessment of cardiovascular disease risk supported by biomarker analysis is a primary requirement to stratify those at high-risk and for optimized treatment of patients.
Large cohort studies are crucial for cardiovascular disease risk estimation with the use of biomarkers, and confirmation of results in independent populations is desirable. Many results from different studies have become available over time, which makes it challenging to assess those markers that consistently keep a predictive value. Meta-analyses combine the results from different studies and present one aggregate score for a risk marker in question, but these studies have also been performed in large numbers. This systematic review presents a comprehensive overview of serological biomarkers for cardiovascular disease events and stroke in cardiovascular disease naïve populations (being primary cardiovascular events), and cardiovascular disease events and stroke in populations with a history of cardiovascular disease (being secondary cardiovascular events) investigated in meta-analyses of the past 24 years [2]. It compares the relevance of the most commonly studied biomarkers used to assess the risk for ischemic cardiovascular event and stroke. The selection of meta-analyses was restricted to prospective studies only, as pooled results from cross-sectional and retrospective case-control studies overestimate the risk for the marker in question. To our knowledge, this is the first systematic review on meta-analyses for biomarkers of atherothrombosis.
Materials and Methods
A literature search on published studies from 1988 to 2011 has been performed in Medline (using the advanced search option in Pubmed), and Embase (see flow diagram in Figure 1). A protocol for the search and data abstraction was set up and discussed with one skilled epidemiologist, and three established investigators for consensus. The search terms were related to “arterial ischemic events” and “meta-analyses”, and were set up broadly to reduce the possibility that publications that use trivial nomenclature would be missed (see Supplementary Methods in File S1). We have taken the PRISMA statements as a framework for reporting the systematic review [3].
Figure 1. PRISMA flow diagram.
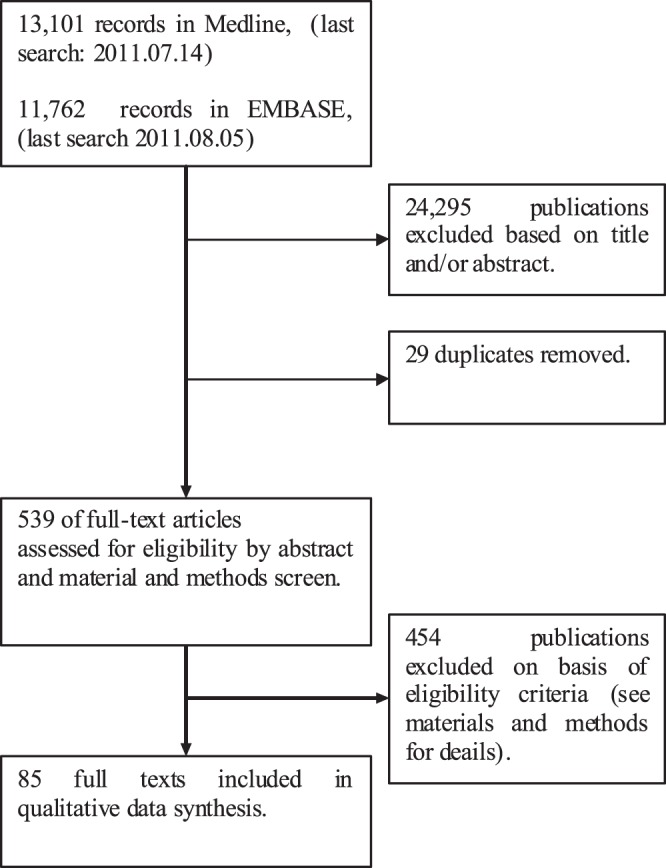
Titles and abstracts were screened on “meta-analyses of prospective studies”, “arterial ischemic disease”, and “levels of circulating markers” (Figure 1, Step 1.). Duplicates were removed from the search results (Figure 1, Step 2.). Eligibility of the papers was assessed by reading the abstracts and material and methods section of the publications (Figure 1, Step 3.). Studies were considered as “not eligible” if they were not prospective (e.g. cross-sectional, retrospective studies), reported other than levels of circulating markers (e.g. alleles, or prediction models), investigated the risk relating to all cause death or hemorrhagic stroke, did not explicitly report the pooled results in the text, figures, or tables; did not pool data from 2 prospective studies or more, did not report risk in relation to levels of the investigated marker (e.g. comparison of marker levels in case and control group), were unpublished reports (e.g. abstracts, posters), or that were not available online via either Medline or Embase (in total 11 meta-analyses were irretrievable online).
After the first selection round, manuscripts were selected for a full text screen. The result of the meta-analysis was extracted, which was reported either by odds ratio (OR), relative risk (RR), relative risk ratio (RRR), or hazard ration (HR), together with the follow up period. In addition, the following parameters were abstracted: the type of investigated end-point(s), to what group the risk applied and how this group was defined (e.g. tertiles, cut-offs), nature of pooled studies (e.g. individual patient data (IPD), cohorts), whether there had been adjustment for other risk factors, presence of statistical heterogeneity or heterogeneity mentioned by the authors, how the pooling was performed (either by regression, Cox-regression, random effects model, fixed effects model, or inverse variance weighted combined risks), number of patients (the amount of cases within the pooled cohorts was preferred but if this was not present the total cohort size was given), number of pooled cohorts, and which population was represented in the results (a population with or without pre-existing cardiovascular disease, a specific subgroup population, or the general population).
If both unadjusted and adjusted results were reported, the adjusted results were abstracted. If risks for more quantiles were reported, only the most extreme was used. If subgroup meta-analyses were reported in one publication (e.g. different age groups or specific gender), these were abstracted unless these were excluded according to the earlier specified criteria. If the number of cases was not reported in the manuscript, calculations were performed by hand. Whenever in the meta-analysis review it was stated that heterogeneity is present between the cohorts, the heterogeneity was recorded as yes. When it was reported that heterogeneity was absent or it was not mentioned, the heterogeneity was recorded as no. If heterogeneity between cohorts was reported and both random effects and fixed effects analysis was performed, the random effects results were abstracted. Stratification of the meta-analyses was performed on population based studies, cohorts without pre-existing cardiovascular disease, cohorts with pre-existing cardiovascular disease, pooled results from populations with and without pre-existing cardiovascular disease, and specific subgroups.
The evaluation criteria for novel risk markers as described by Hlatky et al. 2009 were used as guide to set up evaluation parameters for meta-analyses [4]. We consider the following parameters indicative for the clinical value and quality of the different meta-analyses:
There should be relevant stratification of the researched individuals. Groups with, or without previous cardiovascular disease are clinically more relevant than groups representing the general population, or meta-analyses where cohorts with and without cardiovascular disease were pooled for one result.
The prediction is preferred to be expressed as a hazard ratio, rather than an odds ratio, relative risk, or relative risk ratio as it considers the event rate, and not the difference in number of events at one specific time point.
Pooled end-points should not be too diverse, or at least clinically relevant. Pooling of diverse end-points complicates the interpretation of the results.
The novel risk marker should be able to predict risk beyond the established risk markers, and therefore it should add statistical value in a model where other risk factors are included.
The result of a meta-analysis becomes more reliable with increasing number of events and is even more convincing when a large number of cohorts are used, especially when in absence of heterogeneity between the pooled cohorts.
If heterogeneity of the results is present, this should be addressed by conservative pooling of the results using a random effects model (see http://www.cochrane.org/). Statistical power of risk assessment depends on the number of outcome events, and therefore reporting of the number of events rather than total study size is preferred.
Results
A total of 24.863 publications were screened, which were available online in the period June the 10th of 2011 to August the 5th of 2011. After a screen on title and abstract and subsequent removal of 29 duplicates, a total of 539 publications remained (Figure 1). After monitoring the abstracts and material and methods, 85 publications remained with 214 meta-analyses. On basis of cohort characteristics and end point 9 different types of meta-analyses were identified, which are summarized in Table 1, Table 2, Table 3 and Tables S1–9 in File S1. Meta-analyses for cardiovascular disease events that were performed with studies from groups without pre-existing cardiovascular disease are presented in Table 1 and Table S1 in File S1. Meta-analyses for cardiovascular disease events that were performed with studies from groups with pre-existing cardiovascular disease are presented in Table 2 and Table S2 in File S1. Meta-analyses reported for stroke events in populations without cardiovascular disease are presented in Table 3 and Table S3 in File S1. Pooled results for stroke events in populations with pre-existing cardiovascular disease are provided in Table S4 in File S1. Results from studies with heterogeneous populations being general populations, populations with and without pre-existing disease, and miscellaneous groups for either cardiovascular or stroke events are summarized in the Tables S5–9 in File S1. The tables are organized into categories of markers (e.g. markers related to hemostasis), and per category in descending order of result. The studies reporting on populations only without pre-existing cardiovascular disease, reporting on populations only with pre-existing cardiovascular disease, for either cardiovascular disease or stroke (Table 1–3, Table S1–3 in File S1) are considered most clinically relevant, and therefore are discussed in this review. Meta-analyses reporting on stroke in populations only with pre-existing cardiovascular disease are not discussed in this review, as only two meta-analyses were found in this category and are too few to draw any conclusions upon.
Table 1. Selection of meta-analyses of cohorts without pre-existing cardiovascular disease on markers for cardiovascular disease risk.
| Marker | Risk Applies To | Risk | Results | 95% ci | N Patients | N Cohorts | Reference |
| Diabetes related | |||||||
| Glucose post load | Above: 7.8 mmol/L | RR | 1.58 | 1.19–2.10 | 1,467 cases | 7 | [31] |
| Glycated hemoglobine (HBA(1c)) | HbA1c level: 0.7 | RR | 1.58 | 1.22–2.06 | 1,366 cases | 7 | [32] |
| Hemostasis | |||||||
| Fibrinogen | 1 g/L increase | HR | 2.33 | 1.91–2.84 | 992 cases | 31 | [5] |
| Fibrinogen | 1 g/L increase | HR | 1.93 | 1.79–2.08 | 7,118 cases | 31 | [5] |
| Hormones | |||||||
| Vitamin D (serum 25-OH D) | Decrease in different predefined categories | HR | 1.83 | 1.19–2.80 | 2,007 cases | 5 | [9] |
| Vitamin D (serum 25-OH D) | Decrease in different predefined categories | HR | 1.54 | 1.22–1.95 | 756 cases | 4 | [9] |
| Inflammation | |||||||
| CRP1 | Top vs bottom tertile | RR | 2.43 | 2.10–2.83 | 3,181 cases | 12 | [6] |
| CRP | Top vs bottom tertile | OR | 1.58 | 1.48–1.68 | 7,068 cases | 22 | [33] |
| Lipids | |||||||
| ApoB2 | Top vs bottom tertile | RR | 1.99 | 1.65–2.39 | 6,920 cases | 19 | [7] |
| ApoB/ApoAI ratio | Top vs bottom tertile | RR | 1.86 | 1.55–2.22 | 3.730 cases | 7 | [7] |
| HDL3 | 0.33 mmol/L decrease | HR | 1.83 | 1.65–2.03 | 1,198 cases | 23 | [8] |
| Triglycerides | Top vs bottom tertile | OR | 1.72 | 1.56–1.90 | 10,158 cases | 29 | [34] |
| HDL | 0.33 mmol/L decrease | HR | 1.63 | 1.44–1.85 | 764 cases | 23 | [8] |
| ApoAI | Bottom vs top tertile | RR | 1.62 | 1.43–1.83 | 6,333 cases | 21 | [7] |
| Non-HDL cholesterol | 43 mg/dL increase | HR | 1.59 | 1.36–1.85 | 12,785 cases | 68 | [35] |
| ApoB | 29 mg/dL increase | HR | 1.58 | 1.39–1.79 | 4,499 cases | 22 | [35] |
| Non-HDL cholesterol | 1.53 unit increase | HR | 1.50 | 1.38–1.62 | 4,499 cases | 22 | [35] |
| Non-HDL cholesterol | 1 mmol/L decrease | HR | 0.66 | 0.61–0.71 | 1,198 cases | 23 | [8] |
| Cholesterol/HDL | 1.33 units decrease | HR | 0.60 | 0.56–0.64 | 1,198 cases | 23 | [8] |
| Cholesterol | 1 mmol/L decrease | HR | 0.58 | 0.56–0.61 | 5,561 cases | 61 | [8] |
| Non-HDL cholesterol | 1 mmol/L decrease | HR | 0.57 | 0.52–0.62 | 764 cases | 23 | [8] |
| Cholesterol/HDL | 1.33 units decrease | HR | 0.56 | 0.51–0.60 | 764 cases | 23 | [8] |
| Cholesterol | 1 mmol/L decrease | HR | 0.44 | 0.42–0.48 | 1,309 cases | 61 | [8] |
CRP: C-reactive protein.
Apo: apolipoprotein.
HDL: high density lipoprotein.
Table 2. Selection of meta-analyses of cohorts with pre-existing cardiovascular disease on markers for cardiovascular disease risk.
| Marker | Risk Applies to | Risk | Results | 95% ci | N Patients | N Cohorts | Reference |
| Hemostasis | |||||||
| vWF4 | Top vs bottom tertile | OR | 1.6 | 1.0–2.5 | 723 cases | 8 | [36] |
| Inflammation | |||||||
| Hs5-CRP | 1 mg/L>hs-CRP>3 mg/L | OR | 5.65 | 1.71–18.73 | 477 total | 4 | [13] |
| hs-CRP | 1 mg/L>hs-CRP>3 mg/L | OR | 2.76 | 1.38–5.55 | 386 total | 3 | [13] |
| CRP | Top vs bottom tertile | RR | 1.97 | 1.78–2.17 | 6,485 cases | 83 | [10] |
| CRP | Top vs bottom tertile | RR | 1.5 | 1.1–2.1 | 604 cases | 3 | [37] |
| Ischemia | |||||||
| cTn6T+cTnI | Above: cTnT 0.1–0.2 ng/mL;cTnI 0.1–3.1 ng/mL | OR | 9.39 | 6.46–13.67 | 160 cases | 10 | [12] |
| BNP7+NT8-proBNP | Above: BNP 116 gp/mL, NT-proBNP 227.5 pg/mL | OR | 7.9 | 4.7–13.3 | 75 cases | 5 | [38] |
| cTnI | Above: unknown | RR | 5.7 | 1.8–19 | 882 cases | 4 | [39] |
| cTnI | Above: different per study | OR | 4.94 | 3.9–6.2 | 1,168 cases | 13 | [40] |
| cTnT+cTnI | Above: cTnT 0.1–0.2 ng/mL;cTnI 0.1–0.6 ng/mL | OR | 4.93 | 3.77–6.45 | 1,602 cases | 16 | [12] |
| cTnT | Above: 0.1–0.2 ng/mL | OR | 4.58 | 3.8–5.5 | 1,965 cases | 16 | [40] |
| cTnT | Above: 0.1–0.2 ug/L | OR | 4.4 | 3.0–6.5 | 163 cases | 4 | [41] |
| cTnT | Above: 0.1–0.2 ug/L | OR | 4.3 | 2.8–6.8 | 96 cases | 7 | [41] |
| cTnI | Above: 0.03 ug/L–3.1 ug/L | RR | 4.2 | 2.7–6.4 | n.a. | 9 | [42] |
| cTnT | Above: unknown | RR | 3.8 | 2.6–5.5 | 1,292 cases | 12 | [39] |
| cTnT+cTnI | Above: cTnT 0.1–0.2 ng/mL; cTnI 0.1–3.1 ng/mL | OR | 3.11 | 2.59–3.74 | 201 cases | 21 | [12] |
| cTnT | Above: 0.1–0.2 ng/mL | OR | 2.86 | 2.35–3.47 | 1,330 cases | 3 | [12] |
| cTnT+cTnI | Above: cTnT 0.1–0.2 ng/mL;cTnI 0.6 ng/mL | OR | 2.79 | 2.17–3.58 | 322 cases | 5 | [12] |
| cTnT | Above: 0.1–0.25 ug/L | RR | 2.7 | 2.1–3.4 | n.a. | 12 | [42] |
| cTnT+cTnI | Above: cTnT 0.1–0.2 ng/mL; cTnI:unknown | OR | 2.5 | 2.0–3.1 | 241 cases | 10 | [43] |
| cTnT+cTnI | Above: 0.1–1.5 ng/mL | OR | 2.27 | 1.62–3.16 | 2,401 total | 3 | [44] |
| cTnI | Above: 2.3–0.026 ng/mL | OR | 1.77 | 1.36–2.30 | 1,174 cases | 16 | [45] |
| cTnT | Above: 0.1–0.03 ng/mL | OR | 1.77 | 1.29–2.45 | 293 cases | 6 | [45] |
| cTnT+cTnI | Above: cTnT 0.03–0.1 ng/mL; cTnI 2.3–0.08 ng/mL | OR | 1.59 | 1.29–1.95 | 6,885 total | 15 | [46] |
| Kidney function | |||||||
| Serum creatine (eGFR9) | Reference value vs15–29 ml/min/1.73 m2 | HR | 3.98 | 3.02–5.24 | 266,975 total | 6 | [14] |
| Cystatin C | Top vs bottom quintile | RR | 2.62 | 2.05–3.37 | 2,321 cases | 13 | [11] |
| Serum creatine (eGFR) | Reference value vs 30–44 ml/min/1.73 m2 | HR | 2.50 | 2.10–2.97 | 266,975 total | 6 | [14] |
| Cystatin C | Top vs bottom tertile | RR | 1.72 | 1.27–2.34 | 741 cases | 4 | [11] |
| Serum creatine (eGFR) | Reference value vs 45–59 ml/min/1.73 m2 | HR | 1.63 | 1.22–2.18 | 266,975 total | 6 | [14] |
vWF: von Willebrand factor.
hs: high sensitivity.
cTn: cardiac troponin.
BNP: brain natriuretic peptide.
NT-pro: amino terminal prohormone of
eGFR: estimated glomerular filtration rate.
Table 3. Selection of meta-analyses of cohorts without pre-existing cardiovascular disease on markers for stroke.
In total, 61 meta-analyses were found for cardiovascular disease events in populations without pre-existing cardiovascular disease. In these populations, the highest risk for cardiovascular disease is reported for markers associated with hemostasis, inflammation and lipids. These include, from highest to lower result: C-reactive protein (CRP) (RR: 2.43, 95% confidence interval (ci): 2.10–2.83), fibrinogen (HR: 2.33, 95%ci: 1.91–2.84), cholesterol (HR: 0.44, 95%ci: 0.42–0.48), apolipoprotein (Apo) B (RR: 1.99, 95%ci: 1.65–2.39), ApoA/ApoB ratio (RR: 1.86, 95%ci: 1.55–2.22), high density lipoprotein (HDL) (HR: 1.83, 95%ci: 1.65–2.03), and Vitamin D (HR: 1.83, 95%ci: 1.19–2.80) [5]–[9] (Figure 2, Table 1, Table S1 in File S1).
Figure 2. Plot of the results of meta-analyses on CVD events in populations without pre-existing CVD.
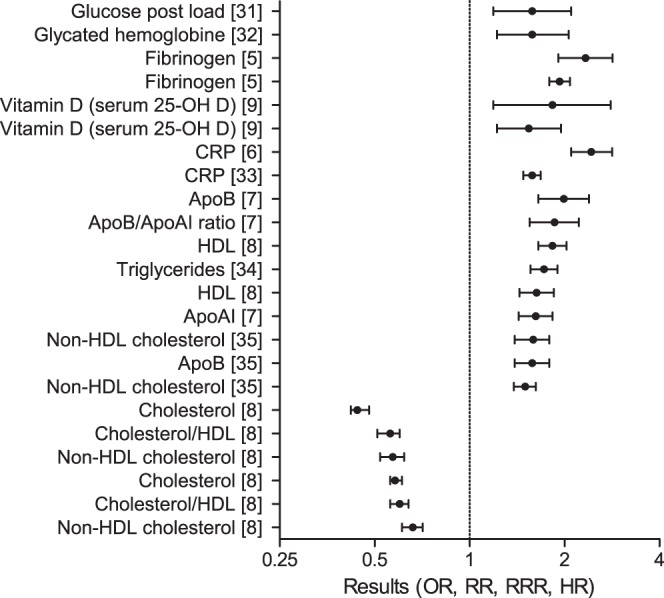
The results of a selection of meta-analyses on CVD events in populations without pre-existing CVD with a result over 1.5 or under 0.66 are graphically represented. Details of the studies are described in Table 1 and Table S1 in File S1. Abbreviations: CRP: C-reactive protein, Apo: apolipoprotein, HDL: high density lipoprotein.
For populations with pre-existing cardiovascular disease, 43 meta-analyses were found reporting on markers for cardiovascular disease events. Markers with high prognostic value were associated with hemostasis, ischemia, inflammation and kidney function. These include, from highest to lower result: cardiac troponin (cTn) I and T (OR: 9.39, 95%ci: 6.46–13.67), high sensitivity (hs) CRP (OR: 5.65, 95%ci: 1.71–18.73), serum creatinine (HR: 3.98, 95%ci: 3.02–5.24), and cystatin C (RR: 2.62, 95%ci: 2.05–3.37) [10]–[14] (Figure 3, Table 2, Table S2 in File S1).
Figure 3. Plot of the results of meta-analyses on CVD events in populations with pre-existing CVD.
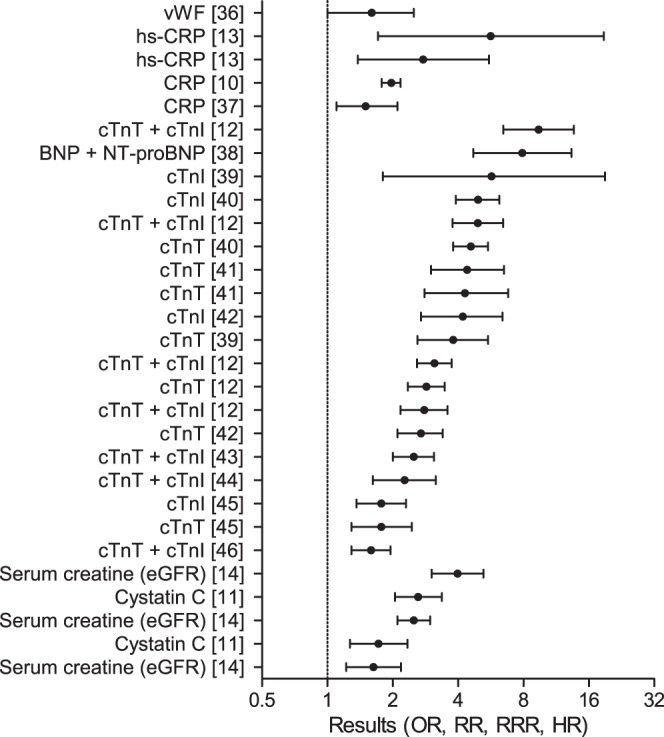
The results of a selection of meta-analyses on CVD events in populations with pre-existing CVD with a result over 1.5 or under 0.66 are graphically represented. Details of the studies are described in Table 2 and Table S2 in File S1. Abbreviations: vWF: von Willebrand Factor, (hs)-CRP: (high sensitivity) C-reactive protein, cTnT/I: cardiac troponin T/I, (NT-pro)BNP: (amino terminal prohormone of) brain natriuretic peptide, eGFR: estimated glomerular filtration rate.
For ischemic stroke events in individuals without pre-existing cardiovascular disease, 18 meta-analyses were found. These were related to hemostasis and kidney function, being fibrinogen (HR: 1.75, 95%ci: 1.55–1.98), and serum uric acid (RR: 1.47, 95%ci: 1.19–1.76) [5], [15] (Figure 4, Table 3, Table S3 in File S1).
Figure 4. Plot of the results of meta-analyses on stroke events in populations without pre-existing CVD.
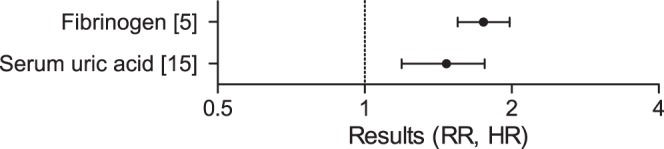
The results of a selection of meta-analyses on stroke events in populations without pre-existing CVD are graphically represented. Details of the studies are described in Table 3 and Table S3 in File S1.
Discussion
Cardiovascular disease is one of the major causes of death world-wide. Studies that evaluate the predictive value of serological biomarkers for cardiovascular disease have grown in large numbers, which has made it difficult to keep track on the overall predictive value of specific biomarkers. Meta-analyses summarize the results of different cohort studies and present one aggregate score per biomarker, but a general overview presenting the overall results of different biomarkers described in the literature is still lacking. This systematic review of meta-analyses on levels of serological biomarkers for atherothrombosis was performed to provide a comprehensive overview of the state of art, and to compare the relevance of the most commonly studied biomarkers. We conclude that for primary cardiovascular events, markers with strong predictive potential are mainly associated with lipids. For secondary cardiovascular events, markers with strong predictive potential are associated with ischemia. Fibrinogen has strong predictive potential for primary stroke.
The clinical relevance of a marker depends not only on its risk prediction strength, but also on the setup of the investigations (e.g. case-control versus cohort study). The quality of the reporting of results (e.g. reporting of adjustment for other risk factors) is another important aspect. It is attractive to use a score to assess the quality and clinical value of meta-analyses as it gives means to rank the reports. Conversely, a score to assess the quality and clinical value requires assigning weight to different factors that influence the results, which is difficult and hard to motivate. Therefore, we have abstracted aspects of the meta-analyses that may have influenced their results without assigning scores. These aspects, summarized in the methods, were adapted from Hlatky et al., 2009 and are reported in the columns of the tables. These rankings provide the reader with insight into the quality and clinical relevance of the markers.
Many of the markers listed in the tables are well known, and some are established risk markers that are applied in the clinic as a risk marker for cardiovascular disease, for example cholesterol. This review also presents markers with strong predictive value that are not used in the clinic for cardiovascular disease risk prediction, such as fibrinogen, vitamin D, and cystatin C. Such markers, which are associated with high risk but which are without clinical application in cardiovascular disease risk prediction as of yet are of special interest as these may prove to be valuable biomarkers in the future. To have clinical utility, these biomarkers should be able to predict risk independently of other established risk markers. In addition, there should be an established assay that is specific and sensitive in measuring the markers [16], [17]. The possibility to intervene therapeutically based on the levels of risk marker, associated with a reduced risk for cardiovascular disease enables the option to use it to evaluate the efficacy of a therapeutic intervention. With these aspects in mind, we will discuss the clinical utility of fibrinogen, vitamin D, and cystatin C in cardiovascular disease management.
Fibrinogen is one of the strongest markers for both predicting stroke and cardiovascular disease in populations without pre-existing cardiovascular disease. It is involved in hemostasis and blood viscosity. Moreover it is known as an acute phase reactant [18]. Age, sex and cohort corrected results remained significant for cardiovascular disease events and stroke [5].
There are 40 different assays to measure fibrinogen, and although they are reported to be relatively accurate, there is much to gain on assay standardization for overall comparability of measurements [19]. In addition, there is great variation in results between different laboratories, with concentrations ranging from 121 to 437 mg/dL for one specific sample [19]. Improvement in assay standardization would make fibrinogen an interesting biomarker.
Specific members of the fibrate class bezafibrate and clofibrate are able to lower fibrinogen levels besides improving high density lipoprotein and triglyceride levels [20]. However, they have not been shown to be of any benefit in reducing cardiovascular disease risk in relation to their fibrinogen lowering levels [21]. Lowering fibrinogen with bezafibrate also has no effect on occurrence of secondary stroke [22]. A causal relationship of high fibrinogen levels and increased cardiovascular disease risk is unclear, as only some of the polymorphisms that influence the level of fibrinogen are associated with increased cardiovascular disease risk [18]. Two genetic variants that affect the levels of fibrinogen are related to the risk for ischemic stroke, but not for myocardial infarction [23].
Low levels of vitamin D are an independent risk factor for cardiovascular death in populations without pre-existing cardiovascular disease [9]. Systematic reviews on interventional vitamin D supplementation and cardiovascular disease risk reported that vitamin D supplementation had no effect on cardiovascular disease risk, indicating a lack of a causal relationship [24], [25].
Serum vitamin D level is widely measured in diagnostic laboratories to assess vitamin D status in a number of clinical conditions such as rickets, osteomalacia, osteoporosis, hyperparathyroidism, chronic kidney disease or pregnancy [26]. The main type of assays are either competitive immunoassays, or direct detection methods with high performance liquid chromatography or liquid chromatography combined with tandem mass spectrometry [26]. There is considerable variation between the results obtained with the various methods, as well as between laboratories [26]. A standard for vitamin D measurements (SRM 972) is available to increase comparability across laboratories, but as of yet it is unclear how comparability has improved. Immunoassays are less sensitive and specific for vitamin D measurements than high performance liquid chromatography, and liquid chromatography combined with tandem mass spectrometry. The latter two techniques are less attractive in aspects of high throughput and required training of staff [26].
For secondary cardiovascular events, cystatin C is one of the strongest risk predictors. Plasma cystatin C is a marker for chronic kidney disease, a disease strongly associated with an increased risk for cardiovascular disease [27], [28]. The contribution of cystatin C in a multivariate model remains significant, which indicates its added value to established risk factors [11]. The reason for the incremental prognostic information given by cystatin C is still unknown, but it is likely to be related to the sensitivity of cystatin C to detect preclinical kidney dysfunction [28].
Because of the association of renal dysfunction with cardiovascular disease, it is unclear whether cystatin C is a direct marker of cardiovascular disease or merely a marker for renal failure, which has implications for therapeutic intervention. In addition, no therapy has been evaluated to date that aimed to treat patients for cardiovascular disease on stratification by cystatin C values [28]. Cystatin C is measured by immunoassays, using particles coated with cystatin-C specific antibodies, and subsequent turbidometry or nephelometry [29]. The assays are precise, as both detection methods provide coeffients of variation ranging from 2 to 8% [30].
This systematic review is subject to some limitations. This review has included only meta-analyses, so the novelty of reported markers is limited. Also, risk markers are absent in this review when they have not been included in a meta-analysis. Some of the meta-analyses are smaller in size than some single cohort studies. The advantage of a meta-analysis compared with a single large cohort study is that the results represent the ability of a marker to predict events in different cohorts, which increases reliability. Heterogeneity among the meta-analyses exists also in the adjustment for other prognostic factors, and in the methods used to pool the results. This limits the comparability of the different risk markers. Lastly, there is no widely acknowledged search strategy, neither for prognostic studies, nor for meta-analyses. We therefore have applied a broad search strategy, but still some meta-analyses may have been missed.
With cardiovascular disease being one of the major causes of death worldwide, there is an ongoing need for new biomarkers that are able to assist in clinical decision making. Markers such as fibrinogen, vitamin D, and cystatin C have a strong association with cardiovascular disease but as of yet have not been implemented in the clinic. Other emerging types of biomarkers for cardiovascular disease risk prediction may prove their value in the future. A novel initiative in cardiovascular risk prediction is the Circulating Cells Consortium that investigates the information present in circulating cells such as platelets and leukocytes in relation to cardiovascular disease events. As these cells interact with the vessel wall, their responsiveness may convey clinical relevant information on cardiovascular disease risk.
Supporting Information
Tables S1–S9.
(DOC)
Funding Statement
This research was performed within the framework of CTMM, the Center for Translational Molecular Medicine (www.ctmm.nl), project CIRCULATING CELLS (grant 01C-102), and supported by the Netherlands Heart Foundation. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Alwan A, Armstrong T, Bettcher D, Branca F, Chisholm D, et al. (2011) Burden: mortality, morbidity and risk factors. Global Status Report on Noncommunicable Diseases 2010: 9–32. [Google Scholar]
- 2. Oxman AD, Cook DJ, Guyatt GH (1994) Users' guides to the medical literature. VI. How to use an overview. Evidence-Based Medicine Working Group. Jama 272: 1367–1371. [DOI] [PubMed] [Google Scholar]
- 3.Moher D, Liberati A, Tetzlaff J, Altman DG (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med 151: 264–269, W264. [DOI] [PubMed]
- 4. Hlatky MA, Greenland P, Arnett DK, Ballantyne CM, Criqui MH, et al. (2009) Criteria for evaluation of novel markers of cardiovascular risk: a scientific statement from the American Heart Association. Circulation 119: 2408–2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Danesh J, Lewington S, Thompson SG, Lowe GD, Collins R, et al. (2005) Plasma fibrinogen level and the risk of major cardiovascular diseases and nonvascular mortality: an individual participant meta-analysis. Jama 294: 1799–1809. [DOI] [PubMed] [Google Scholar]
- 6. Shah T, Casas JP, Cooper JA, Tzoulaki I, Sofat R, et al. (2009) Critical appraisal of CRP measurement for the prediction of coronary heart disease events: new data and systematic review of 31 prospective cohorts. Int J Epidemiol 38: 217–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Thompson A, Danesh J (2006) Associations between apolipoprotein B, apolipoprotein AI, the apolipoprotein B/AI ratio and coronary heart disease: a literature-based meta-analysis of prospective studies. J Intern Med 259: 481–492. [DOI] [PubMed] [Google Scholar]
- 8. Lewington S, Whitlock G, Clarke R, Sherliker P, Emberson J, et al. (2007) Blood cholesterol and vascular mortality by age, sex, and blood pressure: a meta-analysis of individual data from 61 prospective studies with 55,000 vascular deaths. Lancet 370: 1829–1839. [DOI] [PubMed] [Google Scholar]
- 9. Grandi NC, Breitling LP, Brenner H (2010) Vitamin D and cardiovascular disease: systematic review and meta-analysis of prospective studies. Prev Med 51: 228–233. [DOI] [PubMed] [Google Scholar]
- 10. Hemingway H, Philipson P, Chen R, Fitzpatrick NK, Damant J, et al. (2010) Evaluating the quality of research into a single prognostic biomarker: a systematic review and meta-analysis of 83 studies of C-reactive protein in stable coronary artery disease. PLoS Med 7: e1000286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lee M, Saver JL, Huang WH, Chow J, Chang KH, et al. (2010) Impact of elevated cystatin C level on cardiovascular disease risk in predominantly high cardiovascular risk populations: a meta-analysis. Circ Cardiovasc Qual Outcomes 3: 675–683. [DOI] [PubMed] [Google Scholar]
- 12. Ottani F, Galvani M, Nicolini FA, Ferrini D, Pozzati A, et al. (2000) Elevated cardiac troponin levels predict the risk of adverse outcome in patients with acute coronary syndromes. Am Heart J 140: 917–927. [DOI] [PubMed] [Google Scholar]
- 13. Padayachee L, Rodseth RN, Biccard BM (2009) A meta-analysis of the utility of C-reactive protein in predicting early, intermediate-term and long term mortality and major adverse cardiac events in vascular surgical patients. Anaesthesia 64: 416–424. [DOI] [PubMed] [Google Scholar]
- 14. van der Velde M, Matsushita K, Coresh J, Astor BC, Woodward M, et al. (2011) Lower estimated glomerular filtration rate and higher albuminuria are associated with all-cause and cardiovascular mortality. A collaborative meta-analysis of high-risk population cohorts. Kidney Int 79: 1341–1352. [DOI] [PubMed] [Google Scholar]
- 15. Kim SY, Guevara JP, Kim KM, Choi HK, Heitjan DF, et al. (2009) Hyperuricemia and risk of stroke: a systematic review and meta-analysis. Arthritis Rheum 61: 885–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Libby P, Ridker PM (1999) Novel inflammatory markers of coronary risk: theory versus practice. Circulation 100: 1148–1150. [DOI] [PubMed] [Google Scholar]
- 17. Ridker PM (1999) Evaluating novel cardiovascular risk factors: can we better predict heart attacks? Ann Intern Med 130: 933–937. [DOI] [PubMed] [Google Scholar]
- 18. Tousoulis D, Papageorgiou N, Androulakis E, Briasoulis A, Antoniades C, et al. (2011) Fibrinogen and cardiovascular disease: genetics and biomarkers. Blood Rev 25: 239–245. [DOI] [PubMed] [Google Scholar]
- 19.Cushman M, Ballantyne CM, Levy D, Rifai N, Cooper GR, et al.. (2009) Inflammation Biomarkers and Cardiovascular Disease Risk. In: Meyers GL, editor. Emerging Biomarkers for Primary Prevention of Cardiovascular Disease and Stroke: The National Academy of Clinical Biochemistry. pp. 7–24.
- 20. Maison P, Mennen L, Sapinho D, Balkau B, Sigalas J, et al. (2002) A pharmacoepidemiological assessment of the effect of statins and fibrates on fibrinogen concentration. Atherosclerosis 160: 155–160. [DOI] [PubMed] [Google Scholar]
- 21. The BIP Study Group (2000) Secondary prevention by raising HDL cholesterol and reducing triglycerides in patients with coronary artery disease: the Bezafibrate Infarction Prevention (BIP) study. Circulation 102: 21–27. [DOI] [PubMed] [Google Scholar]
- 22. Tanne D, Benderly M, Goldbourt U, Boyko V, Brunner D, et al. (2001) A prospective study of plasma fibrinogen levels and the risk of stroke among participants in the bezafibrate infarction prevention study. Am J Med 111: 457–463. [DOI] [PubMed] [Google Scholar]
- 23. Siegerink B, Rosendaal FR, Algra A (2009) Genetic variation in fibrinogen; its relationship to fibrinogen levels and the risk of myocardial infarction and ischemic stroke. J Thromb Haemost 7: 385–390. [DOI] [PubMed] [Google Scholar]
- 24. Elamin MB, Abu Elnour NO, Elamin KB, Fatourechi MM, Alkatib AA, et al. (2011) Vitamin D and cardiovascular outcomes: a systematic review and meta-analysis. J Clin Endocrinol Metab 96: 1931–1942. [DOI] [PubMed] [Google Scholar]
- 25. Wang L, Manson JE, Song Y, Sesso HD (2010) Systematic review: Vitamin D and calcium supplementation in prevention of cardiovascular events. Ann Intern Med 152: 315–323. [DOI] [PubMed] [Google Scholar]
- 26. Wallace AM, Gibson S, de la Hunty A, Lamberg-Allardt C, Ashwell M (2010) Measurement of 25-hydroxyvitamin D in the clinical laboratory: current procedures, performance characteristics and limitations. Steroids 75: 477–488. [DOI] [PubMed] [Google Scholar]
- 27. Fassett RG, Venuthurupalli SK, Gobe GC, Coombes JS, Cooper MA, et al. (2011) Biomarkers in chronic kidney disease: a review. Kidney Int 80: 806–821. [DOI] [PubMed] [Google Scholar]
- 28. Taglieri N, Koenig W, Kaski JC (2009) Cystatin C and cardiovascular risk. Clin Chem 55: 1932–1943. [DOI] [PubMed] [Google Scholar]
- 29.Myers GL (2009) Markers of Renal Function and Cardiovascular Disease Risk. In: Meyers GL, editor. Laboratory Medicine Practice Guidelines Emerging Biomarkers for Primary Prevention of Cardiovascular Disease and Stroke: The National Academy of Clinical Biochemistry. 43–50. [DOI] [PubMed]
- 30. Laterza OF, Price CP, Scott MG (2002) Cystatin C: an improved estimator of glomerular filtration rate? Clin Chem 48: 699–707. [PubMed] [Google Scholar]
- 31. Coutinho M, Gerstein HC, Wang Y, Yusuf S (1999) The relationship between glucose and incident cardiovascular events. A metaregression analysis of published data from 20 studies of 95,783 individuals followed for 12.4 years. Diabetes Care 22: 233–240. [DOI] [PubMed] [Google Scholar]
- 32. Santos-Oliveira R, Purdy C, da Silva MP, dos Anjos Carneiro-Leao AM, Machado M, et al. (2011) Haemoglobin A1c levels and subsequent cardiovascular disease in persons without diabetes: a meta-analysis of prospective cohorts. Diabetologia 54: 1327–1334. [DOI] [PubMed] [Google Scholar]
- 33. Danesh J, Wheeler JG, Hirschfield GM, Eda S, Eiriksdottir G, et al. (2004) C-reactive protein and other circulating markers of inflammation in the prediction of coronary heart disease. N Engl J Med 350: 1387–1397. [DOI] [PubMed] [Google Scholar]
- 34. Sarwar N, Danesh J, Eiriksdottir G, Sigurdsson G, Wareham N, et al. (2007) Triglycerides and the risk of coronary heart disease: 10,158 incident cases among 262,525 participants in 29 Western prospective studies. Circulation 115: 450–458. [DOI] [PubMed] [Google Scholar]
- 35. Di Angelantonio E, Sarwar N, Perry P, Kaptoge S, Ray KK, et al. (2009) Major lipids, apolipoproteins, and risk of vascular disease. Jama 302: 1993–2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Whincup PH, Danesh J, Walker M, Lennon L, Thomson A, et al. (2002) von Willebrand factor and coronary heart disease: prospective study and meta-analysis. Eur Heart J 23: 1764–1770. [DOI] [PubMed] [Google Scholar]
- 37. Danesh J, Whincup P, Walker M, Lennon L, Thomson A, et al. (2000) Low grade inflammation and coronary heart disease: prospective study and updated meta-analyses. Bmj 321: 199–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Rodseth RN, Lurati Buse GA, Bolliger D, Burkhart CS, Cuthbertson BH, et al. (2011) The predictive ability of pre-operative B-type natriuretic peptide in vascular patients for major adverse cardiac events: an individual patient data meta-analysis. J Am Coll Cardiol 58: 522–529. [DOI] [PubMed] [Google Scholar]
- 39.Heidenreich PA, Go A, Melsop KA, Alloggiamento T, McDonald KM, et al.. (2000) Prediction of risk for patients with unstable angina. Evid Rep Technol Assess (Summ): 1–3. [PMC free article] [PubMed]
- 40. Fleming SM, Daly KM (2001) Cardiac troponins in suspected acute coronary syndrome: a meta-analysis of published trials. Cardiology 95: 66–73. [DOI] [PubMed] [Google Scholar]
- 41. Wu AH, Lane PL (1995) Metaanalysis in clinical chemistry: validation of cardiac troponin T as a marker for ischemic heart diseases. Clin Chem 41: 1228–1233. [PubMed] [Google Scholar]
- 42. Olatidoye AG, Wu AH, Feng YJ, Waters D (1998) Prognostic role of troponin T versus troponin I in unstable angina pectoris for cardiac events with meta-analysis comparing published studies. Am J Cardiol 81: 1405–1410. [DOI] [PubMed] [Google Scholar]
- 43. Heidenreich PA, Alloggiamento T, Melsop K, McDonald KM, Go AS, et al. (2001) The prognostic value of troponin in patients with non-ST elevation acute coronary syndromes: a meta-analysis. J Am Coll Cardiol 38: 478–485. [DOI] [PubMed] [Google Scholar]
- 44. Wu AH, Boden WE, McKay RG (2002) Long-term follow-up of patients with increased cardiac troponin concentrations following percutaneous coronary intervention. Am J Cardiol 89: 1300–1302. [DOI] [PubMed] [Google Scholar]
- 45. Feldman DN, Kim L, Rene AG, Minutello RM, Bergman G, et al. (2011) Prognostic value of cardiac troponin-I or troponin-T elevation following nonemergent percutaneous coronary intervention: a meta-analysis. Catheter Cardiovasc Interv 77: 1020–1030. [DOI] [PubMed] [Google Scholar]
- 46. Nienhuis MB, Ottervanger JP, Bilo HJ, Dikkeschei BD, Zijlstra F (2008) Prognostic value of troponin after elective percutaneous coronary intervention: A meta-analysis. Catheter Cardiovasc Interv 71: 318–324. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S9.
(DOC)


