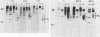Abstract
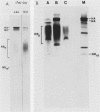
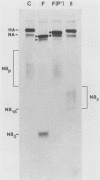
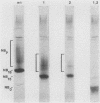
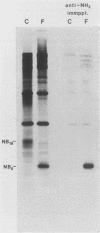
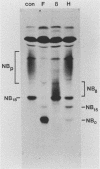
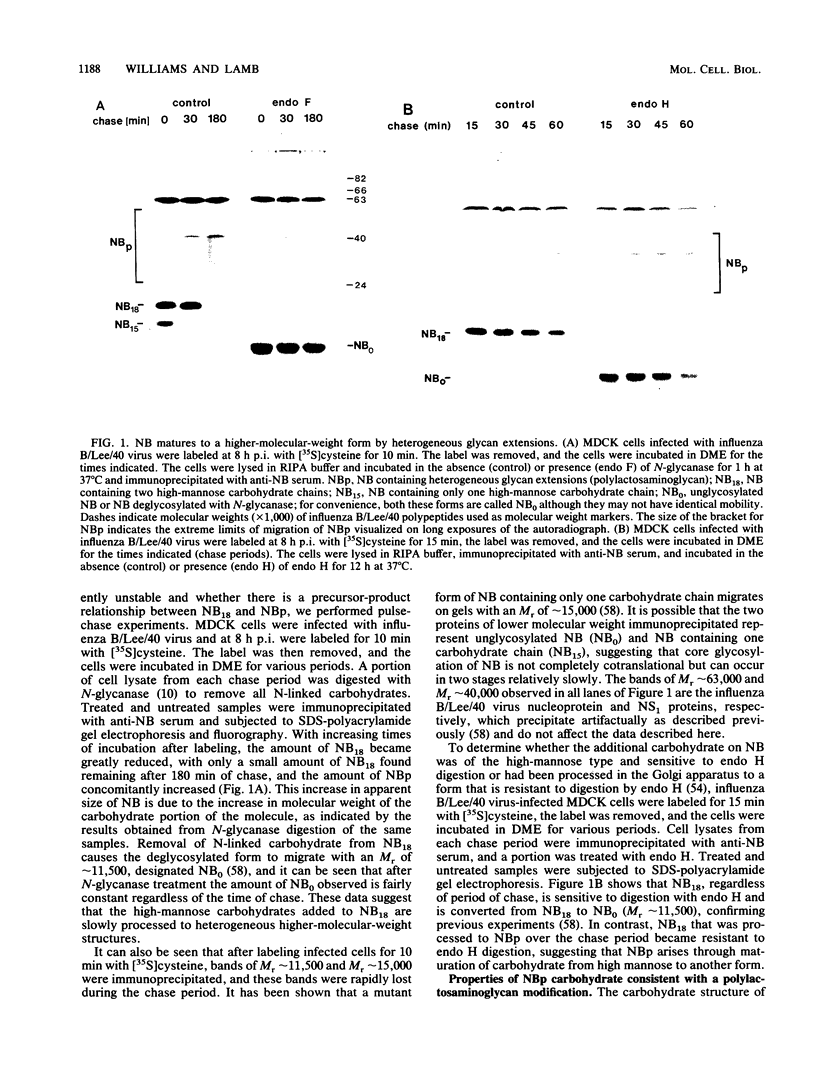
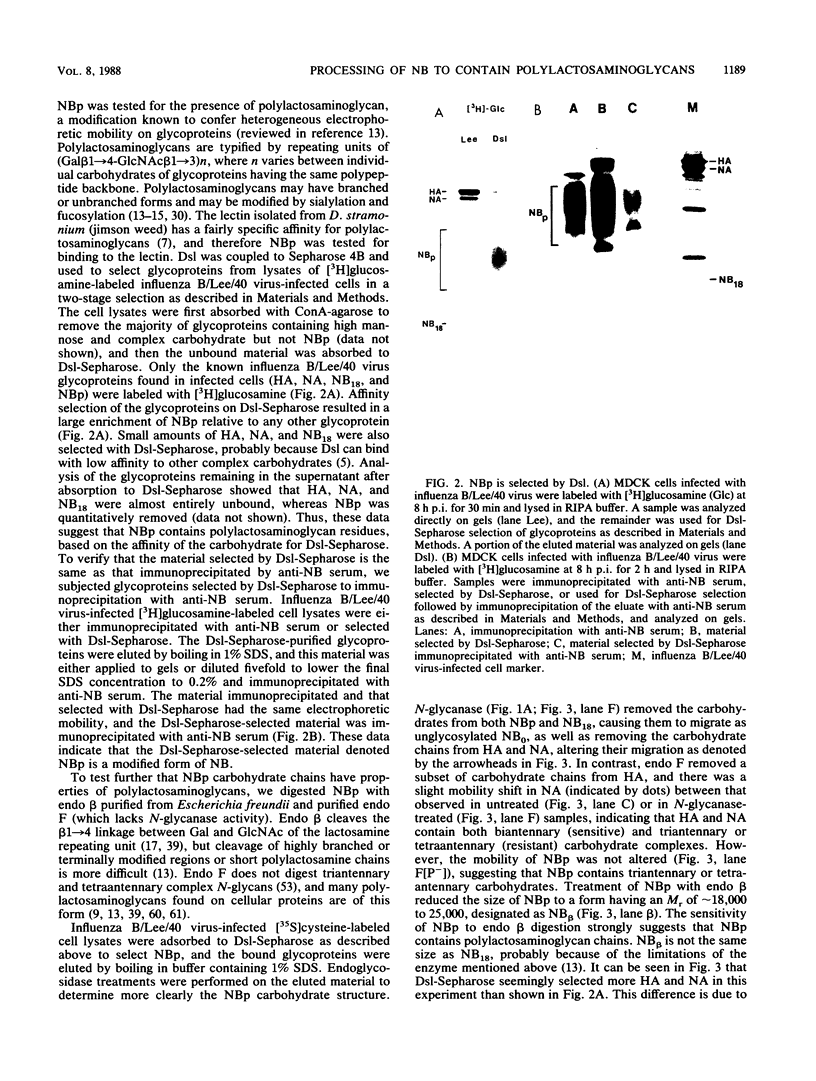
The structure of the carbohydrate components of NB, the small integral membrane glycoprotein of influenza B virus, was investigated. The carbohydrate chains of NB are processed from the high-mannose form (NB18) to a heterogeneous form of much higher molecular weight, designated NBp. Selection of this carbohydrate-containing form of NB with Datura stramonium lectin, its susceptibility to digestion by endo-beta-galactosidase, and determination of the size of NBp glycopeptides by gel filtration chromatography suggested that the increase in molecular weight is due to processing to polylactosaminoglycan. Investigation of the polypeptides produced by influenza B/Lee/40 virus infection of several cell types and another strain of influenza B virus suggested that the signal for modification to polylactosaminoglycan is contained in NB. Expression of mutants of NB lacking either one or both of the normal N-terminal sites of asparagine-linked glycosylation indicated that both carbohydrate chains are modified to contain polylactosaminoglycan. NBp and a small amount of unprocessed NB18 are expressed at the infected-cell surface, as determined by digestion of the surfaces of intact cells with various endoglycosidases. Unglycosylated NB, expressed either in influenza B virus-infected cells treated with tunicamycin or in cells expressing the NB mutant lacking both N-linked glycosylation sites, was expressed at the cell surface, indicating that NB does not require carbohydrate addition for transport.
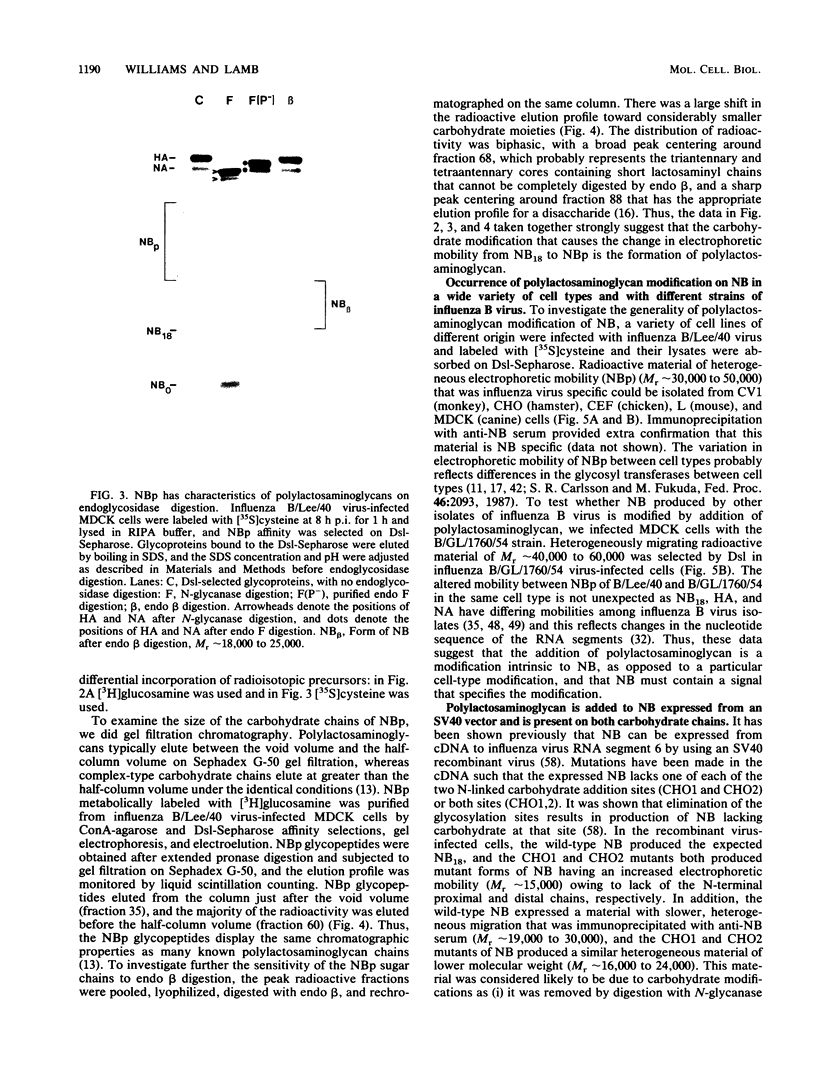
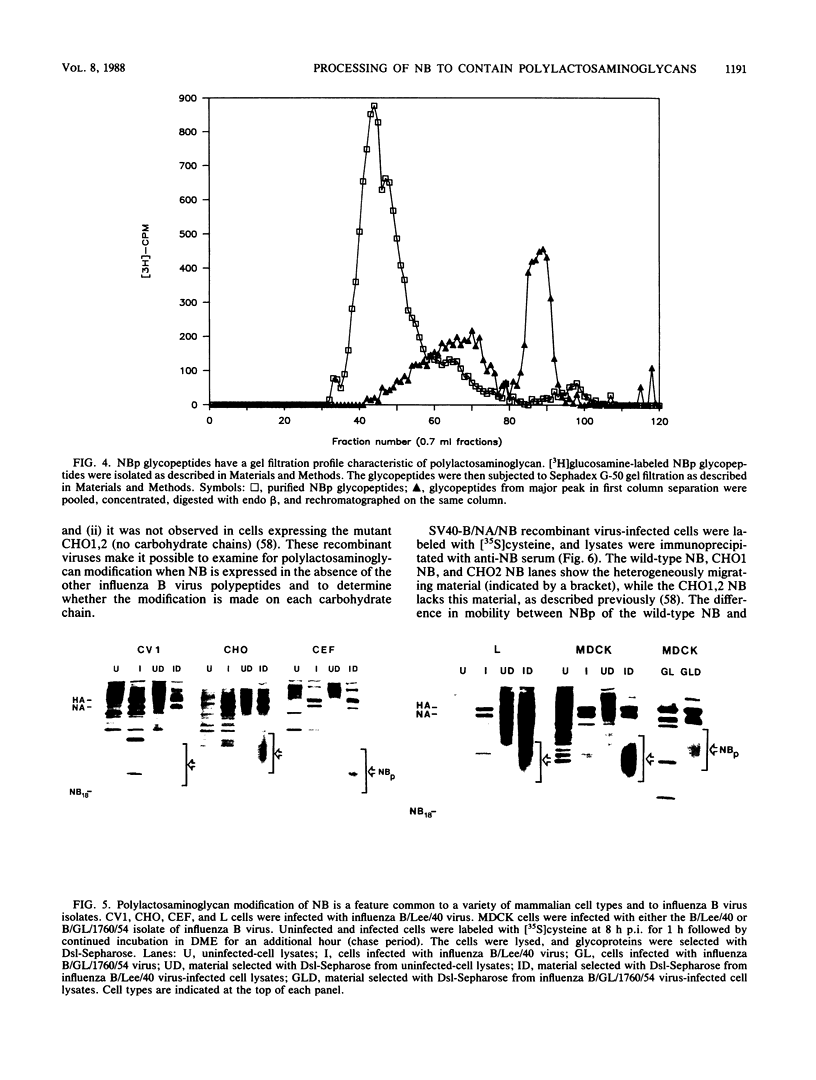
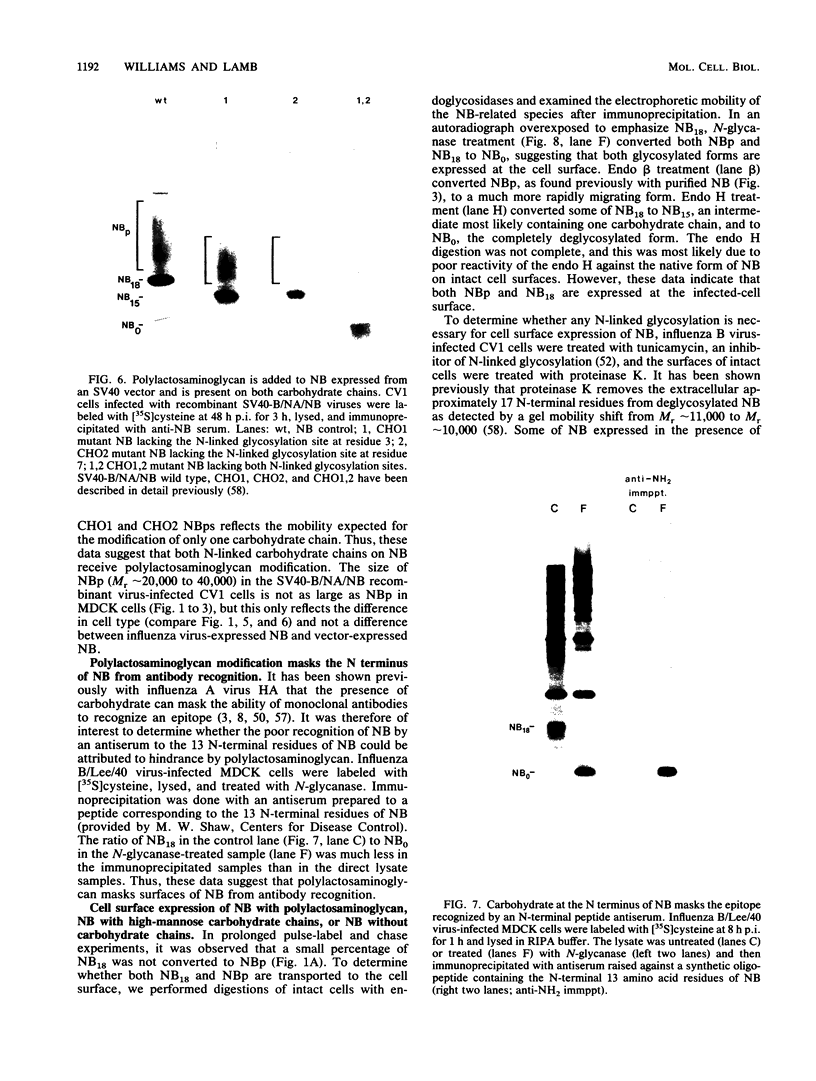
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bassiri M., Privalsky M. L. Mutagenesis of the avian erythroblastosis virus erbB coding region: an intact extracellular domain is not required for oncogenic transformation. J Virol. 1986 Aug;59(2):525–530. doi: 10.1128/jvi.59.2.525-530.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brock C. J., Tanner M. J., Kempf C. The human erythrocyte anion-transport protein. Partial amino acid sequence, conformation and a possible molecular mechanism for anion exchange. Biochem J. 1983 Sep 1;213(3):577–586. doi: 10.1042/bj2130577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caton A. J., Brownlee G. G., Yewdell J. W., Gerhard W. The antigenic structure of the influenza virus A/PR/8/34 hemagglutinin (H1 subtype). Cell. 1982 Dec;31(2 Pt 1):417–427. doi: 10.1016/0092-8674(82)90135-0. [DOI] [PubMed] [Google Scholar]
- Copeland C. S., Doms R. W., Bolzau E. M., Webster R. G., Helenius A. Assembly of influenza hemagglutinin trimers and its role in intracellular transport. J Cell Biol. 1986 Oct;103(4):1179–1191. doi: 10.1083/jcb.103.4.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowley J. F., Goldstein I. J., Arnarp J., Lönngren J. Carbohydrate binding studies on the lectin from Datura stramonium seeds. Arch Biochem Biophys. 1984 Jun;231(2):524–533. doi: 10.1016/0003-9861(84)90417-x. [DOI] [PubMed] [Google Scholar]
- Cummings R. D., Kornfeld S. Fractionation of asparagine-linked oligosaccharides by serial lectin-Agarose affinity chromatography. A rapid, sensitive, and specific technique. J Biol Chem. 1982 Oct 10;257(19):11235–11240. [PubMed] [Google Scholar]
- Cummings R. D., Kornfeld S. The distribution of repeating [Gal beta 1,4GlcNAc beta 1,3] sequences in asparagine-linked oligosaccharides of the mouse lymphoma cell lines BW5147 and PHAR 2.1. J Biol Chem. 1984 May 25;259(10):6253–6260. [PubMed] [Google Scholar]
- Eckhardt A. E., Goldstein I. J. Isolation and characterization of a family of alpha-D-galactosyl-containing glycopeptides from Ehrlich ascites tumor cells. Biochemistry. 1983 Nov 8;22(23):5290–5297. doi: 10.1021/bi00292a007. [DOI] [PubMed] [Google Scholar]
- Elder J. H., Alexander S. endo-beta-N-acetylglucosaminidase F: endoglycosidase from Flavobacterium meningosepticum that cleaves both high-mannose and complex glycoproteins. Proc Natl Acad Sci U S A. 1982 Aug;79(15):4540–4544. doi: 10.1073/pnas.79.15.4540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etchison J. R., Holland J. J. Carbohydrate composition of the membrane glycoprotein of vesicular stomatitis virus grown in four mammalian cell lines. Proc Natl Acad Sci U S A. 1974 Oct;71(10):4011–4014. doi: 10.1073/pnas.71.10.4011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda M. N., Fukuda M., Hakomori S. Cell surface modification by endo-beta-galactosidase. Change of blood group activities and release of oligosaccharides from glycoproteins and glycosphingolipids of human erythrocytes. J Biol Chem. 1979 Jun 25;254(12):5458–5465. [PubMed] [Google Scholar]
- Fukuda M., Bothner B., Ramsamooj P., Dell A., Tiller P. R., Varki A., Klock J. C. Structures of sialylated fucosyl polylactosaminoglycans isolated from chronic myelogenous leukemia cells. J Biol Chem. 1985 Oct 25;260(24):12957–12967. [PubMed] [Google Scholar]
- Fukuda M. Cell surface glycoconjugates as onco-differentiation markers in hematopoietic cells. Biochim Biophys Acta. 1985;780(2):119–150. doi: 10.1016/0304-419x(84)90002-7. [DOI] [PubMed] [Google Scholar]
- Fukuda M., Dell A., Fukuda M. N. Structure of fetal lactosaminoglycan. The carbohydrate moiety of Band 3 isolated from human umbilical cord erythrocytes. J Biol Chem. 1984 Apr 25;259(8):4782–4791. [PubMed] [Google Scholar]
- Fukuda M., Dell A., Oates J. E., Fukuda M. N. Structure of branched lactosaminoglycan, the carbohydrate moiety of band 3 isolated from adult human erythrocytes. J Biol Chem. 1984 Jul 10;259(13):8260–8273. [PubMed] [Google Scholar]
- Fukuda M., Fukuda M. N., Hakomori S. Developmental change and genetic defect in the carbohydrate structure of band 3 glycoprotein of human erythrocyte membrane. J Biol Chem. 1979 May 25;254(10):3700–3703. [PubMed] [Google Scholar]
- Gahmberg C. G., Jokinen M., Karhi K. K., Andersson L. C. Effect of tunicamycin on the biosynthesis of the major human red cell sialoglycoprotein, glycophorin A, in the leukemia cell line K562. J Biol Chem. 1980 Mar 10;255(5):2169–2175. [PubMed] [Google Scholar]
- Gething M. J., McCammon K., Sambrook J. Expression of wild-type and mutant forms of influenza hemagglutinin: the role of folding in intracellular transport. Cell. 1986 Sep 12;46(6):939–950. doi: 10.1016/0092-8674(86)90076-0. [DOI] [PubMed] [Google Scholar]
- Guan J. L., Machamer C. E., Rose J. K. Glycosylation allows cell-surface transport of an anchored secretory protein. Cell. 1985 Sep;42(2):489–496. doi: 10.1016/0092-8674(85)90106-0. [DOI] [PubMed] [Google Scholar]
- Hannink M., Donoghue D. J. Cell surface expression of membrane-anchored v-sis gene products: glycosylation is not required for cell surface transport. J Cell Biol. 1986 Dec;103(6 Pt 1):2311–2322. doi: 10.1083/jcb.103.6.2311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannink M., Sauer M. K., Donoghue D. J. Deletions in the C-terminal coding region of the v-sis gene: dimerization is required for transformation. Mol Cell Biol. 1986 Apr;6(4):1304–1314. doi: 10.1128/mcb.6.4.1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickman S., Kornfeld S. Effect of tunicamycin on IgM, IgA, and IgG secretion by mouse plasmacytoma cells. J Immunol. 1978 Sep;121(3):990–996. [PubMed] [Google Scholar]
- Jennings M. L., Adams-Lackey M., Denney G. H. Peptides of human erythrocyte band 3 protein produced by extracellular papain cleavage. J Biol Chem. 1984 Apr 10;259(7):4652–4660. [PubMed] [Google Scholar]
- Jennings M. L., Nicknish J. S. Erythrocyte band 3 protein: evidence for multiple membrane-crossing segments in the 17 000-dalton chymotryptic fragment. Biochemistry. 1984 Dec 18;23(26):6432–6436. doi: 10.1021/bi00321a024. [DOI] [PubMed] [Google Scholar]
- Keller R. K., Swank G. D. Tunicamycin does not block ovalbumin secretion in the oviduct. Biochem Biophys Res Commun. 1978 Nov 29;85(2):762–768. doi: 10.1016/0006-291x(78)91226-3. [DOI] [PubMed] [Google Scholar]
- Kopito R. R., Lodish H. F. Primary structure and transmembrane orientation of the murine anion exchange protein. Nature. 1985 Jul 18;316(6025):234–238. doi: 10.1038/316234a0. [DOI] [PubMed] [Google Scholar]
- Kornfeld R., Kornfeld S. Assembly of asparagine-linked oligosaccharides. Annu Rev Biochem. 1985;54:631–664. doi: 10.1146/annurev.bi.54.070185.003215. [DOI] [PubMed] [Google Scholar]
- Lamb R. A., Choppin P. W. Segment 8 of the influenza virus genome is unique in coding for two polypeptides. Proc Natl Acad Sci U S A. 1979 Oct;76(10):4908–4912. doi: 10.1073/pnas.76.10.4908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb R. A., Choppin P. W. The synthesis of Sendai virus polypeptides in infected cells. III. Phosphorylation of polypeptides. Virology. 1977 Sep;81(2):382–397. doi: 10.1016/0042-6822(77)90154-4. [DOI] [PubMed] [Google Scholar]
- Lamb R. A., Etkind P. R., Choppin P. W. Evidence for a ninth influenza viral polypeptide. Virology. 1978 Nov;91(1):60–78. doi: 10.1016/0042-6822(78)90355-0. [DOI] [PubMed] [Google Scholar]
- Lamb R. A., Lai C. J. Spliced and unspliced messenger RNAs synthesized from cloned influenza virus M DNA in an SV40 vector: expression of the influenza virus membrane protein (M1). Virology. 1982 Dec;123(2):237–256. doi: 10.1016/0042-6822(82)90258-6. [DOI] [PubMed] [Google Scholar]
- Lamb R. A., Zebedee S. L., Richardson C. D. Influenza virus M2 protein is an integral membrane protein expressed on the infected-cell surface. Cell. 1985 Mar;40(3):627–633. doi: 10.1016/0092-8674(85)90211-9. [DOI] [PubMed] [Google Scholar]
- Li E., Gibson R., Kornfeld S. Structure of an unusual complex-type oligosaccharide isolated from Chinese hamster ovary cells. Arch Biochem Biophys. 1980 Feb;199(2):393–399. doi: 10.1016/0003-9861(80)90295-7. [DOI] [PubMed] [Google Scholar]
- Machamer C. E., Florkiewicz R. Z., Rose J. K. A single N-linked oligosaccharide at either of the two normal sites is sufficient for transport of vesicular stomatitis virus G protein to the cell surface. Mol Cell Biol. 1985 Nov;5(11):3074–3083. doi: 10.1128/mcb.5.11.3074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K., Compans R. W. Effects of glucosamine, 2-deoxyglucose, and tunicamycin on glycosylation, sulfation, and assembly of influenza viral proteins. Virology. 1978 Feb;84(2):303–319. doi: 10.1016/0042-6822(78)90250-7. [DOI] [PubMed] [Google Scholar]
- Nakamura K., Compans R. W. Host cell- and virus strain-dependent differences in oligosaccharides of hemagglutinin glycoproteins of influenza A viruses. Virology. 1979 May;95(1):8–23. doi: 10.1016/0042-6822(79)90397-0. [DOI] [PubMed] [Google Scholar]
- Parekh R. B., Tse A. G., Dwek R. A., Williams A. F., Rademacher T. W. Tissue-specific N-glycosylation, site-specific oligosaccharide patterns and lentil lectin recognition of rat Thy-1. EMBO J. 1987 May;6(5):1233–1244. doi: 10.1002/j.1460-2075.1987.tb02359.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollack L., Atkinson P. H. Correlation of glycosylation forms with position in amino acid sequence. J Cell Biol. 1983 Aug;97(2):293–300. doi: 10.1083/jcb.97.2.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth M. G., Fitzpatrick J. P., Compans R. W. Polarity of influenza and vesicular stomatitis virus maturation in MDCK cells: lack of a requirement for glycosylation of viral glycoproteins. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6430–6434. doi: 10.1073/pnas.76.12.6430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabban E., Marchesi V., Adesnik M., Sabatini D. D. Erythrocyte membrane protein band 3: its biosynthesis and incorporation into membranes. J Cell Biol. 1981 Dec;91(3 Pt 1):637–646. doi: 10.1083/jcb.91.3.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schatzman R. C., Evan G. I., Privalsky M. L., Bishop J. M. Orientation of the v-erb-B gene product in the plasma membrane. Mol Cell Biol. 1986 Apr;6(4):1329–1333. doi: 10.1128/mcb.6.4.1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw M. W., Choppin P. W., Lamb R. A. A previously unrecognized influenza B virus glycoprotein from a bicistronic mRNA that also encodes the viral neuraminidase. Proc Natl Acad Sci U S A. 1983 Aug;80(16):4879–4883. doi: 10.1073/pnas.80.16.4879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw M. W., Choppin P. W. Studies on the synthesis of the influenza V virus NB glycoprotein. Virology. 1984 Nov;139(1):178–184. doi: 10.1016/0042-6822(84)90338-6. [DOI] [PubMed] [Google Scholar]
- Skehel J. J., Stevens D. J., Daniels R. S., Douglas A. R., Knossow M., Wilson I. A., Wiley D. C. A carbohydrate side chain on hemagglutinins of Hong Kong influenza viruses inhibits recognition by a monoclonal antibody. Proc Natl Acad Sci U S A. 1984 Mar;81(6):1779–1783. doi: 10.1073/pnas.81.6.1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Struck D. K., Siuta P. B., Lane M. D., Lennarz W. J. Effect of tunicamycin on the secretion of serum proteins by primary cultures of rat and chick hepatocytes. Studies on transferrin, very low density lipoprotein, and serum albumin. J Biol Chem. 1978 Aug 10;253(15):5332–5337. [PubMed] [Google Scholar]
- Tarentino A. L., Gómez C. M., Plummer T. H., Jr Deglycosylation of asparagine-linked glycans by peptide:N-glycosidase F. Biochemistry. 1985 Aug 13;24(17):4665–4671. doi: 10.1021/bi00338a028. [DOI] [PubMed] [Google Scholar]
- Tarentino A. L., Maley F. Purification and properties of an endo-beta-N-acetylglucosaminidase from Streptomyces griseus. J Biol Chem. 1974 Feb 10;249(3):811–817. [PubMed] [Google Scholar]
- Wickner W. T., Lodish H. F. Multiple mechanisms of protein insertion into and across membranes. Science. 1985 Oct 25;230(4724):400–407. doi: 10.1126/science.4048938. [DOI] [PubMed] [Google Scholar]
- Wiley D. C., Wilson I. A., Skehel J. J. Structural identification of the antibody-binding sites of Hong Kong influenza haemagglutinin and their involvement in antigenic variation. Nature. 1981 Jan 29;289(5796):373–378. doi: 10.1038/289373a0. [DOI] [PubMed] [Google Scholar]
- Williams M. A., Lamb R. A. Determination of the orientation of an integral membrane protein and sites of glycosylation by oligonucleotide-directed mutagenesis: influenza B virus NB glycoprotein lacks a cleavable signal sequence and has an extracellular NH2-terminal region. Mol Cell Biol. 1986 Dec;6(12):4317–4328. doi: 10.1128/mcb.6.12.4317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winterburn P. J., Phelps C. F. The significance of glycosylated proteins. Nature. 1972 Mar 24;236(5343):147–151. doi: 10.1038/236147a0. [DOI] [PubMed] [Google Scholar]
- Yamashita K., Ohkura T., Tachibana Y., Takasaki S., Kobata A. Comparative study of the oligosaccharides released from baby hamster kidney cells and their polyoma transformant by hydrazinolysis. J Biol Chem. 1984 Sep 10;259(17):10834–10840. [PubMed] [Google Scholar]
- Yoshima H., Takasaki S., Kobata A. The asparagine-linked sugar chains of the glycoproteins in calf thymocyte plasma membrane. Structural studies of acidic oligosaccharides. J Biol Chem. 1980 Nov 25;255(22):10793–10804. [PubMed] [Google Scholar]
- Zebedee S. L., Richardson C. D., Lamb R. A. Characterization of the influenza virus M2 integral membrane protein and expression at the infected-cell surface from cloned cDNA. J Virol. 1985 Nov;56(2):502–511. doi: 10.1128/jvi.56.2.502-511.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Figura K., Hasilik A. Lysosomal enzymes and their receptors. Annu Rev Biochem. 1986;55:167–193. doi: 10.1146/annurev.bi.55.070186.001123. [DOI] [PubMed] [Google Scholar]